Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Waldenström macroglobulinemia (WM) is a lymphoid neoplasm resulting from the accumulation, predominantly in the bone marrow (BM), of a clonal population of lymphocytes, lymphoplasmacytic cells, and plasma cells, which secrete a monoclonal immunoglobulin (Ig)M. WM corresponds to lymphoplasmacytic lymphoma (LPL) as defined in the World Health Organization classification system. Most cases of LPL are WM; less than 5% of cases secrete IgA, IgG, kappa, lambda, or can be non-secretory LPL.
In 1944, Jan Waldenström, a Swedish physician-scientist, reported in Acta Medica Scandinavica three cases of a disease he presciently thought was related to myeloma, but for the absence of skeletal bone involvement and the scarcity of plasma cells in a marrow infiltrated with small lymphocytic cells. He noted an increase in plasma protein concentration, marked increased serum viscosity, exaggerated bleeding, and retinal hemorrhages. In collaboration with a colleague, he showed, using ultracentrifugation and electrophoresis, that the abundant abnormal protein had a molecular weight of approximately 1 million and was not an aggregate of smaller proteins. The disease, which he described with such thoroughness, was later named in his honor.
The age-adjusted incidence rate of WM in the United States is 3.4 per 1 million among males and 1.7 per 1 million among females. The incidence of WM increases with age. The incidence rate is higher among Americans of European descent. Americans of African descent represent approximately 5% of all patients.
Genetic factors play a role in the pathogenesis of WM. Approximately 20% of patients with WM are of Ashkenazi Jewish ethnic background. Familial disease has been reported commonly, including multigenerational clustering of WM and other B-cell lymphoproliferative diseases. Approximately 20% of 257 sequential patients with WM presenting to a tertiary referral center had a first-degree relative with either WM or another B-cell disorder. Familial clustering of WM with other immunologic disorders, including hypogammaglobulinemia and hypergammaglobulinemia (particularly polyclonal IgM), autoantibody production (particularly to the thyroid), and manifestation of hyperactive B cells, has also been reported in relatives without WM. Increased expression of the BCL2 gene with enhanced survival has been observed in B cells from familial patients and their family members.
The role of environmental factors is uncertain; however, chronic antigenic stimulation from infections and certain drug or chemical exposures have been considered but have not reached a level of scientific certainty. Hepatitis C virus (HCV) infection was implicated in WM causality in some series, but no association was found in a study of 100 consecutive patients with WM in whom serologic and molecular diagnostic studies for HCV infection were performed.
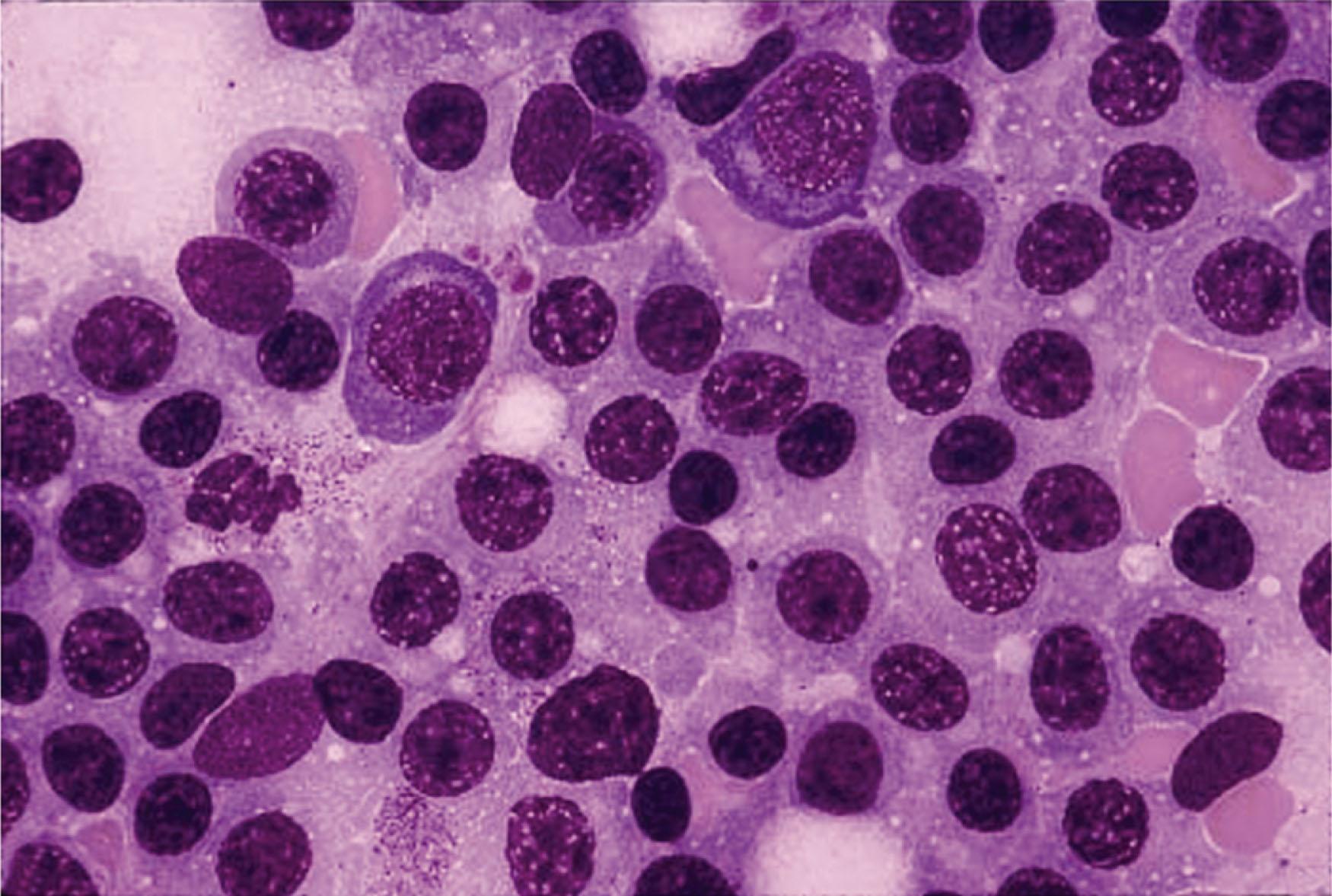
