Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vitamin E, a micronutrient with antioxidant functions, has important actions in both intrauterine and postnatal life. Even in pregnant women with adequate vitamin stores, it seems that there is a transplacental barrier that limits its transfer to the fetus. Thus, physiologic concentrations of vitamin E are lower at birth, which makes newborns (especially premature infants) more susceptible to development of vitamin E deficiency (VED). This nutritional deficiency can have repercussions in the neonatal period (retinopathy, intraventricular hemorrhage, and others), or later, as evidenced by its adverse impact on cognitive performance in childhood. Thus, in this chapter we will discuss vitamin E and its role in pregnancy and lactation, to understand the repercussions of vitamin E for the health of the newborn.
Vitamin E is an antioxidant that reacts with free radicals, preventing lipid peroxidation of cell membranes. This nomenclature represents eight forms synthesized by plants (four tocopherols and four tocotrienols) that vary according to the number of methyl groups in the chromanol ring, being trimethyl (α-), dimethyl (β- or γ-), or monomethyl (δ-). Tocopherols are characterized by a saturated side chain attached to the chromanol ring, while tocotrienols have three unsaturated bonds in the side chain at carbons C (3′), C (7′), and C (11′) ( Fig. 29.1 ). Of the eight naturally occurring forms, α-tocopherol is the most abundant isomer of vitamin E found in human plasma and tissues and is therefore the most studied.
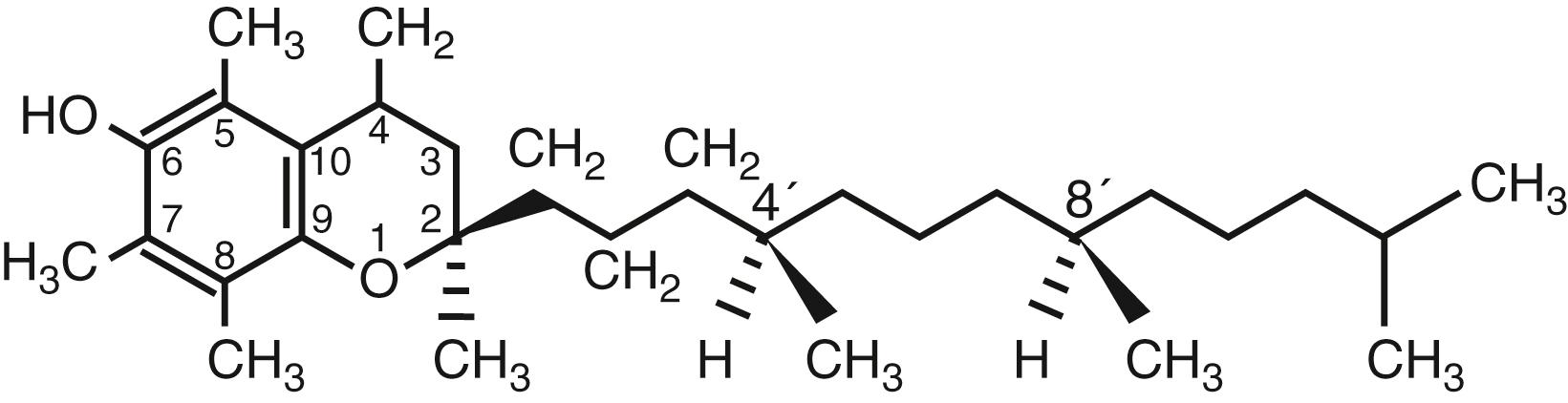
All tocopherols that occur naturally in food have RRR- conformation on the side chain, while the synthesized α-tocopherol (racemic) contains eight stereoisomers [RRR, RSR-, RSS-, and RRS- (form 2R-) and SRR-, SRS-, SSR-, SSS-]. The various forms of vitamin E are not interconvertible in humans and therefore do not have the same metabolism.
