Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An adequate supply of vitamins and trace elements is critical in maintaining optimum health. Measurements of vitamin and trace element concentrations are frequently helpful in nutritional assessment and may be a requisite in suspected deficiency or toxicity, and in the management of patients with cystic fibrosis (CF), bariatric surgery, and for those on nutritional support in the intensive or critical care units. There is also great public interest in and many misconceptions about vitamins and trace elements.
This chapter describes the chemistry, dietary sources, absorption, transport, metabolism, excretion, functions, and recommended intakes of the essential vitamins and trace elements required in humans. These include the fat-soluble vitamins A, E, K with the exception of D; the water-soluble vitamins B 1 , B 2 , B 6 , B 12 , C, folate, biotin, niacin, and pantothenic acid; and the trace elements chromium (Cr), cobalt (Co), copper (Cu), iodine (I), manganese (Mn), molybdenum (Mo), selenium (Se), and zinc (Zn). Free radicals, their measurement, and the trace elements fluoride (F), boron (B), silicon (Si), and vanadium (V) are briefly discussed. The causes and effects of vitamin and trace element deficiency and toxicity are outlined and the laboratory assessment of status, preanalytical variables effecting the methods, and suggested reference intervals are critically evaluated. Some illustrative cases are included. For methodologic details, readers are invited to access the given original references provided in this chapter.
Ancient Egyptians knew that eating ox liver could cure night blindness. During ocean voyages to discover new lands and trade, sailors endured prolonged periods without fresh fruits and vegetables that resulted in vitamin deficiencies. In 1747, the Scottish surgeon James Lind discovered that citrus fruits could prevent scurvy, a disease that caused poor wound healing, bleeding of gums, and a typical perifollicular hemorrhagic rash among others symptoms in sailors. He recommended using lemons and limes, which was adopted by the British Royal Navy that led to the nickname “Limey” for their sailors. In parts of Asia where polished white rice is a staple food, lack of vitamin B 1 resulted in beriberi. In 1884, a Japanese navy physician, Takaki Kanehiro, made the observation that beriberi was endemic in the low-ranking crew who only ate rice. With the support of the Japanese Navy, he undertook experiments using two crews; one fed only white rice and the other a mixed diet and observed that the former had a higher incidence of beriberi. Unfortunately, he concluded that insufficient protein was the cause. It was not until 1897 when Christiaan Eijkman discovered that feeding unpolished rice rather than the polished variety to chickens helped prevent beriberi in them. Hopkins and Eijkman were awarded the Nobel Prize for Physiology and Medicine in 1929 for their discovery of several vitamins. Around the turn of the 20th century, it was recognized that nutritional deficiencies caused diseases, in addition to the then prevailing germ theory of disease. Until the 1930s, when the first commercial yeast extract vitamin B complex and synthetic vitamin C tablets were first sold, vitamins were obtained solely through food. Today vitamin and food supplements are a multi-billion-dollar industry, but it has been argued that they offer very little benefit beyond consuming a healthy balanced diet. There is an increasing need for improved regulation in the vitamin industry that is also plagued with false medical claims. Specialist clinical laboratories have expanded their test repertoire to include the measurement of vitamins and trace elements. In this chapter, the biochemistry and clinical application of the commonly measured vitamins and trace elements are discussed. For a list of suggested readings on vitamins and trace elements, see the Selected References list included at the end of this chapter.
An adequate supply of micronutrients (vitamins and trace elements) is critical in maintaining health. A vitamin is an essential nutrient that cannot be synthesized in the body and must be provided in the diet. The general principle regarding assessment of nutritional status is to determine the extent to which the metabolic demand for nutrients has been or is currently being met by the supply. In clinical practice, this requires balancing supply and demand.
Accurate assessment of supply and intake is a complex process. In practice, a crude estimate of intake is obtained from a careful clinical history obtained by an experienced practitioner or from a food-frequency questionnaire that summarizes the content of the individual’s diet over several days, depending on how frequently particular typical foods are consumed. A more accurate quantitative assessment usually requires a minimum of 3 days’ recording of a complete dietary diary, which is subsequently analyzed using a computer program with reference tables of the nutritional contents of most foods. Unfortunately, estimates of portion size, amounts consumed, and actual nutritional composition of the food consumed may be inaccurate. In addition, disease processes associated with vitamin deficiency must be considered.
Requirements for most micro- and macronutrients that are required to maintain health have been characterized and made available in reports from the Institute of Medicine (IOM) of The National Academies. , However, the effects of disease may increase physiologic demands for such nutrients. For example, hypermetabolism, as a result of trauma or infection, increases the requirements for protein, energy, and micronutrients. Increased losses of nutrients from the gut, kidney, and skin, or through dialysis, may also increase overall nutrient demands.
Table 39.1 summarizes the Recommended Dietary Allowance (RDA) , used in the United States and the Population Reference Intakes (from the European community) for micronutrients. Table 39.1 also summarizes the amounts present in 2000 kcal of most tube-feeds used in most nutritional support therapy. Nutrients consumed tend to be greater than the recommended oral amounts in order to meet increased needs resulting from preexisting deficiencies or due to the disease processes. Doses recommended for intravenously administered nutrition are also summarized in Table 39.1 . For trace elements, these doses are generally less than the oral doses and/or enteral requirements, which allow for reduced absorption. For the vitamins, these are usually greater than the oral and/or enteral requirements to compensate for deficiencies due to disease.
| RDA (USA) | PRI (Europe) | Some Natural Food Sources , | Amount in 2000 kcal Tube Feed | IV Intake , , | |
|---|---|---|---|---|---|
| Vitamins | |||||
| A, μg | 900 (men); 700 (women) | 700 | Liver, yellow/orange fruits, leafy vegetables, fish, soya milk, milk | 1000–2160 | 1000 |
| E, mg | 15 (men and women) | 0.4/g PUFA | Fruits and vegetables, meats, vegetable oils, unprocessed cereals, nuts and seeds | 20–64 | 9.1 |
| K, μg | 120 (men); 90 (women) | 100–200 | Leafy green vegetables especially spinach, eggs, liver | 150 | |
| B 1 , mg | 1.2 (men); 1.1 (women) | 1.1 | Brown rice, vegetables, potatoes, liver, eggs | 1.4–3.4 | 6 |
| B 2 , mg | 1.3 (men); 1.1 (women) | 1.6 | Vegetables such as green beans and asparagus, bananas, dairy products | 2–6 | 3.6 |
| B 6 , mg | 1.3 (men & women <50 years); 1.7 (men >50 years); 1.5 (women >50 years) | 1.5 | Meat, bananas, vegetables, nuts | 2–13.8 | 6 |
| B 12 , μg | 2.4 | 1.4 | Meat and animal products | 3–15 | 5 |
| Folate, μg | 400 | 200 | Leafy vegetables, cereal especially if fortified, bread, pasta, liver | 340–880 | 600 |
| C, mg | 90 (men); 75 (women) | 45 | Fruits especially citrus fruits and vegetables | 100–300 | 200 |
| Biotin, μg | 30 a (men and women) | 15–100 | Eggs, liver, kidney, pancreas, yeast, milk | 100–660 | 60 |
| Niacin, mg | 16 (men); 14 (women) | 18 | Meat, fish, eggs, vegetables, mushrooms, nuts | 18–45 | 40 |
| Pantothenic acid, μg | 5 a | 3–12 † | Meat, vegetables like broccoli, avocadoes, grains | 7–20 | 15 |
| Trace Elements | |||||
| Zinc, mg | 11 | 9.5 | Red meats and some seafood | 13–36 | 3.2–6.5 |
| Copper, mg | 0.9 | 1.1 | Organ meats, seafood, nuts, seeds, grains, cocoa products | 2–3.4 | 0.3–1.3 |
| Selenium, μg | 55 | 55 | Organ meats, seafood, nuts, vegetables (dependent of soil selenium content) | 30–130 | 40–100 |
| Chromium, μg | 25 | 30–200 | Cereals, meats, fish | 10–20 | |
| Molybdenum, μg | 45 a | 74–240 | Legumes, grains and nuts | 19 | |
| Manganese, mg | 2–3 a | 1–10 b | Nuts, legumes, tea, grains | 2.4–8 | 0.05–0.2 |
To accurately assess nutritional status, it is crucial to understand laboratory testing limitations that may be challenging in a clinical context of acute illness. Nutritional assessment in health and disease is considered in detail in Chapter 46 .
Although requirements for vitamins and trace elements in health are known (see Table 39.1 ), the effects of illness on these requirements are not fully understood. However, as deficiency progresses, subclinical stages can slowly manifest. For example, subclinical deficiency of folic acid is associated with an increase in serum homocysteine concentration, which has been proposed to be an independent risk factor for coronary artery disease. Furthermore, folate supplements may not reduce this risk. Similarly, subclinical deficiency of chromium may be associated with impaired glucose tolerance and diabetes.
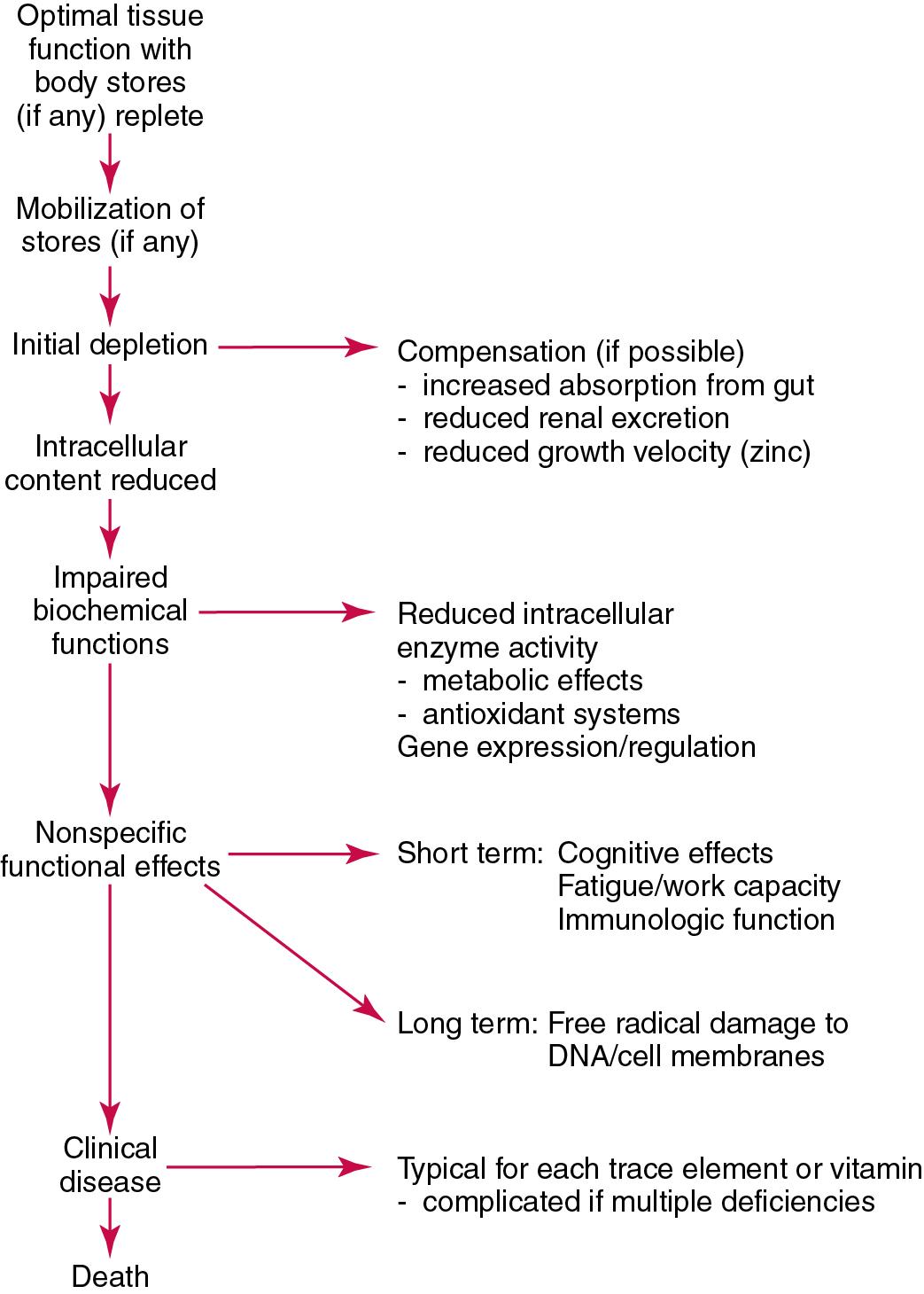
The time course for development of a subclinical deficiency state varies for each individual vitamin and trace element and depends on the nature and quantity of body stores. Moreover, the extent of depletion necessary before significant changes occur is poorly characterized. The consequences of an inadequate intake are illustrated in Fig. 39.1 . This figure shows a progression from optimal tissue status through a period of initial depletion, until a period of subclinical deficiency is reached. In some cases, certain nonspecific histologic changes may lead to risk of tissue damage or neoplastic change. With persistent mismatch of intake and demand, complete clinical deficiency state could develop.
As for all laboratory tests, interpretation of tests of nutritional status requires access to relevant reference intervals and an understanding of factors that may alter them. Different testing methods will yield differing reference intervals. Reference intervals should be used as a guide and considered in the clinical context. Variables such as age, gender, ethnicity, and geographic location might affect the choice of typical diet, thereby varying the concentration of vitamins. These factors introduce both within- and between-subject biological variation. Seasonal variations may also alter the observed concentrations for vitamins such as vitamins D and A.
Several enzymes are dependent upon specific vitamins for functionality and may therefore be used as a surrogate measure of the vitamin’s status. These include erythrocyte enzymes such as transketolase (a marker of thiamine status), glutathione reductase (for riboflavin status), or transaminase (for pyridoxine status). Glutathione peroxidase measured in plasma or erythrocytes may be used as an index of selenium status. These are discussed further in corresponding vitamin and trace element sections.
Concentrations of vitamins and trace elements are measured most often in plasma or serum; this provides a reliable index of status for only a few of them (e.g., vitamin B 12 and vitamin D). For others (e.g., folate), their concentrations may reflect only the adequacy of recent intake. For some vitamins and trace elements, serum measurement is of limited value, especially in seriously ill patients. In part, this is a result of the lack of correlation between the amount of nutrient in the plasma compartment and the amount within the intracellular compartment in most body tissue. For example, substantial stores of particular vitamins or trace elements may be present in individual tissue (e.g., vitamin A in the liver), but mobilization into the plasma is affected by the availability of appropriate binding proteins or by altered metabolism during the acute phase of an illness. Also, differences in the content of individual vitamins have been noted among tissues, but this may not be reflected in serum concentrations.
Tissue concentrations of vitamins or trace elements are rarely measured in nutritional assessment as this involves biopsy; however, when such tissue is available, measurement may be helpful (e.g., copper analysis on liver biopsy of patients with suspected Wilson disease). More commonly, certain types of cells may be obtained from blood samples and can provide useful information. For example, red cell folate is often used as a marker of folate status, and leukocyte ascorbic acid is considered to be a better indicator of body stores than plasma ascorbate, but its use has not been widely adopted because of the large sample volume requirement, the difficulty involved in automating the analysis, the influence of fluctuating leukocyte numbers, and the relative difficulty of the analysis. ,
For most vitamins, their measurement in urine is rarely helpful because most are not under homeostatic control, and excretion may be a direct reflection of recent intake rather than active retention in the face of whole-body deficiency. Urine measurements indicate loss, particularly of water-soluble low-molecular-weight complexes (free or non–protein bound) and may be more useful in assessing overall removal and/or exposure. High concentrations of excretion of certain water-soluble vitamins or trace elements may indicate ingestion of large quantities of supplements and not necessarily reflect a deficiency or toxicity. However, pantothenic acid is measured primarily in urine. Some metabolites of vitamin metabolism may be measured in urine, for example, the assessment of niacin status has been based on measurement of the two urinary metabolites, N ′-methylnicotinamide and N ′-methyl-2-pyridone-5-carboxamide. Urinary excretion of biotin and 3-hydroxyisovaleric acid also appear to be better indicators of biotin status than blood concentrations alone. The presence of low urinary iodide indicates iodide intake from a poor diet and is recommended by the WHO as a marker of iodine status.
The concentration in plasma of various vitamins and trace elements can alter significantly when a systemic inflammatory response syndrome (SIRS; previously known as the acute-phase response [APR]) results from trauma or infection. , This usually occurs independently of tissue stores. , The associated changes may be a result of variations in the binding proteins in plasma such as albumin or retinol-binding protein (RBP), which decrease as part of SIRS. Studies in patients with SIRS as categorized on the basis of increased C-reactive protein (CRP) concentrations have documented decreases in vitamin A, , E, , , B 2, B 6, C, , D, and carotenoids, and changes in the concentrations of many trace elements.
In patients who are relatively stable, with minimal SIRS after injury, infection, or other inflammatory disease, it may be possible to interpret the plasma concentrations of trace elements or of vitamins. One study in 1303 patients whose samples were referred to the Scottish Trace Element and Micronutrient Reference Laboratory for assessment of their micronutrient status examined the effect of the magnitude of SIRS on plasma micronutrient concentrations with the aim of providing guidance on the interpretation of results. The findings are shown in Table 39.2 . The study concluded that the degree of inflammatory response affected the interpretation of plasma micronutrient concentrations and demonstrated that a reliable clinical assessment could be made only if the CRP was less than 10 mg/L for vitamins A and D or less than 5 mg/L for vitamins B 6 and C. There have been guidelines issued on nutrition support by national bodies such as the National Institute for Health and Care Excellence (NICE) in the UK that recommend the monitoring of micronutrient concentrations in patients receiving total parenteral nutrition (TPN) ; therefore when implementing these guidelines it is important to take the effect of SIRS into account when deciding on nutritional support. See illustrative case in Box 39.1 .
| CRP Concentration (mg/L) | Vitamin A (μmol/L) | Vitamin D (nmol/L) | Vitamin B6 (μmol/L) | Vitamin C (μmol/L) |
|---|---|---|---|---|
| ≤5 | 2.0 | 34 | 48 | 23 |
| >5–10 | 2.0 | 33 | 27 | 18 |
| >10–20 | 1.8 | 31 | 32 | 17 |
| >20–40 | 1.6 | 27 | 24 | 8 |
| >40–80 | 1.4 | 23 | 18 | 6 |
| >80 | 1.0 | 20 | 15 | 5 |
A 45-year-old man, who had been previously fit and well, was admitted to intensive trauma unit (ITU) after a car accident resulting in multiple fractures and subdural hematoma. He underwent craniotomy and decompression. He remained hemodynamically stable over the next few days with continuous ventilation. He was fed enterally via a nasogastric tube. He had no history of alcohol or recreational drug use and no significant past medical history. After 5 days in ITU a micronutrient screen together with routine blood tests were ordered. The results for vitamins and selected trace elements were as follows:
| Micronutrient | Result (Local Reference Interval) | Result (Local Reference Interval) |
|---|---|---|
| Vitamin A | 0.5 μmol/L (1.0–2.8) | 14 μg/dL (29–80) |
| Vitamin E | 10 μmol/L (15–40) | 0.4 mg/dL (0.6–1.7) |
| Vitamin C | 10 μmol/L (15–90) | 0.17 mg/dL (0.26–1.6) |
| Vitamin B 6 | 13 nmol/L (20–140) | 3.2 ng/mL (5–35) |
| Beta carotene | 0.6 μmol/L (1.6–5.8) | 34 μg/L (90–310) |
| Zinc | 8 μmol/L (12–18) | 52 μg/dL (78–118) |
| Selenium | 0.3 μmol/L (0.8–2.0) | 24 μg/L (63–157) |
| Total protein | 45 g/L (60–80) | 4.5 g/dL (6.0–8.0) |
| Albumin | 16 g/L (35–50) | 1.6 g/dL (3.5–5.0) |
| C-reactive protein (CRP) | 120 mg/L (<3) | 120 mg/L (<3) |
Commentary : There is a significant decrease in the plasma concentration of all the measured vitamins and trace elements, which may suggest the patient has developed a deficiency of micronutrients. CRP is a positive acute-phase reactant and rises with acute inflammation or infection. , Albumin is a negative acute-phase reactant and most patients in the ITU tend to have a low concentration. These results indicate the presence of a systemic inflammatory response and have important implications for interpreting vitamin and trace element results. Most trace elements and vitamins are negative acute-phase reactants and, therefore when measured in the presence of an acute inflammation, will be low and may be interpreted to indicate micronutrient deficiency. In inflammation capillary permeability increases, resulting in leakage of albumin into the extracellular space. As many macronutrients are bound to albumin and other circulating proteins, there is a transient fall in the measured concentration. On full recovery, there is normalization of micronutrient concentrations. Other causes of low micronutrients during the inflammatory response include sequestration in the liver and other organs, increased utilization in the catabolic state and increased renal excretion. , The short duration of acute illness in the patient in this case was not sufficient to result in micronutrient deficiency, especially because of his apparent healthy state prior to the accident. In this case, supplementation was not indicated.
A free radical is any chemical species (atom, ion, or molecule) capable of independent existence that has an unpaired electron in an orbital, usually rendering them highly reactive. The hydrogen atom is the simplest radical since it contains only one electron. Free radicals can be formed by the loss or gain of a single electron from a nonradical, for example the superoxide species (O 2 − ) generated during the reduction of molecular oxygen (O 2 ) as occurs in the electron transport chain. Reactive oxygen species (ROS) are chemically reactive molecules containing O 2 , for example, hydrogen peroxide (H 2 O 2 ), which is formed by the dismutation of O 2 − or direct reduction of O 2 . Free radicals can be formed endogenously in the body by external stimuli such as irradiation, chemicals from air pollutants, or as byproducts of cellular metabolism. Most oxygen radicals are generated in the mitochondria by the electron transport chain. Several heme proteins generate O 2 − in the presence of O 2 catalyzed by transition metal ions, particularly Fe or Cu.
Free radicals have important functions in the body:
Defense against micro-organisms—phagocytic cells such as macrophages and neutrophils use free radicals to destroy infective agents.
Signal transduction—free radicals have been shown to act in numerous signaling pathways such as the nuclear facto-kappa beta (NF-κβ) signaling pathways, which is crucial for immunity, cell development, and growth.
Mitogenic effects—gene expression has also been shown to be altered by free radicals in both a deleterious and desirous manner.
Free radicals have been implicated in various diseases including atherosclerosis, cancer, diabetes mellitus, and neurologic conditions such as Parkinson disease and Alzheimer disease. , Mechanisms involved in these diseases include damage to DNA strands, lipid peroxidation, and protein degradation.
Organisms have evolved intricate ways of combating free radical damage. These include enzymatic and nonenzymatic antioxidants. An antioxidant is a substance produced in sufficient quantity that neutralizes the lone electron of free radicals. Enzymatic antioxidants include peroxidases, for example, glutathione peroxidase, catalase, and superoxide dismutase (SOD). Nonenzymatic antioxidants include ascorbic acid (vitamin C), α-tocopherol (vitamin E), glutathione, carotenoids, flavonoids, urate, and proteins such as albumin, transferrin, and ceruloplasmin. The balance between free radical and antioxidant activity is crucial, as both have important physiologic roles. It is also possible that the reducing agent (antioxidant) may facilitate pro-oxidant activity by regenerating the oxidized forms involved in the reduction process (e.g., the production of hydrogen peroxide and hydroxyl radicals in the presence of Fe 3+ ). Similarly it has been proposed that selenium toxicosis exerts pro-oxidant activity due to methyl-selenide formation with the production of superoxide radicals and induction of oxidative stress.
Because it is not possible to measure all the active antioxidants in human samples, the concept of a “global” assessment of antioxidant capacity has been used. The main approaches to this measurement include: (1) quenched or delayed production of a stable, measurable radical species, for example the total radical-trapping antioxidant parameter (TRAP) assay, which uses the stable radical species 2,2′-azinobis (3-ethylbenzthiazoline sulfonate) (ABTS + ) assay ; (2) the use of reduction properties of antioxidants against a radical cation or a metal ion, for example the ferric reducing ability of plasma (FRAP) ; or (3) the assessment of products of oxidative metabolism as a measure of the functional adequacy of vitamins and trace elements involved in antioxidant pathways, for example malondialdehyde and F 2 isoprostanes, both of which give an indication of oxidation of polyunsaturated fatty acids within cells. Measurements of total antioxidant capacity are usually standardized with the water-soluble vitamin E analog Trolox. It has been suggested that more than one method should be used to validate any analysis as different methods give varying results. Vitamins that contribute to plasma antioxidant capacity include ascorbate (up to 24% of measured capacity) and α-tocopherol (up to 10%).
A disadvantage of many methods of total antioxidant capacity measurement is the variable contribution of common plasma constituents, particularly albumin and urate, to the measured concentration. Changes in circulating concentrations of these molecules caused by acute-phase changes or changes in renal function can alter measured values without reflecting changes in antioxidant vitamin concentration. This problem is typically resolved by the use of the antioxidant gap, a derived value that subtracts the Trolox equivalence of albumin and urate from the measured total antioxidant capacity.
Few clinical studies have demonstrated any significant benefit from provision of increased quantities of antioxidants. One recent randomized control trial concluded that the early provision of antioxidants or glutamine did not improve clinical outcomes but was associated with an increase in mortality among critically ill patients with multi-organ failure. In general, most studies have shown that antioxidant supplementation usually with vitamin C and/or vitamin E had no beneficial effect and may have been harmful when given at levels substantially above those normally found in the diet.
The word vitamin was coined in 1912 by the polish biochemist Casimir Funk derived from “vitamine” or “vital amine” meaning the “amine of life,” as it was thought at the time that the disease-preventing constituents in food were amines, a fact that was true for thiamine but not other vitamins. Vitamins are organic compounds required in trace amounts (microgram to milligram quantities per day) in the diet for health, growth, and reproduction, which is small in contrast to the relatively large amounts of such macronutrients as protein, lipid, and carbohydrate.
Vitamin groups have historically been classified using a letter of the alphabet followed by an Arabic numeral in subscript to designate structural and functional similarity, for example, A 1 —retinol and A 2 —3-dehydroretinol, or to indicate the approximate order in which they were first identified as members of the so-called B-complex, for example, B 1 —thiamine and B 2 —riboflavin. Common or generic chemical names give a better indication of the types of compounds involved. These often reflect the presence of some specific atom ( thia mine), the prime functional group (pyridox amine ), a larger portion of the molecular structure (phyllo quinone ) or reflect the vitamin’s functional properties (chole calciferol ).
Another classification refers to the relative solubility of vitamins. Those of the fat-soluble group include vitamins A, D, E, and K which are more soluble in organic solvents, whereas B-complex group vitamins and vitamin C are water soluble. The fat-soluble vitamins are absorbed, transported, and stored for longer periods of time and in a manner similar to fat and this has implications for supplementation and deficiency as discussed below. Water-soluble vitamins function as coenzymes for several important enzymatic reactions in both mammals and microorganisms; by contrast, fat-soluble vitamins generally do not function as coenzymes and are rarely used by microorganisms.