Examination of the B-cell clone(s) found in the BM of patients with WM reveals a range of differentiation, from small lymphocytes with large focal deposits of surface immunoglobulins, to lymphoplasmacytic cells, to mature plasma cells that contain intracytoplasmic IgM ( Fig. 92.1 ). Circulating clonal B cells are often detectable in patients with WM, though lymphocytosis is uncommon. WM cells express the monoclonal IgM, and some clonal cells also express surface IgD. The characteristic immunophenotypic profile of WM lymphoplasmacytic cells includes the expression of the pan–B-cell markers CD19, CD20 (including FMC7), CD22, and CD79. Expression of CD5, CD10, and CD23 can be present in 10% to 20% of cases, and their presence does not exclude the diagnosis of WM. In addition, multiparameter flow cytometric analysis has also identified CD25 and CD27 as being characteristic of the WM clone, and that a CD22 dim /CD25 + /CD27 + /IgM + population can be observed among clonal B lymphocytes in patients with IgM monoclonal gammopathy of undetermined significance (MGUS) who ultimately progress to WM.
Somatic mutations in immunoglobulin genes are present with increased frequency of nonsynonymous versus silent mutations in complementarity-determining regions, along with somatic hypermutation, thereby supporting a postgerminal center derivation for the WM B-cell clone in most patients. A strong preferential use of VH3/JH4 gene families without intraclonal variation, and without evidence for any isotype-switched transcripts, has also been shown. Taken together, these data support an IgM + and/or IgM + IgD + memory B-cell origin for most cases of WM.
In contrast to myeloma plasma cells, no recurrent translocations have been described in WM, which can help one distinguish IgM myeloma cases that often exhibit t(11;14) from WM. Despite the absence of IgH translocations, recurrent chromosomal abnormalities are present in WM cells. These include deletions in chromosome 6q21–23 in 40% to 60% of patients with WM, with concordant gains in 6p in 41% of patients with 6q deletion. In a series of 174 untreated patients with WM, 6q deletions, followed by trisomy 18, 13q deletions, 17p deletions, trisomy 4, and 11q deletions, were observed. Deletion of 6q and trisomy 4 were associated with an adverse prognosis in this series. Because 6q deletions represent the most recurrent cytogenetic finding in WM cases, there has been great interest in identifying the region of minimal deletion and possible target genes within this region. Two putative gene candidates within this region include TNFAIP3 , a negative regulator of nuclear factor-κB signaling (NFκB), and PRDM1 , a master regulator of B-cell differentiation. The removal of an NFκB-negative regulator is of particular interest because the phosphorylation and translocation of NFκB into the nucleus is a crucial event for WM cell survival. The success of proteasome inhibitor therapy in WM has been postulated to occur because the degradation of negative regulators of NFκB, such as inhibitor of κB (IκB), is blocked.
