Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Most ischemic strokes involve both white matter and gray matter, and 20% of strokes predominantly involve white matter.
Mechanisms of white matter ischemic injury are less well understood than the mechanisms of gray matter ischemic injury and distinctly different from the latter.
Axons and oligodendrocytes are most vulnerable to ischemic injury in white matter: axons suffer loss of their sodium gradient and accumulate toxic levels of calcium, whereas oligodendrocytes and their myelin suffer non– N -methyl- d -aspartate-mediated glutamate excitotoxicity.
Potential therapeutic approaches include modulation of ionic disruptions, suppression of glutamate-mediated damage, and recruitment of endogenous repair mechanisms.
The dream of meaningful stroke neuroprotection can be realized only when the different pathophysiologies of white matter and gray matter injury are both effectively addressed.
Work in the authors’ laboratories has been supported in part by grants from the National Institutes of Health (BRR, SB, MPG, KA), Eastern Paralyzed Veterans Association (BRR), American Heart Association (MPG, SB), and the Juvenile Diabetes Research Foundation (MPG). Selva Baltan has published previously as Selva Tekkök.
Anoxia or ischemia of the mammalian central nervous system (CNS), including the secondary vascular embarrassment that frequently accompanies traumatic brain and spinal cord insults, , damages both gray and white matter ( Fig. 9.1 ). In fact, 20% of ischemic strokes involve predominantly white matter, as a result of occlusion of small penetrating arteries that supply the deep areas of the cerebral hemispheres (see Chapter 27 ). Clinically, damage to white matter can result in serious disability, as seen in stroke, spinal cord and traumatic brain injury, some forms of vascular dementia, and hypoglycemia. , , The glaring failure of stroke clinical trials testing “neuroprotective” drugs may be due, at least in part, to a lack of benefit for injured white matter. In spite of these facts, how white matter is injured by ischemia has received far less attention than is the case for gray matter. Two reasons are foremost in explaining this neglect: first, the brain of rodents, which is most often used to study stroke, has very little white matter (far less than the human brain), and second, there is a tendency to think that protection of neuron cell bodies alone is sufficient to rescue stroke-imperiled brain tissue.
Great progress has been made in understanding the pathophysiology of anoxic-ischemic white matter injury in the past decade, and the pace of this work is increasing. Models have been developed that allow white matter to be studied independently of gray matter. Basic knowledge about the ionic and molecular events initiated by ischemia in white matter is spawning testable hypotheses for protecting this unique part of the brain during stroke. Most significant is the fact that there is a growing awareness of the importance of this topic in achieving the goal of effective, early treatment of ischemic stroke. This chapter reviews what is currently known about the cellular and molecular events triggered by anoxia or ischemia in white matter and how these events lead to loss of function and irreversible injury.
White matter of the mammalian CNS consists of afferent and efferent axonal tracts that interconnect cortical and nuclear areas of the brain and spinal cord that contain neuronal cell bodies. White matter contains no neuronal cell bodies or synapses. It consists of tightly packed glial cells and myelinated and unmyelinated axons; the presence of myelin lends a white appearance to this tissue. White matter regions vary widely with regard to the ratio of myelinated to unmyelinated axons; for example, all the axons of the optic nerve are myelinated, but of those in the corpus callosum, only about 30% are myelinated. , The anatomy, physiology, and metabolism of myelinated axons are highly specialized and unique compared with those of unmyelinated axons. , It is not surprising, therefore, that regional differences have been noted in the pathophysiology of white matter injury.