The main function of vitamin E is to be a potent antioxidant, capable of neutralizing free radicals by donating electrons from its chromanol ring, regardless of the presence of cofactors. It protects polyunsaturated fatty acids (PUFAs, mainly arachidonic acid [ARA; 20:4 ω-6] and docosahexaenoic acid [DHA; 22: 6 ω-3]) from the lipid peroxidation chain reaction by reducing peroxyl radicals as they are formed, so it is essential in any life cycle. In the presence of vitamin E, peroxyl radicals (ROO•) react more quickly with the vitamin (hydroxyl tocopherol, or vit E-OH) than with PUFAs, to form the corresponding hydroxide and the tocopheroxyl radical (vit EO•), as shown below:
The resulting tocopheroxyl radical reacts with vitamin C or other electron donors to return to its reduced state (recycling of vitamin E).
Vitamin E is known for its important role in reproduction, making a major contribution to fetal antioxidant capacity, since other antioxidant enzyme systems are still developing in the embryonic phase. In addition to prevention of oxidative damage to DNA, proteins, and lipids, other research suggests that adequate levels of vitamin E may be required for optimal intrauterine growth. ,
Thus, for both fetus and newborn, adequate body reserves of α-tocopherol are important, particularly at the critical moment of birth, which abruptly increases oxidative stress. The transition from the relatively hypoxic intrauterine environment to the relative hyperoxia of postnatal life favors imbalance between production of free radicals and antioxidant, especially in situations of prematurity.
Other functions attributed to vitamin E are believed to be—at least in part—consequences of its antioxidant role in maintaining the integrity of cell membranes. Vitamin E is essential in brain functioning, protecting neuronal membranes against lipid peroxidation, which could result in neuronal loss, DNA damage, and decline in memory and learning. This function has been supported by clinical or observational studies documenting benefits of vitamin E on cognitive development and neurodegenerative diseases. Vitamin E also has antiinflammatory activities, and α-tocopherol appears to suppress expression of adiponectin in neonates.
Specific cellular functions have been attributed to α-tocopherol that are independent of its antioxidant capacity. These actions complement the vitamin’s role in several processes, such as protein C kinase inhibition and transcriptional gene modulation through direct effects on gene transcription. ,
Although gamma-tocopherol (γ-tocopherol) is the predominant form of the vitamin in the diet, , , the Institute of Medicine of the USA in 2000 advised that nutritional requirements for vitamin E in humans should be met only by the RRR-α-tocopherol form in foods or by 2R-α-tocopherol isomers present in fortified foods or supplements. This decision was based on the specific affinity of the liver protein α-tocopherol transfer protein (α-TTP) for α-tocopherol, which primarily secretes this form into the circulation with consequent uptake by tissues, while the other vitamin counterparts are preferentially metabolized and excreted. In addition, at that time, scarcity of scientific knowledge about the other elements of vitamin E and their health benefits also contributed to this determination.
The reference values for nutrient intake are established according to characteristics of sex and stage of life of healthy individuals. Currently, the Dietary Reference Intakes (DRIs) provide four categories of suggested nutrient intake recommendations to assess the food consumption of individuals or population groups, among other applications. The recommendations for nutrient intake for vitamin E are shown in Table 29.1 . These vitamin E intake recommendation categories were established based on observations of reversal of deficiency symptoms in humans using supplements containing 2R-α-tocopherol, the amounts of α-tocopherol needed to correct erythrocyte hemolysis in vitro, or serum concentrations of α-tocopherol needed to prevent peroxide-induced erythrocyte hemolysis. The estimated average requirement (EAR) was based on experiments with individuals with VED resulting from prolonged insufficient vitamin E consumption. The recommended dietary allowance (RDA) was estimated to meet the needs of a nutrient for 97% to 98% of healthy individuals. For lactating women, it was defined based on the average amount of vitamin E secreted in human milk each day (4 mg/780 mL) plus the EAR for nonlactating women (12 mg), which totals 16 mg/day of α-tocopherol.
| Life-Stage Group | AI | EAR | RDA | UL |
|---|---|---|---|---|
| Infants | ||||
| Preterm (<37 wk gestation) | 21 a | |||
| 0–6 mo | 4 | |||
| 7–12 mo | 5 | |||
| Children | ||||
| 1–3 yr | 5 | 6 | 200 | |
| 4–8 yr | 6 | 7 | 300 | |
| 9–13 yr | 9 | 11 | 600 | |
| 14–18 yr | 12 | 15 | 800 | |
| Adults ≥ 19 yr | 12 | 15 | 1000 | |
| Pregnancy 14–50 yr | 12 | 15 | 1000 | |
| Lactation | 16 | 19 | 1000 | |
a From Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev . 2003;(4):CD003665.