A highly recurrent somatic mutation (MYD88 L265P ) was first identified in patients with WM by whole-genome sequencing (WGS) and confirmed by multiple studies through Sanger sequencing and/or allele-specific polymerase chain reaction (PCR) assays. MYD88 L265P is expressed in 90% to 95% of WM cases when more sensitive allele-specific PCR is employed using both CD19-sorted and unsorted BM cells. By comparison, MYD88 L265P was absent in myeloma samples, including IgM myeloma, and was expressed in a small subset (6% to 10%) of patients with marginal zone lymphoma, who surprisingly have WM-related features. By PCR assays, 50% to 80% of patients with IgM MGUS also express MYD88 L265P , and expression of this mutation was associated with increased risk for malignant progression. The presence of MYD88 L265P in patients with IgM MGUS suggests a role for this mutation as an early oncogenic driver, and other mutations and/or copy number alterations leading to abnormal gene expression are likely to promote disease progression.
The impact of MYD88 L265P to growth and survival signaling in WM cells has been addressed in several studies ( Fig. 92.2 ). Knockdown of MYD88 decreased survival of MYD88 L265P -expressing WM cells, whereas survival was enhanced by knock-in of MYD88 L265P versus wild-type (WT) MYD88 . The discovery of a mutation in MYD88 is of significance, given its role as an adaptor molecule in Toll-like receptor (TLR) and interleukin-1 receptor (IL-1R) signaling. All TLRs except for TLR3 use MYD88 to facilitate their signaling. Following TLR or IL-1R stimulation, MYD88 is recruited to the activated receptor complex as a homodimer, which then complexes with interleukin receptor-associated kinase 4 (IRAK4) and activates IRAK1 and IRAK2. Tumor necrosis factor receptor-associated factor 6 is then activated by IRAK1, leading to NFκB activation via IκBα phosphorylation. Use of inhibitors of the MYD88 pathway led to decreased IRAK1 and IκBα phosphorylation, as well as to survival of MYD88 L265P -expressing WM cells. These observations are of particular relevance to WM because NFκB signaling is important for WM growth and survival. Bruton’s tyrosine kinase (BTK) is also activated by MYD88 L265P . Activated BTK coimmunoprecipitates with MYD88, which could be abrogated by use of a BTK inhibitor, and overexpression of MYD88 L265P but not WT MYD88 triggers BTK activation. Knockdown of MYD88 by lentiviral transfection or use of a MYD88 homodimerization inhibitor also abrogated BTK activation in MYD88 L265P -mutated WM cells.
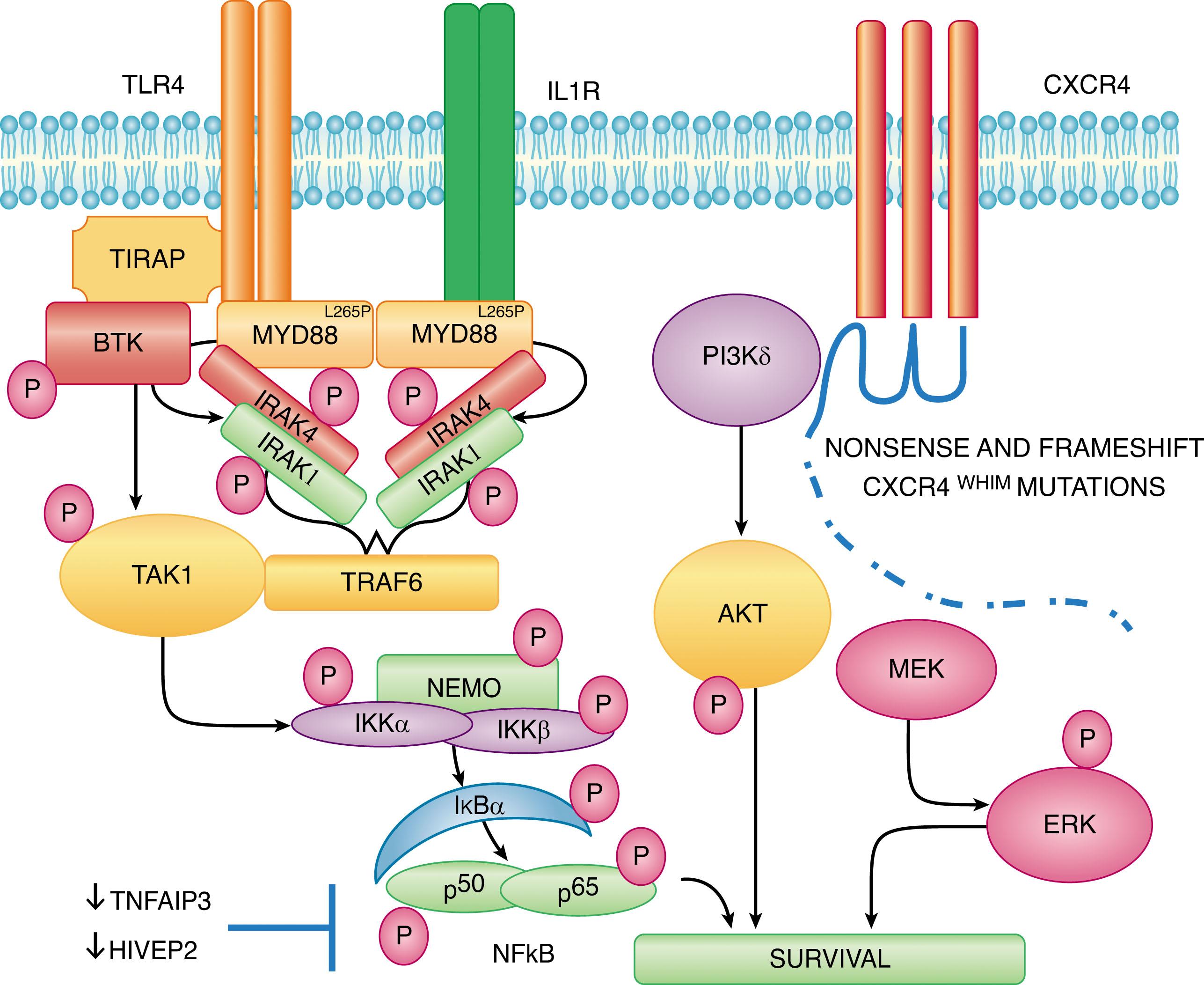
The second most common somatic mutation after MYD88 L265P revealed by WGS was found in the C-terminus of the C-X-C chemokine receptor type 4 (CXCR4) receptor. These mutations are present in 30% to 35% of patients with WM, and they impact serine phosphorylation sites that regulate CXCR4 signaling by its only known ligand, SDF-1α (CXCL12). The location of somatic mutations found in the C-terminus of CXCR4 in WM are similar to those observed in the germline of patients with WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis, retention of neutrophils within the marrow) syndrome, a congenital immunodeficiency disorder characterized by chronic noncyclic neutropenia. Patients with WHIM syndrome exhibit impaired CXCR4 receptor internalization following SDF-1α stimulation, which results in persistent CXCR4 activation and myelokathexis.
