Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The gastrointestinal (GI) tract must maintain a barrier to food antigens and the microbial communities within the gut, while continually producing an array of specialized cell types to carry out digestive, absorptive, and regulatory functions unique to each regional segment. To fulfil this diversity of roles, the intestinal epithelium has evolved to maximize epithelial surface area through the formation of villi, which are finger-like projections that protrude into the intestinal lumen. The villi are covered in epithelial cells that take part in nutrient absorption and innate defense. Given the high burden placed on the intestinal epithelium, it is replaced rapidly, with the entire epithelial surface turning over approximately every 3–5 days. To accommodate this rapid turnover, the intestine possesses a specialized compartment at the base of the villi called the crypt of Lieberkühn, which houses highly proliferative stem cells at its base and transit-amplifying progenitor cells closer to the lumen. Cells within the intestine are born and die in a very ordered manner, when the intestinal stem cell (ISC) divides, one of the daughters moves luminally toward the villus and becomes a rapidly proliferating transit-amplifying cell, followed by terminal differentiation near the crypt opening. Subsequently, differentiated cells continue to move toward the villus tip like a conveyer belt until they undergo programmed detachment and cell death as they are shed into the lumen ( Fig. 7.1 ). The architecture of intestinal crypts sequesters and protects stem cells from gut microbe-derived metabolites that otherwise might inhibit stem cell proliferation. It has been speculated that crypts may have coevolved with microbial communities in our intestine as a way to physically protect stem cells from microbial metabolites that may be detrimental to ISC function.
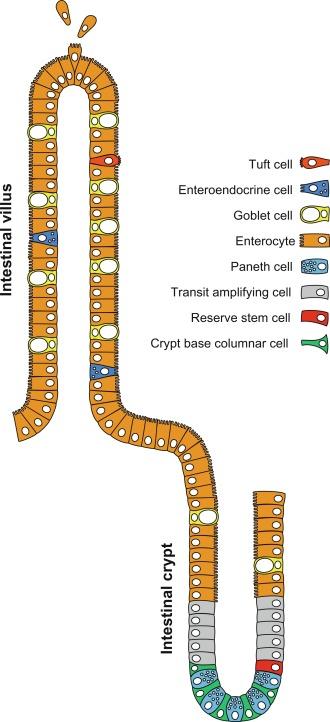
Somatic stem cells, such as those found in the intestine, are required to both maintain normal tissue homeostasis and to repair tissue following injury or damage. In order for any cell to be considered a stem cell, two key requirements must be met: the stem cell must self-renew throughout life; and, it must give rise to daughter cells which can give rise to differentiated, specialized cell types. Stem cells and their progeny are frequently described as following a hierarchy in which stem cells sit at the top, where they undergo self-renewal, giving rise to highly proliferative transit-amplifying progenitor cells, each of which can then differentiate into one of multiple cell lineages. However, as in most biological systems, the intestine does not perfectly conform to the stereotype, and a remarkable amount of plasticity has been demonstrated within the crypt. Normal epithelial homeostasis in the intestine is maintained by a population of highly proliferative cells called the “crypt base columnar” (CBC) stem cells and the hierarchical model of stem cell dynamics is largely satisfied in this context. However, following a physiological stress, cells that have left the crypt and become committed progenitors to the secretory or absorptive lineages can revert back, once again adhering to the definition of a stem cell. In addition to this reversion, populations of “reserve” stem cells have been identified that are resistant to different types of damage, such as radiation injury.
The intestine has been a powerful model system to study stem cell dynamics, due to its organized tissue architecture; however, it has also been an intense focus of study due to a significant number of diseases that affect this system, such as inflammatory bowel diseases and intestinal cancers. Through these studies, as described throughout this chapter, WNT signaling has emerged as a key effector in both normal ISC homeostasis, and in disease. This chapter provides a brief historical perspective on our understanding of WNT signaling, a broad introduction to the WNT signaling network, and then discusses how WNT signaling plays a role in the intestine, during embryonic development, in the normal adult intestine, and in disease.