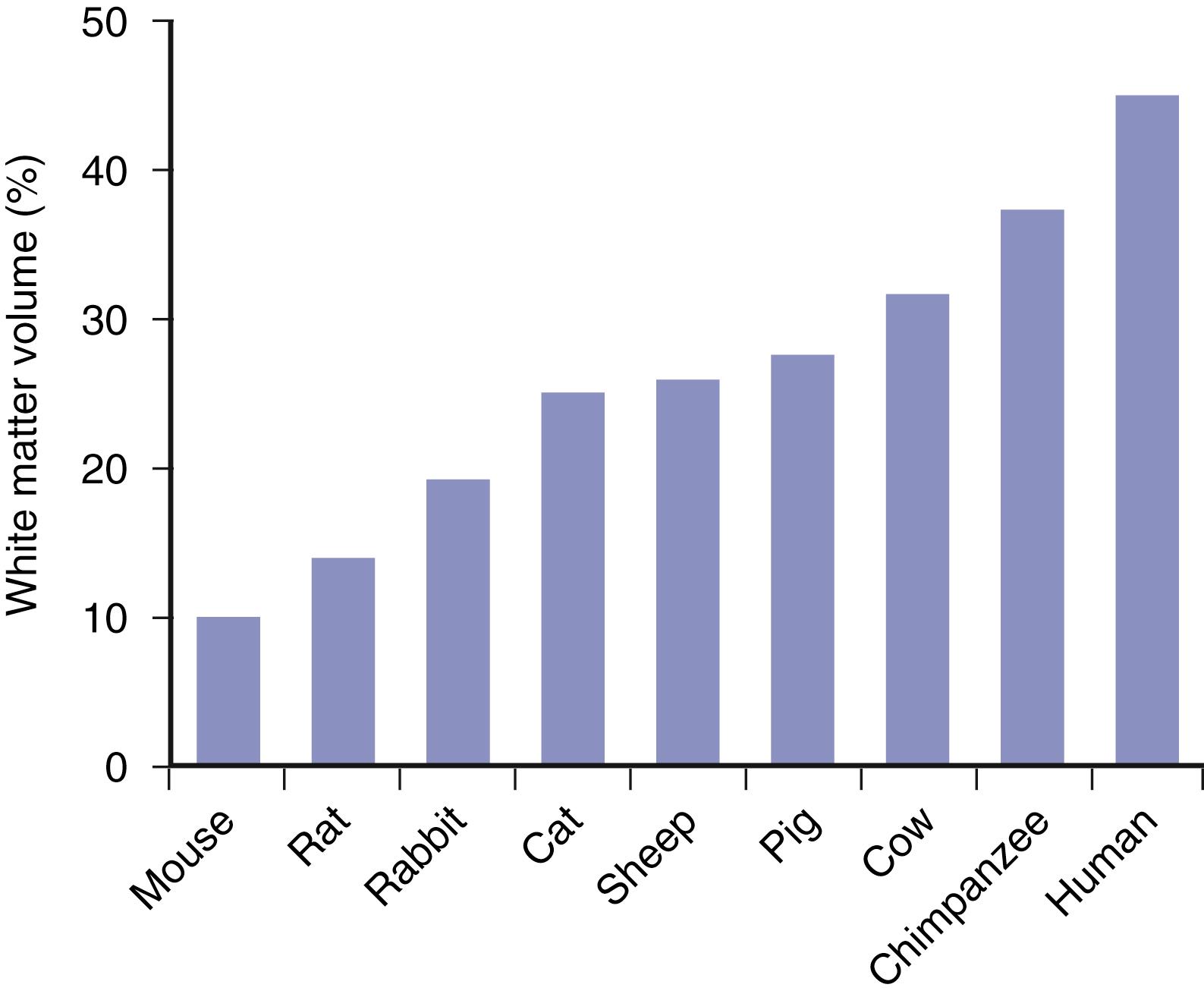
Most axons in cerebral white matter provide connections within cortical regions. These connections include short fiber bundles between adjacent cortical regions (U fibers) and longer axons projecting between contralateral hemispheres (callosal fibers) or distinct brain areas (association fibers). Output or input projections to basal ganglia, brainstem, or spinal cord are only a small proportion of total CNS axons. Because most white matter axons connect cortical regions, the massive growth in cortical area from small lissencephalic animals to animals with larger, gyrencephalic brains is associated with a great and disproportionate expansion in white matter volume ( Fig. 9.2 ). White matter constitutes only a small fraction of forebrain volume in rodents (for mice, approximately 10% white matter; total forebrain volume 125 mm 3 ) but occupies a large proportion of the human brain (approximately 50%; total volume 1,000,000 mm 3 ) (see Fig. 9.2 ). This massive, greater than fourfold expansion in the percent volume of brain occupied by white matter means that the human brain has far more white matter at risk during ischemia. It may also help to explain why successful therapies in animal models of stroke have not yet proven to be successful in humans.
