Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This work was supported in part by grants DK62713 from the National Institutes of Health (to ASD) and CeDAR (Center for vitamin D receptor Activator Research) grant (to ASD) from Massachusetts General Hospital.
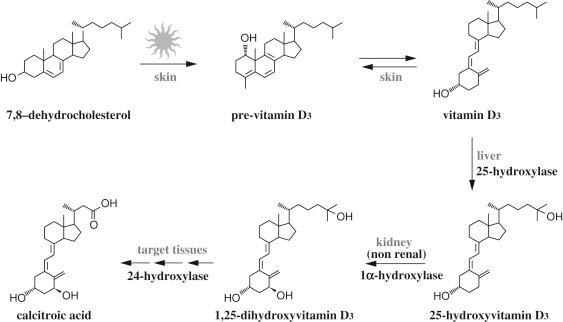
Vitamin D is essential for bone health, as has been recognized since the 1930s when vitamin D fortification of milk eradicated rickets, a skeletal disorder characterized by undermineralized bone. In recent years, strong epidemiological associations in the normal population between vitamin D deficiency and higher risk of fractures and osteoporosis, and also of cardiovascular disease, hypertension, diabetes, tuberculosis, autoimmune disease, and cancer have unraveled novel vitamin D biological actions critical in preventing these devastating disorders. However, vitamin D, which can be either obtained from the diet or formed from 7-dehydrocholesterol in the skin via a nonenzymatic, ultraviolet light–dependent reaction, has little intrinsic activity. To exert its plethora of biological effects, vitamin D must be metabolized ( Fig. 19.1 ). The first step in vitamin D bioactivation is its hydroxylation in carbon 25, which occurs primarily in the liver and renders the most abundant metabolite in circulation, 25-hydroxyvitamin D. Serum levels of 25-hydroxyvitamin D directly correlate with vitamin D concentrations, thus constituting the best indicator of vitamin D status. The kidney proximal convoluted tubule is the principal site for the final and most critical step in vitamin D bioactivation: 1α-hydroxylation of 25-hydroxyvitamin D to the potent calcitropic steroid hormone 1,25-dihydroxyvitamin D (1,25(OH) 2 D or calcitriol). However, numerous nonrenal sources of calcitriol have been identified throughout the body. Under normal physiological conditions, nonrenal calcitriol production appears to serve cell-specific autocrine/paracrine functions in the microenvironment where it is generated, rather than contribute to serum calcitriol levels. The cytochrome p450 containing enzymes that catalyze 25- and 1α-hydroxylations are CYP2R1 and CYP27B1.
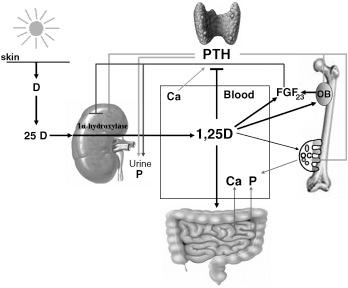
Similar to other steroid hormones, most calcitriol actions are mediated by a high affinity, intracellular receptor, the vitamin D receptor (VDR), which acts as a ligand-activated transcription factor either to increase or suppress the rate of transcription of vitamin D target genes by RNA-polymerase II. Vitamin D maintenance of a healthy skeleton requires calcitriol/VDR regulation of the expression of numerous genes involved not only in calcium and phosphate fluxes but also in the hormonal interactions between the kidney, the parathyroid gland, the intestine, and bone. These genes control transepithelial calcium transport, the expression of the calcium-sensing receptor (CaSR) and, consequently, cellular responses to extracellular calcium, phosphate absorption and resorption, the coupling of bone forming and resorbing activities, and the tight loops between the rates of renal calcitriol synthesis and catabolism, parathyroid cell growth, and the synthesis and secretion of parathyroid hormone (PTH), and bone synthesis of the phosphatonin FGF23. Fig. 19.2 summarizes the central role of the calcitriol/VDR complex in coordinating with PTH and FGF23 the numerous hormonal feedback loops required for the maintenance of calcium and phosphate homeostasis and skeletal health in normal individuals. A decrease in serum calcium is sensed by the parathyroid glands which, to restore calcium balance, rapidly enhance the secretion and/or synthesis of PTH. Elevations in serum PTH induce calcium resorption from bone and stimulate renal 1α-hydroxylase activity to increase calcitriol production. Increased serum calcitriol induces intestinal calcium absorption, and, upon calcium normalization, both calcium and calcitriol close the loop by suppressing PTH synthesis and renal 1α-hydroxylase activity. Calcitriol also elevates serum phosphate levels by promoting intestinal absorption, renal reabsorption, and bone resorption. Calcitriol also induces the synthesis of the phosphaturic hormone FGF23 in bone. FGF23, in turn, suppresses renal calcitriol synthesis through direct inhibition of 1α-hydroxylase expression and lowers serum phosphate levels by inhibiting renal phosphate reabsorption. Thus whereas calcitriol-induced increases in serum calcium and phosphate ensure appropriate bone mineralization, calcitriol-coordinated regulation of PTH and FGF23 prevents the excesses of these minerals that could lead to overmineralization of bone or ectopic calcification.