The term vitamer refers to chemical compounds that generally have a similar molecular structure and that belong to a common or generic vitamin group. For example, vitamin A includes the vitamers: carotenoids (α-carotene, β-carotene, γ-carotene and the xanthophyll β-cryptoxanthin), retinoic acid, retinol, and retinal. Typically, vitamers serve similar functions but have varying potency dependent on differences in absorption and interconversions from one form to another. Table 39.3 gives a list of 13 known vitamins and vitameric groups essential to humans.
| Common or Generic Name | Vitamer or Common Chemical Name | Function | Symptoms and Causes of Deficiency or Associated Diseases | Currently Used Methods | Reference Intervals |
|---|---|---|---|---|---|
| Fat-Soluble Vitamins | |||||
| Vitamin A | Retinol, retinal, retinoic acid, carotenoids | Antioxidant, role in vision, gene expression, embryonic development, immune and reproductive functions | Nyctalopia (night blindness), Bitot spot, xerophthalmia, keratomalacia. Common in infants and children especially in less developed countries; due to fat malabsorption, cystic fibrosis and may occur due to retinol binding protein deficiency as a results of protein malnutrition, liver disease and zinc deficiency | HPLC, LC-MS/MS |
|
| Vitamin E | Tocopherols, tocotrienols | Antioxidant (prevents the peroxidation of unsaturated fatty acids), role in gene transcription, immunity, inhibits platelet aggregation, recently been implicated in bone physiology | Lipid peroxidation, red blood cell fragility causing hemolytic anemia especially in premature infants. May occur due to fat malabsorption, cystic fibrosis | HPLC, LC-MS/MS | Premature neonates: 0.1–0.5 mg/dL (2.3–11.6 μmol/L) 1–12 years: 0.3–0.9 mg/dL (7–21 μmol/L) 13–19 years: 0.6–1.0 mg/dL (14–23 μmol/L) >19 years: 0.5–1.8 mg/dL (12–42 μmol/L) As a ratio of cholesterol: 3.5 to 9.5 μmol/mmol cholesterol |
| Vitamin K | Phylloquinones (K 1 ), menaquinones (K 2 ), menadiones (K 3 ) | Coagulation, bone metabolism | Increased clotting time, hemorrhagic disease of the newborn. Also due to fat malabsorption, cystic fibrosis, liver disease | Prothrombin time, PIVKA, HPLC, LC-MS/MS | 0.2–2.2 nmol/mmol triglyceride |
| Water-Soluble Vitamins | |||||
| Vitamin B 1 | Thiamine, aneurin | Forms the coenzyme thiamine pyrophosphate (TPP) required for decarboxylation reactions involved in carbohydrate metabolism, and nerve function | Beriberi, Wernicke-Korsakoff syndrome in alcoholics, rare thiamine-responsive IOM | Erythrocyte transketolase, HPLC, LC-MS/MS | Erythrocyte transketolase activity: 0.75–1.30 U/g Hb (48.4–83.9 kU/mol Hb) Percent TPP effect (activation): Normal: 0–15% Marginal: 16–25% Deficient: >25% TPP concentration: 173–293 nmol/L or 280–590 ng/g Hb (erythrocytes). 90–140 nmol/L or 275–675 ng/g Hb (whole blood) |
| Vitamin B 2 | Riboflavin | Essential component of coenzymes involved in reduction-oxidation (redox) reactions in the body | Angular stomatitis, dermatitis, photophobia, riboflavin-dependent IOM | Erythrocyte glutathione reductase, HPLC, LC-MS/MS, | Erythrocyte glutathione reductase activation by FAD: Adequacy: 1.20 Marginal: 1.21–1.40 Deficiency: >1.41 Serum or plasma concentrations of riboflavin (median [range]): plasma FAD: 101 [57–170] nmol/L plasma FMN: 6.3 [3.3–14.1] nmol/L plasma riboflavin: 11 [4–34] nmol/L erythrocyte FAD: 1.9 [0.7–3.8] pmol/g Hb erythrocyte FMN: 0.11 [0.04–0.44] pmol/g Hb erythrocyte riboflavin: 0.02 [0.01–0.13] pmol/g Hb |
| Vitamin B 3 | Niacin, nicotinic acid, nicotinamide | Coenzyme or cosubstrate in many biological redox reactions, and thus for energy metabolism. | Pellagra (dermatitis, dementia, diarrhea). Seen in communities with corn-based staple diets, in carcinoid syndrome (precursor tryptophan diverted to serotonin formation), Hartnup disease (unable to absorb tryptophan), and medications such as isoniazid |
HPLC & LC-MS/MS for urine metabolite, nicotinamide coenzymes | 2.4–6.4 mg/day (17.5–46.7 μmol/day) or 1.6–4.3 mg/g creatinine (11.7–31.4 μmol/g creatinine) |
| Vitamin B 5 | Pantothenic acid, panthenol, pantethine | General metabolism, acetyl and acyl transfer | Burning feet syndrome | Microbiological, CPB, HPLC, LC-MS, GC-MS | Whole blood or serum: 344–583 μg/L (1.57 to 2.66 μmol/L) Urinary excretion: 1–15 mg/d (5–68 μmol/day) |
| Vitamin B 6 | Pyridoxine, pyridoxal, pyridoxamine | the active form pyridoxal phosphate is required for synthesis, catabolism of various amino acids | Epileptiform convulsions, dermatitis, anemia, medications such as penicillamine and isoniazid decrease it, pyridoxine-responsive IOM notably homocystinuria, and hyperhomocysteinemia (together with vitamin B 12 and folate deficiencies) | Aspartate transaminase, HPLC, LC-MS/MS | Plasma PLP: 9.5–24 ng/mL (39–98 nmol/L); Erythrocyte PLP: 250–680 pmol/g Hb |
| Vitamin B 7 | Biotin, vitamin H | Coenzyme for carboxylation reactions involved in gluconeogenesis, lipogenesis and catabolism of branched-chain amino acids; roles in cell signaling, epigenetic regulation of genes and chromatin structure | Dermatitis, developmental delay. Seen with excessive raw egg consumption, those on parenteral nutrition and IOM notably biotinidase deficiency |
Microbiological, CPB, carboxylases, avidin binding, urinary metabolites | 0.5–2.20 nmol/L |
| Vitamin B 9 | Pteroylglutamic acid, folic acid, folate | Required for the interconversions of amino acids such as homocysteine to methionine and the biosynthesis of purines and pyrimidines, required for DNA synthesis | Megaloblastic anemia, neural tube defects. Caused by gut sterilization, malabsorption, decreased intake, increased requirements, e.g., pregnancy, medications, e.g., methotrexate, anti-convulsants. Deficiency linked to hyperhomocysteinemia, cancer and stroke |
Red blood cell and serum folate, CPB, microbiological, homocysteine | >3 μg/L (7.0 nmol/L) for serum folate and less than 150 μg/L (340 nmol/L) for RBC folate |
| Vitamin B 12 | Cyanocobalamin, hydroxocobalamin, methylcobalamin | Required for erythropoiesis, methylation processes necessary for DNA and cell metabolism, and is cofactor for various enzymes notably those involved in the metabolism of methylmalonic acid and homocysteine | Pernicious and megaloblastic anemia, peripheral neuropathy. Caused by decreased intake (vegetarians), short bowel syndrome (loss of distal ileum), malabsorption syndromes, medications (N 2 O, phenytoin, methotrexate, and proton pump inhibitors), and Imerslund-Grasbeck syndrome. Deficiency results in methylmalonic aciduria and homocysteinemia |
CPB, immunometric, microbiological, methylmalonate, homocysteine, holotranscobalamin | WHO consultation defined a serum vitamin B 12 concentration less than 203 ng/L (150 pmol/L) as deficient |
| Vitamin C | Ascorbic acid | Connective tissue formation, antioxidant | Scurvy, infantile scurvy (Barlow disease). Linked with osteoporosis, anemia, diabetes mellitus, cancer |
Spectrophotometric-enzymatic methods, HPLC | 0.4 and 1.5 mg/dL (23–85 μmol/L) or 20–53 μg/10 8 leukocytes (1.14–3.01 fmol/leukocyte) |
Vitamin A serves an important role in vision, is required for gene expression, embryonic development, and immune and reproductive functions, and is an antioxidant. An illustrative case of vitamin A deficiency has recently been reported ( Box 39.2 ).
A 13-year-old boy was referred by his primary care physician to the local hospital with a few months’ history of progressively “fuzzy” vision, particularly at night. Apart from a history of “fussy eating” habits, his past medical history was unremarkable. On preliminary assessment, he was systemically well, interacting appropriately and clinical examinations were all normal. His height and weight were on the 50th centile for age. On ophthalmologic examination, he was found to have reduced visual acuity in the left eye with no perception of light. He had normal vision in the right eye. An MRI brain scan showed normal appearances of the optic nerves and chiasm. Electrodiagnostic testing was carried out and an electroretinogram (ERG) demonstrated a complete absence of rod function but nearly normal cone function. This pattern was in keeping with that seen in vitamin A deficiency. Selected blood test results are shown below. An extremely low vitamin A concentration of less than 0.3 μmol/L (reference interval: 0.9 to 2.5) was found. Other routine blood test results not shown were all within reference limits. His dietary history was evaluated. The patient had an extremely selective eating pattern, consuming only potato chips, French fries, custard, and diluting juice since the age of 2 years. He was commenced on oral vitamin A supplementation and is showing continued improvement in his degree of visual loss. Ongoing care issues include improving nutritional status with dietetic and psychological input and repeat ophthalmology and electrodiagnostic testing to monitor progress.
| Result (Local Reference Interval) | ||
|---|---|---|
| Vitamin A | <0.3 μmol/L (0.9–2.5) | <8.6 μg/dL (26–72) |
| α-Carotene | <0.2 μmol/L (0.3–1.1) | <10 μg/L (14–60) |
| Vitamin B 12 | 176.8 pmol/L (190–900) | 241 pg/mL (259–1227) |
| Vitamin D | 61 nmol/L (25–170) | 24 ng/mL (10–68) |
| Vitamin E | 14 μmol/L (13–24) | 0.6 mg/dL (0.56–1.0) |
| Adjusted calcium | 2.14 mmol/L (2.20–2.70) | 8.6 mg/dL (8.8–10.8) |
Commentary: Blindness secondary to vitamin A deficiency is common in developing countries. However, sporadic cases can occur in developed countries due to nutritional insufficiency secondary to food faddism in otherwise healthy children. The condition has a variable course. Permanent visual damage is possible in cases of prolonged or severe visual loss. Rod function appears to recover most quickly and completely and central cones, if affected (as in this case), have slower recovery. , This case highlights the importance of checking for visual problems in fussy eaters and those at risk of nutritional deficiencies, thereby ensuring that appropriate management is undertaken to prevent permanent visual loss.
Vitamin A is the nutritional term for the group of compounds with a 20-carbon structure containing a methyl-substituted cyclohexenyl ring (β-ionone ring) and an isoprenoid side chain ( Fig. 39.2 ), with a hydroxyl group (retinol), an aldehyde group (retinal), a carboxylic acid group (retinoic acid), or an ester group (retinyl ester) at the terminal C15.
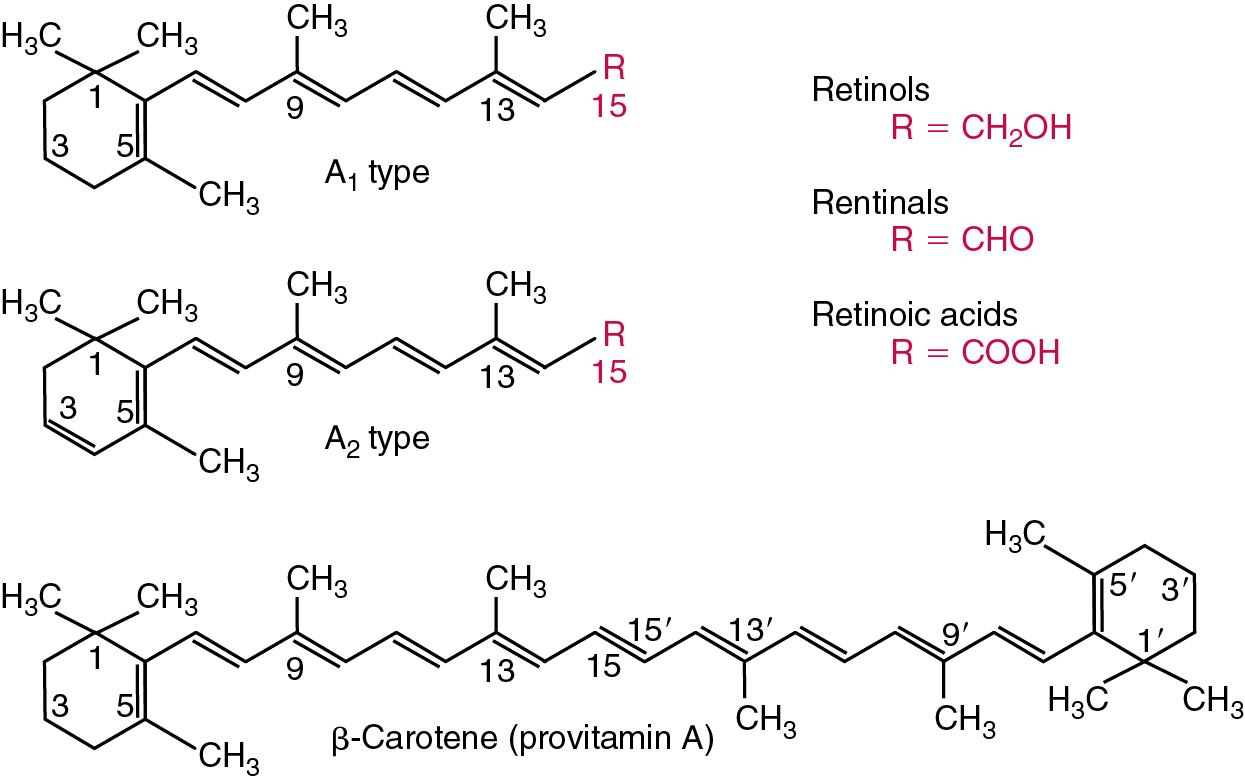
Retinol, the principal vitamin A vitamer, can be oxidized reversibly to retinal—which shares all the biological activity of retinol—or further oxidized to retinoic acid, which shows some of its biological activity. The principal storage forms of vitamin A are retinyl esters, particularly palmitate. The term retinoids refers to retinol, its metabolites, and synthetic analogs with similar structure. Included in the vitamin A family are some dietary carotenoids (C40 polyisoprenoid compounds) that are classified as provitamin A because they are cleaved biologically to yield retinol. Although around 1000 compounds with carotenoid structure have been identified, only about 50 possess provitamin A activity, with the principal dietary compounds being β-carotene, α-carotene, and β-cryptoxanthin. Carotenoids are vitamin A precursors but have no vitamin A activity themselves. Vitamin A compounds are yellowish oils or low-melting-point solids (depending on isomeric purity) that are practically insoluble in water but are soluble in organic solvents and mineral oil. Vitamin A is sensitive to oxygen and to ultraviolet (UV) light, which induces a greenish fluorescence with an absorbance peak at 325 nm. The structure for the most common and effective provitamin A, β- carotene, is illustrated in Fig. 39.2 . This compound is an orange-to-purple, water-insoluble solid that is oxidized in air to inactive products. The other carotenes, cryptoxanthin and β-apocarotenals, are asymmetric with only one β-ionone ring and yield less vitamin A activity.
Preformed vitamin A is obtained from animal-derived foods, such as liver, offal, and fish oils. Other sources are full cream milk, butter, and fortified margarines. The provitamin A carotenoids are obtained from yellow to orange fruits and vegetables and from green leafy vegetables; they have no vitamin A activity themselves but are converted to vitamin A in the body. Good sources are pumpkin, carrots, tomatoes, apricots, grapefruit, lettuce, and most green vegetables. The US National Health and Nutrition Examination Survey (NHANES II) indicated that approximately 25% of the vitamin A requirement was provided by carotenoids and about 75% by preformed retinol.
Preformed vitamin A, most often in the form of retinyl esters or carotenoids, are subject to emulsification and mixed micelle formation by the action of bile salts before they are transported into the intestinal cell. Here the retinyl esters are moved across the mucosal membrane and hydrolyzed to retinol within the cell to then be re-esterified by cellular RBP II and packaged into chylomicrons, which then enter the mesenteric lymphatic system and pass into the systemic circulation. A small amount of the ingested retinoid is converted into retinoic acid in the intestinal cell. The efficiency of absorption of preformed vitamin A is high (70 to 90%).
Carotenoids, also in micellar form, are absorbed into the duodenal mucosal cells by passive diffusion. The efficiency of absorption of carotenoids is much lower than for vitamin A (9 and 22%), and is subject to more variables, including carotenoid type, the amount in the meal, matrix properties, nutrient status, and genetic factors. Once inside the mucosal cell, β-carotene is principally converted to retinal by the enzyme β-carotene-15,15′-dioxygenase. Retinal is converted by retinal reductase to retinol and subsequently esterified. β-Carotene can also be cleaved eccentrically to β-apocarotenals, which can be further degraded to retinal or retinoic acid. The newly synthesized retinyl esters form both preformed vitamin A and carotenoids, along with exogenous lipids and nonhydrolyzed carotenoids, then pass with chylomicrons via the lymphatic system to the liver, where uptake by parenchymal cells again involves hydrolysis. In the liver, retinol is bound with RBP (MW ≅ 21,000 Da) and transthyretin (thyroxine-binding prealbumin) (MW ≅ 55,000 Da) in a 1:1:1 complex of sufficient size to prevent loss by glomerular filtration and is returned to the circulation, or stored as esters within the stellate cells. Delivery of retinol to the tissue is controlled by the availability of the vitamin A–protein complex in the circulation, although this control mechanism can be bypassed by large doses of retinol.
Retinoic acid from the intestinal mucosa is transported bound to serum albumin via the portal vein. Retinoic acid cannot be significantly reduced to retinal but is rapidly metabolized in tissue, such as liver, to yield more polar catabolites (e.g., 5,6-epoxyretinoic acid) and conjugates, such as retinoyl β-glucuronide, that are excreted. A small amount of retinoic acid undergoes enterohepatic circulation after intestinal hydrolysis of the glucuronide, which is excreted in bile.
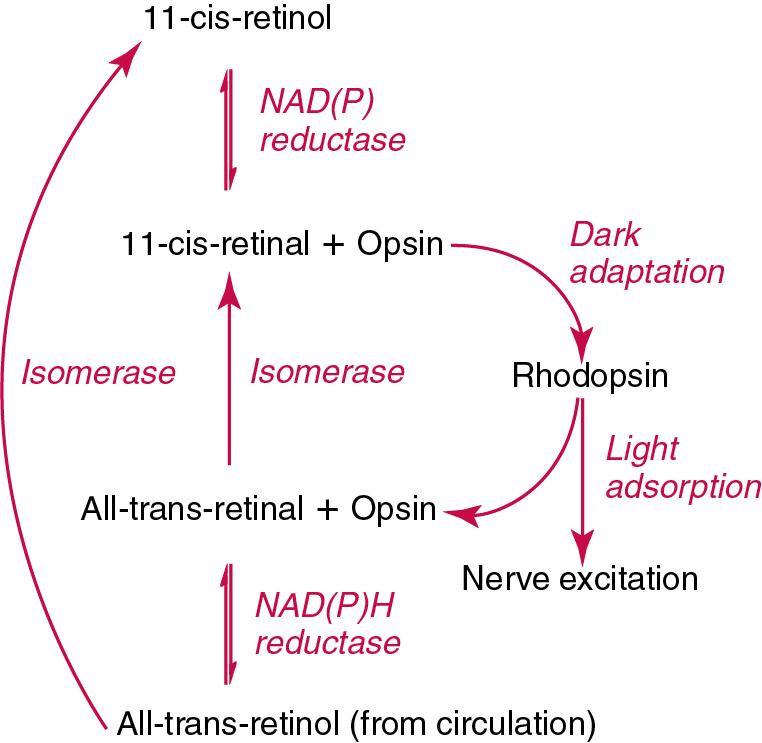
The participation of retinal in vision is considered the most important physiologic function of vitamin A. All- trans- retinol is the predominant circulating form of vitamin A. Cells of the retina isomerize this to the 11 -cis alcohol that is reversibly dehydrogenated to 11 -cis retinal. This sterically hindered geometrical isomer of the aldehyde combines as a lysyl-linked Schiff base with suitable proteins (e.g., opsin) to generate photosensitive pigments, such as rhodopsin. Illumination of such pigments causes photoisomerization and the release of all- trans- retinal and the protein, a process that couples the large conformational change with ion flux and optic nerve transmission. The all- trans- retinal is isomerized to the 11- cis isomer, which combines with the liberated protein to reconstitute the photo pigment in a visual cycle, as shown in Fig. 39.3 . The pyridine nucleotide–dependent dehydrogenase (reductase) can also reduce the all- trans- retinal to all -trans- retinol.
Other functions of vitamin A include its role in reproduction, growth, embryonic development, and immune function ; many of these functions are mediated through the binding of retinoic acid to specific nuclear receptors that regulate genomic expression. In normal growth, and in maintenance of the integrity of epithelial cells, retinoic acid acts through the activation of retinoic acid receptors (RARs) and retinoid X receptors (RXRs) in the nucleus to regulate various genes that encode for structural proteins, enzymes, extracellular matrix proteins, RBPs, and receptors. Vitamin A deficiency impairs innate immunity by impeding normal regeneration of mucosal barriers damaged by infection, and by diminishing the function of neutrophils, macrophages, and natural killer cells. Vitamin A is also required for adaptive immunity and plays a role in the development of both T-helper cells and B cells. Retinol and its metabolites and synthetic retinoids provide protective effects against the development of certain types of cancer by blocking tumor promotion, inhibiting proliferation, inducing apoptosis, inducing differentiation, or by performing a combination of these actions. , Finally, synthetic retinoids have been used successfully, both topically and systemically, to treat severe acne and other skin disorders of abnormal keratinization. However, caution is required regarding the use of vitamin A or β-carotene supplements in the general population as they have been shown to be teratogenic.
In the older system of international units (IU), now largely redundant, a ratio for equivalence of activity of 1:2:4 for retinol: β-carotene: other provitamin A carotenoids was used, but this was superseded in 1967 by the retinol equivalent (RE), devised by a Food and Agriculture/World Health Organization (WHO) Expert Committee and proposing an equivalence ratio of 1:6:12. However, studies using stable isotopes of β-carotene led the Food and Nutrition Board of the US Institute of Medicine to recommend the retinol activity equivalent (RAE) as the basis of calculation of retinol intake. In this system, 1 RAE = 1 μg retinol, 12 μg β-carotene, or 24 μg carotenoids. With this system, current Recommended Daily Allowances (RDAs) for vitamin A are 900 μg RAE for men 19 years and older; 700 μg RAE for women 19 years and older, with up to 770 μg RAE/day in pregnancy and up to 1300 μg RAE/day in lactation; 300 to 900 μg RAE for children 1 to 18 years, dependent on age and gender; and an adequate intake (AI) of 400 μg RAE at 0 to 6 months and 500 μg RAE from 7 to 12 months for infants.
The recommended provision of vitamin A to adults during intravenous nutrition (IVN), whether this is partial or TPN, is 1000 μg retinol. This is usually provided as retinol palmitate and may be supplied with other fat-soluble vitamins in a mixture dissolved in a fat emulsion for intravenous (IV) feeding, or may be designed to be compatible with a mixture of all vitamins suitable for addition to other water-soluble nutrients. ,
Vitamin A deficiency primarily affects infants and children, and its prevalence is subject to WHO surveillance. Risk factors include poverty, low birth weight, poor sanitation, malnutrition, infection, and parasitism. As hepatic accumulation of vitamin A occurs during the last trimester of pregnancy, preterm infants are relatively vitamin A deficient at birth.
Neonatal vitamin A supplementation has been a matter of some controversy due to mixed results in trials studying various populations. Evidence from one systematic review of randomized trials showed benefit of vitamin A supplementation for children aged 6 to 59 months, reducing all-cause mortality by 23 to 30%. However, in children less than 6 months the results have been mixed ranging from no benefit to possible harm prompting calls for further large trials. To address this issue, the WHO, supported by the Bill & Melinda Gates Foundation, commissioned three large, double-blind, placebo-controlled, randomized trials in selected localities in India, Ghana, and Tanzania to examine the effects of neonatal vitamin A supplementation using a standardized protocol. These three studies randomly assigned 99,938 newborn babies to receive one dose of 50,000 IU of vitamin A or placebo within 72 hours of birth. The results were somewhat mixed showing some evidence of benefit for survival to 6 months of age in India (risk ratio [RR] 0.90, 95% confidence interval [CI] 0.81 to 1.00), but no benefit for survival in Tanzania or Ghana. There was a suggestion of increased risk of mortality by 6 months of age in the African countries (in Tanzania, RR 1.10, 95% CI 0.95 to 1.26, P = .193; in Ghana, RR 1.12, 95% CI 0.95 to 1.33, P = .183). , In addition, there was evidence of increased risk of bulging fontanelle in vitamin A-supplemented neonates. A closer examination of the data reveals that evidence of maternal vitamin A deficiency may be an important factor; in India almost 25% of all women studied were vitamin A deficient, in Ghana this was less than 3% and in Tanzania 5 to 8%. It may be inferred from this that maternal deficiency may be a potential predictor for neonatal supplementation but at the present time there is no strong evidence for a global policy for neonatal vitamin A supplementation.
In general, providing a daily oral intake of vitamin A that meets the RDA of 400 μg RAE is sufficient. Infants with birth weights of less than 1500 g (those under 30 weeks of gestation) have virtually no hepatic vitamin A stores and are at risk of vitamin A deficiency. Various researchers have observed that (1) bronchopulmonary dysplasia (BPD), a debilitating, chronic lung disease that mimics some histologic features of vitamin A deficiency, is common in premature infants; (2) intramuscular injections of 630 μg RAE every 2 days can reduce the incidence of BPD; (3) blood concentrations of vitamin A decline during TPN, often reaching concentrations of 10 to 15 μg/dL (normal, 20 to 65 μg/dL) unless adequate supplements are given; and (4) vitamin A (retinol) delivered in TPN solutions may be absorbed into the inner walls of plastic administration sets; however, this loss can be minimized by the use of ethylene vinyl acetate rather than polyvinyl chloride.
Fat malabsorption, particularly caused by celiac disease or chronic pancreatitis, and protein-energy malnutrition predispose to vitamin A deficiency. Liver disease diminishes RBP synthesis, and ethanol abuse leads to both hepatic injury and competition with retinol for alcohol dehydrogenase, which is necessary for the oxidation of retinol to retinal and retinoic acid. Vitamin A deficiency may lead to anemia, although the precise mechanism is not known.
Clinical features of vitamin A deficiency include degenerative changes in eyes and skin and poor dark adaptation or night blindness (nyctalopia) followed by degenerative changes in the retina. Xerophthalmia is seen to occur when the conjunctiva becomes dry with small gray plaques with foamy surfaces (Bitot spots). These lesions are reversible with vitamin A administration. More serious effects of deficiency are known as keratomalacia and cause ulceration and necrosis of the cornea that lead to perforation, prolapse, endophthalmitis, and blindness. Usually, associated skin changes include dryness, roughness, papular eruptions, and follicular hyperkeratosis. The general change consists of atrophy of certain specialized epithelia, followed by metaplastic hyperkeratinization.
Toxic effects of hypervitaminosis A occur mainly as a result of ingestion of excess vitamin or as a side effect of inappropriate therapy. Hypervitaminosis A occurs after liver storage of retinol and its esters exceeds 3000 μg/g tissue, with ingestion of more than 30,000 μg/day for months or years, or if plasma vitamin A concentrations exceed 140 μg/dL (4.9 μmol/L). The elderly are more susceptible to vitamin A toxicity at lower doses, as exposure to retinyl esters is longer because of delayed postprandial clearance of lipoproteins.
Symptoms of acute toxicity present as abdominal pain, nausea, vomiting, severe headaches, dizziness, sluggishness, and irritability, followed within a few days by desquamation of the skin and recovery. Chronic toxicity from moderately high doses taken for protracted periods is characterized by bone and joint pain, hair loss, dryness and fissures of the lips, anorexia, benign intracranial hypertension, weight loss, and hepatomegaly. Administration of doses up to threefold the RDA for several years resulted in classic histologic changes of hepatotoxicity in 41 patients. In addition, it has been shown that osteoporosis and hip fracture are associated with vitamin A intakes only twice the RDA. Infants given excess vitamin A over months to years can develop intracranial features, typically bulging fontanelle, and skeletal abnormalities at doses of 5500 to 6750 μg/day.
Epidemiologic and experimental evidence has supported the view that high vitamin A intake in humans, acting via 13- cis -retinoic acid, is teratogenic. , The critical period of susceptibility is the first trimester of pregnancy, and primary abnormalities derive from the cranial neural crest (CNC) cells. A 1995 study of almost 23,000 pregnant women found that those who ingested more than 4500 μg/day of pre-formed vitamin A were at greater risk of delivering infants with malformations of CNC cell origin than were women consuming less than 1500 μg/day. A further intriguing association, supported in part by epidemiologic studies, is that observed between excessive vitamin A intake and reduction in bone mineral density (BMD). Studies of Scandinavian women show that consistent loss of BMD at four sites was associated with increased intake of preformed vitamin A, whereas other studies have showed no increase in bone mineral loss with preformed vitamin A intakes of up to 2000 μg/day. Hypervitaminosis A is also a known cause of hypercalcemia, especially in chronic kidney disease. ,
Carotenemia results from chronic excessive intake of carotene-rich foods, principally carrots and is usually reported in infants and children. This condition, in which yellowing of the skin is observed, is benign because the excess carotene is deposited rather than converted to vitamin A. There is a role for the measurement of β-carotene in the differential diagnosis of specific cases of jaundice in children. There have been reports of impaired activity of the enzyme β-carotene-15,15′-dioxygenase in children leading to accumulation of β-carotene, especially when consuming carotene-rich foods, but it is a benign condition. Carotenemia has also been linked to amenorrhea, but the mechanism behind this association remains unknown. Increased concentrations have also been found in hypothyroid patients, in whom conversion to vitamin A is decreased, and in patients with hyperlipemia associated with diabetes mellitus.
Although measurement of the plasma concentration of vitamin A is the most convenient and widely used assessment of vitamin A status, it is not an ideal indicator because it does not decline until liver stores become critically depleted, which is thought to occur at a concentration of approximately 20 μg/g liver.