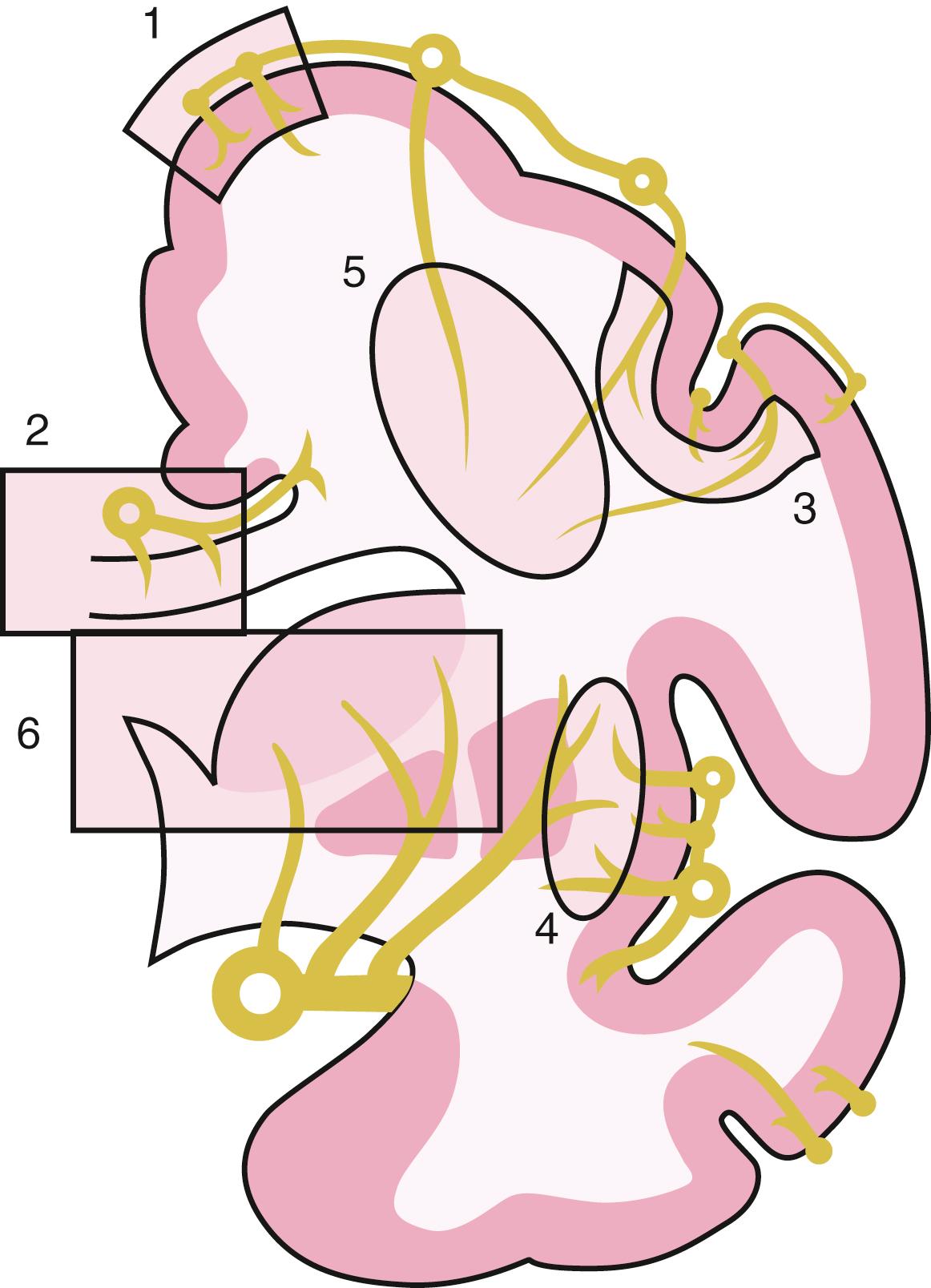
Although our understanding of the roles of glial cells in CNS functions continues to evolve, , it is clear that astrocytes are crucial for ionic homeostasis of the brain’s extracellular space (ECS), glutamate uptake, and synaptogenesis. Astrocytes have a complicated but central role in supporting antioxidant synthesis in the brain. Only astrocytes contain glycogen, and they can provide neurons and axons (and possibly oligodendrocytes) with usable energy substrate in the form of lactate when glucose is restricted. Because astrocytes are natural anaerobes and contain glycogen, they are the only cells in the brain that are able to maintain enough adenosine triphosphate (ATP) to function, at least temporarily, during ischemia. , Oligodendrocytes provide myelin for CNS axons; conduction fails in myelinated axons with injury to oligodendrocytes or their myelin. Ischemia will, of course, eventually affect all the elements in white matter, leading to axon–glial interactions that are important for understanding how injury occurs. These complex cellular interactions during ischemia or other disruptions in energy metabolism have just begun to be explored in white matter. For example, microglial cells are thought to produce damaging free radical species during ischemia, and astrocytes are the key cell type capable of defending against free radical–mediated injury. Little is known, however, about these topics as they pertain to anoxic-ischemic injury in white matter.
It is essential to understand that an axon’s energy metabolism is independent from that of its cell body of origin. Axons extend for great distances from their cell bodies and depend on the local production of ATP to maintain ion gradients and sustain energy-consuming functions. This metabolic isolation also means that axons suffer energy deprivation in a manner that is independent of neuronal cell bodies. The metabolic rate of white matter is about half that of gray matter on the basis of oxygen consumption. Axons, more than glial cells, are calculated to contribute to this high metabolic rate. The adult mammalian CNS is generally presumed to fail rapidly in the absence of oxygen. There are few actual data on this question, however, because making an animal anoxic without compromising blood supply is challenging. Surprisingly, about 25% of myelinated axons in young adult rodent optic nerve can function anaerobically, but this ability declines and then is lost in older mice. This finding implies that some axons in younger adult white matter could tolerate prolonged periods of pure anoxia without permanent injury.