As it derives from EAR, this recommendation does not apply to neonates and children under 1 year of age. Because of the lack of functional criteria to establish reversal of VED symptoms in infants, intake recommendations are based on the adult EAR only for infants and children over 1 year of age. For neonates and children under 1 year of age, recommendations are formulated in terms of adequate intake (AI), based on estimates of vitamin E intake of breastfeeding children (0 to 6 months of age). The vitamin E AI for children from 0 to 6 months of exclusive breastfeeding was defined based on the average volume of breast milk consumed by children of this age (780 mL/day), regardless of the gestational age of delivery, with an estimated content of 4 mg of α-tocopherol. Finally, the tolerable upper intake level (UL) defines the highest value of prolonged daily intake of a nutrient that poses no apparent risk of adverse health effects in almost all individuals of any stage of life or sex. For premature and very low-birth-weight children (<37 gestational weeks and <1500 g), the intake limit of 21 mg/day has been adopted, due to evidence demonstrating that excess vitamin E supplementation at this stage of life may increase the risk of sepsis.
Insufficient consumption of vitamin E can result in a nutritional status of inadequacy or deficiency, especially during stages of life characterized by increasing requirements. It is therefore essential to identify risk situations to support future intervention strategies to combat nutritional deficiencies.
Although the various forms of vitamin E are found in foods, α-tocopherol is the main form to be evaluated in the chemical composition tables of foods, being found in foods that are sources of fats, including vegetable oils (such as olive oil, sunflower, and canola), wheat germ, sunflower seeds, nuts (such as hazelnut, peanut, Brazil nut, almond, pistachio), dark green leaves (such as spinach and kale), and some fruits ( Table 29.2 ).
| Food | Vitamin E (α-Tocopherol) mg/100 g a |
|---|---|
| Sunflower oil | 41.08 |
| Nuts, almonds | 25.63 |
| Olive oil | 14.35 |
| Canola oil | 17.46 |
| Soy oil | 8.18 |
| Brazil nuts | 7.14 |
| Peanut | 6.91 |
| Canned tuna | 2.50 |
| Mango | 1.11 |
The absorption of vitamin E is strongly influenced by the amount of fat available in the meal. Because of its fat-soluble nature, intestinal absorption occurs through solubilization in micelles, which reach the liver through the remaining chylomicrons. , Vitamin E’s bioavailability appears to be greater when it is found in long-chain triglyceride emulsions than when it is in medium-chain triglyceride emulsions. The addition of milk and iron does not seem to alter the bioavailability of α-tocopherol.
A high intake of vitamin A can reduce bioavailability of vitamin E, reflecting the antioxidant role of vitamin E in protecting vitamin A during absorption. Other nutrients may also interfere with bioavailability of vitamin E. Intestinal α-tocopherol transporters—such as scavenger proteins class B type I (SR-BI), CD36 molecule (CD36), NPC1-like transporter 1 (NPC1L1), and ATP-binding cassettes A1 and G1 (ABCA1 and ABCG1)—are also carriers of cholesterol, γ-tocopherol, vitamin D, vitamin K, and carotenoids, among other nutrients and phytochemicals, which could lead to competition and reduced transport. However, more research is needed to answer questions that permeate bioavailability and recommendations for vitamin E intake.
As previously anticipated, digestion and absorption of vitamin E is closely associated with the same steps as fats. Dietary vitamin E (mainly α- and γ-tocopherol) requires bile salts and pancreatic secretions to form micelles, with consequent uptake by intestinal epithelial cells (distal part) and release into the circulation in chylomicrons. , Chylomicrons are rapidly hydrolyzed by lipoprotein lipase (LPL), producing chylomicron remnants. Although this stage is not limited by the absence of fat or by fasting, it is enhanced by fat ingestion, suggesting that the absorption of α-tocopherol is highly dependent on the chylomicron assembly processes.