Vitamin D actions unrelated to mineral homeostasis or skeletal health and critical in disease prevention require calcitriol/VDR regulation of the expression of numerous genes with potent effects on proliferation, differentiation, and function in many cell types including skin, pancreas, and muscle, as well as the hematopoietic, immune, and nervous systems.
Comprehensive studies of mineral and skeletal homeostasis in mice lacking the VDR and/or 1α-hydroxylase have further validated the obligatory role of VDR in calcitriol signaling and revealed not only which VDR responses are the most pathophysiologically relevant but also the existence of 1,25(OH) 2 D 3 -independent actions of the VDR as well as VDR-independent actions of 1,25(OH) 2 D 3 . In addition to the evidence from transgenic mouse models, kidney disease underscores the pathophysiological repercussions of defective renal calcitriol synthesis causing systemic calcitriol deficiency and reduced calcitriol/VDR complex formation. These patients not only develop secondary hyperparathyroidism and the resulting disorders in skeletal remodeling known as renal osteodystrophy but also cardiovascular disorders, hypertension, diabetes, as well as immunological and neuromuscular abnormalities that greatly enhance morbidity and mortality rates. Importantly, calcitriol/analog therapy, even at doses insufficient to correct either high serum PTH or mineral abnormalities, increases survival, thereby supporting the relevance of nonclassical calcitriol/VDR actions and their distinct sensitivity to therapy.
Most of 1,25(OH) 2 D 3 biological activities require the VDR, an ancient member of the superfamily of nuclear receptors for steroid hormones. The biological relevance of the VDR in mediating the myriad gene-regulatory effects of calcitriol in mineral and skeletal homeostasis is underscored by the phenotypes of hypocalcemia, severe skeletal growth disorders, secondary hyperparathyroidism, and variable hypophosphatemia that are similar to those in vitamin D deficiency rickets, in patients with hereditary hypocalcemic vitamin D-resistant rickets (HVDRR, a syndrome which results from loss-of-function mutations in the VDR gene causing functional defects in the VDR), and also in transgenic mice in which the VDR has been ablated. Intriguingly, however, clinical evidence from HVDRR patients and studies in the VDR-ablated transgenic models suggested a “dispensable” role for vitamin D in bone metabolism. Bone mineralization defects in these patients were completed resolved by calcium infusion. Similarly, in the VDR-null mice, both the parathyroid and skeletal phenotypes of rickets were rescued through feeding a diet high in calcium, phosphate, and lactose, which allows normalization of serum calcium by facilitating vitamin D-independent intestinal calcium absorption. These findings confirmed that the induction of intestinal calcium absorption is the dominant biological action of the calcitriol/VDR complex for skeletal health, especially when there is a physiologic stress from the requirement of bone mineralization under conditions of low calcium availability.
Recent comprehensive studies by Panda and collaborators using a double 1α-hydroxylase- and VDR-null mice model, challenged the concept of a “dispensable” role of vitamin D in the calcium/PTH/bone axis. They conclusively demonstrated that not only for calcium absorption but also for longitudinal bone growth, osteoblast, and osteoclast activity, both calcitriol and the VDR are essential. They confirmed the findings in the VDR-null mice that bone mineralization can occur by providing a high ambient calcium concentration, which can also very effectively suppress PTH secretion. However, to prevent parathyroid hyperplasia, both calcium and calcitriol are necessary. In addition, more recent reports using a double 1α-hydroxylase and PTH-null mice model also revealed that calcitriol uses intestinal and renal, but not skeletal, mechanisms to elevate serum calcium and also that calcitriol-induced increases in endochondral and appositional bone are independent of endogenous PTH. Clearly, although calcium and calcitriol have discrete and overlapping functions in mineral and skeletal homeostasis, calcium cannot entirely substitute for calcitriol. Indeed, calcitriol/VDR-regulated genes are involved in the induction of intestinal and renal transepithelial calcium transport, in bone anabolism and catabolism, and in the tight interactions between the parathyroid gland and the kidney that mediate the maintenance of PTH/calcitriol/calcium axis, and also between bone and the kidney in the control of the FGF23/calcitriol/phosphate axis for phosphate and bone metabolism.