Vitamin A status is assessed by the measurement of retinol concentration. Retinol circulates in plasma as a 1:1:1 complex with RBP and transthyretin, forming a complex preventing glomerular filtration. The circulating concentration of RBP is determined by dietary protein and zinc, which are necessary for RBP synthesis. Thus protein malnutrition, liver disease, and zinc deficiency resulting in RBP deficiency will lead to hypovitaminosis A. In contrast, renal failure resulting in decreased excretion of RBP has been reported to result in hypervitaminosis A. As previously discussed, another confounding factor in the assessment of vitamin A status is the effect of inflammation. , , , Both RBP and transthyretin are negative acute-phase proteins; thus inflammatory changes will result in transient falls in both proteins and plasma retinol. To distinguish inflammatory from nutritional causes of reduced plasma retinol concentrations, it may be necessary to measure CRP. It is important to bear in mind that beta-carotenes are a useful adjunct in assessing deficiency but should not be measured in isolation in this context.
Early chemical methods which are rarely used include the Carr-Price photometric method, which uses antimony trichloride in chloroform as the reagent, and the later Neeld-Pearson method, which uses trifluoroacetic acid to produce a blue pigment with the conjugated double bonds of vitamin A (and the carotenoids). To improve specificity and sensitivity, later methods used high-performance liquid chromatography (HPLC) after solvent extraction and other separation techniques with fluorometric or spectrophotometric detection. , , HPLC has brought enhanced specificity, lowered limits of detection, improved accuracy using primary standards, reference materials, and quality assurance schemes, and made acceptable reproducibility achievable (between batch coefficients of variation [CVs] of less than 15% for both vitamin A and β-carotene). Both normal and reverse-phase HPLC have been used. In the normal-phase HPLC, compounds to be separated are adsorbed to microparticulate silica gel and are eluted in the order of least polar to most polar. Acceptable separation and quantitative yields of neutral and charged retinoids are obtained. Reversed-phase HPLC is preferable for acid-sensitive compounds such as 5,6-epoxyretinoic acid. Photometric, electrochemical, and mass spectrophotometric detectors have all been used. Refer to chapter 19 for general principles of chromatography and extraction. Briefly, serum is deproteinized with ethanol containing internal standards, centrifuged, and extracted with hexane. This is followed by evaporation to dryness and the residue is redissolved in tetrahydrofuran. An aliquot of this solution is injected into a silica-coated (C 18 ) reversed phase chromatographic column and detected photometrically with the absorbance measured at 325 nm for vitamin A and 450 nm for carotenes. Peak height ratios are used for quantification with normalization using the internal standards. HPLC-mass spectrometry methods have also been developed and are increasingly being used given the widespread adoption of this technique by clinical laboratories.
Because circulating retinol concentrations do not always correlate with total body stores of vitamin A, indirect tests have been used to assess these stores. The relative-dose-response test, described first by Loerch and associates, requires two blood samples to be collected—one before and one 5 hours after a physiologic dose of vitamin A. In vitamin A–depleted subjects, a rapid, large, and sustained rise in serum retinol concentration contrasts with a lower rise in vitamin A–sufficient subjects. A modified relative-dose-response test using 3,4-didehydroretinyl (DR) acetate rather than retinyl acetate, and measuring the DR: retinol ratio after 5 hours has been used by other workers to assess the vitamin A status. , The quantitative assessment of total body stores of vitamin A can also be undertaken using deuterated retinol dilution techniques but is rarely necessary.
Recent advances in vitamin measurement involve the use of supported liquid extraction (SLE) methodology for sample preparation using modified diatomaceous earth (natural fossilized biominerals containing high silica content) packed into columns or 96-well plates. The method is similar to the traditional liquid-liquid extraction but instead of two immiscible phases, the aqueous phase is immobilized onto an inert diatomaceous earth-based support material and the solvent immiscible organic phase flows through the support. This method can be used for the extraction of a range of analytes including fat- and water-soluble vitamins from aqueous samples such as blood. It gives excellent recovery; lower limits of quantification and good analytical sensitivity; good reproducibility; removes matrix interferences such as proteins and phospholipids; and improved throughput with good amenability for automation with hyphenation to immunoassay, HPLC, and tandem mass spectrometry methods.
There have been recommendations regarding the harmonization of the measurement of vitamin A, E, and carotenoids in blood. It is well known that there is wide variation in the methodology for the fat-soluble vitamins and how results are reported to clinicians. The harmonization (and standardization when a certified reference material is available) of the methods used by clinical laboratories is one step forward in providing universal reference intervals.
Plasma, serum, or whole blood specimens are all suitable for retinol measurements. Fasting samples are recommended, especially if a patient is taking oral or parenteral vitamin A supplementation. A sample should be taken at least 8 hours post-supplementation if fasting is not possible. Vitamin A samples are light-sensitive and should be protected from light as much as is possible by wrapping in foil. Vitamin A showed good stability in whole blood collected into tubes containing lithium heparin for up to 48 hours at room temperature and without light protection. Another study reported that vitamin A was stable for up to 72 hours in whole blood samples kept at 32 °C and up to 14 days in serum stored at 11 °C.
Guidance reference intervals for serum vitamin A are 20 to 40 μg/dL (0.70 to 1.40 μmol/L) for 1- to 6-year-old children; 26 to 49 μg/dL (0.91 to 1.71 μmol/L) for 7- to 12-year-old children; 26 to 72 μg/dL (0.91 to 2.51 μmol/L) for 13- to 19-year-old adolescents; and 30 to 80 μg/dL (1.05 to 2.80 μmol/L) for adults. Values above 30 μg/dL (1.05 μmol/L) are associated with appreciable reserves in the liver and correlate well with vitamin A intake. Within the reference interval, values for men are generally about 20% higher than those for women. By HPLC with UV detection, the reference interval for serum α-carotene is 14 to 60 μg/L (26 to 112 nmol/L), β-carotene is 90 to 310 μg/L (167 to 577 nmol/L), lutein is 80 to 200 μg/L (140 to 352 nmol/L), and lycopene 100 to 300 μg/L (186 to 559 nmol/L). However, it must be borne in mind that reference intervals will be dependent on the local population diet. For more information, refer to the Appendix on Reference Intervals. Laboratories should verify that these ranges are appropriate for use in their own settings.
As discussed in Chapter 54 , vitamin D plays an essential role as a hormone in the control of calcium and phosphorous metabolism and bone physiology.
Vitamin E is an antioxidant that acts to prevent the peroxidation of unsaturated fatty acids by free radicals. It also has a role in gene transcription and immunity, inhibits platelet aggregation, and has recently been implicated in bone physiology.
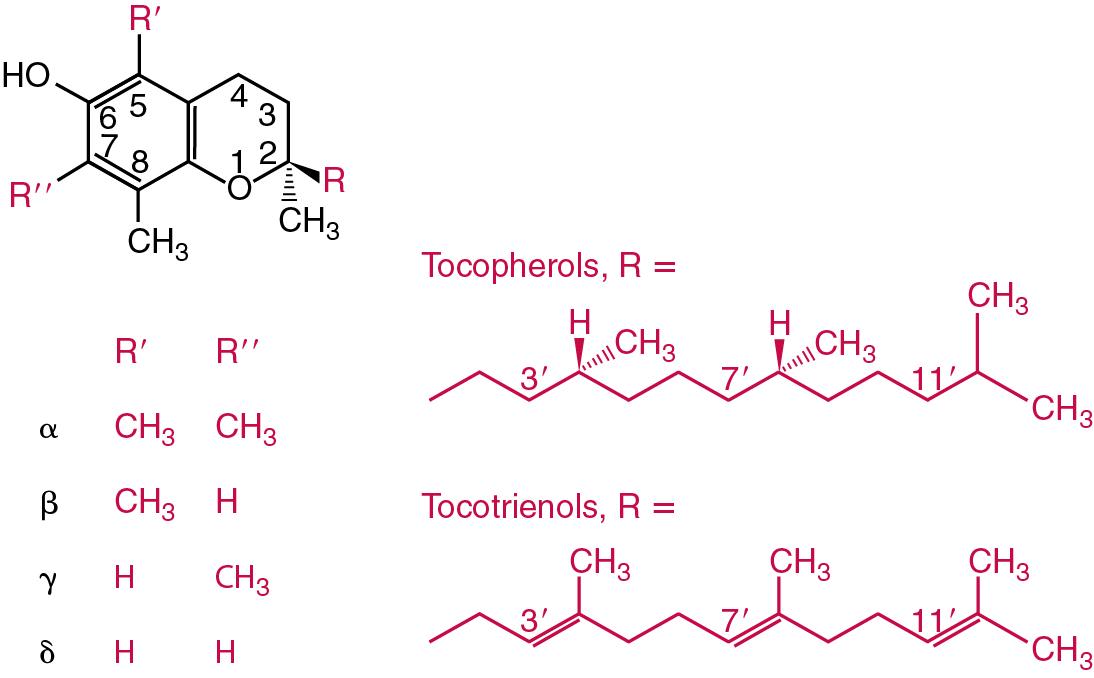
Vitamin E is the nutritional term for the group of tocopherols and tocotrienols that have biological activity similar to the naturally occurring form RRR-α-tocopherol (formerly d -α-tocopherol). Both groups have a common 6-chromanol nucleus substituted with methyl groups at positions 2 and 8 and with a phytyl tail of isoprenoid units at position 2. The isoprenoid chain is saturated in the tocopherols but is unsaturated at positions 3′, 7′, and 11′ for tocotrienols ( Fig. 39.4 ). The Greek letter prefixes α, β, γ, and δ indicate the presence or absence of methyl groups at positions 5 and 7. The tocopherols have three asymmetric carbon atoms in the isoprenoid chain, giving eight optical isomers. The naturally occurring tocopherols occur as the RRR forms, whereas the synthetic compounds found in foods and supplements are of various racemic forms (RRR-, RSR-, RRS-, and RSS-α-tocopherol) and are less biologically active. Tocopherol and tocotrienols are viscous oils at room temperature, soluble in fat solvents, and insoluble in aqueous solutions, although there exists a water-soluble analog (trolox-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid). Also, tocopherol and tocotrienols are stable to acid and heat in the absence of oxygen but are labile to oxygen in alkaline solutions and to UV light.
The principal sources of dietary vitamin E are oils and fats, particularly wheat germ oil and sunflower oil, grains, and nuts. Meats, fruits, and vegetables contribute little vitamin E. Gamma-tocopherol is the major form of vitamin E in many plant seeds in the US diet, but it is present at only one quarter to one tenth of the concentration of α-tocopherol in human plasma.
In the presence of bile, vitamin E is absorbed from the small intestine. Most forms of vitamin E are absorbed nonselectively and are secreted in chylomicron particles along with triacylglycerol and cholesterol. Some of this chylomicron-bound vitamin E is transported and delivered to the peripheral tissue (mainly adipose tissue) with the aid of lipoprotein lipase. The liver takes up the chylomicron remnants where α-tocopherol is incorporated into very low-density lipoproteins (VLDLs) by α-tocopherol transfer protein (α-TTP), enabling further distribution of α-tocopherol throughout the body. Plasma vitamin E is further delivered to the tissue by low-density lipoprotein (LDL) and high-density lipoprotein (HDL). The specificity of α-TTP for α-tocopherol is probably responsible for its preferential storage in most tissue. Vitamin E is excreted via the bile, in the urine as tocopheronic acid and as a β-glucuronide conjugate in the form of carboxyethyl hydroxychromans (CEHC), and by other unknown routes.
The inhibition of free radical mediated lipid peroxidation is the main role for vitamin E. This occurs mainly within the polyunsaturated fatty acids of membrane phospholipids. Tocopherols and tocotrienols inhibit lipid peroxidation largely because they scavenge lipid peroxyl radicals faster than the radical can react to adjacent fatty acid sidechains or membrane proteins. The resultant tocopheryl or tocotrienol radicals may then react with additional peroxyl radicals to produce tocopherones (nonradicals), or they may be regenerated by transfer of an electron to ascorbate to form the ascorbyl radical. Thus vitamins E and C act synergistically to reduce lipid peroxidation ( Fig. 39.5 ). Some epidemiologic surveys have shown an association between reduced vitamin E intakes (and other dietary factors) and increased incidence of chronic disease, particularly cardiovascular disease and cancer, although intervention studies have produced mixed results. , The Women’s Antioxidant Cardiovascular Study confirmed the lack of effect of antioxidants on cardiovascular events and also in slowing the rate of cognitive decline. Vitamin E also has no proven effect in reducing the incidence of various cancers. ,
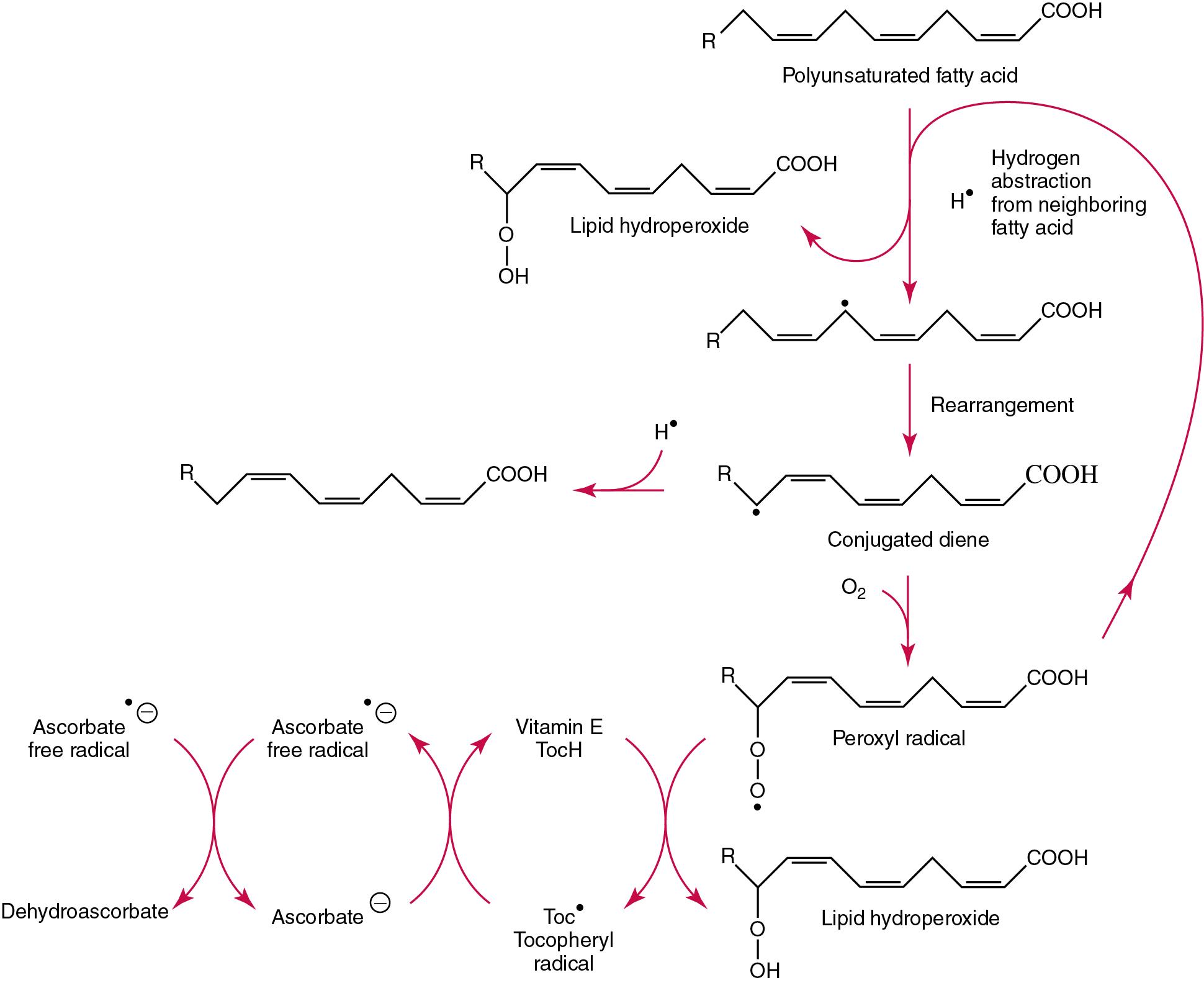
Beyond its antioxidant properties, α-tocopherol inhibits protein kinase C and 5-lipoxygenase and activates protein phosphatase 2A and diacylglycerol kinase. Some genes (coding for CD36, α-TTP, α-tropomyosin, and collagenase) are affected by α-tocopherol at the transcriptional level. α-Tocopherol also induces inhibition of cell proliferation, platelet aggregation, and monocyte adhesion, which are thought to be the result of direct interaction of α-tocopherol with cell components. , There is some evidence that vitamin E may have anti-inflammatory properties.
A recent study has shown that serum vitamin E is a determinant of bone mass by stimulating osteoclast fusion. In that study, mice deficient in α-TTP (Ttpa −/− ), a mouse model of genetic vitamin E deficiency, had high bone mass as a result of a decrease in bone resorption. The authors showed that vitamin E could stimulate osteoclast fusion via an intricate mechanism resulting in loss of bone mass. The authors suggest that given the widespread use of vitamin E, a large, randomized-controlled trial examining the effect of vitamin E on human bone mass may be warranted.
The requirement for vitamin E is related to the polyunsaturated fatty acid content of cellular structures and therefore depends on the nature and quantity of dietary fat that affect such composition. Hence the minimum adult requirement for vitamin E is not certain but is probably not more than 3 to 4 mg (4.5 to 6 IU) of RRR - α-tocopherol/day for those who ingest a diet containing the minimum of essential fatty acids (3% of calories). Because vitamin E activity is derived from a series of tocopherols and tocotrienols in usual mixed diets, the α-tocopherol equivalent is used based on the abundance and activity relative to the biologically most active RRR - α-tocopherol. The α-tocopherol equivalent is the sum of α-tocopherol, β-tocopherol (multiplied by a factor 0.5), γ-tocopherol (multiplied by 0.1), and α-tocotrienol (by 0.3). It has been estimated that a range of 7 to 13 mg of α-tocopherol equivalents (10 to 20 IU) can be expected in balanced diets supplying 1800 to 3000 kcal. This intake will maintain plasma concentrations of total tocopherols within the reference interval of 0.5 to 1.2 mg/dL (12 to 28 μmol/L), which ensures an adequate concentration in all tissue. Some investigators claim that the ratio of circulating α-tocopherol to total lipids (or triglycerides or β-lipoproteins) is a more accurate indicator of tissue vitamin E status than circulating α-tocopherol alone.
In the year 2000, the RDA for vitamin E for both male and female adults was increased by 50% from 10 to 15 mg/day (35 μmol/day or 21 IU) by the US Food and Nutrition Board. Most European reference intakes are related to the polyunsaturated fatty acid intake. Critics have argued that without supplementation, this amount could not be met by the usual North American diet. There has also been concern raised that given the anti-aggregatory effect of vitamin E on platelets, the widespread use of aspirin may have an additive effect and result in increased incidence of bleeding and hemorrhagic stroke. , Another departure in the newer recommendations was that the daily requirement must be met by RRR-α-tocopherol alone, as the other forms of vitamin E are not converted to α-tocopherol and are poorly recognized by the α-tocopherol transfer protein in the liver.
The recommended amount of vitamin E to be supplied intravenously to adults as α-tocopherol is 9.1 mg/day or 10 IU/day. , This is lower than the oral dose but accounts for complete delivery into the bloodstream.
Premature and low-birth-weight infants are particularly susceptible to vitamin E deficiency because placental transfer is poor and infants have limited adipose tissue where much of the vitamin is normally stored. Signs of deficiency include (1) irritability, (2) edema, and (3) hemolytic anemia. Anemia reflects the shortened life span of erythrocytes with fragile membranes; it does not respond to iron therapy, which may aggravate the condition. Although symptoms of vitamin E deficiency are rare in children and adults, deficiency can occur in some conditions such as fat malabsorption states including CF and chronic cholestasis in children, and can cause neuropathy and hemolytic anemia. The genetic disorder abetalipoproteinemia (vitamin E is transported on lipoproteins) can also confer vitamin E deficiency (the Bassen-Kornzweig syndrome ). Variants of the gene coding for α-TTP lead to very low plasma α-tocopherol concentrations and cause neurologic symptoms including cerebellar ataxia, requiring treatments with large amounts (up to 2 g/day or 3 IU) of vitamin E. Hypovitaminosis E may be present asymptomatically and only manifest acutely as a result of oxidative stress, as in major trauma or SIRS.
Vitamin E toxicity only results from excessive supplementation. Such supplementation is contraindicated in subjects with coagulation defects caused by vitamin K deficiency and in those receiving anticoagulant drugs. The US Food and Nutrition Board has recommended a tolerable upper limit (UL) of 1000 mg/day (1430 IU/day) of vitamin E for adults 19 years and older, based on the absence of hemorrhagic toxicity in animal models, although this has been challenged on the grounds that in those regularly taking aspirin, this intake may be high and may be associated with increased risk of bleeding. A comprehensive review of tolerance and safety of vitamin E suggested that intakes up to 3000 mg/day (4285 IU/day) were safe, and reversible side effects of gastrointestinal symptoms, increased creatinuria, and impairment of blood coagulation are seen at intakes of 1000 to 3000 mg/day (1430 to 4285 IU/day). However, as noted earlier, long-term use of intakes greater than 400 mg/day (572 IU/day) may cause increased mortality.
Assessment of vitamin E status has been achieved by functional methods such as (1) protection of erythrocyte hemolysis on addition of peroxide, (2) inhibition of formation of lipid peroxidation products (malondialdehyde, thiobarbituric acid–reactive substances [ethane or pentane]), or (3) direct measurement of vitamin E concentration in tissues (erythrocytes, lymphocytes, or platelets) or serum. , Early direct methods used photometric or fluorometric measurement often based on the Emmerie-Engel procedure, in which tocopherol is oxidized to tocopheryl quinone by FeCl 3 , and the resultant Fe 2+ is coupled with α,α′-dipyridyl to form a red color. Later, chromatographic methods were used, including thin layer and gas liquid chromatography, which had the ability to separate the tocopherols and the tocotrienols, but these methods were labor intensive and time consuming. HPLC is currently the method of choice for quantification of tocopherols in serum, as it offers the advantages of accuracy (through the use of primary standards) and reproducibility (between-batch CVs of less than 5%) and the ability to quantitate multiple analytes, including vitamin A and some carotenoids, in a single analytical run. , Both α- and γ-tocopherols are the principal vitamers seen, although others may be detected with minor modifications to the analytical conditions. HPLC-mass spectrometry methods have also been developed and are increasingly being used given the widespread adoption of this technique by clinical laboratories.
Plasma, serum, or whole blood specimens are all suitable, but it is recommended that local laboratories should be consulted for their preferred sample type. Vitamin E is light sensitive, and samples should be protected from light as much as is possible by wrapping in foil. Vitamin E showed good stability in whole blood collected in lithium heparin tubes for up to 48h at room temperature and without light protection, while another study reported that vitamin E was stable for up to 72 hours in whole blood samples kept at 32 °C and up to 14 days in serum stored at 11 °C.
Guidance reference intervals for serum or plasma (heparin) vitamin E are 0.1 to 0.5 mg/dL (2.3 to 11.6 μmol/L) for premature neonates; 0.3 to 0.9 mg/dL (7 to 21 μmol/L) for children (1 to 12 years) ; 0.6 to 1.0 mg/dL (14 to 23 μmol/L) for adolescents (13 to19 years); and 0.5 to 1.8 mg/dL (12 to 42 μmol/L) for adults. As vitamin E circulates mainly bound to lipoproteins it has been shown that correcting for the concentration of cholesterol gives a better reflection of vitamin E status. As a ratio of cholesterol, the reference range is 3.5 to 9.5 μmol/mmol cholesterol. For more information, refer to the Appendix on Reference Intervals. Laboratories should verify that these ranges are appropriate for use in their own settings. In addition, as discussed under the section on vitamin A, given the wide variation in the methods for measuring fat-soluble vitamins, recommendations have been published to harmonize these methods.
Vitamin K has important roles in coagulation and bone metabolism.
Vitamin K is the common generic name for a group of compounds with a methylated naphthoquinone structure (2-methyl-1,4-napthoquinones) which are substituted with sidechains at carbon 3. Phylloquinone (K 1 type) synthesized in plants and menaquinones (K 2 type) of bacterial origin are the two principal natural classes of vitamin K ( Fig. 39.6 ). The principal vitamin K 1 (phylloquinone) bears a saturated, phytol, 20-carbon side chain derived from four isoprenoid units; this is the main K vitamin produced by plants and is the major dietary form for humans. , K 2 shows greater variation, but an all- trans- farnesylgeranylgeranyl, 35-carbon chain of 7 isoprenoid units is typical; these are produced in humans by large bowel bacteria, although their contribution to vitamin K status remains a matter of dispute. Several synthetic analogs and derivatives have been used in human nutrition; most relate to or derive from menadione (K 3 ), which lacks a side chain substituent at position 3, but can be converted to menaquinone (MK) (e.g., MK-4, where 4 is the number of isoprenoid sidechains) through addition of the side chain in the liver. The K vitamins are insoluble in water but dissolve in organic fat solvents. They are destroyed by alkaline solutions and reducing agents and are sensitive to UV light.
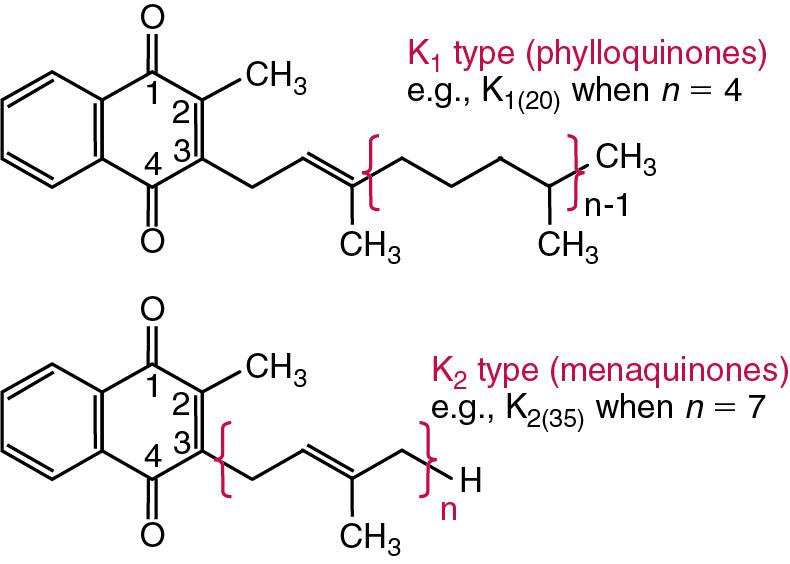
The main dietary sources of the phylloquinones are green vegetables, margarines, and plant oils, whereas some MKs can be obtained from cheese, other milk products, and eggs.
As for other fat-soluble vitamins, the absorption of natural vitamin K from the small intestine into the lymphatic system is facilitated by bile. The efficiency of absorption varies from 15 to 65%, as reflected by recovery in lymph within 24 hours. Vitamins K 1 and K 2 are bound to chylomicrons for transport from mucosal cells to the liver. Menadione (K 3 ) is more rapidly and completely absorbed from the gut before entering the portal blood. In liver, intracellular distribution is seen mostly in the microsomal fraction, where phenylation of menadione to form K 2 occurs. Release of vitamin K to the bloodstream allows association with circulating β-lipoproteins for transport to other tissue. Significant concentrations of vitamin K have been noted in the spleen and skeletal muscle.
Within metabolically active and vitamin K–dependent tissue, especially liver, a microsomal vitamin K cycle exists ( Fig. 39.7 ). The vitamin (quinone) is normally reduced by a thiol-sensitive flavoprotein system to hydroquinone, which then can couple to the oxygen and carbon dioxide with the use of γ-carboxylation of glutamyl residues in specific proteins (e.g., prothrombin). The 2,3-epoxide of vitamin K that is subsequently formed is reduced to the starting vitamin K quinones—a process that can be antagonized by vitamin K antagonists such as warfarin.