In patients with WM, two classes of CXCR4 mutations occur in the C-terminus. These include nonsense (CXCR4 NS ) mutations that truncate the distal 15– to 20–amino acid region and frameshift (CXCR4 FS ) mutations that comprise a region of up to 40 amino acids in the C-terminal domain. Nonsense and frameshift mutations are almost equally divided among patients with WM with CXCR4 somatic mutations, and over 30 different types of CXCR4 mutations have been identified in patients with WM. Preclinical studies with WM cells engineered to express nonsense and frameshift CXCR4 -mutated receptors have shown enhanced and sustained AKT and extracellular signal-regulated kinase signaling following SDF-1α relative to wild type CXCR4 ( CXCR4 WT ) (see Fig. 92.2 ), as well as increased cell migration, adhesion, growth, and survival, and also drug resistance of WM cells.
Many copy number alterations have been revealed in patients with WM that impact growth and survival pathways. Frequent loss of HIVEP2 (80%) and TNAIP3 (50%) genes that are negative regulators of NFκB expression (see Fig. 92.2 ), as well as LYN (70%) and IBTK (40%) that modulate B-cell receptor signaling have been revealed by WGS. WGS has also revealed common defects in chromatin remodeling, with somatic mutations in ARID1A present in 17% and loss of ARID1B in 70% of patients with WM. Both ARID1A and ARID1B are members of the SWI/SNF family of proteins, and are thought to exert their effects via p53 and cyclin-dependent kinase inhibitor 1A regulation. TP53 is mutated in 7% of sequenced WM genomes, whereas PRDM2 and TOP1 that participate in TP53-related signaling are deleted in 80% and 60% of patients with WM, respectively. Taken together, somatic events that contribute to impaired DNA damage response are also common in WM.
The importance of MYD88 and CXCR4 mutations in the clinical presentation of patients with WM has been established. Significantly higher BM involvement, serum IgM levels, hyperviscosity syndrome, and acquired von Willebrand disease were observed in the patients with MYD88 L265P CXCR4 NS mutations. Patients with MYD88 L265P CXCR4 /FS or MYD88 L265P CXCR4 WT had intermediate BM and serum IgM levels; those with MYD88 WT CXCR4 WT showed the lowest BM disease burden. Fewer patients with MYD88 L265P and CXCR4 /FS or NS than with MYD88 L265P CXCR4 WT presented with adenopathy, further delineating differences in disease tropism based on CXCR4 status. Despite the more aggressive presentation associated with CXCR4 NS genotype, risk of death was not impacted by CXCR4 mutation status. Risk of death and histological transformation to more aggressive lymphoma were found to be higher in patients with the MYD88 WT versus the MYD88 L265P genotype.