Because white matter is less metabolically active than gray matter, it requires a lower blood flow (per volume of tissue). The blood flow in cerebral white matter averages 30 mL/100 mg/min compared with 50 mL/100 mg/min in gray matter. White matter has a much less dense capillary network than gray matter. Much of the cerebral white matter is perfused by penetrating arterioles that originate from larger pial vessels. Deeper regions of the subcortical white matter are supplied by the striate arteries that arise from the circle of Willis. The most important of these are the medial striate arteries, which arise directly from the internal carotid artery and supply much of the internal capsule as it courses through the basal ganglia. As white matter tracts descend through the brainstem, they are perfused by penetrating arteries from the vertebral and basilar arteries or their circumferential branches.
Different regions of white matter have distinct patterns of vascular supply ( Fig. 9.3 ). For example, in the centrum semiovale, the blood supply consists of long arterioles, which are characteristically terminal vessels with few anastomoses; occlusion of one of these vessels results in an area of ischemia that cannot be rescued by the redistribution of blood flow because there are no anastomotic connections with neighboring vessels. In contrast, the immediately subcortical association bundles (U fibers), corpus callosum, external capsule-claustrum, and extreme capsule are supplied by interdigitating arterioles derived from two or more pial vessels. This dual vascular supply may account for the relative resistance of these white matter areas to damage after anoxia or hypoperfusion.
Research in white matter anoxia or ischemia requires experimental systems that appropriately model the cell biology and physiology of myelinated axons and glia. Improved understanding of the pathways leading to white matter injury can lead to therapeutic approaches that can be tested in progressively more complex in vitro and in vivo systems and, ultimately, in clinical trials. However, it is important to understand the potential strengths and limitations of each model system and to ensure that experiments appropriately examine specific facets of injury and recovery.
Cell culture models provide the opportunity to examine individual cellular elements of white matter in isolation, separate from the effects of perfusion and vascular supply. Experiments in primary cultures allow assessment of enriched populations of brain cells to effects of energy deprivation, which is typically produced by transient removal of oxygen and glucose. Under these conditions, the relative vulnerabilities of cells, from greatest to least, are approximately as follows: neurons > oligodendrocytes = microglia > endothelial cells > astrocytes. Within the oligodendrocyte lineage, immature oligodendrocytes are more vulnerable than slightly more mature cells to energy deprivation. Reasons proposed for the high vulnerability of oligodendrocytes to ischemic damage include their high metabolic demands, poor resistance to oxidative stress, and vulnerability to extracellular glutamate. ,
Cell culture models provide excellent systems for studying the molecular biology, pharmacology, and neurochemistry of ischemic injury, but important limitations must be considered. Most cultured cells are derived from the brains of perinatal animals and so may reflect immature phenotypes not found in the adult brain. Relatively few studies of cultured central axons physically isolated from their neuronal cell bodies, as occurs in vivo, have been performed. More importantly, culture models generally exclude the important cell–cell interactions that characterize intact white matter. It is possible to address some of these concerns because systems for isolating axons are well described and oligodendrocytes selectively myelinate axons in coculture. With the use of such a compartmented chamber system, for example, it was possible to determine that ischemic injury to isolated axons (cell bodies in another compartment) is mediated primarily by influx of Ca 2 + and Na+ but is independent of glutamate receptor activation.
Many of the limitations of cell cultures are avoided in models that use immediately isolated, perfused preparations of intact white matter. Considerable progress in understanding white matter injury comes from studies of the isolated rodent optic nerve, a CNS white matter tract consisting solely of myelinated axons. Other preparations of CNS white matter regions include isolated spinal cord dorsal column and brain slices, including corpus callosum. These ex vivo white matter tract preparations offer several advantages for studying the mechanisms of white matter injury, including the capacity to quantitatively assess axonal function with the use of electrophysiology and to perform confocal imaging of cellular components. , Importantly, each white matter tract offers intact, three-dimensional (3D) interactions between glial cells and axons. The use of different white matter tracts facilitates the detection of region-specific differences in the mechanisms of white matter injury. , Although these preparations are not suitable for studies of long-term outcomes after hypoxic or ischemic injury, the axons remain electrically functional for at least several hours in vitro, permitting the assessment of short-term recovery. It is imperative to emphasize the importance of studying white matter injury at normal body temperature. Brain slices are commonly studied at several degrees below body temperature because they survive better at these cooler temperatures. Anoxic-ischemic injury of white matter, however, is very sensitive to temperature ; the degree of injury decreases markedly with cooling. The real pitfall is that lower temperatures alter injury mechanisms, which can lead to false conclusions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here