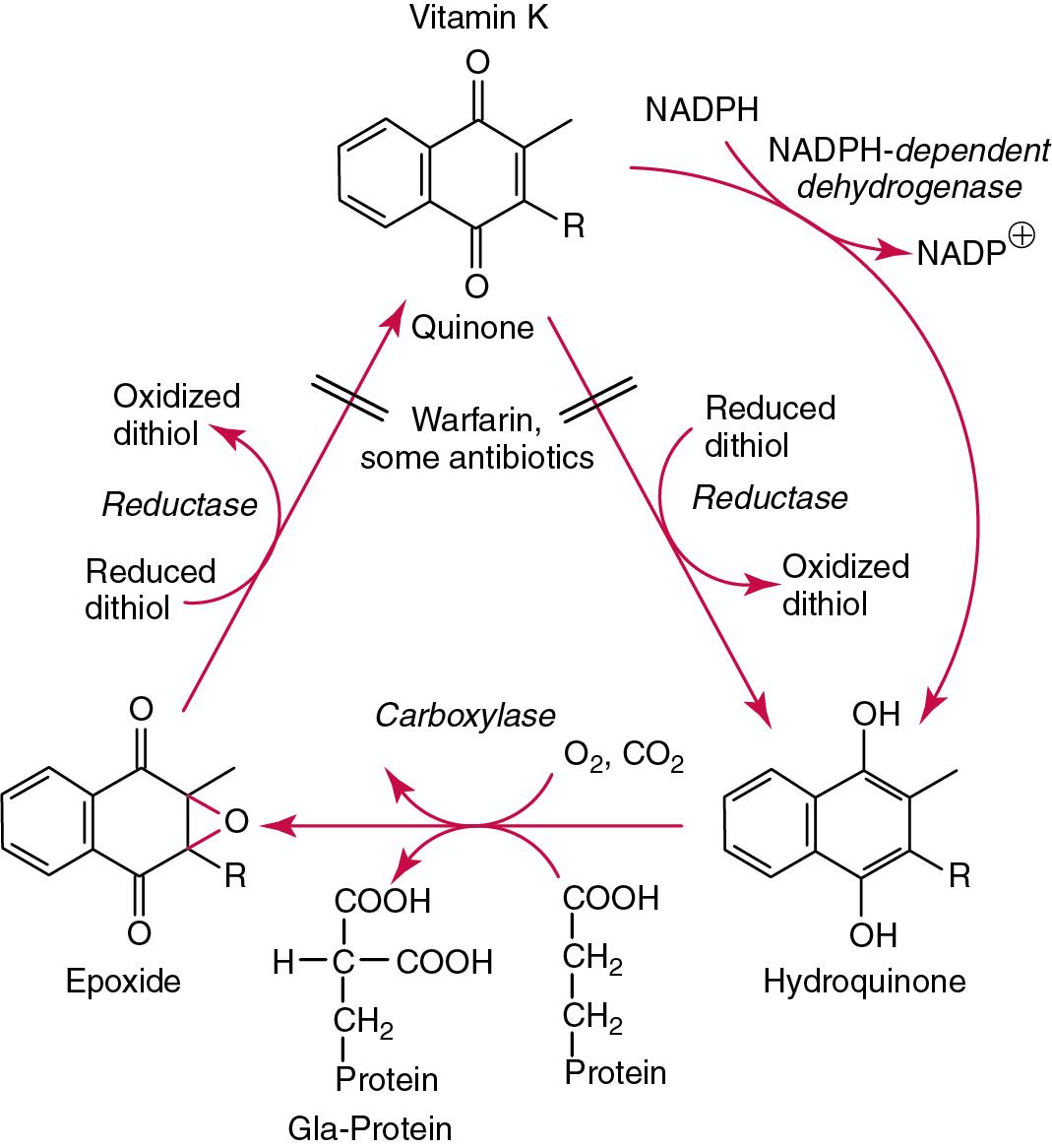
Only traces of metabolites of vitamins K 1 and K 2 appear in urine; a considerable portion of vitamin K 3 (menadione) is conjugated at the hydroquinone concentration to form β-glucuronide and sulfate esters, which are excreted.
The essential and most thoroughly defined role of vitamin K is as a cofactor to vitamin K–dependent carboxylase, an enzyme necessary for the post-translational conversion of specific glutamyl residues in target proteins to γ-carboxyglutamyl (Gla) residues. This γ-carboxylation increases the affinity of these proteins for calcium. , The antihemorrhagic function of vitamin K depends on the formation of the Gla proteins prothrombin (factor II), proconvertin (factor VII), plasma thromboplastin component (factor IX), and Stuart factor (factor X), which, together with two other hemostatic vitamin K–dependent proteins, proteins C and S, and Ca 2+ , initiate a process to form thrombin that then catalyzes the conversion of fibrinogen to a fibrin clot.
Proteins that contain γ-carboxyglutamyl are also abundant in bone tissue, with osteocalcin accounting for up to 80% of the total γ-carboxyglutamyl content of mature bone. Epidemiologic studies have shown an association between low vitamin K intakes and hip fracture risk. Intervention studies have shown that vitamin K 1 can increase BMD in osteoporotic subjects and can reduce fracture rates. Evidence indicates that vitamins K and D may act synergistically in maintaining bone density.
A further major Gla protein, matrix Gla protein (MGP)—containing five residues of γ-carboxyglutamic acid—is found in vascular smooth muscle, bone, and many soft tissues (heart, kidney, and lungs). , It is thought that MGP accumulates at sites of calcification, including calcified aortic valves and bone, and is a potent inhibitor of calcification. In experimental studies with mice lacking the gene coding for MGP, calcification of the arteries was observed that led to hemorrhagic death of the animals as a result of blood vessel rupture. Several other Gla proteins have been identified, and putative roles have been assigned.
Although the human gut bacteria synthesize large quantities of MKs, and such compounds are found in the liver in concentrations up to 10 times those of phylloquinones, absorption of these compounds has been difficult to demonstrate, and dietary restriction of vitamin K leads to evidence of inadequacy, as demonstrated by undercarboxylation of vitamin K–dependent proteins. Thus dietary reference intakes (DRIs) for vitamin K have been revised by the Food and Nutrition Board of the US Institute of Medicine. Current recommendations are 120 μg/day for men older than 18 years; 90 μg/day for women older than 18 years, including those pregnant or lactating; 30 to 75 μg/day for children 1 to 18 years, dependent on age; 2.0 μg/day for infants up to 6 months; and 2.5 μg/day for infants between 7 and 12 months, with the latter requirements met by breast milk.
In the United States, it remains controversial whether vitamin K should be included in preparations of vitamins for use in TPN. Although this has been standard in Europe for many years, the long-standing recommendation from the American Medical Association was not to include vitamin K, because this would complicate the provision of adequate warfarin therapy in those patients who require anticoagulation. However, the 2003 requirements of the US Food and Drug Administration (FDA) specified that vitamin K should be included in vitamin supplements for both infants and adults, making the judgment that the physiologic and practical benefits of regular provision outweigh any problems in readjusting warfarin dosage. The recommended IV adult dose is 150 μg/day, which is provided as phytonadione.
Although vitamin K deficiency in the adult is uncommon, the risk is increased with fat malabsorption states such as (1) bile duct obstruction, (2) CF, and (3) chronic pancreatitis and liver disease. , Risk is also increased by the use of drugs that interfere with vitamin K metabolism, such as the coumarin anticoagulants (e.g., warfarin) and antibiotics containing the N -methylthiotetrazole side chain (e.g., cephalosporin). Other at-risk groups are hospitalized patients with poor nutrient intakes or those receiving TPN, when fat-soluble vitamin supplements may not fully meet requirements. Conversely, ingestion of supraphysiologic doses of vitamins A and E has been reported to induce vitamin K deficiency, probably through competitive mechanisms. Defective blood coagulation and demonstration of abnormal noncarboxylated prothrombin are currently the only well-established signs of vitamin K deficiency.
Hemorrhagic disease of the newborn , can develop readily because of (1) poor placental transfer of vitamin K, (2) hepatic immaturity leading to inadequate synthesis of coagulation proteins, and (3) the low vitamin K content of early breast milk. Prothrombin concentrations during this period are only about 25% of adult concentrations. Severe diarrhea and antibiotics used to suppress diarrhea readily exacerbate the situation, so prothrombin concentrations can drop below 5% of the adult concentration and bleeding can occur. This condition is routinely prevented by the prophylactic administration of phylloquinone intramuscularly, or orally, immediately after birth.
The use of high doses of naturally occurring vitamin K (K 1 and K 2 ) appears to have no known toxic effect; however, menadione (K 3 ) treatment can lead to the formation of erythrocyte cytoplasmic inclusions known as Heinz bodies and hemolytic anemia. With severe hemolysis, increased bilirubin formation and undeveloped capacity for its conjugation may produce kernicterus in the newborn.
Because no adverse effects associated with vitamin K consumption from food or supplements have been reported in humans or animals, the US Institute of Medicine has reported that a quantitative risk assessment cannot be performed, and thus an UL cannot be derived for vitamin K.
A wide range of biochemical and functional tests are available for vitamin K status. Because of its relatively low plasma concentration (approximately 50 times lower than vitamin D and at least 10 3 times lower than vitamin A or E), vitamin K has long presented an analytical challenge. For this reason, vitamin K status has traditionally been assessed by functional methods, primarily by its effect on clotting time. The prothrombin time (PT) is assessed by adding a portion of tissue thromboplastin to recalcified plasma and measuring the clotting time against a normal control sample (see Chapter 81 ). In vitamin K deficiency, the PT rises at least 2 seconds beyond the control time and may rise above 30 seconds (normal, 10 to 14 seconds). Attempts at cross-laboratory standardization led to the introduction of the International Normalized Ratio (INR), by which PT can be expressed as a fraction of the control time.
A more sensitive (1000-fold) assessment of vitamin K status with respect to prothrombin can be made by the immunoassay of des-γ-carboxy prothrombin, or undercarboxylated prothrombin, PIVKA-II (protein induced by vitamin K absence or antagonism). , PIVKA-II has proved to be a useful marker of subclinical vitamin K deficiency. Another measurement of deficient γ-carboxylation, plasma undercarboxylated osteocalcin, has been shown to correlate individually with PIVKA-II and plasma phylloquinone concentrations and has a better correlation with plasma phylloquinone than PIVKA-II. In this study of biochemical indices of vitamin K nutritional status in a healthy adult population, the urinary γ-carboxyglutamic acid : creatinine ratio was measured by derivatization, HPLC separation, and fluorometric detection and was shown to be sensitive to changes in dietary phylloquinone intake. This marker may have advantages in epidemiologic surveys as a less invasive sample.
Direct measurement of plasma phylloquinone is probably the best indicator of vitamin K status and has been shown to correlate with intake. HPLC methods are the mainstay of vitamin K measurement and typically require 0.2 to 2.0 mL of serum or plasma. Protein precipitation and lipid extraction (often into hexane) followed by solvent evaporation, preparative HPLC (to isolate vitamin K from other lipids), re-evaporation of the vitamin K–rich fraction, dilution in the mobile phase, and further HPLC, with electrochemical or fluorometric detection, often after postcolumn reduction, , are required. HPLC-mass spectrometry methods have also been developed. In general, typical between-batch imprecision values are CVs of 11 to 18% with limits of detection lower than 50 pmol/L. An External Quality Assessment Scheme (EQAS) is available.
Plasma, serum, or whole blood specimens are all suitable, but it is recommended that local laboratories should be consulted for their preferred sample type. Vitamin K is light-sensitive and samples should be protected from light as much as is possible by wrapping in foil. Vitamin K showed good stability in whole blood collected into tubes containing clot activator for up to 72 hours at room temperature and without light protection.
As plasma vitamin K concentration is influenced by plasma triglyceride owing to the association of circulating vitamin K 1 with VLDL, it is expressed as a ratio of the triglyceride concentration. It is also affected by the acute phase response in SIRS likely as a result of inflammation-dependent plasma lipid redistribution. The suggested reference interval for plasma vitamin K is 0.2 to 2.2 nmol/mmol triglyceride. , For more information, refer to the Appendix on Reference Intervals. Laboratories should verify that these ranges are appropriate for use in their own settings.
Vitamin B 1 , also known as thiamine or aneurin, forms the coenzyme thiamine pyrophosphate (TPP). It is required for the essential decarboxylation reactions catalyzed by the pyruvate and 2-oxoglutarate complexes ( Box 39.3 ).
A 38-year-old white Scottish man with mild learning difficulties presented with a 3-day history of diplopia and agitation, after 7 days of presumed viral gastroenteritis. On admission, he was agitated, mildly confused, tachycardic, and tachypneic. There were no chest signs or peripheral edema. He had complete bilateral sixth cranial nerve palsies and horizontal nystagmus, with dilated, slowly reacting pupils. Limb movements were clumsy, with moderate cerebellar signs and dysdiadochokinesis, but no tremor. He was clinically jaundiced. Electrocardiography (ECG) showed inferolateral T wave inversion. His heart size was at the upper limit of normal on chest radiography. On specific questioning, he gave a history of lifelong avoidance of alcohol, but of 34 kg weight loss over the preceding three months. This information was corroborated by his parents and practice nurse. At an initial weight of 127 kg (body mass index 42.4), he had received dietary advice from his practice nurse. He proceeded to lose weight, which fell rapidly from 123 kg at 4 weeks, to 110 kg at 8 weeks, 104 kg at 11 weeks, and finally 93 kg (body mass index 31) on admission. More recently, pursuing greater weight loss, he had eliminated all bread, cereals, and fats, on a diet considered starvation by his parents, without nutritional supplements. Selected biochemical, hematologic, vitamin, and trace element results available on the stated days are shown:
| Test | Reference Range (Conversion Factor to Traditional Units) | DAY | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 50 | ||
| Calcium (adjusted) | 2.10–2.60 mmol/L (1 mmol/L = 4 mg/dL) | 2.67 | 2.52 | |||
| Magnesium | 0.70–1.00 mmol/L (1 mmol/L = 2 mEq/L) | 0.94 | 0.89 | |||
| Ferritin | 10.0–275.0 μg/L (1 μg/L =2.25 pmol/L) | 224 | 25.0 | |||
| CRP | <10 mg/L (1 mg/L = 9.52 mmol/L) | 11 | 13 | 12 | 6.7 | 2.7 |
| Albumin | 32–45 g/L | 40 | 37 | 38 | 37 | 41 |
| Bilirubin | <20 μmol/L (1 μmol/L = 0.06 mg/dL) | 48 | 38 | 35 | 24 | 16 |
| Alkaline phosphatase | 40–150 U/L (1 U/L = 0.02 μcat/L) | 140 | 126 | 130 | 116 | 93 |
| Aspartate aminotransferase | <40 U/L ( 1 U/L = 0.02 μcat/L) | 34 | 17 | |||
| Alanine aminotransferase | <50 U/L (1 U/L = 0.02 μcat/L) | 70 | 57 | 59 | 62 | 18 |
| γ-Glutamyl transferase | <70 U/L ((1 U/L = 0.02 μcat/L) | 111 | 86 | 87 | 74 | |
| Hemoglobin | 130–180 g/L | 152 | 149 | 153 | 144 | 139 |
| Vitamin B 1 | 275–675 ng/g hemoglobin | 132 | 453 | |||
| RBC vitamin B 2 | 1.0–3.4 nmol/g hemoglobin | 1.6 | 2.9 | |||
| RBC vitamin B 6 | 250–680 pmol/g hemoglobin | 139 | 619 | |||
| Vitamin B 12 | 200–900 pg/mL (1 pg/mL = 0.74 pmol/L) | 481 | 328 | |||
| Folate | 3.1–20.0 ng/mL (1 ng/mL = 2.27 nmol/L) | 1.4 | 3.3 | |||
| Vitamin C | 15–90 μmol/L (1 μmol/L = 0.02 mg/dL) | 3 | 27 | |||
| Vitamin A | 1–3 μmol/L (1 μmol/L = 28.65 μg/dL) | 0.5 | 0.9 | |||
| Vitamin E | 15–45 μmol/L (1 μmol/L = 0.04 μg/dL) | 20 | 20 | |||
| Copper | 10.0–22.0 μmol/L (1 μmol/L = 6.37 μg/dL) | 14.4 | 17.1 | |||
| Manganese | 70–280 nmol/L (1 nmol/L = 0.05 μg/L) | 182 | 176 | |||
| Selenium | 0.8–2.0 μmol/L (1 μmol/L = 78.74 μg/L) | 0.66 | 0.74 | |||
| Zinc | 10.7–18.0 μmol/L (1 μmol/L = 6.54 μg/dL) | 15.1 | 10.8 | |||
Commentary: Given the presence of agitation and cerebellar signs the most likely cause of the ophthalmoplegia and electrocardiographic manifestation is Wernicke encephalopathy as a result of acute vitamin B 1 (thiamine) deficiency. Praising the patient for losing weight inadvertently motivated him to try harder, which inevitably led him to starvation and consequently malnutrition. The classic triad of Wernicke encephalopathy is ophthalmoplegia, ataxia, and mental confusion. Low concentrations of red blood cell (RBC) thiamine diphosphate (TDP), the active form of thiamine, confirmed the diagnosis. TDP reflects body thiamine stores and correlates with transketolase functional testing (discussed below). Vitamins A, C, B 6 , folate, and selenium were all low without a marked systemic inflammatory response, as evidenced by the only marginally raised C-reactive protein (CRP). Vitamin B 1 deficiency causes inhibition of carbohydrate metabolism and accumulation of acetaldehyde, which affects astrocytes within cranial nerve nuclei. Magnesium is required for vitamin B 1 action. Despite deficiencies in other vitamins, there were no associated features. The immediate treatment for thiamine deficiency in alcoholics or malnourished patients is intravenous thiamine, commonly administered with water soluble vitamins B and C as Pabrinex (thiamine 250 mg) before switching to oral thiamine supplementation (refer to local guidance for dosing regimens). Ophthalmoplegia usually resolves rapidly with treatment. The most important learning point is that without thiamine repletion, carbohydrate administration would precipitate Wernicke-Korsakoff syndrome and permanent anterograde amnesia. Magnesium repletion is also required to allow thiamine to function.
The signs of cardiomegaly and ECG abnormalities indicated wet beriberi in addition to the classic neurologic signs of dry beriberi, which are both as a result of thiamine deficiency. If left untreated this condition progresses to high-output heart failure, which is a reported cause of sudden death from starvation or anorexia nervosa (discussed in text).
Obstructive jaundice and ultrasonography showed several small gallstones within the gallbladder. Symptomatic cholelithiasis is a recognized complication of consuming a low-fat diet or of extreme weight loss.
The patient was treated with 5 mL intravenous Pabrinex, which contains anhydrous glucose (1 g), ascorbic acid (500 mg), nicotinamide (160 mg), pyridoxine hydrochloride (50 mg), riboflavin (4 mg), and thiamine hydrochloride (250 mg) three times daily for 3 days, resumed normal feeding, and made a good recovery with eventual normal findings on ECG, chest radiography, echocardiography, and normal neurologic signs.
The structure of thiamine [3-(4-amino-2-methyl-pyrimidyl-5-methyl)-4-methyl-5-(β-hydroxyethyl)thiazole] is that of a pyrimidine ring, bearing an amino group, linked by a methylene bridge to a thiazole ring ( Fig. 39.8 ). The thiazole has a primary alcohol side chain at C5, which can be phosphorylated in vivo to produce thiamine phosphate esters, the most common of which is TPP (also known as thiamine diphosphate [cocarboxylase]). Monophosphate and triphosphate esters also occur. The basic vitamin is isolated or synthesized and handled as a solid thiazolium salt (e.g., thiamine chloride hydrochloride). Thiamine is somewhat heat labile, particularly in alkaline solutions, where base attacks occur at C2 of the thiazolium ring.
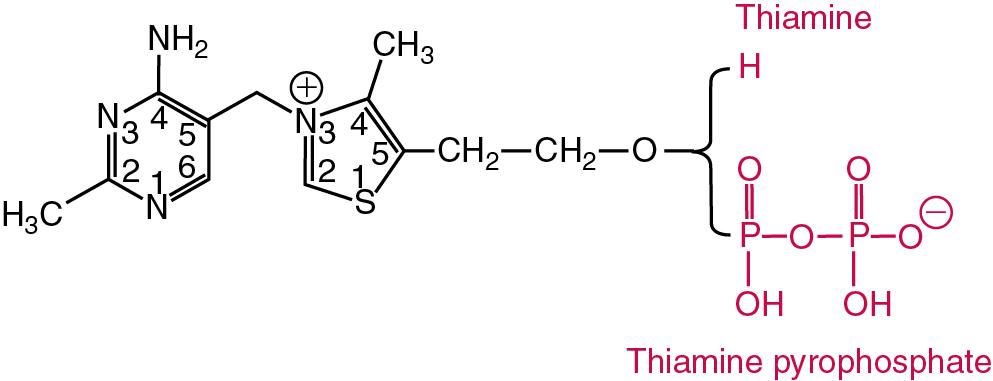
Small amounts of thiamine and its phosphates are present in most plant and animal tissues, but more abundant sources include unrefined cereal grains, liver, heart, kidney, and lean cuts of pork. The enrichment of flour and derived food products, particularly breakfast cereals, has considerably increased the availability of this vitamin.
Thiamine absorption occurs primarily in the proximal small intestine by a saturable (thiamine transporter) process at low concentration (1 μmol/L or lower) and by simple passive diffusion beyond that, although percentage absorption diminishes with increased dose. Absorbed thiamine undergoes intracellular phosphorylation, mainly to the pyrophosphate, but at the serosal side, 90% of transferred thiamine is present in the free form. Thiamine uptake is enhanced by thiamine deficiency and is reduced by thyroid hormone, diabetes, and ethanol ingestion. The gene for the specific thiamine transporter has been identified, and the transporter cloned. Thiamine is carried by portal blood to the liver. The free vitamin is present in plasma, but the coenzyme, TPP, is the primary cellular component. Approximately 30 mg is stored in the body, with 80% as pyrophosphate, 10% as triphosphate, and the rest as thiamine and its monophosphate. About half of body stores are found in skeletal muscle, with much of the remainder in heart, liver, kidneys, and nervous tissues (including the brain, which contains most of the triphosphate).
The three tissue enzymes known to participate in the formation of phosphate esters are (1) thiaminokinase (a pyrophosphokinase), which catalyzes formation of TPP and adenosine monophosphate (AMP) from thiamine and adenosine triphosphate (ATP); (2) TPP-ATP phosphoryl-transferase (cytosolic 5′-adenylic kinase), which forms the triphosphate and adenosine diphosphate (ADP) from TPP and ATP; and (3) thiamine triphosphatase, which hydrolyzes TPP to the monophosphate. Although thiaminokinase is widely distributed in the body, phosphoryl transferase and the membrane-associated triphosphatase are found mainly in nervous tissue.
With the use of labeled thiamine probes, a study of thiamine metabolism at normal loads produced an estimated half-life of thiamine of 9.5 to 18.5 days and showed a large number of breakdown products in the urine. Several of these urinary catabolites are shown in Fig. 39.9 .
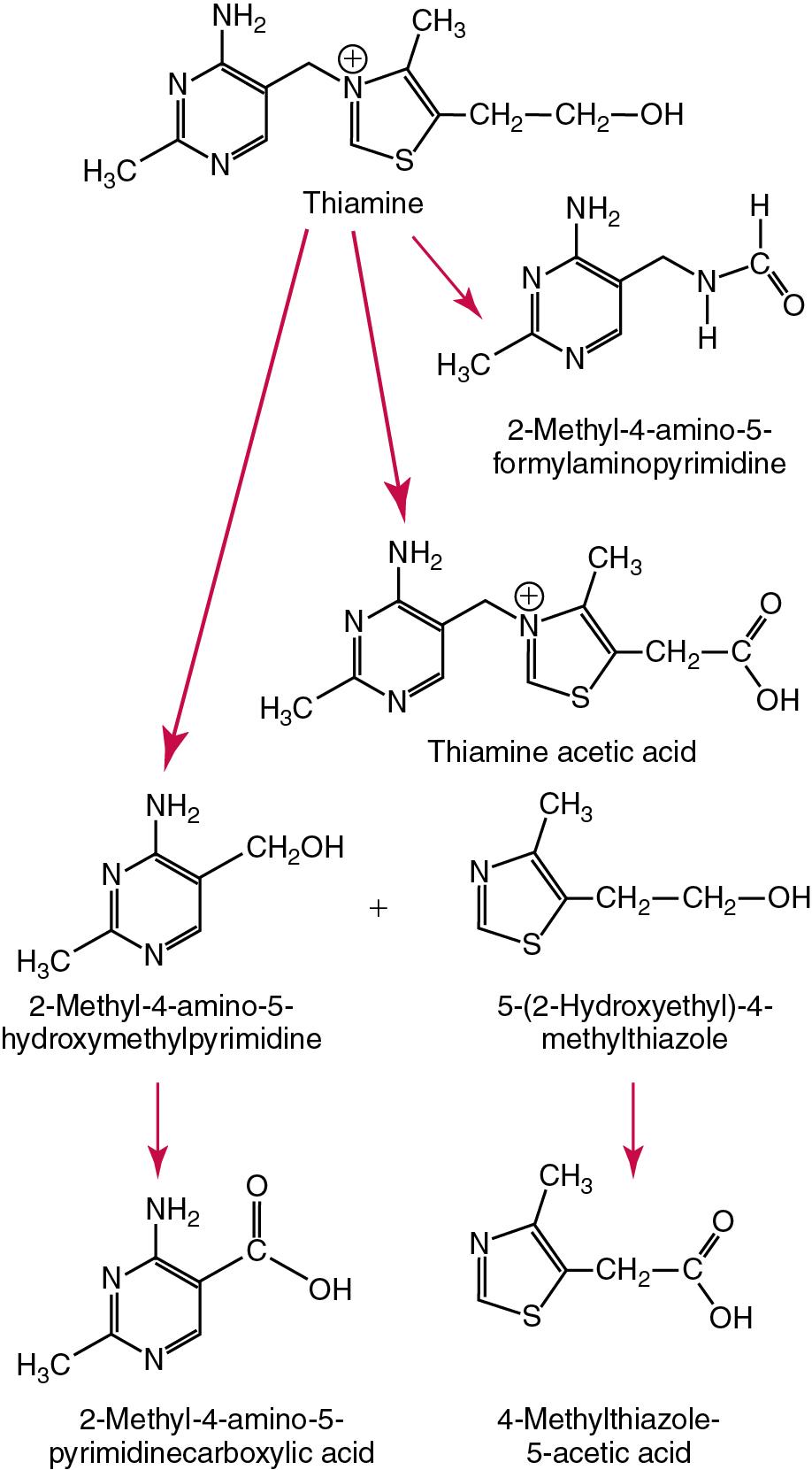
Thiamine is required by the body as the pyrophosphate (TPP) in two general types of reactions: (1) the oxidative decarboxylation of 2-oxo acids catalyzed by dehydrogenase complexes, and (2) the formation of α-ketols (ketoses) catalyzed by transketolase and as the triphosphate (TTP) within the nervous system. TPP functions as the Mg 2+ -coordinated coenzyme for so-called active aldehyde transfers in multienzyme dehydrogenase complexes that affect decarboxylative conversion of α-keto (2-oxo) acids to acyl-coenzyme A (acyl-CoA) derivatives, such as pyruvate dehydrogenase and α-ketoglutarate dehydrogenase. These are often localized in the mitochondria, where efficient use in the Krebs tricarboxylic acid (citric acid) cycle follows.
Three types of subunit proteins constitute such dehydrogenase complexes: (1) a TPP-dependent decarboxylase, which converts the 2-oxo acid to an α-hydroxyalkyl–TPP complex; (2) a transacylase core, which contains lipoyl residues that are acylated by the α-hydroxyalkyl–TPP; and (3) a flavin adenine dinucleotide (FAD)-dependent dihydrolipoyl dehydrogenase, which reoxidizes the reduced lipoyl residues produced after transfer of their acyl functions to reduced CoA. In addition to energy and an ultimate ATP supply derived from reactions in the Krebs cycle, the initial pyruvate dehydrogenase–catalyzed step provides acetyl-CoA as a biosynthetic precursor to other essential compounds, such as lipids and acetylcholine of the parasympathetic nervous system.
Transketolase is a TPP-dependent enzyme found in the cytosol of many tissues, especially liver and blood cells, in which principal carbohydrate pathways exist. In the pentose phosphate pathway, which additionally supplies reduced nicotinamide-adenine dinucleotide (NAD) phosphate (NADPH) necessary for biosynthetic reactions, this enzyme catalyzes the reversible transfer of a glycolaldehyde moiety from the first two carbons of a donor ketose phosphate to the aldehyde carbon of an aldose phosphate.
Although thiamine as its pyrophosphate contributes to nervous system composition and function in such essential reactions as energy production and biosynthesis of lipids and acetylcholine, a further specific, noncofactor role for thiamine has been proposed in excitable cells. Here, TTP is thought to be involved in the regulation of ion channels, specifically, chloride channels of large unitary conductance, the so-called maxi-Cl channels. , TTP may also have more basic metabolic functions, including acting as a phosphate donor for the phosphorylation of proteins, suggesting a potential role in cell signaling. A subacute necrotizing encephalomyelopathy is seen in patients with Leigh syndrome, resulting from the presence of an inhibitor of TPP-ATP phosphoryl transferase and consequent reduction in TTP concentration.