In patients with WM, increased numbers of mast cells are found in the BM, where they are usually admixed with tumor cell aggregates ( Fig. 92.3 ). The role of mast cells in WM has been investigated in one study in which coculture of primary autologous or mast cell lines with WM lymphoplasmacytic cells resulted in dose-dependent WM cell proliferation and/or tumor colony formation through CD40 ligand (CD40L) signaling. WM cells release soluble CD27 (sCD27), which may be triggered by cleavage of membrane bound CD27 by matrix metalloproteinase 8. sCD27 levels are elevated in the serum of patients with WM and follow disease burden in mice engrafted with WM cells, as well as in patients with WM. sCD27 triggers the upregulation of CD40L as well as a proliferation-inducing ligand on mast cells derived from patients with WM, and mast cell lines through its receptor CD70. Modeling in mice treated with a CD70-blocking antibody shows inhibition of tumor cell growth, suggesting that WM cells require a microenvironmental support system for their growth and survival. High levels of CXCR4 and very late antigen-4 (VLA-4) are expressed by WM cells. In blocking experiment studies, CXCR4 was shown to support migration of WM cells, whereas VLA-4 contributed to adhesion of WM cells to BM stromal cells.
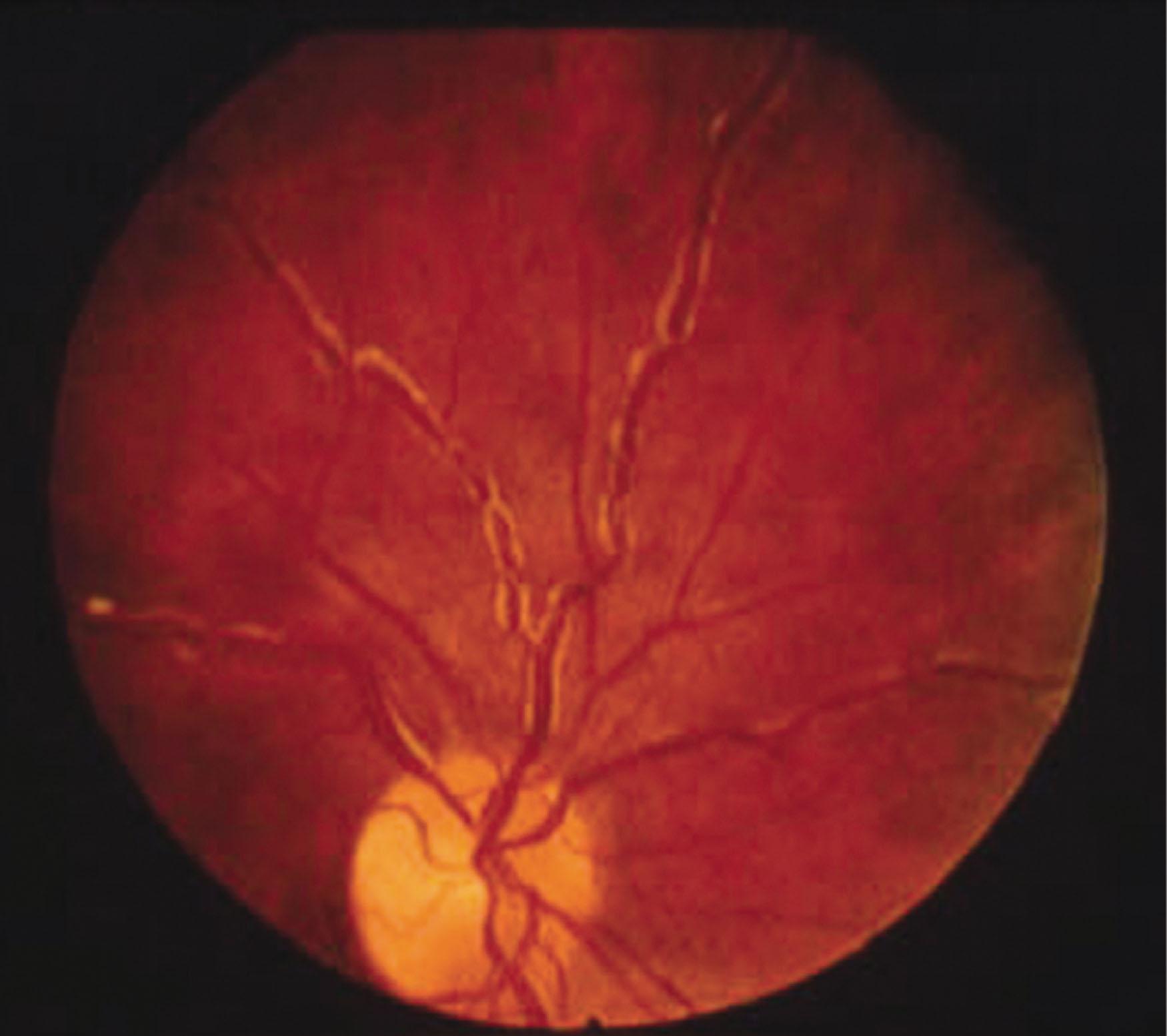
Table 92.1 provides the clinical and laboratory features at the time of diagnosis of patients with WM in one large institutional study. Unlike most indolent lymphomas, splenomegaly and lymphadenopathy are uncommon (≤15%). Purpura is frequently associated with cryoglobulinemia and in rare circumstances with light-chain (AL) amyloidosis. Hemorrhagic and neuropathic manifestations are multifactorial (see IgM-Related Neuropathy section below). The morbidity associated with WM is caused by the co-occurrence of two main components: tissue infiltration by neoplastic cells and, importantly, the physicochemical and immunologic properties of the monoclonal IgM. As shown in Table 92.2 , the monoclonal IgM can produce clinical manifestations through several different mechanisms related to its physicochemical properties, nonspecific interactions with other proteins, antibody activity, and tendency to deposit in tissues.