The first WNT homolog, Wingless ( wg ), was described in Drosophila melanogaster in 1976, and was shown to be critical for wing development. Nüsslein-Volhard and Wieschaus subsequently identified wg in a forward genetic screen as a gene required for normal segmentation and polarity of the embryo. Around the same time, Nusse and Varmus found that a mouse mammary tumor virus induced tumors by integrating into the chromosome and activating the expression of an unknown gene that was named Int1 (integration 1). Int1 was then demonstrated to be a spontaneous loss-of-function mutat allele in the mouse that was described in 1967 which resulted in mice that lacked part of the cerebellum. Int1 and wg were subsequently identified as homologues, and gene-targeting experiments in mice confirmed that genetic deletion of Int1 led to aberrant cerebellar development. A portmanteau of the words wg and Int1 lead to coining the gene as “ Wnt ”.
Following the discovery of the initial WNT proteins, many more WNT pathway components were identified in forward genetic screens carried out in Drosophila , and mutations were identified that caused similar polarity and patterning defects to that of wg mutations. These components included armadillo (β-catenin), disheveled, porcupine, and zeste white 3 ( GSK3β ).
A connection between WNT signaling and the intestine was made with the discovery of a mouse with a dominant mutation that predisposed the animal to multiple intestinal neoplasia (MIN), which was subsequently demonstrated to be a mutation in the adenomatous polyposis coli ( Apc ) gene, and which makes up part of the destruction complex (see the next section). Null mutations in Apc lead to stabilization of β-catenin (encoded by the Ctnnb1 gene, we will use β-catenin throughout for both gene and protein), its subsequent nuclear localization and constitutive transcriptional activation. Following these discoveries, it was demonstrated that β-catenin bound with TCF/LEF transcription factors to modulate gene expression, that TCF4 was critical for mediating β-catenin-dependent transcription in the intestine, and that stabilizing β-catenin mutations led to colon cancer. Much of this early work helped shape the current understanding of the importance of WNT signaling in the ISC .
Historically, two types of ISCs were identified: CBCs cells and label-retaining (or “+ 4”) cells. These studies used techniques such as tracking of radiolabeled particles that were phagocytosed or incorporation of radioactive 3H-thymidine incorporation into DNA (label-retaining) in order to mark and follow these cells over time . It was previously thought that the + 4 cell was a largely quiescent stem cell that sat atop of the stem cell hierarchy and rarely divided, but ultimately would give rise to CBCs, which were more proliferative . More recently, however, it has been accepted that the + 4 cell is likely not a specialized reserve population of stem cells, rather, the evidence demonstrates that this cell is a secretory progenitor cell that has the capability of reverting into a stem-like state upon injury to the CBCs ( Fig. 7.1 ).
CBC cells have been defined using molecular markers and transgenic lineage tracing techniques (see Section 7.5 ), and are the proliferative engines that drive cellular production in the crypts, dividing daily with frequent turnover. The + 4 cells have been redefined as “reserve ISCs” (rISC) and appear to have a phenotype that is similar to an enteroendocrine progenitor cell, dividing infrequently under homeostatic conditions but being called upon to produce new CBCs in response to injury or other stimuli. Evidence also suggests that there is a large amount of plasticity among the cells in the crypts, particularly in the context of injury, and in addition to the rISCs, both secretory and absorptive progenitor cells can revert and give rise to new CBCs following injury.
WNT signaling is mediated by secreted WNT ligands binding to receptors on the cell surface. This binding starts a complex cascade of events downstream of the ligand-receptor interaction, typically referred to as a “signal transduction pathway”. The term “pathway” invokes the notion of a linear path from one point to another, and it is worth noting that this concept far oversimplifies the complex events that actually occur downstream of the ligand-receptor interactions. Conceptually, it is more accurate to envision that there is a broad network of events that take place downstream of the ligand-receptor interaction, and that this network of events will be different in different cellular contexts; for example, the cascade of events in the ISC may be far different from those that occur in a different cell or tissue, and may be distinct from other cells within the crypt. However, the network of events that takes place downstream of the ligand-receptor interactions are incompletely characterized, and so for the purpose of this chapter, an overview of the basic elements of the WNT signaling “pathway” is provided. For additional reading, many excellent reviews cover this topic at length.