Because thiamine is necessary mainly for the metabolism of carbohydrates, fats, and alcohol, a direct correlation has been noted between physiologic requirements and the amount of metabolizable food intake. A greater requirement is present under situations in which metabolism is increased (e.g., in normal conditions of increased muscular activity, pregnancy, and lactation; in abnormal cases of protracted fever, after trauma, and hyperthyroidism). Clinical signs of deficiency in adults can be prevented with intakes of thiamine above 0.15 to 0.2 mg/1000 kcal, but 0.35 to 0.4 mg/1000 kcal may be closer to a concentration necessary to maintain urinary excretion and TPP-dependent erythrocyte transketolase activity within normal reference intervals. , With further consideration of average caloric intakes and activities in different age groups, the most recent recommendations of RDA are 1.2 mg/day for males 19 years and older and 1.1 mg/day for females 19 years and older. The requirement for pregnant women increases early in pregnancy and then remains constant; 1.4 mg/day is recommended. The lactating woman secretes 0.1 to 0.2 mg of thiamine/day in milk, so 1.4 mg/day is suggested. Based on the thiamine content of human milk and with an increment considered to provide a margin of safety, 0.2 mg/day is the allowance for infants up to 6 months, and 0.3 mg/day for infants 7 to 12 months. For children owing to growth, up to 3 years, 0.5 mg/day is suggested and between 4 to 8 years 0.6 mg/day is suggested.
Traditionally, the IV recommendation was 3 mg/day for adults, usually provided as thiamine hydrochloride, but also as thiamine mononitrate or tetrahydrate. In the 2000 FDA recommendations, this was increased to 6 mg/day, with recognition of the likelihood of increased demands for thiamine caused by hypercatabolism in such patients and the very serious potential complications of deficiency. ,
See Box 39.3 for an illustrative case of thiamine deficiency.
Causes of thiamine deficiency include inadequate intake caused by diets largely dependent on milled, nonenriched grains such as rice and wheat, or the ingestion of raw fish containing thiaminases, which hydrolytically destroy the vitamin in the gastrointestinal tract. Tea may also contain anti-thiamine factors. , Chronic alcoholism often leads to thiamine deficiency caused by reduced intake, impaired absorption, impaired use, and reduced storage, and may lead clinically to the Wernicke-Korsakoff syndrome. , Other at-risk groups include those receiving parenteral nutrition without adequate thiamine supplementation, elderly patients taking diuretics, and patients undergoing long-term renal dialysis.
Beriberi (origin: Sinhalese from a word meaning weakness) is the disease resulting from thiamine deficiency. , Clinical signs of thiamine deficiency primarily involve the nervous and cardiovascular systems. In the adult, symptoms most frequently observed include mental confusion, anorexia, muscular weakness, ataxia, peripheral paralysis, ophthalmoplegia, edema (wet beriberi), muscle wasting (dry beriberi), tachycardia, and an enlarged heart. In infants, symptoms appear suddenly and severely, often involving cardiac failure and cyanosis. Commonly, the distinction between wet (cardiovascular) and dry (neuritic) manifestations of beriberi relate to duration and severity of the deficiency, the degree of physical exertion, and caloric intake. The wet or edematous beriberi results from severe physical exertion and high carbohydrate intake, whereas the dry or polyneuritic beriberi , stems from relative inactivity with caloric restriction during the chronic deficiency. The three major physiologic derangements that typically involve the cardiovascular system are peripheral vasodilatation leading to a high-output state, biventricular myocardial failure, and retention of sodium and water, leading to edema. Nervous system involvement includes peripheral neuropathy, Wernicke encephalopathy, and the amnesic psychosis of Korsakoff syndrome. More rarely, but especially in seriously ill patients in hospitals, an acute form of cardiac failure has been described (Shoshin beriberi), which may be fatal, but can be successfully and rapidly reversed with high-dose IV thiamine. ,
Several thiamine-responsive disorders are caused by genetic variant. In thiamine-responsive megaloblastic anemia (TRMA), the gene has been mapped and cloned and designated “ SLC19A2 ” as a member of the solute carrier gene superfamily. Variants of this gene, the product of which is a membrane protein that transports thiamine with submicromolar affinity, have been found in all TRMA kindreds studied. Thiamine-responsive pyruvate dehydrogenase complex deficiency, presenting with lactic acidosis, can be caused by a point variant within the TPP–binding region, and a thiamine-responsive branched-chain keto acid dehydrogenase complex deficiency, presenting as a form of maple syrup urine disease, is caused by variants in the E1 α-subunit of the enzyme complex. Therapeutic doses of 5 to 20 mg of thiamine daily have proved beneficial in these cases.
Because no reports have described adverse effects from consumption of excess thiamine from food and supplements (supplements of 50 mg/day are widely available without prescription), and because the data are inadequate for a quantitative risk assessment, no tolerable upper intake levels (ULs) have been defined for thiamine. However, because stimulators of transketolase enzyme synthesis, such as thiamine, support the high rate of nucleic acid ribose synthesis necessary for tumor cell survival, chemotherapy resistance, and proliferation, some concern has been expressed that thiamine supplementation of common food products may contribute to increased cancer rates in the Western world. However, little evidence is available to support this assumption. Rarely, individuals given high-dose IV thiamine in the treatment of beriberi have developed anaphylaxis (frequency of about 1:100,000).
As thiamine deficiency develops, rapid loss of the vitamin is seen from all tissues except the brain. The decrease of TPP in the erythrocyte roughly parallels the decrease of this coenzyme in other tissue. During this time, thiamine concentrations in urine fall to near zero; urinary metabolites remain high for some time before decreasing.
Historically, assessment of thiamine status was performed using animal bioassay (correction of bradycardia in thiamine-deficient rats). Later, it was performed by microbiological assays; some bacterial microbiological assays are still in use in the food industry. Early chemical methods were often based on the production of a fluorophore, thiochrome, when thiamine is oxidized with ferricyanide in alkaline solution—a property that is used in some modern chromatographic methods.
Because the basic biological function of thiamine is to act as the pyrophosphate cofactor in several enzyme systems, two differing approaches to assessment of status have become available. The analyte, free or phosphorylated, can be measured directly in a suitable body fluid or tissue, or its properties as an enzymatic cofactor can be exploited in a functional assay. Both approaches have their advantages and disadvantages, and consensus as to which is the more useful has not been achieved; the two are probably complementary, each supplying some, but not all, of the information necessary to assess thiamine adequacy ( Table 39.4 ).
| Erythrocyte Thiamine Pyrophosphate | Erythrocyte Transketolase Activation | |
|---|---|---|
| Advantages | Pure standard available Precise and robust method More stable when frozen Depletes at rates similar to other organs Method (HPLC) allows measurement of other forms of thiamine Can detect tissue accumulation |
May correlate better with clinical conditions in repleted patients Large database established |
| Disadvantages | May normalize very early with parenteral treatment | Depletion of apoenzyme may be non-nutritional related, e.g., liver disease, diabetes Variants may have abnormal binding May be influenced by cofactor deficiencies, e.g., magnesium Difficult to standardize, less robust Derived activation coefficient reduces precision |
The most used enzyme for the functional assay is transketolase. Transketolase catalyzes two reactions in the pentose phosphate pathway ( Fig. 39.10 ). As an enzyme within the erythrocyte, transketolase is independent of nonspecific changes in the extracellular plasma. As vitamin B 1 deficiency becomes more severe, (1) thiamine becomes limiting in the body cells, (2) the amount of the coenzyme is depleted, and (3) transketolase activity subsequently diminishes. The TPP effect measures the extent of depletion of the transketolase enzyme for coenzyme by assaying enzyme activity before and after TPP supplementation. The percent increase in activity is defined as the TPP effect, or the activation coefficient. Several methods are available to measure transketolase activity. In the Brin procedure, activities of holo forms and apo forms of transketolase in erythrocyte hemolysates are measured before and after addition of TPP, by spectrophotometric determinations of the amount of ribose-5′-phosphate used or hexose-6-phosphate formed. This method is reliable but time-consuming. In an alternative method, the rate of formation of glyceraldehyde-3-P is measured indirectly by a coupled reaction in a system containing excess triosephosphate isomerase (TIM), glycerolphosphate dehydrogenase (GD), and NADH. Glyceraldehyde-3-P is converted by TIM to dihydroxyacetone-P, which, in the presence of GD and NADH, is reduced to glycerol-1-P. The rate of NADH oxidation, measured at 340 nm, is proportional to the transketolase activity. Kinetic methods such as these have been automated with consequent improvements in throughput and precision.
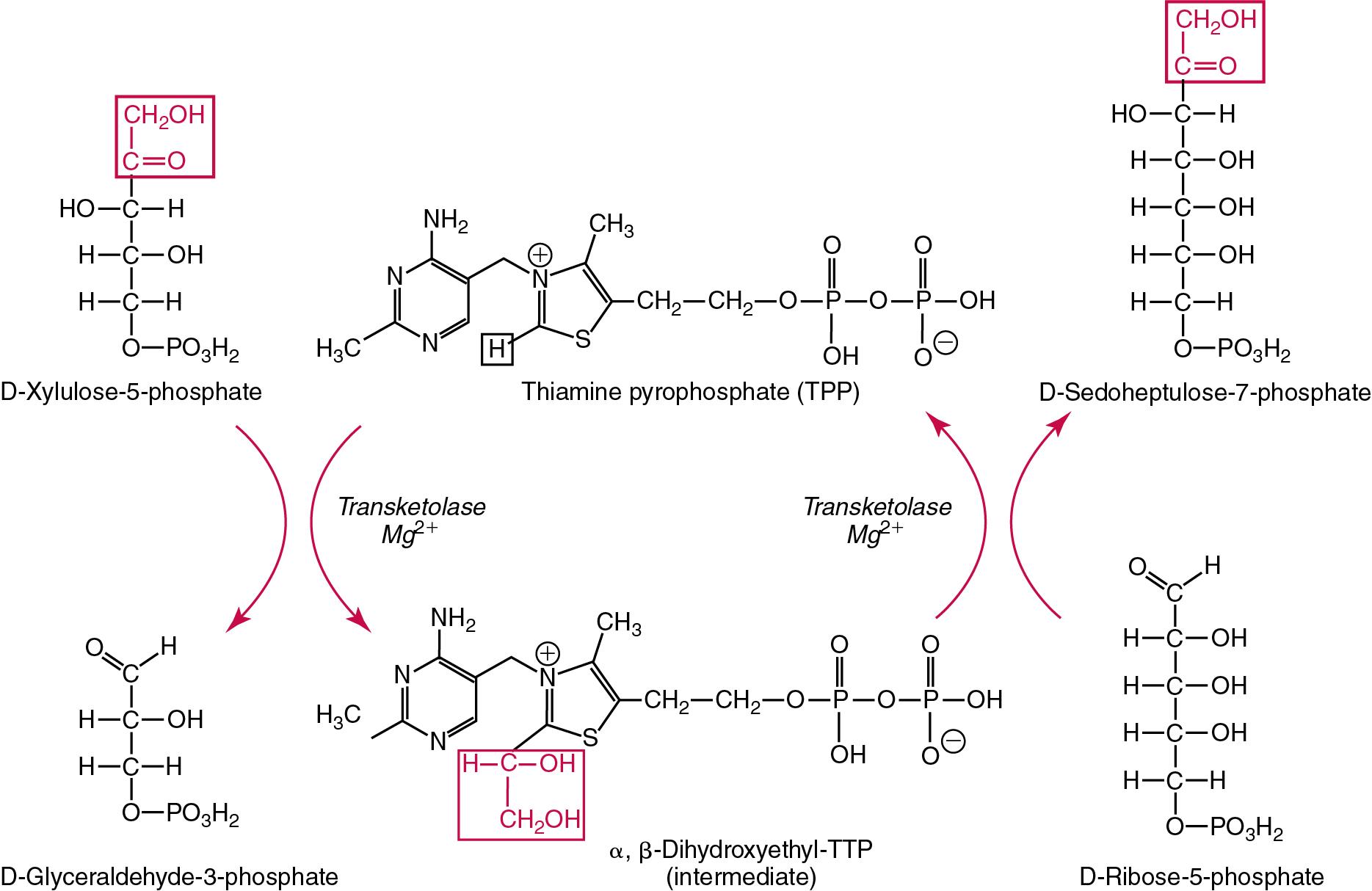
The transketolase activation test basically consists of two tests: (1) measurement of basal activity and (2) assessment of the degree to which basal activity can be increased by exogenous TPP; each may be influenced by different factors. There is some evidence that chronic deficiency states of thiamine may down-regulate synthesis of the apoenzyme. In comparison studies against erythrocyte TPP concentrations, better correlations were obtained with basal activity rather than the activation coefficient.
Other potential disadvantages of the transketolase test include reductions in apoenzyme synthesis in diseases other than thiamine deficiency such as diabetes and liver disease, reduced apoenzyme-to-coenzyme binding with apotransketolase variants, lack of stability relative to TPP on processing and storage, lack of a standard or EQAS, and variations in published ULs for the activation coefficient from 15.5 to 40%. The main advantages of the transketolase test are that it is widely used, has a relatively large database and body of experience, and is claimed to correlate better with clinical conditions in alcoholic patients being repleted with thiamine.
Circulating thiamine concentration may be directly measured in plasma, erythrocytes, or whole blood. The plasma (or serum) concentration is thought to reflect recent intake and is mainly unphosphorylated thiamine at low concentration (around 10 to 20 nmol/L). Because the erythrocyte contains approximately 80% of the total thiamine content of whole blood (mainly as the pyrophosphate) and erythrocyte thiamine stores deplete at a similar rate to other major organs, HPLC measurement of TPP in erythrocytes is a good indicator of body stores. Typical HPLC methods include a protein precipitation step; precolumn or postcolumn formation of the fluorophore; thiochrome, usually with alkaline ferricyanide; and isocratic separation. The method is easily standardized with pure TPP; it has good precision (CVs of less than 5%) and acceptable limits of detection (around 10 nmol/L), and the analyte is stable at −70 °C for at least 7 months and at room temperature for 48 hours. Whole blood samples may be analyzed in a similar manner to washed erythrocytes and may provide the advantage of simpler sample handling, but they are subject to variable plasma dilution. However, a good correlation has been obtained between erythrocyte and whole blood TPP concentrations, particularly when whole blood TPP included a correction for hemoglobin (Hb). A rapid HPLC method for measuring both thiamine and its phosphate esters has been described.
Determination of the urinary excretion of thiamine in a 4-hour specimen, especially with comparison of excretion before and after a test load, is helpful in differentiating among extremes of thiamine status. However, as with most assessments based on the quantity of water-soluble vitamins in urine, excretion can be influenced considerably by dietary intake, absorption, and other factors. Measurements of certain urinary metabolites, notably thiamine acetic acid, have been suggested as reflecting thiamine status but are not routinely requested.
Whole blood collected into containers with the preservatives lithium heparin or EDTA is recommended, but local laboratories should be consulted for their preferred sample type. A recent study using HPLC with fluorimetric detection reported that vitamin B 1 showed good stability up to 72 hours at room temperature.
Reference intervals for thiamine and its esters depend on whether (1) erythrocytes, whole blood, or plasma is used as a sample; (2) cellular concentrations are expressed per liter of packed red cells or grams of Hb; and (3) mass or SI units are used. Some guidance intervals are as follows: for erythrocyte transketolase activity, 0.75 to 1.30 U/g Hb (48.4 to 83.9 kU/mol Hb) is used; for percent TPP effect (activation), 0 to 15% is normal, 16 to 25% is marginally deficient, and more than 25% is severely deficient with clinical signs. For direct TPP concentration measurements, typical intervals are 173 to 293 nmol/L erythrocytes and 90 to 140 nmol/L whole blood, 280 to 590 ng/g Hb in erythrocytes and 275 to 675 ng/g Hb in whole blood with less than 150 ng/g Hb indicating clinical deficiency. , For more information, refer to the Appendix on Reference Intervals. Laboratories should verify that these ranges are appropriate for use in their own settings.
Vitamin B 2, also known as riboflavin, is an essential component of coenzymes that are involved in reduction-oxidation (redox) reactions in the body.
Flavins are a family of yellow-colored compounds with the basic structure of 7,8-dimethyl-10-alkylisoalloxazine. Riboflavin, commonly known as vitamin B2, is the precursor of all biologically important flavins, notably flavin mononucleotide, FMN (riboflavin-5′-phosphate) and FAD ( Fig. 39.11 ). Riboflavin and its related metabolites act as cofactors to several reduction-oxidation enzymes. FMN is formed from riboflavin by flavokinase-catalyzed phosphorylation, and FAD is formed from FMN and ATP by the action of FAD synthetase, also called pyrophosphorylase . FAD is further converted by covalent bonding to form various tissue flavoproteins. Flavins are stable during exposure to heat but are decomposed by light, which causes photodegradation of the d -ribitol side chain at position 10 of the isoalloxazine ring system to ultimately yield lumiflavin (7,8,10-trimethylisoalloxazine) under alkaline conditions and lumichrome (7,8-dimethylalloxazine) at all pH values, especially in neutral-to-acidic solutions. Flavins are chemically and biologically reduced to nearly colorless compounds that rapidly reoxidize on exposure to air (oxygen).
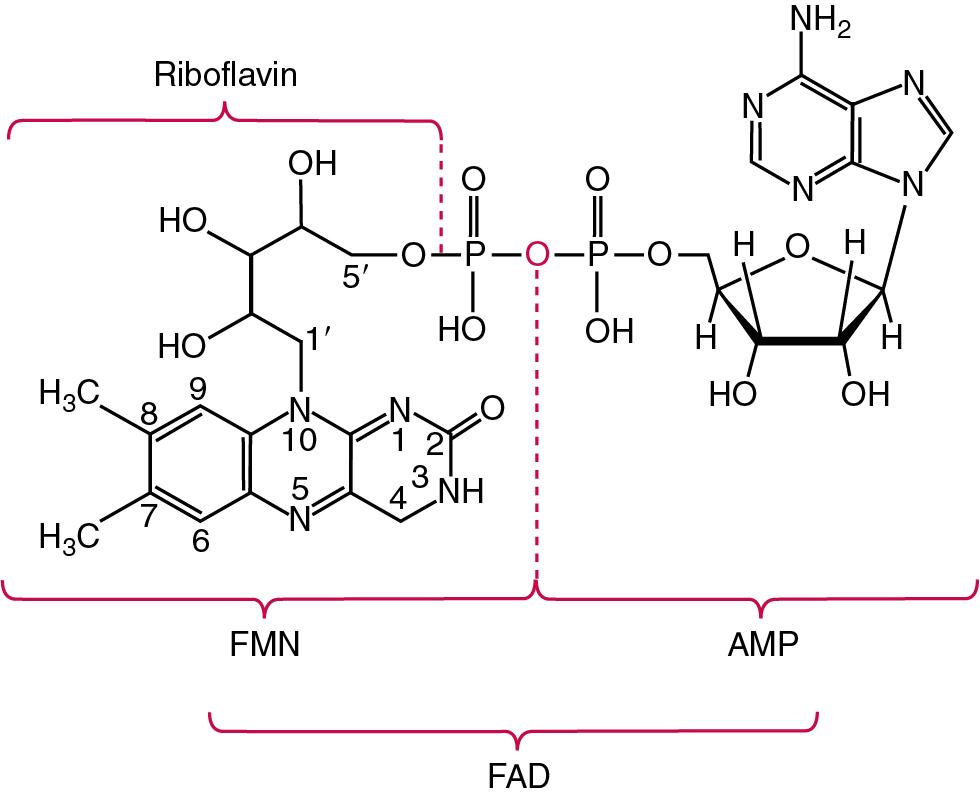
Rich sources of the coenzyme forms of the vitamin include liver, kidney, and heart. Many vegetables are also good sources, but cereals are low in flavin content. Current practices of fortification and enrichment of cereal products have made these significant contributors to the daily requirement. Milk from cows and from humans are a good source of the vitamin and probably the main source in western diets, , but considerable loss can occur from exposure to light during pasteurization and bottling, or as a result of irradiation to increase vitamin D content.
Most dietary riboflavin is taken in as a complex of proteins with the coenzymes FMN and FAD. These coenzymes are released from noncovalent attachment to proteins because of gastric acidification. Nonspecific action of pyrophosphatase and phosphatase on the coenzyme occurs in the upper gut. The vitamin is primarily absorbed in the proximal small intestine by a saturable transport system that is rapid and proportional to intake before leveling off at doses near 27 mg riboflavin/day. Bile salts appear to facilitate uptake, and a modest amount of the vitamin circulates via the enterohepatic system. Active transport at lower concentrations of intake was thought to be sodium ion–dependent and to involve phosphorylation, although later work has suggested that uptake is independent of sodium ions. , The transport of flavins in human blood involves loose binding to albumin and tight binding to numerous globulins, with major binding noted to several classes of immunoglobulins (IgA, IgG, and IgM). Pregnancy increases the concentration of carrier protein for riboflavin, which results in a higher rate of riboflavin uptake at the maternal surface of the placenta. Uptake of riboflavin into the cells of organs such as liver is facilitated, possibly requiring a specific carrier at physiologic concentrations, but it can occur by diffusion at higher concentrations. Metabolic interconversions of flavins at the cellular concentration are outlined in Fig. 39.12 .
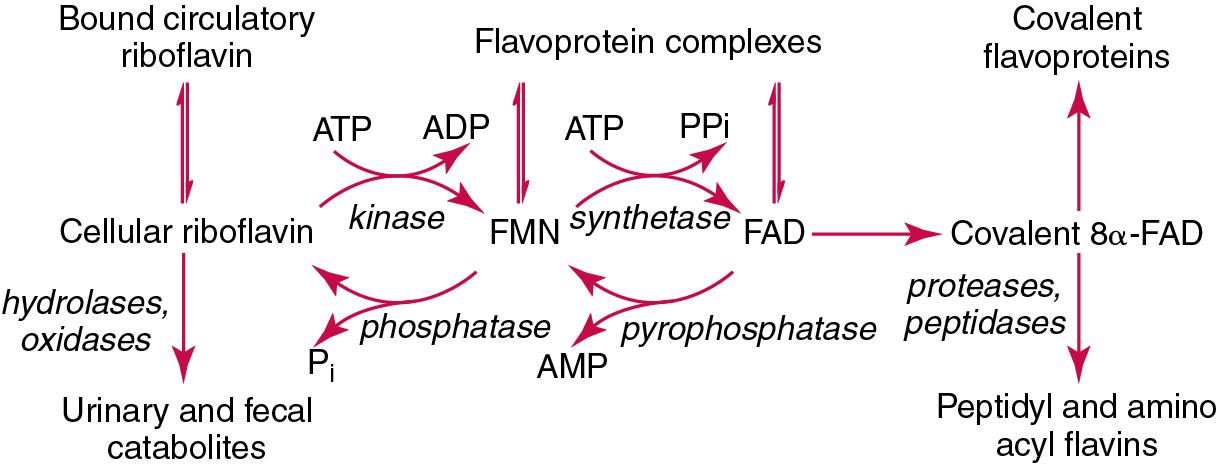
Conversion of riboflavin to coenzymes occurs within the cellular cytoplasm of most tissues but particularly in the small intestine, liver, heart, and kidney. The obligatory first step is the ATP-dependent phosphorylation of the vitamin catalyzed by flavokinase. The FMN product can be complexed with specific apoenzymes to form several functional flavoproteins, but the larger quantity is further converted to FAD in a second ATP-dependent reaction catalyzed by FAD synthetase (pyrophosphorylase). Biosynthesis of flavocoenzymes, particularly at the flavokinase step, is likely tightly regulated. Thyroxine and triiodothyronine stimulate FMN and FAD synthesis in mammalian systems. , FAD is the predominant flavocoenzyme present in tissue, where it is complexed mainly with numerous flavoprotein dehydrogenases and oxidases. Some FAD (<10%) can become covalently linked to any of five specific amino acid residues of a few important apoenzymes. Examples include 8α- N (3)-histidyl FAD within succinate dehydrogenase and 8α- S -cysteinyl FAD within monoamine oxidase, both of mitochondrial localization. Turnover of covalently attached flavocoenzymes requires intracellular proteolysis, and further degradation of the coenzymes involves nonspecific pyrophosphatase cleavage of FAD to FMN and AMP, and further action by nonspecific phosphates on FMN and AMP. Because there is little storage of riboflavin as such, urinary excretion reflects dietary intake. Milk contains reasonable quantities of the vitamin and lesser amounts of coenzyme, principally FMN. Smaller quantities of side chain degradation products such as lumichrome, 10-formylmethylflavin and 10-(2′-hydroxyethyl)flavin, and ring-altered compounds are excreted; this may largely result from the action of intestinal microorganisms. Traces of 8α-flavin peptides and catabolites are found in urine and feces.
Riboflavin and its coenzyme derivatives are involved in a large variety of chemical reactions. These derivatives are capable of one- and two-electron transfer processes and play a pivotal role in coupling the two-electron oxidation of most organic substrates to the one-electron transfer of the respiratory chain, thus being involved in energy production. They also function as electrophiles and nucleophiles, with covalent intermediates of flavin and substrate frequently involved in catalysis. Flavoproteins catalyze dehydrogenation reactions, hydroxylations, oxidative decarboxylations, deoxygenations, and reductions of oxygen to hydrogen peroxide. , The chemical versatility of the flavoproteins is clearly controlled by specific interactions with the proteins with which they are bound. Other major functions of riboflavin include drug metabolism in conjunction with the cytochrome P450 enzymes and lipid metabolism.
Flavins also have pro-oxidative and antioxidative functions. They are thought to contribute to oxidative stress through their ability to produce superoxide and to catalyze the production of hydrogen peroxide. As an antioxidant, FAD is a coenzyme to glutathione reductase in the regeneration of reduced glutathione from oxidized glutathione, which is necessary for the removal of lipid peroxides. Riboflavin deficiency is associated with increased lipid peroxidation. Flavins have also been linked with apoptosis and have homocysteine-lowering properties ; FAD is a cofactor to methylenetetrahydrofolate reductase (MTHFR) in the remethylation of homocysteine. An interaction between folate, riboflavin, and the genotype of MTHFR is apparent, especially in colorectal cancer. Other possible therapeutic uses of riboflavin include prophylaxis of migraine attacks and treatment of lactic acidosis caused by the use of nucleoside reverse transcriptase inhibitors in patients with the acquired immunodeficiency syndrome (AIDS) or by genetic defects in the mitochondrial respiratory chain, as seen in Leigh disease. Riboflavin is also effective in treating the lipid storage myopathy associated with variants of ETFDH (electron-transferring flavoprotein dehydrogenase). Antagonism of riboflavin metabolism has been used as an anti-infective agent notably in malaria treatment. ,
Riboflavin status has been assessed based on the relationship of dietary intake to overt signs of hyporiboflavinosis, urinary excretion of the vitamin, erythrocyte riboflavin content, and erythrocyte glutathione reductase activity. Calculations have been based on protein allowances, energy intakes, and body size, but these do not differ significantly because they are interdependent. At least 0.5 mg of riboflavin/1000 kcal is required by the adult, and 0.6 mg/1000 kcal constitutes the allowance suggested for all ages. Based on considerations such as these, the current RDA has been set at 1.3 mg/day for men 19 to 70 years of age and older, and 1.1 mg/day for women in the same age group. Children 1 to 3 years old have an RDA of 0.5 mg/day, increasing to 0.6 mg/day up to age 8. From 8 to 18 years, RDAs progressively approach adult concentrations. Because pregnant women tend to excrete less riboflavin as pregnancy progresses and additionally exhibit FAD stimulation of erythrocyte glutathione reductase activity, recommended allowances call for an additional 0.3 mg/day during pregnancy. During lactation, between 18 and 80 μg of riboflavin is secreted daily into every 100 mL of human milk. If it is assumed that an infant will ingest an average of 750 mL of milk/day during its first 6 months and 600 mL/day for the next 6 months, this secretion rate translates into an ingestion of between 100 and 600 μg riboflavin/day. Further, if it is assumed that 70% of maternally ingested riboflavin is used for milk production, these data suggest that the present RDA for lactating women should be increased by an additional 400 to 500 μg/day. Accordingly the RDA for lactating women has been set at 1.6 mg/day.
The recommended IV supply of riboflavin in adults is 3.6 mg/day. , Riboflavin in TPN mixtures may be subject to degradation under exposure to UV light, so bags containing riboflavin should contain fat emulsion or should be covered to provide protection from light.
Although riboflavin has a wide distribution in foodstuffs, many people live for long periods on low intakes; consequently, minor signs of deficiency are common in many parts of the world. In addition to poor intake, functional deficiency can be induced by diseases such as hypothyroidism and adrenal insufficiency, which inhibit the conversion of riboflavin to its coenzyme derivatives, by drugs such as chlorpromazine, imipramine, and amitriptyline, which have a similar tricyclic structure to riboflavin, by the anticancer drug doxorubicin and the antimalarial quinacrine. Excess ethanol ingestion interferes with both digestion and absorption of riboflavin.