| Median | Range | Normal Reference Range | |
|---|---|---|---|
| Age (years) | 58 | 32–91 | NA |
| Sex (male/female) | 215/141 | NA | |
| Marrow involvement (% of area on slide) | 30 | 5–95 | NA |
| Adenopathy (% of patients) | 15 | NA | |
| Splenomegaly (% of patients) | 10 | NA | |
| IgM (mg/dL) | 2620 | 270–12,400 | 40–230 |
| IgG (mg/dL) | 674 | 80–2770 | 700–1600 |
| IgA (mg/dL) | 58 | 6–438 | 70–400 |
| Serum viscosity (cp) | 2.0 | 1.1–7.2 | 1.4–1.9 |
| Hematocrit (%) | 35 | 17–45 | 35–44 |
| Platelet count (×10 9 /L) | 275 | 42–675 | 155–410 |
| White cell count (×10 9 /L) | 6.4 | 1.7–22 | 3.8–9.2 |
| β 2 -M (mg/dL) | 2.5 | 0.9–13.7 | 0–2.7 |
| LDH (U/mL) | 313 | 61–1701 | 313–618 |
| Properties of IgM Monoclonal Protein | Diagnostic Condition | Clinical Manifestations |
|---|---|---|
| Pentameric structure | Hyperviscosity | Headaches, blurred vision, epistaxis, retinal hemorrhages, leg cramps, impaired mentation, intracranial hemorrhage |
| Precipitation on cooling | Cryoglobulinemia (type I) | Raynaud phenomenon, acrocyanosis, ulcers, purpura, cold urticaria |
| Autoantibody activity to myelin-associated glycoprotein, ganglioside M 1 , sulfatide moieties on peripheral nerve sheaths | Peripheral neuropathies | Sensorimotor neuropathies, painful neuropathies, ataxic gait, bilateral foot drop |
| Autoantibody activity to IgG | Cryoglobulinemia (type II) | Purpura, arthralgia, renal failure, sensorimotor neuropathies |
| Autoantibody activity to red blood cell antigens | Cold agglutinins | Hemolytic anemia, Raynaud phenomenon, acrocyanosis, livedo reticularis |
| Tissue deposition as amorphous aggregates | Organ dysfunction |
|
| Tissue deposition as amyloid fibrils (light-chain component most commonly) | Organ dysfunction | Fatigue, weight loss, edema, hepatomegaly, macroglossia, organ dysfunction of involved organs (heart, kidney, liver, peripheral sensory and autonomic nerves) |
The increased plasma IgM levels lead to increased blood hyperviscosity and complications. The mechanisms behind the marked increase in the resistance to blood flow and the resulting impaired transit through the microcirculatory system are complex. The main determinants are (1) a high concentration of monoclonal IgMs, which may form aggregates and may bind water through their carbohydrate component; and (2) the interaction of IgMs with blood cells. Monoclonal IgM increases red cell aggregation (rouleau formation) and red cell internal viscosity while reducing red cell deformability. The presence of cryoglobulins contributes to increasing blood viscosity, as well as the tendency to induce erythrocyte aggregation. Serum viscosity is negligible up to an IgM concentration up to 30 g/L, then increases sharply at higher levels, with a median time to symptomatic hyperviscosity of 3 months when IgM levels surpass 60 g/L. Increased plasma viscosity may also contribute to inappropriately low erythropoietin production, which is the major reason for anemia in these patients. Renal synthesis of erythropoietin is inversely correlated with plasma viscosity. Clinical manifestations are related to circulatory disturbances that can best be appreciated by ophthalmoscopy, which shows distended and tortuous retinal veins, hemorrhages, and papilledema ( Fig. 92.4 ). Symptoms usually occur when the monoclonal IgM concentration exceeds 50 g/L or when serum viscosity is greater than 4.0 centipoises (cp), but there is individual variability, with some patients showing no evidence of hyperviscosity even at 10 cp. The most common symptoms are oronasal mucosal bleeding, visual disturbances because of retinal bleeding, and dizziness that rarely may lead to stupor or coma. Heart failure can be aggravated, particularly in the elderly, owing to increased blood viscosity, expanded plasma volume, and anemia. Inappropriate red cell transfusions can exacerbate hyperviscosity and may precipitate cardiac failure.