WNT signaling takes place through a module made up of receptors, transducers, and effectors ( Fig. 7.2 ). WNT ligands bind to the Frizzled (FZD) family of G-coupled protein receptors along with a member of the LRP family of coreceptors. WNT-ligand binding to the FZD-LRP receptor complex initiates intracellular events that transduce the ligand-receptor interaction. The signaling strength of the ligand-receptor interaction is mediated, in part, by the ability of the receptors to accumulate at the cell surface. Accumulation of the FZD-LRP receptor complex is controlled by membrane bound E3-ubiquitin ligases ZNRF3 and RNF43, which act to stimulate ubiquitination, internalization, and degradation of the FZD-LRP complex. A second ligand-receptor interaction, which involves the R-spondin(RSPO) family of secreted ligands and LGR receptors, act to potentiate WNT signaling by binding to ZNRF3/RNF43. The LGR-RSPO-ZNRF3/RNF43 complex is then ubiquitinated in an E3 ligase-dependent manner, internalized, and targeted for degradation. This prevents the ubiquitination and internalization of the FZD-LRP receptor complex, allowing receptors to accumulate on the cell surface, thereby amplifying the number of WNT ligand-receptor complexes that form in order to stimulate downstream signaling ( Fig. 7.2 ).
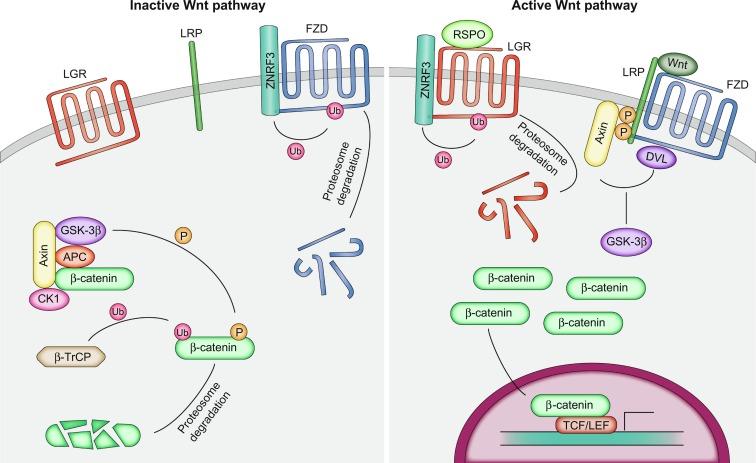
One of the main transducers of WNT signaling in the ISC is β-catenin, a protein that is tightly regulated in both the inactive and active states. In the absence of WNT ligand (inactive state), β-catenin is primarily found at adherens junctions where it plays an important role in epithelial cell:cell adhesion . Free β-catenin that is not incorporated into adherens junctions is sequestered in the cytoplasm and actively targeted for degradation by a protein machine referred to as the destruction complex, which phosphorylates β-catenin near its amino terminus thus targeting it for ubiquitin- dependent proteasomal degradation. The destruction complex includes glycogen synthase kinase 3β GSK3β, CK1, AXIN and APC tumor suppressor proteins, where AXIN and APC function as scaffolds and interact with β-catenin directly. CK1 and GSK3β are Ser/Thr kinases that phosphorylate β-catenin, which is subsequently recognized by β-TrCP, a component of an E3 ubiquitin ligase complex. As a consequence, once ubiquitinated, β-catenin is degraded rapidly by the 26S proteasome. Following WNT ligand binding and dimerization of the FZD-LRP receptors, β-catenin accumulates in the cytoplasm and translocates into the nucleus, where it acts as a transcription factor; however, the mechanism by which β-catenin is stabilized is not entirely clear, and several models for this regulation exist. One study suggested that active WNT signaling inhibits β-catenin ubiquitination within the destruction complex, saturating the destruction complex with phosphorylated β-catenin and thus allowing newly synthesized β-catenin to accumulate in the cytoplasm and translocate to the nucleus. A second model proposed that phosphorylation of AXIN by GSK3 β kept AXIN active (“open”) in order to bind β-catenin. Upon ligand-receptor interactions, AXIN formed a complex with LRP, and the AXIN-LRP interaction subsequently promoted AXIN dephosphorylation and inactivated (“closed”) the AXIN complex through an intramolecular interaction. Inactivation of AXIN diminished its association with β-catenin and inhibited β-catenin phosphorylation. Yet another layer of complexity is added to the regulation of the destruction complex as AXIN itself forms a multimeric complex that is stabilized by APC, thus increasing the ability of the destruction complex to process β-catenin.