Because flavin coenzymes are widely distributed in intermediary metabolism, the consequences of deficiency may be widespread. Riboflavin coenzymes are involved in the metabolism of vitamin B 12 and folic acid (irreversible reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate [5-MTHF]) and therefore are a determinant of plasma homocysteine concentration; pyridoxine (conversion to pyridoxal 5-phosphate); and, niacin (conversion of 5-hydroxytryptamine to tryptophan that is required for niacin synthesis). , Therefore deficiency will affect enzyme systems other than those requiring flavin coenzymes per se. With increasing riboflavin deficiency, tissue concentrations of FMN and FAD will fall, as does flavokinase activity, thus further decreasing FMN concentrations. FMN concentrations are decreased proportionately more than FAD concentrations. Decreases in the activities of enzymes requiring FMN generally follow the fall in tissue concentrations, whereas FAD-dependent enzymes are more variably affected.
The deficiency syndrome is characterized by (1) sore throat; (2) hyperemia; (3) edema of the pharyngeal and oral mucous membranes; (4) cheilosis; (5) angular stomatitis; (6) glossitis (magenta tongue); (7) seborrheic dermatitis; and (8) normochromic, normocytic anemia associated with pure red blood cell (RBC) aplasia of the bone marrow. , , However, some of these symptoms, such as glossitis and dermatitis, when encountered in the field may be seen to have resulted from other complicating deficiencies.
As riboflavin-derived cofactors are essential for the function of numerous dehydrogenases, they have been linked to genetic defects such as Brown-Vialetto-Van Laere and Fazio-Londe syndromes. Variants in the electron transferring flavoprotein genes ( ETFA/ETFB ) and its dehydrogenase ( ETFDH ) are causative for multiple acyl-CoA dehydrogenase deficiency. Variants in ACAD9, encoding the acyl-CoA dehydrogenase 9 protein were recently reported in mitochondrial disease with respiratory chain complex I deficiency. These conditions are responsive to riboflavin therapy.
Probably as a result of its limited solubility and limited gastric absorption, no adverse effects have been associated with ingestion of riboflavin appreciably above RDA amounts. One study reported no short-term side effects in 49 patients treated with 400 mg/day of riboflavin with meals for at least 3 months. Because of lack of data for risk assessment, no tolerable upper intake amount has been proposed for riboflavin.
Riboflavin status can be assessed by (1) determination of urine riboflavin excretion, (2) by a functional assay measuring the erythrocyte glutathione reductase activation coefficient (EGRAC), which is the ratio between enzyme activity determined with and without the addition of the cofactor FAD, or (3) direct measurement of riboflavin or its metabolites in plasma or erythrocytes. The advantages and disadvantages of functional and direct methods were discussed in the section on thiamine.
Urinary riboflavin has been measured using fluorometric and microbiological procedures, but for specificity, HPLC combined with fluorometric detection is the method of choice. , Under conditions of AI, the amount excreted per day is more than 120 μg or 80 μg/g creatinine. The rate of excretion expressed as μg/g creatinine is greater for children than for adults. Conditions causing negative nitrogen balance and the administration of antibiotics and certain psychotropic drugs (phenothiazine derivatives) increase urinary riboflavin because of tissue depletion and displacement. A load return test may augment reliability but is more cumbersome.
Riboflavin status is commonly assessed by the determination of FAD-dependent glutathione reductase activity in freshly lysed erythrocytes. , This enzyme-based assay has been chosen for most surveys of riboflavin status. Most methods measure the rate of change of absorbance at 340 nm caused by the oxidation of NADPH and have been automated to give rapid throughputs and CVs of less than 2% within the run, although some have used fluorescence detection with increased sensitivity. Potential problems include that (1) in long-standing riboflavin deficiency, apoenzyme activity may be reduced, possibly leading to a misleading activation coefficient calculation; and (2) in patients with glucose-6-phosphate deficiency, a misleadingly low activation coefficient may be measured, possibly caused by enhanced binding of FAD to the apoenzyme. Thus methodologic variation can lead to substantial differences in results.
Direct measurement of riboflavin, FMN, and FAD in plasma or erythrocytes is undertaken by HPLC, usually with fluorescence detection after protein precipitation, or by capillary zone electrophoresis with laser-induced fluorescence detection (CZE-LIF). In a study of riboflavin status, with FMN and FAD concentrations in plasma and erythrocytes from elderly subjects at baseline and after low-dose riboflavin supplementation obtained using activation coefficient measurements and CE-LIF, it was concluded that concentrations of all B 2 vitamers except plasma FAD are potential indicators of vitamin B 2 status, and that plasma riboflavin and erythrocyte FMN may be useful in assessment of vitamin B 2 status in population studies. In critically ill patients, plasma albumin-bound FAD may fall as a result of the systemic inflammatory response (SIR) and due to redistribution of FAD because of increased tissue requirement; red cell FAD is unaffected. , , Therefore the measurement of red cell FAD is more sensitive and recommended especially in critically ill patients.
Whole blood collected into containers with the preservatives lithium heparin or EDTA is recommended but local laboratories should be consulted for their preferred sample type. Ideally a fasting sample should be collected, especially if the patient is receiving oral or parenteral vitamin B 2 supplementation. Otherwise, sampling should be undertaken at least 8 hours post-supplementation. As vitamin B 2 is light-sensitive, samples should be protected from light by wrapping with foil. However, a recent study using HPLC with fluorimetric detection reported that vitamin B 2 showed good stability up to 72 hours at room temperature.
Guidance reference intervals for the activation coefficient of erythrocyte glutathione reductase by FAD are 1.20 (adequacy), 1.21 to 1.40 (marginal deficiency), and 1.41 and above (deficiency). The reference interval for serum or plasma concentrations of riboflavin has previously been quoted as 4 to 24 μg/dL (106 to 638 nmol/L); however, lower intervals have been reported using liquid chromatography with tandem mass spectrometry or using the established HPLC with fluorometric detection method. Indeed one group studying 119 apparently healthy individuals using HPLC with fluorometric detection obtained the following results [median (range)]: plasma FAD—101 (57 to 170) nmol/L; plasma FMN—6.3 (3.3 to 14.1) nmol/L; plasma riboflavin—11 (4 to 34) nmol/L; erythrocyte FAD—1.9 (0.7 to 3.8) pmol/g Hb; erythrocyte FMN—0.11 (0.04 to 0.44) pmol/g Hb; erythrocyte riboflavin 0.02 (0.01 to 0.13) pmol/g Hb. For the recommended test of vitamin B 2 sufficiency—erythrocyte FAD—using HPLC with fluorometric detection, the suggested reference interval is 1.0 to 3.4 nmol/g Hb. , For more information, refer to the Appendix on Reference Intervals. Laboratories should verify that these ranges are appropriate for use in their own settings.
Pyridoxine (pyridoxol), pyridoxamine, and pyridoxal are the three natural forms of vitamin B 6 . They are converted to the active form pyridoxal phosphate, which is required for synthesis, catabolism, and interconversions of various amino acids.
The vitamin B 6 group comprises three natural forms: pyridoxine (pyridoxol) (PN), pyridoxamine (PM), and pyridoxal (PL), which are 4-substituted 2-methyl-3-hydroxyl-5-hydroxymethyl pyridines ( Fig. 39.13 ). During metabolic conversion, each vitamer becomes phosphorylated at the 5-hydroxymethyl substituent. Although both pyridoxamine-5′-phosphate (PMP) and pyridoxal-5′-phosphate (PLP; P-5′-P) interconvert as coenzyme forms during aminotransferase (transaminase)-catalyzed reactions, PLP is the coenzyme form that participates in a large number of B 6 -dependent enzyme reactions.
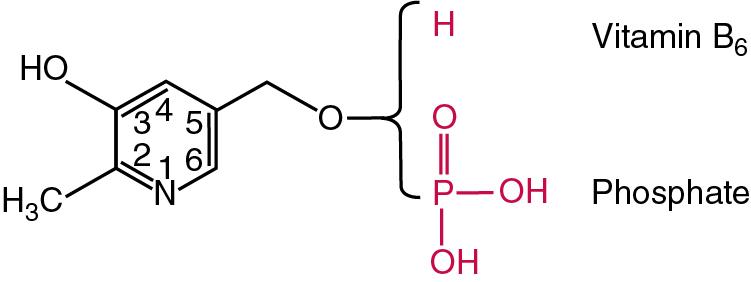
Vitamin B 6 is widely distributed in animal and plant tissues, where the phosphorylated forms, particularly PLP, predominate. Meats, poultry, and fish are good sources, as are yeast, certain seeds, bran, and bananas; somewhat more limited sources are milk, eggs, and green leafy vegetables. In the United States and in some other countries, fortified ready-to-eat cereals are the main dietary source of vitamin B 6 . The common commercial form of the vitamin is pyridoxine hydrochloride, which is a water-soluble, white, crystalline solid. Solutions of B 6 vitamers are decomposed by light, especially in the UV region, at neutral to alkaline pH, and there is significant loss during thermal processing of foods.
Food sources of animal origin contain mainly PLP with some PMP, whereas plant sources also contain pyridoxine-5′-glucoside, which is absorbed in a different manner. The phosphorylated sources are hydrolyzed by the intraluminal action of intestinal alkaline phosphatase, but pyridoxine-5′-glucoside is less effectively hydrolyzed by nonspecific glycosidase within cells, and some pyridoxine-5′-glucoside can be absorbed intact and hydrolyzed in various tissues. The nonphosphorylated vitamers are readily absorbed by the mucosal cells through a process of passive diffusion, which does not appear to be limited by load, although a carrier mechanism may also exist. Here, as in other cells requiring vitamin B 6 , the unphosphorylated vitamers may be “metabolically trapped” as phosphorylated forms by cytoplasmic pyridoxal kinase, which is responsible for catalyzing the ATP-dependent phosphorylation of all three vitamin forms. Transport to the liver via the portal vein is done by the unphosphorylated form.
Fig. 39.14 shows the intracellular metabolism of vitamin B 6 . Most cells contain a cytosolic FMN-dependent, pyridoxine (pyridoxamine)-5′-phosphate oxidase responsible for catalyzing the oxygen-dependent conversion of pyridoxine phosphate and pyridoxamine phosphate to PLP (and hydrogen peroxide). PLP can enter directly into subcellular organelles such as hepatocyte mitochondria and can bind for catalytic function with numerous specific apoenzymes throughout the cell. In addition, the erythrocyte traps PLP as a conjugate Schiff base with hemoglobin. Vitamin B 6 in muscle accounts for 80% of body stores, mostly as PLP bound to glycogen phosphorylase. Total body stores of vitamin B 6 are thought to be about 1 mmole.
Release of free vitamin, mainly pyridoxal, occurs when physiologic nonsaturating concentrations of vitamin are absorbed. Here the phosphates are hydrolyzed by nonspecific alkaline phosphatase located on the plasma membrane of cells. Some PLP is released into the circulation by the liver. PLP is the principal tissue form of vitamin B 6 , whereas pyridoxal constitutes much of the circulating vitamin. The main catabolite excreted in urine is 4-pyridoxic acid (4-PA), which is formed by the action of the FAD-dependent general liver aldehyde oxidase, and NAD-specific aldehyde dehydrogenase, which is found in most tissues. ,
As coenzyme PLP, vitamin B 6 functions in more than 100 reactions that embrace the metabolism of macronutrients, such as proteins, carbohydrates, and lipids. PLP-dependent enzymes that are involved in amino acid metabolism are especially diverse. Aminotransferases affect breaking of the α-hydrogen bond with the ultimate formation of a 2-oxo acid and pyridoxamine-5′-phosphate; this reversible reaction provides an interface between amino acid metabolism and that of ketogenic and glucogenic reactions.
Other examples of PLP-requiring enzymes are the amino acid decarboxylases that lead to formation of amines, including several that are functional in nervous tissue (e.g., epinephrine, norepinephrine, serotonin, γ-aminobutyrate); cysteine desulfhydrase and serine hydroxymethyltransferase, which use PLP to effect the loss or transfer of amino acid sidechains; phosphorylase, which catalyzes phosphorolysis of the α-1,4-linkages of glycogen; and cystathione β-synthase in the transsulfuration pathway of homocysteine. Additionally, the biosynthesis of heme depends on the early formation of 5-aminolevulinate from PLP-dependent condensation of glycine and succinyl-CoA, followed by decarboxylation; an important role in lipid metabolism is the PLP-dependent condensation of l-serine with palmitoyl-CoA to form 3-dehydrosphinganine, a precursor of sphingomyelins. Therapeutically, vitamin B 6 has been used for the treatment of some intractable seizures in neonates and infants and for the treatment of other vitamin B 6 –responsive inborn errors of metabolism and the carpal tunnel syndrome.
Requirements for vitamin B 6 are complicated by (1) differences in protein intake, (2) the probable provision of a fraction of the necessary quantity through bacterial synthesis in the intestinal tract, (3) the use of alcohol and oral contraceptives, and (4) the infrequent cases in which extra requirements are apparent. , Estimates of requirements with some margin of safety have been based on the production and cure of clinical signs of deficiency but more often on biochemical parameters. The latter include determination of the urinary excretion of vitamin B 6 and 4-PA or xanthurenic acid after a tryptophan load test, plasma concentrations of PLP, and RBC transaminase activity. A ratio of 0.016 mg of vitamin B 6 /g of protein intake has been suggested for normal adults and may be extrapolated to children and adolescents. Recent recommendations have proposed RDAs of 0.5 mg/day for children 1 to 3 years, 0.6 mg/day for children 4 to 8 years, 1.0 mg/day for children 9 to 13 years, 1.3 mg/day for boys 14 to 18 years, 1.3 mg/day for men to age 50 years, and 1.7 mg/day for men older than 50 years. Girls 14 to 18 years of age have an RDA of 1.2 mg/day; women 19 to 50 years, 1.3 mg/day; and women older than 50 years, 1.5 mg/day. An addition of 0.6 mg B 6 /day is suggested for pregnant women to match the increased protein allowance that occurs during gestation. During lactation, 2.0 mg/day is recommended to accommodate for extra protein intake and to provide a concentration of 0.10 to 0.25 mg/L of the vitamin in milk, which is adequate for the breast-fed infant.
The recommended IV supply of vitamin B 6 for adults has been increased from 4 to 6 mg/day to ensure adequate amounts in patients who are sometimes receiving large amino acid intakes. This is usually provided as pyridoxine hydrochloride.
Vitamin B 6 deficiency in isolation is rare; it is more usual in association with deficits in other vitamins of the B-complex. As with other water-soluble vitamins that function as coenzymes, the relative affinity of the coenzyme for a given apoenzyme and the extent to which a particular holoenzyme-catalyzed reaction is essential are reflected in the progressive symptoms of deficiency of the vitamin. Investigations into the consequences of vitamin B 6 deficiency in the human patient use diets deficient in the vitamin and/or diets containing an antagonist, usually 4′-deoxypyridoxine. However, in some instances, drug interactions have led to hypovitaminosis of B 6 . The antituberculosis drug isoniazid (isonicotinic acid hydrazide) forms hydrazones with pyridoxal and PLP. As with other carbonyl reagents, not only do such compounds cause loss by displacement and urinary excretion, but the Schiff bases formed with pyridoxal inhibit pyridoxal kinase, and the PLP Schiff bases may additionally inhibit some PLP-dependent enzymes. Penicillamine (β-dimethyl cysteine), used in the treatment of patients with Wilson disease in an attempt to decrease the damaging concentrations of copper found in liver, inactivates PLP by forming a thiazolidine derivative. Other drugs that can cause vitamin B 6 deficiency include the antiparkinsonian drugs benserazide and carbidopa, which react by forming hydrazones, and theophylline. ,
Several vitamin B 6 –responsive inborn errors of metabolism , are known. Pyridoxine-responsive epilepsy presenting with cases of infantile convulsions occur as a result of the apoenzyme for glutamate decarboxylase having poor affinity for the coenzyme. If they also occur due to pyridoxine 5’-phosphate oxidase deficiency, the active form of vitamin B 6 cannot be synthesized from dietary or supplemented pyridoxine. There is also a type of chronic anemia where the number but not the morphologic abnormality of erythrocytes is improved by pyridoxine supplementation. Xanthurenic aciduria occurs as a result of the decreased affinity of the mutant enzyme kynureninase for PLP whereas primary cystathioninuria is caused by defective cystathionase. Finally, homocystinuria, which is caused by deficiency of the enzyme cystathionine β-synthetase (or synthase) or due to the enzyme’s decreased activity as a result of vitamin B 6 deficiency both resulting in the accumulation of plasma homocysteine spilling over into urine (homocystinuria). In all of these metabolic derangements, high-dose (200 to 1000 mg/day) vitamin B 6 may be necessary. Low vitamin B 6 status (together with low vitamin B 12 and folate status) in humans has been linked to hyperhomocysteinemia and is an independent risk factor for cardiovascular disease, , although clinical trials have been inconclusive.
Biochemical markers of vitamin B 6 deficiency occur early and become more marked as the deficiency progresses. Plasma concentrations of PLP and urinary output of B 6 and 4-PA are decreased within 1 week of removal of the vitamin from the diet. Because liver kynureninase activity is decreased, xanthurenic acid is increased in urine. Aminotransferase activity in serum and RBCs also decreases. Clinically, electroencephalographic abnormalities appear within 3 weeks, and epileptiform convulsions are a common finding in young vitamin B 6 –deficient subjects. In addition, skin changes occur, including dermatitis with cheilosis and glossitis. Hematologic manifestations may include a decrease in the number of circulating lymphocytes and possibly a normocytic, microcytic, or sideroblastic anemia. ,
Although no adverse effects have been observed with high intakes of vitamin B 6 from food sources, high oral supplemental doses have been found to have neurotoxic and photosensitive effects. The first reported cases in humans were a series of seven patients who had taken between 2 and 6 g pyridoxine/day for up to 40 months. Four of these patients were unable to walk, and all showed severe sensory neuropathy of the extremities, although most of the symptoms were reversed on stopping the pyridoxine. None of the subsequent studies showed any evidence of sensory nerve damage at intakes below 200 mg/day. Based on the end point of development of sensory neuropathy, 1998 recommendations have set a tolerable upper intake amount of 100 mg/day for adults.
As with the other B vitamins that act as coenzymes, biochemical assessment of vitamin B 6 can be made by direct chemical analysis of the vitamer or its metabolites or by functional means. Measurements that have been used are PLP in plasma or red cells, its metabolite 4-PA in urine or plasma, the activity and activation coefficient of the red cell aminotransferases (aspartate and alanine), and the tryptophan load metabolite excretion test. Because no single marker adequately reflects status, a combination of these markers offers the best approach.
Direct assessment was originally performed by microbiological techniques using specific strains of Saccharomyces carlsbergensis for all three natural vitamers, Enterococcus faucium for pyridoxal and pyridoxamine, and Lactobacillus casei for pyridoxal. Concentrations of 20 μg vitamin B 6 /g creatinine in urine are considered indicative of marginal or inadequate dietary intake of the vitamin. Plasma PLP and plasma or urine 4-PA are most commonly measured by HPLC, PLP with fluorescence detection following precolumn fluorophore formation as a semicarbazone , or a pyridoxic acid phosphate, and 4-PA with its natural fluorescence. During deficiency, the concentration of 4-PA will drop well below the normal concentration of at least 0.8 mg/day in urine. Using ion-pair reversed-phase chromatography, plasma vitamin B 6 vitamers (PLP, PL, PN, PMP, PM, and 4-PA) were measured in 90 patients undergoing coronary angiography before and after treatment with pyridoxine 40 mg daily for up to 84 days. PLP, 4-PA, and to a lesser degree PL were found to be the predominant B 6 metabolites in pretreatment plasma. After treatment, PN was also detectable, and PN and PL showed the largest increases in concentration. Increases in plasma concentrations of PLP, PL, and 4-PA occurred within 3 days of supplementation and were steady for the remainder of the study period. In critical illness, plasma PL and PLP are low, and the relationship between them is disturbed, whereas this is less pronounced in red and white blood cells, suggesting that intracellular PLP concentrations are more reliable than plasma measurements in such patients. Other direct measurements have used recombinant enzyme technology. A homogeneous, nonradioactive recombinant enzymatic method for PLP has been described that uses 5 μL of plasma, has a detection limit of 5 nmol/L, and may be applicable to adaptation to an automated analyzer.
Functional assessment of vitamin B 6 status may be made by measuring the activity of red cell aspartate (or alanine) aminotransferase and its activation coefficient on incubation with PLP, although because the apoenzyme is highly unsaturated with PLP, the results obtained have greater variability than those derived by corresponding methods for vitamins B 1 and B 2 and thus are considered less useful. Activation coefficients of less than about 1.5 for aspartate aminotransferase and 1.2 for alanine aminotransferase are considered normal, but this may depend somewhat on the assay method used. Measurement of urinary tryptophan metabolites, particularly xanthurenic acid, following an oral load (2 to 5 g) of l -tryptophan, is one of the most common indices used in studies of vitamin B 6 nutriture, because changes can be recognized early and measurements are relatively easy. Amounts of xanthurenate well above normal (about 25 mg/day) are seen in vitamin B 6 deficiency. Concentrations of other metabolites, such as kynurenic acid and 3-hydroxykynurenine, are increased. A method describing the simultaneous determination of tryptophan and its metabolites including kynurenic and xanthurenic acids by liquid chromatography with diode array, fluorescence, and tandem mass spectrometry detection systems has been published.
Whole blood collected into containers with the preservatives lithium heparin or EDTA is recommended, but local laboratories should be consulted for their preferred sample type. Ideally a fasted sample should be collected, especially if the patient is receiving oral or parenteral vitamin B 6 supplementation. Otherwise, sampling should be undertaken at least 8 hours post-supplementation. As vitamin B 6 is light-sensitive, samples should be protected from light by wrapping with foil. However, a recent study using HPLC with fluorimetric detection reported that vitamin B 6 in whole blood significantly and gradually increased by a mean of 9.9% over basal concentration in 24h when kept at room temperature. It is therefore recommended that if delay in transportation is anticipated, erythrocytes should be prepared by centrifugation and removal of plasma and the white cells in the buffy layer and the cells stored frozen until dispatch. It was suggested that this may be a result of increased instability caused by light exposure or due to release from erythrocytes as a result of hemolysis.
Vitamin B 6 is affected by SIRS as shown in Table 39.2 , which lists the magnitude of change in vitamin B 6 with increasing CRP concentrations. A guidance reference interval given by one source for plasma PLP was 9.5 to 24 ng/mL (39 to 98 nmol/L) and plasma concentrations less than 5 ng/mL (20 nmol/L) adjudged deficient. Another study in 126 apparently healthy subjects derived a reference interval of 5.2 to 34 ng/mL (21 to 138 nmol/L), whereas for erythrocyte PLP, the reference interval given was 250 to 680 pmol/g Hb with a concentration of less than 200 pmol/g Hb indicating at risk of deficiency. , For more information, refer to the Appendix on Reference Intervals. Laboratories should verify that these ranges are appropriate for use in their own settings.
Vitamin B 12 , also known as cyanocobalamin, is a water-soluble vitamin that is required for erythropoiesis, for methylation processes necessary for metabolism, and is a cofactor for enzymes involved in the metabolism of methylmalonic acid (MMA) and homocysteine.
Vitamin B 12 is one of the most structurally complex small molecules produced by nature, and the only known carbon-metal bond (involving cobalt) found in a biologically active molecule. The generic term vitamin B12 refers to a group of physiologically active substances chemically classified as cobalamins or corrinoids . They are composed of tetrapyrrole rings surrounding central cobalt atoms and nucleotide sidechains attached to the cobalt atoms. The cobalamin tetrapyrrole ring, exclusive of cobalt and other sidechains, is called a corrin. All compounds containing this corrin nucleus are corrinoids. The cobalt-corrin complex is termed cobamide. In cobalamins, 5,6-dimethylbenzimidazole riboside is bound to the cobalt atom by one of its imidazole nitrogens, and its 2′-ribose carbon is linked with an ester of aminoisopropanol and propionic acid to the corrin ring ( Fig. 39.15 ).
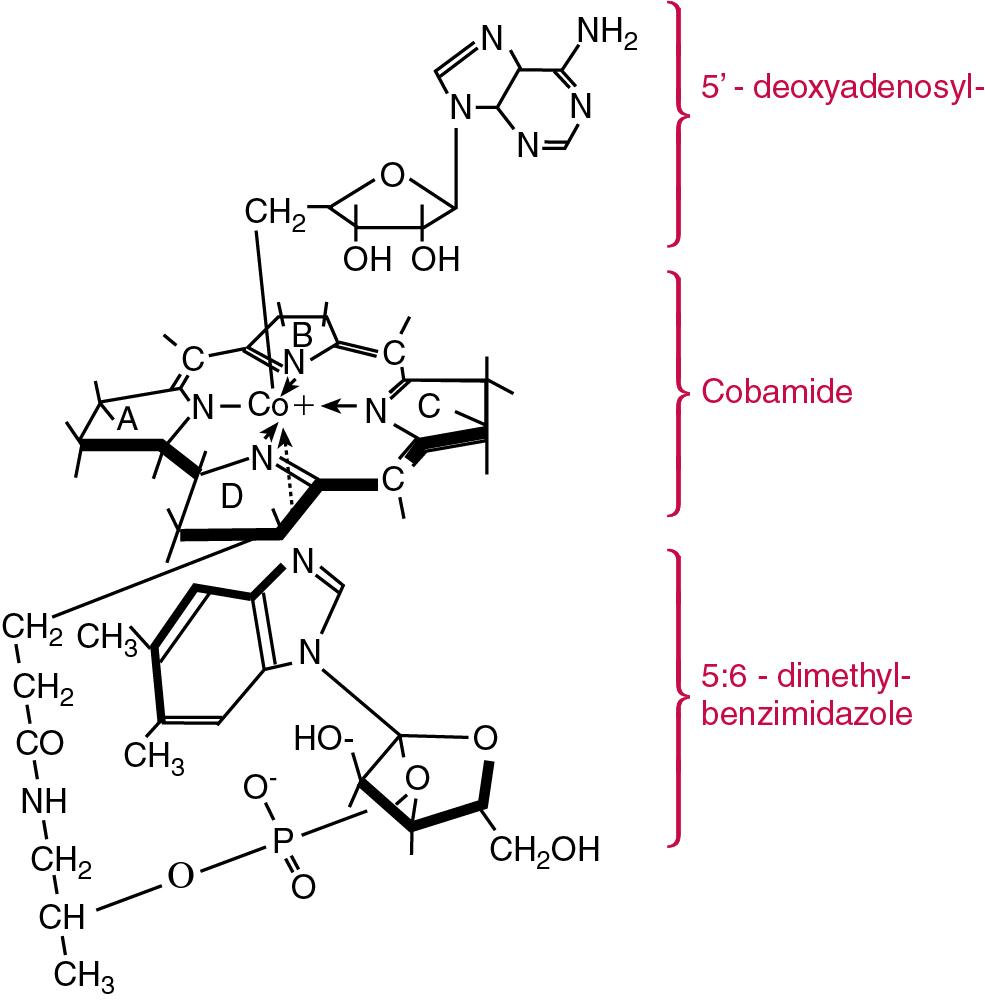
Cobalamins differ in the nature of additional side groups bound to cobalt. Examples include methyl (methylcobalamin), 5′-deoxyadenosine (deoxyadenosyl [short form, adenosyl], cobalamin, or coenzyme B 12 ), hydroxyl (hydroxocobalamin), H 2 O (aquocobalamin, or vitamin B 12b ), and cyanide (cyanocobalamin). Cyanocobalamin is a stable compound that forms dark red, needle-like crystals; it is the reference compound for measuring serum cobalamin concentration. Less stable serum cobalamins may be converted to this compound for quantification. The predominant physiologic form of cobalamin in serum is methylcobalamin, whereas that in the cytosol is adenosylcobalamin. It is recommended that the term vitamin B12 be used as the generic descriptor for all corrinoids exhibiting qualitatively the biological activity of cyanocobalamin. Cyanocobalamin has a molecular weight of 1355 Da and a solubility of 12 g/L in water at 20 °C. It is soluble in lower alcohols and aliphatic acids, but is insoluble in acetone, ether, and chloroform. It is gradually destroyed on exposure to light. Aqueous solutions of cyanocobalamin exhibit a distinctive absorption spectrum with maxima at 278, 361, and 550 nm, and with absorptivity coefficients of 115, 207, and 63, respectively, at these maxima. The spectrum is independent of pH but changes when cyanocobalamin binds to intrinsic factor (IF). Because of its stability in aqueous solutions and its distinct absorption spectrum, accurate concentrations of cyanocobalamin are prepared and used as calibrators for the measurement of serum cobalamin concentrations.
As plants do not use the vitamin, the main dietary sources are meat and meat products, dairy products, fish and shellfish, and fortified ready-to-eat cereals.