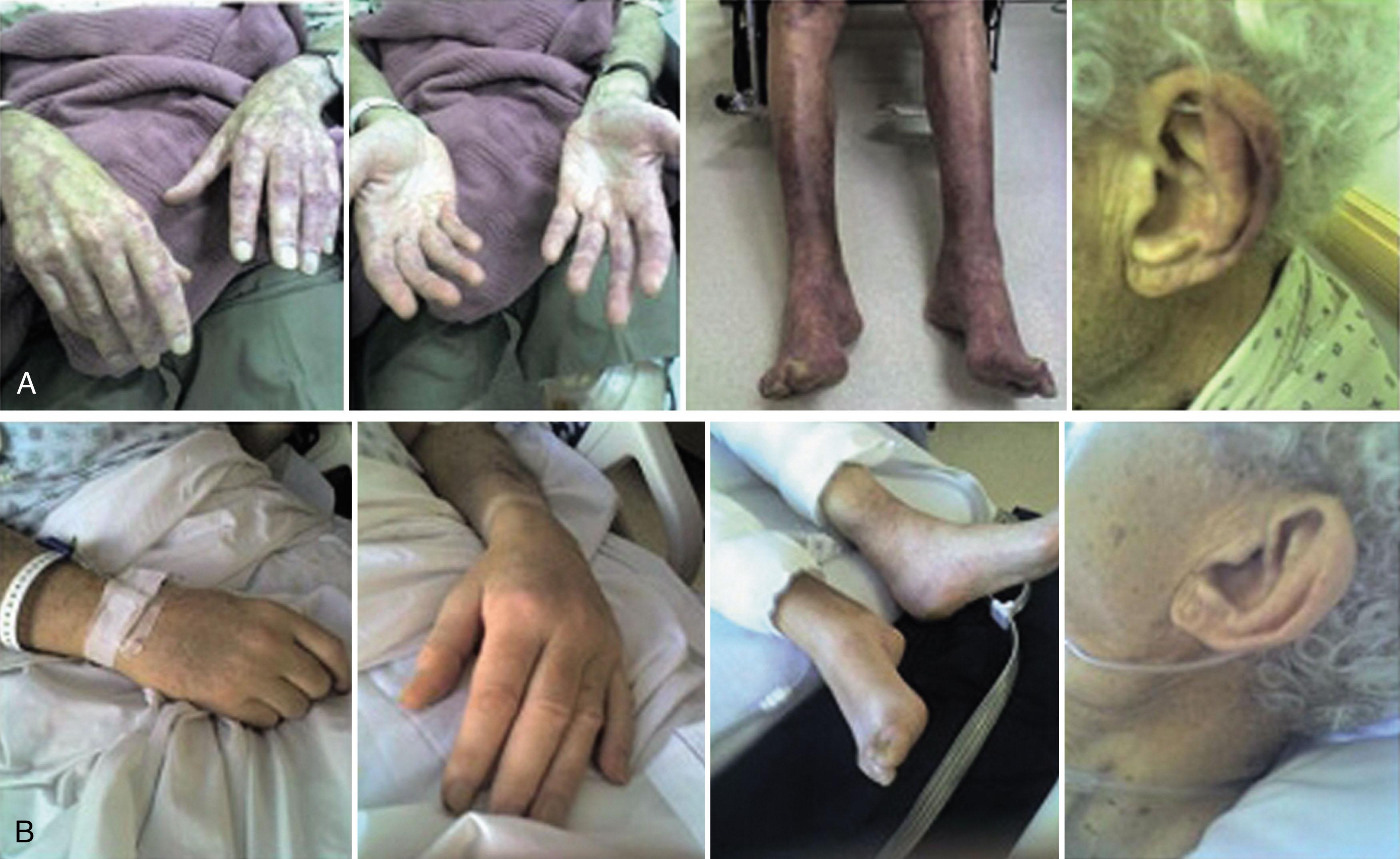
The monoclonal IgM can behave as a type I cryoglobulin in up to 20% of patients, and it leads to no symptoms in most cases. Cryoprecipitation is dependent mainly on the concentration of monoclonal IgM; for this reason, plasmapheresis or plasma exchange is commonly effective in this condition. Symptoms result from impaired blood flow in small vessels and include Raynaud’s phenomenon; acrocyanosis; necrosis of the regions most exposed to cold, such as the tips of the nose, ears, fingers, and toes ( Fig. 92.5 ); malleolar ulcers; purpura; and cold urticaria. Renal manifestations are infrequent. Mixed cryoglobulins (type II) consisting of IgM-IgG complexes may be associated with HCV infection.