The vitamin E molecules released during chylomicron hydrolysis are transferred directly to peripheral tissues, while those remaining in the chylomicron remnants are endocytosed by liver cells through a receptor-mediated mechanism. Other forms of the vitamin are efficiently absorbed and supplied to the liver in chylomicrons, but little of that material is captured into newly secreted lipoproteins for the supply to peripheral tissues. Intestinal fat can also modulate the physiology of α-tocopherol, sequestering it from circulation and decreasing the bioavailability of the vitamin to the liver.
In the liver, these lipoproteins are hydrolyzed and vitamin E released. The α-tocopherol is captured and transported by α-TTP (α-tocopherol transport protein) to the cell membrane, where it is secreted into the circulation by the ABCA1 receptor and incorporated by very low-density lipoproteins (VLDLs). α-TTP is a small hepatic cytoplasmic protein with differential affinity for α-tocopherol, also expressed in other tissues such as the brain and placenta. It is largely responsible for the intracellular transport of α-tocopherol and mediates its secretion into plasma, which explains why α-tocopherol is the most abundant form of the vitamin in the circulation. , Haga and colleagues demonstrated that the liver plays a central role in the regulation of α-tocopherol, with high expression of at least six genes related to blood concentration, distribution, transport, and metabolism of α-tocopherol, including afamin (AFM); class B receiver scavenger, type I (SCARB1); tocopherol-associated protein (SEC14L2) ; α-TTP gene; ABCA1; and cytochrome P450 family 4, subfamily F, polypeptide 2 (CYP4F2).
Similarly to chylomicron particles, VLDL triacylglycerols are catabolized by LPL on the surface of peripheral tissues, which results in the transfer of α-tocopherol to adjacent tissues and to high-density lipoprotein (HDL) particles. VLDL particles (or intermediate density lipoproteins, IDL) with remaining α-tocopherol are removed by the liver. Some vitamin is transferred to other peripheral tissues during catabolism of LDL.
Depending on the metabolism of lipoproteins, exchanges between LDL and HDL favor the transport of α-tocopherol in the circulation and its transfer to reproductive and other tissues, especially the liver, lung, brain, placenta, and mammary gland. The acquisition of α-tocopherol by these tissues appears to be mediated by an SR-BI receptor, located on the surface of cells and capable of binding HDL and LDL to capture lipids.
The largest body store of α-tocopherol is in adipose tissue (about 90%), but it seems that its mobilization is weak in response to dietary VED in adults, , probably due to the redistribution of α-tocopherol from other tissues to fat cells. It is not known how this reserve behaves during lactation.
The body’s ability to sustain high plasma concentrations of α-tocopherol is limited, apparently not because of reduced absorption but due to increased excretion. Fairus and colleagues demonstrated that plasma levels of α-tocopherol peak at 6 to 8 hours after oral supplementation, with return toward baseline after that period. Levels of circulating α-tocopherol are maintained through mechanisms associated with those that control the circulation of lipids, with a positive correlation being found between plasma levels and half-life of α-tocopherol and serum total lipid levels in individuals without dyslipidemia. These findings raise the question of whether this relationship is due to greater vitamin E consumption for antioxidant protection against lipid peroxidation or if it is due to a lower catabolism of circulating lipoproteins that also carry vitamin E.
The main route of elimination is through the feces, resulting from incomplete absorption, secretion by mucus cells, and excretion along with bile salts. In the liver, the other forms of vitamin E (δ-, β- and γ-) and the excess of α-tocopherol are excreted in the bile or metabolized by side-chain degradation via initial enzymatic degradation of cytochrome P450 (ω-oxidation) and subsequent β-oxidation to form carboxyethyl hydroxychromanol (CEHC). , Finally, this main metabolite of tocopherols is largely excreted in the urine, especially in situations of increased dietary intake of vitamin E (>12.8 mg/day) and high circulating α-tocopherol levels. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here