The uptake of vitamin B 12 from the intestine into the circulation is a complex mechanism, involving vitamin B 12 –binding molecules, receptors, and transporters. Vitamin B 12 is tightly bound to proteins and must be released from food by stomach acid and pepsin. The synthetic form is free and therefore readily available for metabolism. Thus acid blocking drugs such as proton pump inhibitors e.g., omeprazole can cause vitamin B 12 deficiency. The free vitamin B 12 molecule is bound to haptocorrin (R protein) and travels with it into the duodenum, where the haptocorrin is digested by pancreatic enzymes. Liberated vitamin B 12 then binds to IF, a glycoprotein with a molecular weight of approximately 50 kDa that is produced by the gastric parietal cells in the fundus and body of the stomach. One molecule of IF binds one molecule of vitamin B 12 . Gastric secretion of IF is stimulated by food, histamine, and gastrin produced by the antrum portion of the stomach; it is inhibited by vagal blockade. , , When the vitamin B 12 –IF complex reaches the distal ileum, it is bound by specific receptors known as the cubam complex, which consists of two subunits, namely cubilin and amnionless on the surface of mucosal epithelial cells and it is internalized. The vitamin B 12 –IF complex is dissociated within the mucosal epithelial cells by lysosomes and released into blood.
In circulation, vitamin B 12 then binds to two circulating binding proteins, haptocorrin (HC, referred to as holohaptocorrin when bound to vitamin B 12 and previously known as transcobalamin I) and transcobalamin (TC, holotranscobalamin when bound to vitamin B 12 and previously known as transcobalamin II) ; however, only the TC-bound fraction has receptor-mediated cellular uptake and is therefore the bioactive fraction. The function of HC that is released by granulocytes is currently unknown, and low haptocorrin concentrations, found in approximately 15% of persons with low serum cobalamin, could be one of the most common causes of low cobalamin concentrations. HC accounts for 80 to 94% of endogenous plasma vitamin B 12 , whereas TC accounts for the remainder, but the latter is the more important vitamin B 12 transport protein in plasma. TC is a β-protein synthesized mainly in the liver and has a molecular weight of approximately 43 kDa; it has a single vitamin B 12 –binding site per molecule. TC transports vitamin B 12 to receptors on cell membranes throughout the body. Binding is very rapid: if TC–vitamin B 12 is injected intravenously, it is almost completely cleared in one passage through tissue, mostly by the liver. The TC–vitamin B 12 complex enters the cell by pinocytosis. Lysosomal proteolysis degrades TC and releases the vitamin B 12 . Unbound vitamin B 12 can enter the tissue cells, but the process is very inefficient.
As previously discussed, only vitamin B 12 bound to TC is capable of cellular uptake, , and as a result malabsorption of TC-bound vitamin B 12 leads to deficiency in a large proportion of cases. Almost all vitamin B 12 (bound to TC) is taken up by hepatocytes as the blood in the portal vein passes through the liver where it is stored and subsequently released in blood to meet physiologic demands. If the quantity of vitamin B 12 exceeds the capacity of hepatocyte receptors, most of the excess is excreted by the kidneys. Normally, approximately 1 mg of vitamin B 12 is stored in the liver—a quantity equivalent to the daily metabolic requirement for 2000 days. Thus when the dietary supply of vitamin B 12 is interrupted or mechanisms of absorption are impaired, vitamin B 12 deficiency does not become evident for several years.
Vitamin B 12 is continually secreted in the bile, but most is reabsorbed and is available for metabolic functions. If circulating vitamin B 12 concentrations exceed the binding capacity of the blood, the excess will be excreted in the urine, but in most circumstances, the highest losses of vitamin B 12 occur through the feces.
Vitamin B 12 is a cofactor or coenzyme for various enzyme systems. In humans it is required by adenosylcobalamin, coenzyme to l -methylmalonyl-CoA mutase in the conversion of l -methylmalonyl CoA to succinyl-CoA ( Fig. 39.16 ). The conversion of l -methylmalonyl-CoA to succinyl-CoA links propionyl-CoA, which is formed from branched-chain amino acids such as valine, isoleucine, and methionine with the tricarboxylic acid (TCA) cycle. Congenital defects of mutase synthesis or inability to synthesize adenosylcobalamin results in life-threatening methylmalonic aciduria and metabolic ketoacidosis. It is also required by methylcobalamin, a coenzyme to methionine synthase in the conversion of homocysteine to methionine. In these reactions (see Fig. 39.16 and Fig. 39.17 ), methylcobalamin serves as an intermediate in the transfer of a methyl group from 5-MTHF to homocysteine for the formation of methionine. Methionine is required for protein synthesis and as the methyl donor, S- adenosylmethionine. Congenital defects in methionine synthase or the synthesis of methylcobalamin results in severe hyperhomocysteinemia. As discussed under the section on Vitamin B 6 , defects in the enzyme cystathionine β-synthase or its cofactor vitamin B 6 also results in hyperhomocysteinemia. Vitamin B 12 is also vital for erythropoiesis; deficiency leads to macrocytosis characterized by an increased mean corpuscular volume (MCV) greater than 98 fL [as seen in the complete/full blood count (CBC/FBC)] and megaloblastic anemia characterized by immature, large and nucleated RBC as a result of defective DNA synthesis that relies on adequate concentrations of vitamin B 12 (and folate).
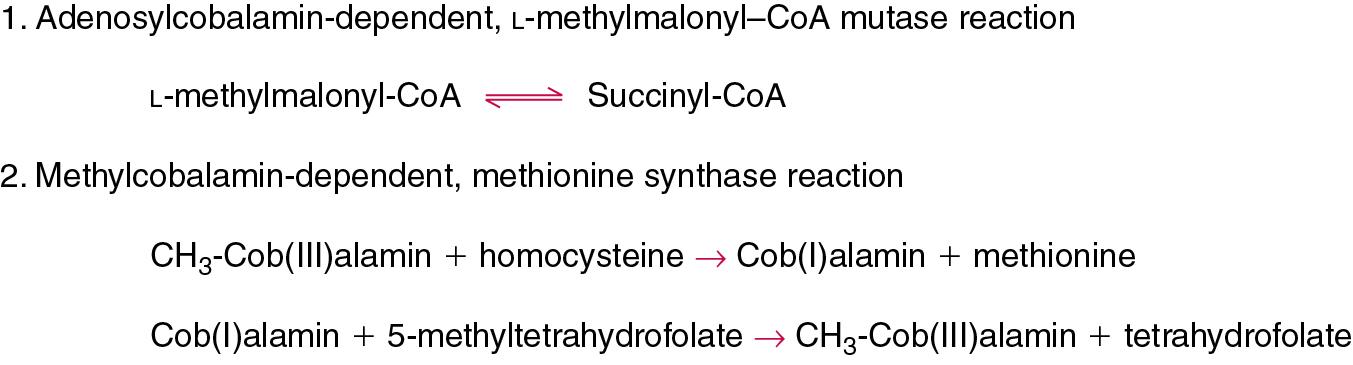
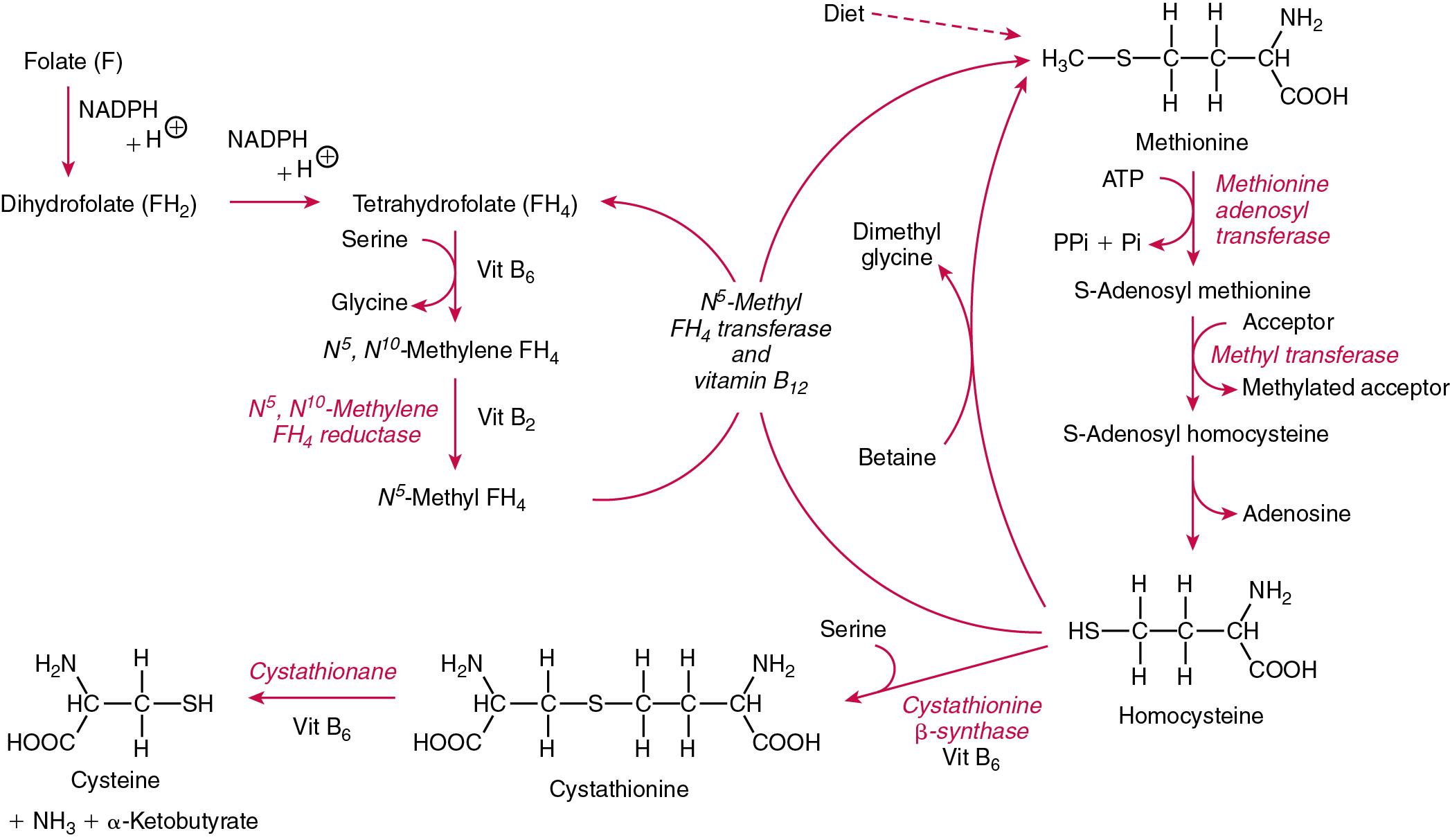
As can be seen in Fig. 37.17 , on the left the remethylation pathway is shown where homocysteine is converted to methionine catalyzed by methionine synthase (N 5 -methyl FH 4 transferase), which requires vitamin B 12 to function. This pathway links the folate cycle to homocysteine metabolism. On the right the transmethylation pathway is depicted showing the metabolism of methionine to homocysteine, which is then converted to cysteine in the transsulphuration pathway.
Total body stores of vitamin B 12 are estimated to be between 2 and 5 mg in the adult man, of which about 1 mg is in the liver and a smaller amount in the kidney. A daily obligatory loss of vitamin B 12 of about 0.1% of body pool is believed to occur, irrespective of size, suggesting that a daily requirement to maintain stores would be 2 to 5 μg. The daily Western diet contains between 5 and 30 μg of vitamin B 12 , with average ingestion of 7 to 8 μg/day by adult men and 4 to 5 μg/day by adult women. Additional small amounts may be available from vitamin B 12 synthesis by intestinal microorganisms. Of the amount ingested, between 1 and 5 μg is absorbed.
The RDA for vitamin B 12 is based on the amount necessary for maintenance of hematologic status and normal serum vitamin B 12 concentrations; it assumes 50% absorbance of ingested vitamin B 12 . The RDA for adults (19 to 50 years) has been set at 2.4 μg/day, with an increase to 2.6 μg/day in pregnancy and to 2.8 μg/day in lactation. RDAs for children are 0.9 μg/day at 1 to 3 years, 1.2 μg/day at 4 to 8 years, 1.8 μg/day at 9 to 13 years, and 2.4 μg/day at 14 to 18 years. Because 10 to 30% of older persons may be unable to absorb naturally occurring vitamin B 12 , it is recommended that those older than 50 years meet their RDA mainly by consuming foods fortified with vitamin B 12 or with a vitamin B 12 –containing supplement.
The recommended IV intake for adults is 5 μg/day as cyanocobalamin—an amount in excess of the oral recommendation that will more than meet requirements.
Deficiency of vitamin B 12 in humans is associated with macrocytosis, megaloblastic anemia, and neuropathy. The most common cause of vitamin B 12 deficiency is pernicious anemia, an autoimmune disease in which chronic atrophic gastritis in the fundus and body region of the stomach results from autoantibodies to gastric parietal cells directed against gastric parietal cell H + /K + -ATPase, which is responsible for secreting acid (H + ) in exchange for potassium (K + ). Loss of parietal cells also leads to decreased production of IF. In addition to IF deficiency, blocking autoantibodies that bind the vitamin B 12 -binding sites of IF prevent the formation of vitamin B 12 -IF complex required for recognition by the cubam complex in the distal ileum. , One population study showed that 1.9% of persons older than 60 years have undiagnosed pernicious anemia, although the diagnosis is made most commonly in young to middle-aged black women and in middle-aged to elderly whites. Pernicious anemia may also occur in children because of failure of IF secretion or secretion of biologically inactive IF. Other groups at risk for vitamin B 12 deficiency include: (1) those older than 65 years of age; (2) with malabsorption; (3) who are vegetarians or vegans; (4) with autoimmune disorders (pernicious anemia usually occurs as part of the autoimmune polyglandular syndrome type 3B that includes autoimmune thyroiditis); and (5) taking prescribed medication known to interfere with vitamin absorption or metabolism, including nitrous oxide (also known as laughing gas that inactivates vitamin B 12 by oxidizing its cobalt atom), phenytoin, dihydrofolate reductase inhibitors, metformin, and proton pump inhibitors; as well as (6) infants with suspected metabolic disorders.
Intestinal malabsorption of vitamin B 12 may be caused by gastrectomy or terminal ileal resection as this is where vitamin B 12 is absorbed. There is an inverse relationship noted between the length of ileum resected and absorption of vitamin B 12 . Other causes of malabsorption include tropical sprue, inflammatory disease of the small intestine, intestinal stasis with overgrowth of colonic bacteria (which consume vitamin B 12 ingested by the host), and human immunodeficiency virus (HIV) infection. Another cause of vitamin B 12 malabsorption is failure to extract cobalamin from food as seen in some cases of patients on proton-pump inhibitors. Some patients fail to absorb cobalamin bound to food, whereas absorption of non–food-bound cobalamin in the Schilling test (see below) is unimpaired. This is particularly a problem in patients with compromised gastric status or early in the course of development of pernicious anemia. ,
Vegetarians have a lower intake of vitamin B 12 than omnivores, and although clinical signs of deficiency are uncommon, biochemical markers of status indicate functional vitamin B 12 deficiency. In a study of 66 lactovegetarians or lacto-ovo vegetarians, 29 vegans, and 79 omnivores, the incidence of low holotranscobalamin II was 77, 92, and 11%, respectively, in the three groups; increased methylmalonic MMA occurred in 68, 83, and 5%, respectively; and increased total homocysteine was found in 38, 67, and 16%, respectively.
A large number of disorders are associated with cobalamin deficiency in infancy or childhood. Of these, the most commonly encountered is the Imerslund-Grasbeck syndrome, a condition that is characterized by inability to absorb vitamin B 12 , with or without IF, and proteinuria. It appears to be due to an inability of intestinal mucosa to absorb the vitamin B 12 –IF complex as a result of variants in cubilin or amnionless. The second most common of these is congenital deficiency of gastric secretion of IF. Very rarely, congenital deficiency of vitamin B 12 in a breast-fed infant is due to deficiency of vitamin B 12 in maternal breast milk resulting from unrecognized pernicious anemia in the mother. This is rare because most women with undiagnosed and untreated pernicious anemia are infertile. , Methylmalonic acidemias (acidurias) and homocysteinemias may result due to vitamin B 12 deficiency and depending on the underlying variant may or may not be responsive to supplementation with the vitamin.
The hematologic effects of vitamin B 12 deficiency are indistinguishable from those of folate deficiency. Classical morphologic changes in the blood, in approximate order of appearance, are: hypersegmentation of neutrophils, macrocytosis, anemia, leukopenia, and thrombocytopenia, with megaloblastic changes in bone marrow accompanying peripheral blood changes. The cause of the hematologic abnormalities is attributable to decreased DNA synthesis with an adequate RNA synthesis caused by the secondary block in folate metabolism caused by vitamin B 12 deficiency and resultant diminished cell differentiation. Many immature cells die in the bone marrow, possibly by apoptosis, leading to the release of bilirubin and lactate dehydrogenase (LD) into the blood. This is termed ineffective erythropoiesis. All bone marrow lesions can be reversed by vitamin B 12 treatment.
In addition to hematologic changes, vitamin B 12 deficiency can lead to a demyelinating disorder of the central nervous system in man. Serious and often irreversible neurologic disorders can occur, such as burning pain or loss of sensation in the extremities, weakness, spasticity and paralysis, confusion, disorientation, and dementia. This condition has been given the name subacute combined degeneration of the spinal cord . Neurologic symptoms may occur without any discernible hematologic changes in the blood ; indeed, an intriguing inverse relationship between the hematologic and the neurologic has been observed. The incidence of neurologic complications is between 75 and 90% of all individuals with clinically observable vitamin B 12 deficiency; in about 25% of cases, these may be the only clinical manifestation of deficiency. , The mechanism of the disorder is uncertain, although indirect evidence suggests that disorders of both enzyme systems requiring vitamin B 12 coenzymes are necessary before neurologic symptoms occur. The response of neurologic symptoms to vitamin B 12 replacement is often dependent on the duration of the symptoms. Vitamin B 12 deficiency may be associated with other mainly gastrointestinal complications, such as glossitis of the tongue, appetite and weight loss, flatulence and constipation, mental changes, and infertility. , ,
No adverse effects have been associated with excess vitamin B 12 intake from food or supplements in healthy people. Daily oral doses of up to 2 mg of cyanocobalamin have been used for treatment of deficiency in those who tolerate oral supplementation. Data in the literature are insufficient to propose a tolerable upper intake amount for vitamin B 12 .
Both direct and indirect (functional) methods are available for assessment of vitamin B 12 status. Indirect tests include assays for urinary and serum concentrations of MMA, plasma homocysteine, the deoxyuridine suppression test, and the vitamin B 12 absorption test. Cytochemical staining of RBC precursors and the test for IF blocking antibodies are other ancillary methods of assessing vitamin B 12 status.
A comprehensive review of methods for measuring vitamin B 12 in various biological samples has been published. Microbiological, competitive protein binding (CPB), and immunometric assays have been used for quantification of serum vitamin B 12 . Microbiological assays have largely been replaced by other, more convenient and precise methods, although they remain reference methods for the determination of biologically active vitamin B 12 . The most widely used procedures use Euglena gracilis, Lactobacillus leishmannii, or a mutant of Escherichia coli, although each of these organisms is susceptible to growth inhibition by antibiotics or other drugs, such as methotrexate, that may be present in a patient’s serum. Furthermore, these assays require at least 24 hours for the establishment of adequate growth of these microorganisms. However, use of microtiter enzyme-linked immunosorbent assay (ELISA) plate technology has enhanced the utility of some microbiological assays.
Commercial kits are available for CPB assays of vitamin B 12 . The vitamin B 12 binder used is often nonhuman IF, usually obtained from hog stomach. If the IF is not highly purified, it may contain R proteins, which bind not only vitamin B 12 but also related metabolically inactive compounds, yielding higher values. IF therefore must be highly purified or must have cobinamide (a vitamin B 12 analog) added to the IF to saturate all binding sites on the R proteins. Cobinamide is not bound by IF.
In a widely used CPB assay, vitamin B 12 (cobalamin) competes with 57 Co-labeled cobalamin for a limited number of binding sites on IF. Some assays require a preliminary step in which the specimen is boiled in a buffered solution containing dithiothreitol, potassium cyanide (KCN), and 57 Co-labeled tracers to release vitamin B 12 from endogenous binding proteins. Alternatively, other procedures irreversibly denature endogenous binding proteins by increasing the pH from 12 to 13 and then readjusting the pH to 9.3 before the binding reagent is added. Subsequent separation of bound and free folate and vitamin B 12 is achieved by contact with dextran-coated charcoal, which absorbs the free (unbound) molecules, leaving protein-bound vitamin B 12 in the solution.
Most immunometric methods use solid-phase separation by immobilizing the IF binder on beads or magnetic particles. The free vitamin B 12 then remains in the supernatant, and the bound analytes become part of the solid-phase suspension. For simultaneous folate/vitamin B 12 measurement, a gamma-scintillation counter that discriminates between the energy levels of 57 Co (for vitamin B 12 ) and 125 I (for folate) must be used.
Multiple automated and semiautomated systems are available for measuring vitamin B 12 and folate, using, for example, chemiluminescence as a signal. The precision of automated systems allows specimens to be analyzed in singlet where CVs less than those found for the mean of duplicates of radioimmunoassays are maintained.
Indirect tests assess the functional adequacy of vitamin B 12 . Serum MMA concentration is increased when lack of adenylcobalamin causes a block in the conversion of methylmalonyl-CoA to succinyl-CoA. It is a sensitive test of status, being often the first analyte to be raised in subclinical vitamin B 12 deficiency. , It has a further advantage in that it is unaffected by folate deficiency. Early methods for MMA lacked sensitivity and specificity; this situation has been resolved by the adoption of gas chromatographic–mass spectrometric methods following derivatization for both urine and serum , or liquid chromatographic–mass spectrometric methods. Unfortunately MMA is increased by renal insufficiency and in persons above the age of 65 years. , Plasma total homocysteine concentration is also a sensitive indicator of vitamin B 12 status because methylcobalamin is required for the remethylation of homocysteine to methionine, but it is not specific, being elevated in deficiencies of folate, vitamin B 12 , vitamin B 2 , and vitamin B 6 . , Plasma concentrations of total homocysteine can be reliably measured by HPLC with fluorescent or electrochemical detection, and with enzymatic and capillary gas chromatography–mass spectroscopy methods. Plasma samples for homocysteine analysis must be obtained soon after venipuncture to reduce preanalytical increases that may occur on standing, although these can be minimized by the use of a fluoride–ethylenediaminetetraacetic acid (EDTA) tube. Increased screening of plasma total homocysteine concentrations as an independent risk factor for cardiovascular disease may lead to identification of additional cases of subclinical vitamin B 12 deficiency. ,
The measurement of holotranscobalamin is potentially useful as a specific marker of biologically available vitamin B 12 , because it is the only vitamin B 12 moiety that is specifically available for uptake by all cells and has been shown to have the best diagnostic accuracy for vitamin B 12 deficiency. , However, assays for holotranscobalamin are not widely available at present but this is changing as manufacturers realize that this is a more accurate and reliable marker of deficiency. Other methods have been described for the measurement of holotranscobalamin in serum, using an immobilized monoclonal antibody to human transcobalamin, followed by measurement of released cobalamin by CPB or an automated assay by enzyme immunoassay termed active B 12 . Another method uses magnetic beads coated with cobalamin to precipitate apotranscobalamin, followed by measurement of holotranscobalamin in the supernatant by ELISA. Although these methods are claimed to be precise and simple to perform, their interpretation has been questioned and their sensitivity and specificity in the diagnosis of vitamin B 12 deficiency remains to be established.
The deoxyuridine suppression test measures the effects of prior addition of deoxyuridine on uptake of radiolabeled thymidine into the DNA of cultured bone marrow cells, peripheral blood lymphocytes, or whole blood. Normal samples that contain vitamin B 12 can convert deoxyuridine to thymidine and therefore do not take up as much thymidine. Samples from patients who are deficient in vitamin B 12 show less suppression than those from normal patients. Because it is relatively time-consuming, the deoxyuridine suppression test is not widely available for use as a diagnostic test.
The Schilling test is primarily a test of vitamin B 12 absorption and not of status, but it permits differentiation of causes of vitamin B 12 deficiency (pernicious anemia or intestinal malabsorption). The proportion absorbed from orally administered 57 Co- or 58 Co-labeled vitamin B 12 is measured by determining radioactivity in feces, urine, or serum or by externally scanning the liver. The usual procedure is to measure radioactivity in a 24-hour urine sample, which is collected after oral administration of 0.5 μg of radioactive Co-labeled vitamin B 12 after an overnight fast. In normal individuals, 8% or more of the dose administered is excreted in the urine, whereas in people with pernicious anemia, less than 7% (often 0 to 3%) is excreted. A confirmatory test for lack of IF requires ingestion of vitamin B 12 and IF.
Spurious increases in vitamin B 12 have been reported in patients with pernicious anemia when using automated analyzers based on the competitive binding of serum vitamin B 12 with reagents using IF. This has been attributed to the high concentrations of IF-blocking autoantibodies in these patients interfering in the assay. Up to 70% of patients with pernicious anemia have IF-blocking antibodies. Assay manufacturers are aware of this problem and have taken steps to inactivate the IF-blocking antibodies. However, at the present time the scale of the problem is unknown and alternative methods should be used if in doubt including the measurement of IF-blocking antibodies.
For vitamin B 12 whole blood collected into container with clot activator to obtain serum is recommended but local laboratories should be consulted for their preferred sample type. Ideally a fasting sample should be collected, especially if the patient is receiving oral or parenteral vitamin B 12 supplementation. One recent study using a radioimmunoassay method reported that vitamin B 12 showed good stability up to 72 h at room temperature corroborating similar data reported earlier using an enzyme immunoassay-based method. There are no special requirements for MMA, but delayed freezing should be avoided.
Because protein consumption can increase homocysteine levels, it is recommended that fasting samples are collected. Homocysteine is bound to albumin; therefore it is decreased in the supine position; more than 3 minutes duration of venous stasis may increase it. However, these factors are not necessarily crucial because they are unlikely to affect the interpretation of the results. EDTA tubes are most widely used but use of serum or citrated or heparinized rather than EDTA plasma will not greatly influence the results. Blood samples should be centrifuged within 1 hour or kept cold by collecting on ice until centrifugation. ,
Depending on the laboratory and the procedure used, reference intervals vary widely. A recent WHO consultation defined a serum vitamin B 12 concentration less than 203 ng/L (150 pmol/L) as deficient. Changes in serum vitamin B 12 concentration as a function of age in healthy adults have been the subject of contradictory reports. Data from a study population in the United States (Framingham Study) showed an increased prevalence (40.5% of 222 subjects) of low serum vitamin B 12 concentration of less than 350 ng/L (<258 pmol/L) in elderly subjects in comparison with a control group of younger subjects (17.9% incidence). Vitamin B 12 concentrations within the reference interval may not necessarily reflect adequate vitamin B 12 status, because serum concentrations may be maintained at the expense of tissue stores. Conversely, low serum vitamin B 12 concentrations may not be indicative of vitamin B 12 deficiency. Most of the vitamin B 12 in serum is bound to haptocorrin, which is released by granulocytes and has no functional role in the transport of vitamin B 12 to cells. , Low serum vitamin B 12 concentration may be due to a reduction in haptocorrin as a consequence of low total granulocyte mass. This has been observed in benign neutropenia, multiple myeloma, and leukemic reticuloendotheliosis and may be expected in other conditions in which the bone marrow is hypoplastic, aplastic, or replaced by malignant cells.
Increased MMA and low holotranscobalamin may be found in patients with normal vitamin B 12 ; hence holotranscobalamin is a better predictor of B 12 than total B 12 . Serum MMA concentrations below 376 nmol/L have been considered acceptable in an elderly US population, as have concentrations below 320 nmol/L in a group of older Dutch subjects. MMA is increased by renal insufficiency and age. , Reference values for holotranscobalamin have been variable but are in the range of 19 to 134 pmol/L and are age and gender dependent. Homocysteine levels less than 15 μmol/L or if older than 65 years, less than 20 μmol/L are considered acceptable. For more information, refer to the Appendix on Reference Intervals. Laboratories should verify that these ranges are appropriate for use in their own settings.
Folic acid, also known as folate, pteroylglutamic acid, or vitamin B 9 , serves as a carrier of one-carbon groups in many metabolic reactions. It is necessary for the interconversions of amino acids such as homocysteine to methionine and the biosynthesis of purines and pyrimidines, required for DNA synthesis.