Monoclonal IgM may exert its pathogenic effects through specific recognition of autologous antigens, the most notable being nerve constituents, immunoglobulin determinants, and red blood cell antigens.
IgM-related peripheral neuropathy is common in patients with WM, with estimated prevalence rates of 5% to 40%. Approximately 8% of idiopathic neuropathies are associated with a monoclonal gammopathy, with a preponderance of IgM (60%), followed by IgG (30%) and IgA (10%). The nerve damage is mediated by diverse pathogenetic mechanisms: (1) IgM antibody activity toward nerve constituents causing demyelinating polyneuropathies; (2) endoneurial granulofibrillar deposits of IgM without antibody activity, associated with axonal polyneuropathy; (3) occasionally by tubular deposits in the endoneurium associated with IgM cryoglobulin; and, rarely, (4) amyloid deposits or neoplastic cell infiltration of nerve structures.
Half of the patients with IgM neuropathy have a distinctive clinical syndrome that is associated with antibodies against a minor 100 kDa glycoprotein component of nerve known as the myelin-associated glycoprotein (MAG). Anti-MAG antibodies are generally monoclonal IgMκ and usually also exhibit reactivity with other glycoproteins or glycolipids that share antigenic determinants with MAG. The anti–MAG-related neuropathy is typically distal and symmetrical, affecting both motor and sensory functions; it is slowly progressive with a long period of stability. Most patients present with sensory complaints (paresthesias, aching discomfort, dysesthesias, or lancinating pains); imbalance and gait ataxia, owing to lack proprioception; and muscle atrophy in advanced stage. Patients with predominantly demyelinating sensory neuropathy in association with monoclonal IgM to gangliosides with disialosyl moieties, such as GD1b, GD3, GD2, GT1b, and GQ1b, have also been reported. Anti-GD1b and anti-GQ1b antibodies were associated with sensory ataxic neuropathy. These antiganglioside monoclonal IgMs present core clinical features of chronic ataxic neuropathy, sometimes associated with ophthalmoplegia and/or red blood cell cold agglutinating activity. The disialosyl epitope is also present on red blood cell glycophorins, thereby accounting for the red cell cold agglutinin activity of anti-Pr2 specificity. Monoclonal IgM proteins that bind to gangliosides with a terminal trisaccharide moiety, including ganglioside M 2 and GalNac-GD1A, are associated with chronic demyelinating neuropathy and severe sensory ataxia, unresponsive to glucocorticoids. Antiganglioside IgM proteins may also cross-react with lipopolysaccharides of Campylobacter jejuni , an infection known to precipitate the Miller Fisher syndrome, a variant of Guillain-Barré syndrome. Thus, molecular mimicry may play a role in this condition. Anti-sulfatide monoclonal IgM proteins, associated with sensory-sensorimotor neuropathy, have been detected in 5% of patients with IgM monoclonal gammopathy and neuropathy. Motor neuron disease has been reported in patients with WM and monoclonal IgM with anti-GM 1 and sulfoglucuronyl paragloboside activity. Polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (the POEMS syndrome) are rare in patients with WM.
Monoclonal IgMs may have cold agglutinin activity; they recognize specific red cell antigens at temperatures below 37°C, producing a chronic hemolytic anemia (see Chapter 47 ). This disorder occurs in less than 10% of patients with WM and is associated with cold agglutinin titers greater than 1:1000 in most cases. The monoclonal component is usually an IgMκ and reacts most commonly with red cell I/i antigens, resulting in complement fixation and activation. Mild to moderate chronic hemolytic anemia can be exacerbated after cold exposure. Hemoglobin levels usually remain above 70 g/L. The hemolysis is usually extravascular, mediated by the removal of C3b opsonized red cells by the mononuclear phagocytes, primarily in the liver. Intravascular hemolysis from complement destruction of red blood cell membrane is infrequent. The agglutination of red cells in the skin circulation also causes Raynaud syndrome, acrocyanosis, and livedo reticularis. Macroglobulins with the properties of both cryoglobulins and cold agglutinins with anti-Pr blood group specificity can occur. These properties may have as a common basis the binding of the sialic acid–containing carbohydrate present on red blood cell glycophorins, which involve the Pr blood group and Ig molecules. Several other macroglobulins with antibody activity toward autologous antigens (e.g., phospholipids, tissue and plasma proteins), and foreign ligands have also been described.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here