Once WNT signaling is active, β-catenin accumulates in the cytoplasm and is translocated to the nucleus where it interacts with the TCF/LEF family transcription factors, including TCF1, TCF3, TCF4 (also known as TCF7L2), and LEF1 in mammals, which guide β-catenin to specific DNA loci (reviewed in Refs. In the absence of β-catenin, TCF/LEF proteins bind to target DNA sequences and interact with Groucho/TLE corepressors to repress transcription of nearby target genes. On entering the nucleus, β-catenin displaces Groucho/TLE recruits additional coactivators to form a TCF-β-catenin complex that can initiate transcription. Genome-wide analysis of DNA binding by β-catenin in the murine intestinal epithelium and in colorectal cancer (CRC) cells demonstrated that the majority of β-catenin bound loci colocalized with TCF4 sites. A second class of β-catenin bound DNA colocalized with a minimal amount of TCF4; however, the latter drove only minimal transcription. When TCF/LEF and β-catenin are bound to DNA, transcriptional corepressors are displaced, and transcriptional coactivators are recruited. There is a large number of coactivators potentially recruited to the DNA; however, one of the major events that takes place is the recruitment of histone acetyltransferases (HATs) including CREB-binding protein (CBP) and its close relative p300, which act to acetylate histones. Increased histone methylation of lysine 4 (H3-K4me3) at various target promoters has also been documented, and both histone modifications are associated with increased transcription of target gene mRNA.
Intestine development is a complex process that includes regional patterning and morphogenetic events that give rise to region-specific functions and structures in the intestine. These early developmental processes rely on epithelial-mesenchymal cross talk to establish the cell-intrinsic regional identity of the epithelium. This signaling also controls an epithelial transition from a simple, relatively smooth, epithelial tube surrounded by mesenchyme to the formation of the stereotypical villus units that have evolved to provide an increased absorptive surface area; it is during this process of villus emergence that the future crypt domain is also established.
Evidence in adult mice has demonstrated that the ISCs are intrinsically programmed with their location/region specific function and gene expression patterns. This is supported by classical embryological studies, which have shown that the mesenchyme has potent inductive properties during early development and is instructive for the regional identity of the endoderm ; however, once the different regions of the intestine are specified (i.e., duodenum jejunum and ileum), the endodermal compartment then retains this positional information. Thus, it is not entirely surprising that when crypts from the duodenum, jejunum, or ileum of the adult were isolated and grown in culture for extended periods of time, they retained their region-specific gene expression programs. How this regional identity is established, is still not entirely clear. Recent evidence has suggested that gradients of growth factor signaling along the developing gastrointestinal tract may be responsible for specifying regional intestinal identity, with increasing levels of WNT and FGF signaling inducing progressively more posterior fates ; however, it is likely that anterior-posterior patterning is far more complicated than currently appreciated. Similarly, how the regional pattern is maintained is also not clear, but ex vivo culture of specific diverse regions of the intestine retain their identity, suggesting that the environment is not required to actively maintain regional identity. It is possible that epigenetic mechanisms play a role in this regional maintenance.