Folate and folic acid are generic terms for a family of compounds that function as coenzymes in the processing of one-carbon units and that are derived from pteroic acid (Pte ), to which one or more molecules of glutamic acid are attached. Pteroic acid is composed of a pteridine ring joined to a p- aminobenzoic acid residue ( Fig. 39.18 ). In basic solution, this substance has absorption maxima at 256, 282, and 365 nm and is fluorescent. When pteroic acid is conjugated with one molecule of l -glutamic acid, pteroylglutamic acid (PteGlu) is formed; this can be reduced to dihydrofolic acid (H 2 PteGlu or DHF/FH 2 ) with hydrogens in positions 7 and 8, or to tetrahydrofolate (H 4 PteGlu or THF/FH 4 ) with hydrogens in positions 5, 6, 7, and 8. Only the reduced forms are biologically active. Other folate derivatives have multiple glutamic acid residues (H 4 PteGlu n ), where n , the number of glutamate residues, may be 1 to 7. Biochemically, these polyglutamates are similar to monoglutamates, but the former function as the natural coenzymes. Multiple forms of folic acid occur with substitutions of functional groups such as methyl, formyl, methylene, hydroxymethyl, and others at nitrogen atoms in the pteroic acid residue, usually N 5 or bridging N 5 and N 10 . Although various forms of folic acid are normally present in human serum and other body fluids, the principal form is 5-MTHF or N 5 -methylFH 4 . This is slowly oxidized in alkaline solution, but the process is reversed by adding ascorbic acid. It is relatively stable in acid solutions but is unstable when exposed to light.
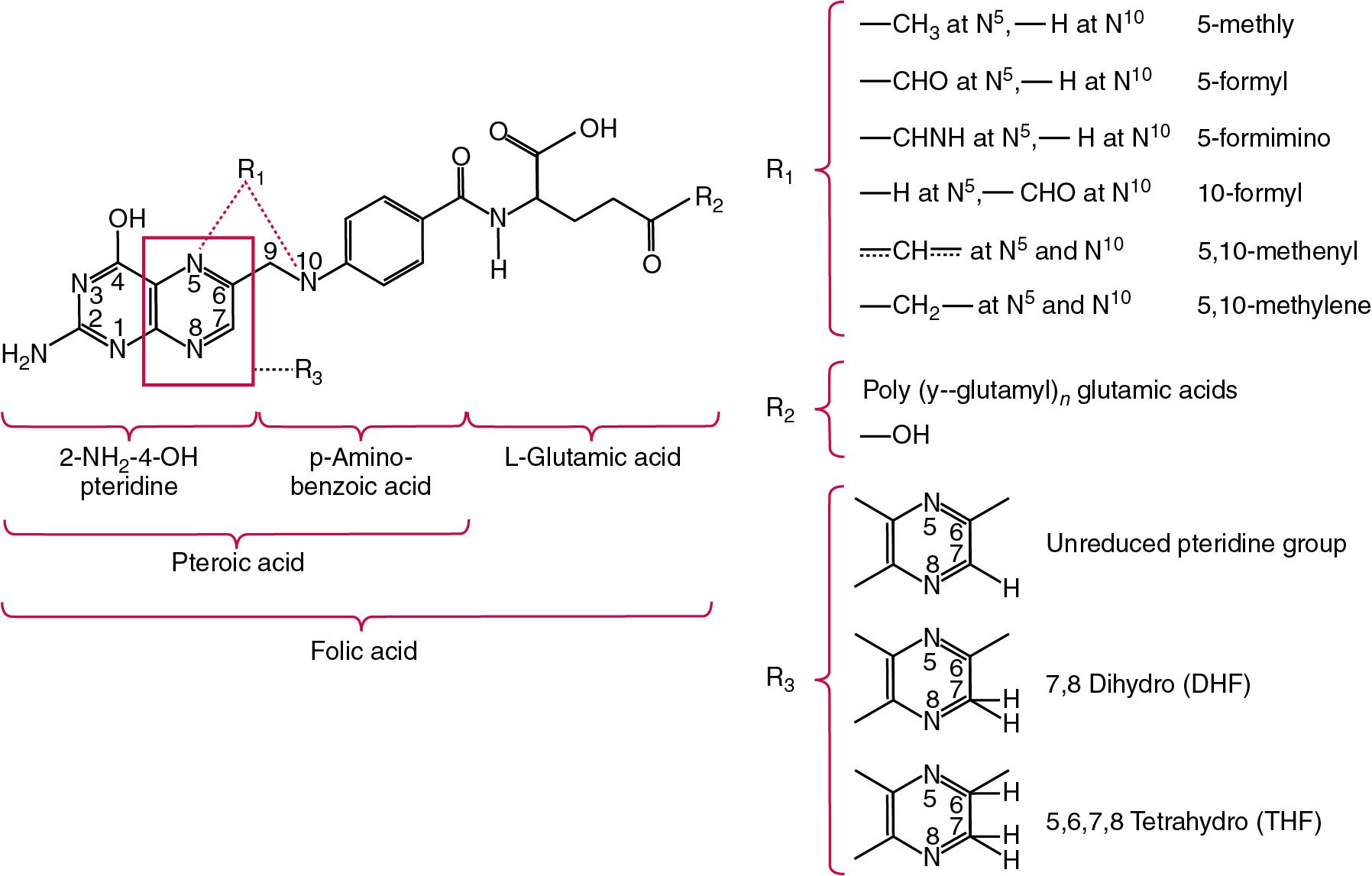
The principal food sources of folate are liver, dark green leafy vegetables such as spinach, legumes such as kidney and lima beans, and orange juice, although in countries where cereal fortification with folate is established, cereal is often the major source of dietary folate. ,
Folate is absorbed from dietary sources such as those listed previously, mainly as reduced methyl- and formyl-tetra-hydropteroylpolyglutamates. The bioavailability of folate from food sources is variable and is dependent on factors such as incomplete release from plant cellular structure, entrapment in food matrix during digestion, inhibition of deglutamation by other dietary constituents, and possibly the degree of polyglutamation. The bioavailability of supplemental folic acid is greater than that of food folate and may be as high as 100% for folic acid supplements taken on an empty stomach compared with about 50% for food folates. Polyglutamate forms of folate present in food are first converted to monoglutamates, by pteroylpolyglutamate hydrolase, in the intestinal mucosa. Absorption of monoglutamyl folates at low concentration occurs through a saturable transport process with an acidic pH optimum (pH around 5), with an additional, apparently nonsaturable absorption mechanism when intestinal folate concentrations exceed 5 to 10 μmol/L. , After cellular uptake, most of the folate is reduced and methylated and enters the circulation as THF, circulating loosely bound to albumin or to a lesser degree to a high-affinity folate-binding protein (FBP). Uptake by certain cells (kidney, placenta, and choroid plexus) occurs by membrane-associated FBPs that act as folate receptors, and the reduced folate carrier, a member of the SLC19 family, facilitates uptake by most tissue. Once within the cell, THF is demethylated and converted to the polyglutamyl form by folylpolyglutamate synthase, which helps to retain folate within the cell. For release into the circulation, the polyglutamates are reconverted to monoglutamates by polyglutamate hydrolase.
Folic acid and vitamin B 12 metabolism are linked by the reaction that transfers a methyl group from 5-MTHF to cobalamin. In cases of cobalamin deficiency, folate is “trapped” as 5-MTHF and is “metabolically dead” (also known as the methyl-trap hypothesis). It cannot be recycled to the active form, which is THF and back into the folate pool to serve as the main one-carbon unit acceptor for many biochemical reactions. Eventually, cellular depletion of 5,10-methylenetetrahydrofolate (5,10-MTHF) ensues (see Fig. 37.17 ), causing a reduction in purine and pyrimidine synthesis and hence DNA resulting in megaloblastic anemia and neuropathies. This concept is supported by the fact that THF corrects megaloblastic anemia in patients with congenital methylmalonic aciduria and homocystinuria, whereas it is not corrected with 5-MTHF. However, some investigators have suggested that vitamin B 12 is required for the conversion of folic acid to the formyl form, and that formyltetrahydrofolates are natural substrates for forming folate polyglutamates.
Protein-free plasma folate is filtered at the glomerulus, and most is reabsorbed by the proximal renal tubules. Consequently, intact urinary folate represents only a small percentage of intake. Folate is predominately excreted by catabolism following cleavage of the C 9 -N 10 bond to produce p -aminobenzoylpolyglutamates, which then are hydrolyzed to monoglutamates and N -acetylated before excretion. Biliary excretion of folate has been estimated at about 100 μg/day, but much of this is reabsorbed in an enterohepatic circulation. Fecal losses have been studied by radiolabeling and have been found to be similar in type and quantity to urinary losses.
Folate coenzymes, together with coenzymes derived from vitamins B 12 , B 6 , and B 2 , are essential for one-carbon metabolism. Biochemically, a carbon unit from serine or glycine is transferred to tetrahydrofolate (THF) to form 5,10-MTHF, which then is (1) used in the synthesis of thymidine (and incorporation into DNA), (2) oxidized to formyl-THF for use in the synthesis of purines (precursors of RNA and DNA), or (3) reduced to 5-MTHF, which is necessary for the methylation of homocysteine to methionine. Much of this methionine is converted to S - adenosylmethionine, a universal donor of methyl groups to DNA, RNA, hormones, neurotransmitters, membrane lipids, and proteins. Some of these reactions are illustrated in Fig. 39.19 . Different folates are involved in these reactions, depending on the chemical state of the single carbon fragments transferred ( Table 39.5 ).
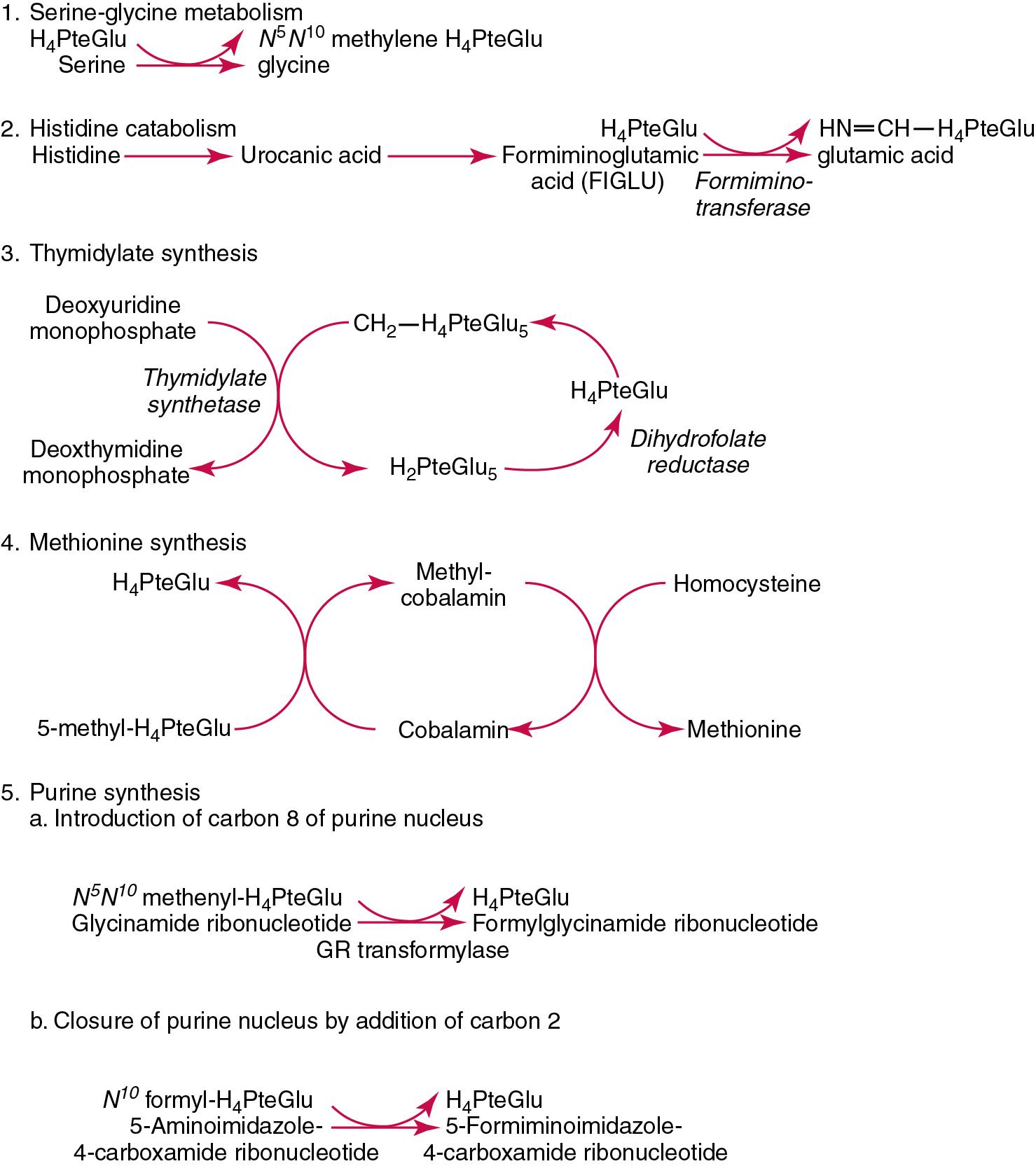
| Reaction | Group Transferred | Folic Acid Derivative |
|---|---|---|
| Serum/glycine metabolism | Methylene (–CH 2 –) | N 5 ,N 10 -methylene THF/FH 4 |
| Histidine catabolism | Formimino (–CHNH) | N 5 -formimino THF/FH 4 |
| Thymidylate synthesis | Methylene (–CH 2 –) | N 5 ,N 10 -methylene THF/FH 4 |
| Methionine synthesis | Methyl (–CH 3 ) | N 5 -methyl THF/FH 4 |
| Purine synthesis | Methenyl (–CH–) | N 5 ,N 10 -methyenyl THF/FH 4 |
| Formyl (–CHO) | N 10 -formyl THF/FH 4 |
Interconversions of these forms of folic acid take place through various electron transfer reactions facilitated by specific enzyme systems and coenzymes, such as reduced forms of flavin-adenine dinucleotide (FADH 2 ) and NADPH. Conversion between the N5 -, N10- methylene form and N10- formyl forms is readily reversible, but the reduction of methylene to methyl and the reduction of free THF to formyltetrahydrofolate are essentially irreversible. Conversion of 5-MTHF back to THF requires cobalamin (see Fig. 39.17 ).
Based on folate concentrations in liver biopsy samples, and given that the liver contains about half of all body stores, total body stores of folate are estimated to be between 12 and 28 mg. Kinetic studies that show both fast-turnover and very-slow-turnover folate pools indicate that about 0.5 to 1% of body stores are catabolized or excreted daily, suggesting a minimum daily requirement of between 60 and 280 μg to replace losses. In calculating nutritional requirements, the concept of dietary folate equivalents (DFEs) has been used to adjust for the nearly 50% lower bioavailability of food folate compared with supplemental folic acid, such that 1 DFE = 1 μg of food folate = 0.6 μg of folic acid from fortified food or as a supplement consumed with food = 0.5 μg folic acid supplement taken on an empty stomach. Before the fortification program of cereal grains with folic acid conducted between 1988 and 1994, the median intake of folate from food in the United States was approximately 250 μg/day; this figure is expected to increase by about 100 μg/day after fortification. Recommendations on DRIs made in 1998 by the US Institute of Medicine have shifted the emphasis away from prevention of deficiency toward the concept of optimal health. Current RDAs of the US Institute of Medicine are 400 μg/day DFE for adults 19 years and older and for adolescents between 14 and 18 years; 300 μg/day DFE for children 9 to 13 years; 200 μg/day DFE for children 4 to 8 years; and 150 μg/day DFE for children 1 to 3 years. AI for infants 0 to 6 months is set at 65 μg/day DFE, and for infants 7 to 11 months, 80 μg/day DFE. Based on maintenance of erythrocyte folate concentrations during pregnancy, the RDA for pregnant women of all ages is set at 600 μg/day DFE and for lactating women of all ages 500 μg/day DFE. ,
The previous adult recommendation for an IV supply of folic acid of 400 μg/day has been increased to 600 μg/day as part of the requirements set by the FDA. ,
Deficiency of folate may result from (1) absence of intestinal microorganisms (gut sterilization), (2) poor intestinal absorption (e.g., after surgical resection, in celiac disease or sprue), (3) insufficient dietary intake (including chronic alcoholism), (4) excessive demands (as in pregnancy, liver disease, and malignancies), (5) administration of antifolate drugs (e.g., methotrexate), and (6) anticonvulsant therapy (that increases folate requirements, especially during pregnancy). Inadequate folate intakes leads first to decreased serum folate concentration, then to a decrease in RBC folate concentration and an increase in plasma homocysteine, and then to megaloblastic changes in the bone marrow and other tissues. Megaloblastic anemia (characterized by large, abnormally nucleated erythrocytes in the bone marrow) is the major clinical manifestation of folate deficiency, although sensory loss and neuropsychiatric changes may also occur. Deficiencies of folate and iron may coexist in malnourished people, in which case the latter may mask the expected macrocytic and megaloblastic changes.
Pregnancy brings increased demand to folate stores because of increased DNA synthesis, and one-carbon transfer reactions and low serum folate concentrations in pregnancy are associated with adverse outcomes, including preterm delivery, infant low birth weight, and fetal growth retardation. Additionally, many observational studies have confirmed a reduction in risk of neural tube defects (NTDs) with periconceptual folic acid supplementation. , In a large controlled intervention trial conducted in two regions of China and involving approximately 250,000 women, a daily supplement of 400 μg of folic acid taken at least 80% of the time was associated with an 85% risk reduction of NTD in an area of high baseline frequency of the NTD, and a 40% reduction in an area of low baseline frequency. Current suggestions are that women planning pregnancy should take at least 400 μg/day until the 12th week of pregnancy, although a daily intake of 5 mg of folic acid is recommended, especially for those with a previous history of NTD. , Since the US FDA program of fortification of all grain products with folic acid (140 μg/100 g) began in 1996, study populations have shown a doubling of mean plasma folate concentrations and significant falls in total homocysteine concentrations, with a substantial reduction in the incidence of NTDs. A recent study designed to determine the optimal RBC folate concentration required to prevent NTDs with 400 μg/day analyzed data from two regions in nonfortified populations of China. The results showed an inverse dose-response relationship of NTDs with RBC folate concentrations with women with the lowest RBC folate concentration having the highest risk of NTDs. The threshold optimal RBC folate concentration for the prevention of NTDs was found to be 1000 nmol/L. These data will help to guide policy decisions with regard to voluntary versus mandatory folate fortification of foods.
Although the cause of NTD is probably multifactorial, involving more than one aspect of folate use, one factor that contributes to this and other folate-requiring conditions is genetic polymorphism. The most extensively studied polymorphic alleles are those of 5,10-methyleneterahydofolate reductase (MTHFR), the enzyme responsible for the irreversible reduction of 5,10-MTHF to 5-MTHF, the methyl donor of homocysteine to methionine. Recent meta-analyses have strongly suggested a significant association of the variant MTHFR C677T and a suggestive association of other polymorphisms with increased risk of NTDs. , A single-point C-to-T variant at base pair 677 (C677T), causing substitution of valine for alanine, leads to a thermolabile protein with reduced enzymatic activity. The homozygous T/T variant has an incidence of around 12% in Asian and white populations with a 50% loss of enzyme activity; the heterozygous C/T variant has an incidence of up to 50% in some populations, with a lesser degree of enzyme inactivity. Although plasma and RBC folate and homocysteine concentrations are associated with MTHFR genotype with those with MTHFR T/T showing lower folate and high homocysteine concentrations, one trial that undertook supplementation with folic acid doses of 100 μg/day or 4000 μg/week did not reduce high homocysteine concentrations in those with the MTHFR T/T genotype.
The role of folic acid in the metabolism of homocysteine has received much interest. Elevation of plasma homocysteine concentration has been postulated to be an independent risk factor for coronary artery disease , , and cerebrovascular disease. , The involvement of folate in its coenzyme forms with homocysteine and methionine metabolism is summarized in Fig. 39.17 . Folate is the principal micronutrient determinant of homocysteine status, and supplementation with folate (0.5 to 5.0 mg/day) has been used as a treatment modality to reduce circulating homocysteine concentrations. , However, the most recent Cochrane meta-analyses of trials of homocysteine-lowering therapy with folate, vitamins B6 or B12 given alone or in combination have not shown a reduction in cardiovascular disease. However, in a recent trial of folate among adults with hypertension in China without a history of stroke or MI, the combined use of enalapril and 0.8 mg folic acid, compared with enalapril alone, significantly reduced the risk of first stroke. This study showed that targeting individuals with low baseline folate and choosing primary end points in trials carefully provided better insight into the intricate interplay of nutrients and disease processes.
Folate appears to have a protective effect on colorectal cancer development but is associated with increased risk of gastric cancer, prostate cancer, and may be associated with lung cancer. One meta-analysis of 10 RCTs showed a borderline significant increase in frequency of overall cancer in the folic acid-supplemented group compared to controls, whereas another showed that cancer incidences were higher in the folic acid-supplemented groups than the non-folic acid-supplemented groups (relative risk =1.21 [95% CI: 1.05 to 1.39]). Another meta-analysis has shown that fortification of foods to prevent NTDs has not been associated with increased cancer incidence during the first 5 years of treatment as the doses used for fortification are a magnitude lower than those used in clinical trials. Even so, it has been suggested that folic acid–supplementation trials should be performed with careful monitoring (of cancer incidence) to help guide future policy decisions.
Lower than normal serum folate concentrations have been reported in patients with psychiatric disorders, but treatment with folate has given mixed results. Limited evidence suggests that folate may have a role as a supplement to other treatments for depression.
No adverse effects have been reported from the consumption of folate-fortified foods; thus any signs of toxicity are associated with supplemental folate. Most of the limited evidence suggests that excessive folate supplementation, typically in doses up to 10 mg/day (although some have given 500 mg/day), can precipitate or exacerbate neuropathy in vitamin B 12 –deficient subjects, and it is this end point that has been used to set a tolerable upper intake concentration of 1 mg/day from fortified foods or supplements for adults. One recognized complication of folate supplementation is that it “masks” vitamin B 12 deficiency, because the associated anemia responds to folate alone. This may delay treatment of the deficiency, allowing neurologic abnormalities to progress. It has been recommended that if a low serum folate is present concomitantly with low vitamin B 12 , the latter should be corrected first. In addition, serum folate, rather than RBC folate, is the preferred marker for folate status in the presence of vitamin B 12 deficiency (discussed further below).
Folate status may be reliably assessed by direct measurement of serum and RBC or whole blood concentrations, and its metabolic function as a coenzyme may be assessed by metabolite concentrations such as plasma homocysteine. Serum folate concentrations are considered indicative of recent intake and not of tissue stores, but serial measurements have been used to confirm AI. Whole blood and RBC folate concentrations are more indicative of tissue stores over the lifetime of RBCs and are therefore a better indicator of longer-term folate status than serum folate. Because folate is taken up only by the developing RBC in the bone marrow and not by the mature cell, RBC concentrations reflect folate status over the 120-day life span of the cell. However, a pathology benchmarking review concluded that serum and RBC folate provide equivalent information regarding folate status. Urine folate excretion is not a recommended indicator of folate status.
CPB assays have now largely replaced microbiological procedures for the measurement of serum, whole blood, or RBC folate, although the use of microtiter 96-well plates has enabled a microbiological assay using L. casei to be partially automated. The binder used in the CPB folate assay is a protein that occurs naturally in milk, called β-lactoglobulin or milk folate binder; it is commonly used together with a radioactive 125 I-folate label, although nonisotopic fluorescence and bioluminescence labels are becoming more popular. One commercial assay uses selective protein binding coupled with ion capture, followed by fluorescence assay. A comparison of frequently used laboratory analyzers shows marked variation. However, because problems of standardization and intermethod agreement persist with CPB assays, more specific analytical techniques have been developed, including HPLC with electrochemical or mass-spectrometric detection.
Several analytes are known to be indicative of folate metabolism. Plasma homocysteine is considered a sensitive indicator of folate status and is strongly correlated with serum folate concentration in the lower range, that is below 10 nmol/L (4.5 μg/L). A homocysteine concentration above 15 μmol/L is indicative of folate deficiency. However, as the concentration of homocysteine is dependent on age, gender, renal function, genetic factors, and the status of other vitamins (B 6 and B 12 ), it is not a specific marker of folate status. Similarly, the methylation of DNA is dependent on adequate MTHFR. A sensitive new method for the rapid detection of abnormal methylation patterns among global DNA patterns has been reported and may have promise as a functional marker, as may measurement of the degree of uracil incorporation into DNA, with 5,10-methylene THF required for the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) by thymidylate synthetase. ,
Whole blood collected into containers with the preservatives lithium heparin or EDTA is the required sample type for RBC folate, whereas tubes containing clot activators are required for serum folate. Local laboratories should be consulted for their preferred sample type. Ideally a fasting sample should be collected, especially if the patient is receiving folate supplementation. A recent study using a radioimmunoassay method reported that RBC folate collected into containers with EDTA showed good stability up to 48 hours when stored at room temperature. However, serum folate significantly and progressively decreased over time with a maximum decrease of 26.8% compared to basal concentration at 72 hours when kept at room temperature and was consistent with a previous report that analyzed serum folate using a microbiologic assay. Due to the instability of folate in serum, it is therefore recommended that if delay in transportation is anticipated, serum should be separated from RBCs by centrifugation and stored frozen until analysis. Because there is a significant amount of folate in RBCs, hemolysis invalidates serum folate results.
Because of methodologic differences, reference intervals for folate are method dependent. Data collected from the National Health and Nutritional Examination Survey (NHANES) of 1988 to 1994 in the United States, in which almost 3000 blood samples were analyzed, revealed reference intervals of 2.6 to 12.2 μg/L (6.0 to 28.0 nmol/L) for serum folate and 103 to 411 μg/L (237 to 945 nmol/L) for RBC folate. However, a recent analysis conducted since mandatory supplementation of cereal grain products began, yielded age-, gender-, and ethnicity-specific reference intervals for both serum and RBC folate. This demonstrates the importance of establishing local, or at least national, reference intervals. Biochemical deficiency has been defined as a concentration of less than 3 μg/L (7.0 nmol/L) for serum folate and less than 150 μg/L (340 nmol/L) for RBC folate. For more information, refer to the Appendix on Reference Intervals. Laboratories should verify that these ranges are appropriate for use in their own settings.
Vitamin C ( l -ascorbic acid) serves as a reducing agent in several important hydroxylation reactions in the body. An illustrative case of vitamin C deficiency has recently been reported.
The term vitamin C refers to all molecules that exhibit antiscorbutic properties (derived from Latin word for scurvy, scorbutus ) in humans and includes both ascorbic acid and its oxidized form, dehydroascorbic acid (DHA) as shown in Fig. 39.20 . The vitamin C redox system comprises these molecules and the free radical intermediate, monodehydroascorbic acid, the product of one-electron oxidation of ascorbic acid. l -ascorbic acid is the enol form of 2-oxo- l -gulofuranolactone, the enolic hydroxyl on ring carbon 3 having a p Ka of 4.2 and conferring its acidic nature. The vitamin is a white, crystalline solid that is readily soluble in water. Acidic solutions (below pH 3) show absorption maximum at 245 nm, whereas solutions of the ionized material (above pH 5) have an absorption peak at 265 nm. Ascorbic acid is a relatively strong reductant with an E ′ 0 (pH 7) of +0.58 volts. The DHA form is more labile than the reduced form to hydrolytic ring opening to yield 2,3-diketo- l -gulonic acid, which is not antiscorbutic.
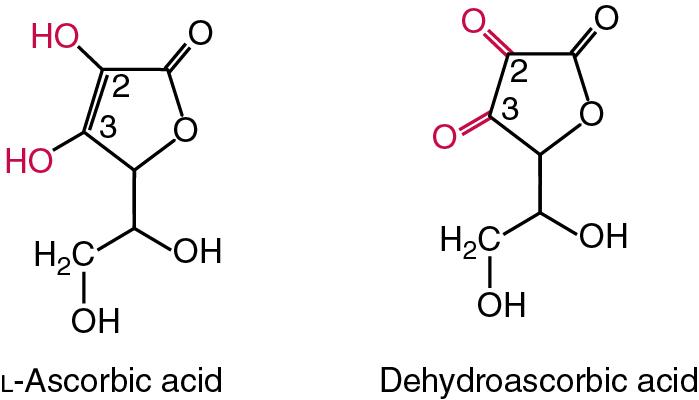
Plants and most animals possess the ability to synthesize the vitamin from d -glucose via the lactones of d-glucuronic and l -gulonic acids; however, some mammals, including humans, lack l -gulonolactone oxidase, the enzyme that catalyzes the formation of 2-keto- l -gulonolactone from d-glucose, which then spontaneously tautomerizes to l -ascorbic acid. Excellent sources of the vitamin include citrus fruits, berries, melons, tomatoes, green peppers, broccoli, brussels sprouts, and leafy green vegetables. Significant loss occurs during processes and exposure to heat and aerobic conditions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here