Formation of the proliferative compartment in the intestine begins concurrently with villus emergence during embryonic development. At this period of time, the flat epithelial tube transitions from a pseudostratified epithelium to a columnar epithelium during villus emergence, and proliferation becomes restricted to the domains between villi called the intervillus domains, which will ultimately deepen and give rise to bona fide crypts in the adult. Recent attention has been placed on understanding the transition from an immature progenitor cell state in the fetal intestine to the adult ISC. Through this work, it has become clear that there are significant differences between the fetal intestinal progenitor cell and the mature, postnatal, ISC, though the significance of these differences is yet to be fully appreciated.
Several studies have demonstrated that the ISC marker, LGR5, is expressed in intestinal progenitor cells as early as embryonic day (E) 8.5 during embryonic development in mice, whereas intervillus domains are not present until around E15.5, and bona fide crypts do not appear until postnatal week 2 in the mouse.
Interestingly, while Lgr5 is known to be a sensitive WNT-signaling target gene in adult ISCs, it appears that WNT-signaling is dispensable for regulation of Lgr5 in the early fetal intestine, before intervillus domains are present, at a time when Lgr5 gene expression is robust. Indeed, genetic studies have demonstrated that blocking WNT signaling prior to E15.5 has little effect on proliferation, whereas loss of WNT signaling following villus formation at E15.5 is catastrophic. Interestingly, while Lgr4 and Lgr5 play redundant roles to potentiate WNT signaling in the adult (see Section 7.5.2 ), loss of Lgr5 has only modest effects on cytodifferntiation when genetically deleted, and Lgr4 appears to be the dominant receptor in the developing intestine. Consistent with the notion that stem/progenitor cells are significantly different in the developing and adult intestine, studies have used transcriptional profiling (RNA sequencing) to show that the transcriptome is vastly different when comparing Lgr5 + cells in the fetal and adult intestine. Mechanistically, it appears that the transcription factor ID2 is expressed in the early fetal intestine and acts to repress WNT-signaling target genes, and genetic deletion of Id2 leads to precocious activation of target genes.
In addition to regional patterning and emergence of the stem cell domain (intervillus domain, crypt), it is also clear that there are major changes along the developmental continuum leading to maturation of intestinal epithelial cells into functional cell types. In this context, maturation is defined as the ability of the epithelium to express genes required for digesting nutrients in postnatal life. In mice, maturation is partly controlled by a transcription factor, BLIMP1 (PRDM1), which functions to repress genes expressed in the postnatal intestine, and thus Blimp1 is progressively downregulated as development progresses. In the adult intestine, a similar maturation must take place as ISC daughters transition from a proliferative state toward a differentiated cell type responsible for a specific cellular function, where the cell acquires the ability to perform specific cellular tasks through the expression of specific genes and proteins (i.e., an enterocyte must express digestive enzymes). In this context, it has been demonstrated that the stem cells retain their ability to make different cell fate choices by maintaining an accessible chromatin state. This allows access of cell-specific transcription factors to the necessary chromatin in order to drive appropriate transcriptional programs. In addition, the intestine-specific transcription factor CDX2 plays an important role in the transition from a stem cell state to a differentiated state by shifting the sites where it is bound to chromatin. That is, CDX2 is selectively bound to a smaller number of genomic loci in the stem cell state, but during differentiation into mature functional cell types, CDX2 binding expands to include more genomic loci where genes reside whose expression is required for cell-specific function.
Through studies of the developing immature and adult intestine (see also Section 7.5 ), we have begun to appreciate that there are major changes throughout an organism’s lifespan, and that intestinal stem/progenitor cells are regulated by multiple mechanisms across that time. It has recently been demonstrated that the fetal and adult gastric epithelium also changes across developmental time, but that during injury and repair of the adult stomach, a “fetal program” is reactivated. Thus, it is likely that insights into intestine development will inform our understanding of how regeneration and injury-repair takes, and changes associated with aging take place in the adult.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here