As indicated earlier, the calcitriol/VDR complex also regulates the expression of genes unrelated to mineral or skeletal homeostasis. The complex controls cell proliferation, differentiation, and survival in numerous cell types, including cells of the immune system, regulates specific cell function such as the suppression of the renin–angiotensin system, the enhancement of glucose-mediated insulin secretion, the induction of the expression of the natriuretic peptide receptor, and induction of cytochromes p450 that mediate xenobiotic detoxification. Indeed, VDR-null mice elicited uterine hypoplasia, mammary gland hyperplasia, hypertension, enhanced sensitivity to skin carcinogens, defective myocyte development, and impaired Th1 responses due to defective macrophage cytokine synthesis. The nonclassical calcitriol/VDR actions suggest that calcitriol deficiency in kidney disease could partially account for the development of hypertension, diabetes, immunological disorders, and neuromuscular defects, important comorbid conditions in this patient population. The next section presents our current understanding of the basic mechanisms underlying VDR mediation of calcitriol regulation of gene transcription.
Like the other members of the steroid receptor family, the VDR acts as a ligand-activated transcription factor. Fig. 19.3 depicts the two critical domains of the VDR molecule involved in VDR control of gene transcription: the classical zinc finger motif for DNA binding and a multifunctional C-terminal domain for ligand binding, heterodimerization with the VDR partner, the retinoid X receptor (RXR), and the interface for recruiting of VDR-interacting nuclear proteins (coregulators), which markedly enhance or suppress the rate of gene transcription by the VDR. The VDR molecule lacks, however, the long N-terminal extension known as activation function 1 (AF1), typical of the traditional steroid hormone receptors.
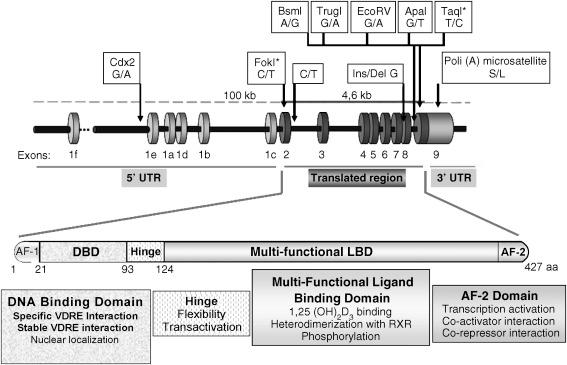
The DNA-binding domain (DBD), extending from 22 in amino acid to 110 in human VDR, is highly conserved among nuclear steroid receptors. The DBD is organized into two zinc-nucleated modules, the zinc finger DNA-binding motifs that are responsible for high-affinity interaction with specific DNA sequences in the promoter region of 1,25(OH) 2 D 3 target genes, called vitamin D-responsive elements (VDREs). Natural mutations in the zinc finger region of the human VDR result in defective DNA binding and the most severe clinical phenotypes of vitamin D resistance, thus providing compelling evidence not only for the obligatory role of the VDR in vitamin D actions but also for the absolute requirement for DNA binding as a mechanism for VDR signaling. Four nuclear localization signals (NLS), short stretches of basic amino acids, exist in the DBD of the VDR. Two of them, at amino acids 49–50 and 102–104, show coidentity in contacting DNA and as NLS. Point mutations in these NLS cause both defective cytoplasmic-to-nuclear translocation and the phenotype of HVDRR. Interestingly, basic residues at equivalent position are also important for the nuclear translocation of the VDR protein partner, the retinoid X receptor, RXR. PKC phosphorylation of the VDR at serine 51, in close vicinity with this dual NLS-DNA-binding sequence, markedly impairs nuclear localization and DNA-binding affinity.
The hinge region between the DBD and the ligand-binding domain is thought to make the VDR flexible. The function of the hinge region in gene regulation by the VDR is still unfolding.
The ligand-binding domain (LBD) is located in the carboxyterminal portion of the VDR molecule and mediates the high-affinity binding of 1,25(OH) 2 D 3 (Kd = 10 −10 –10 −11 M). In contrast, 25(OH)D 3 , the precursor of calcitriol and the most abundant metabolite in the circulation, binds the VDR nearly 100 times less avidly. The A ring containing the 1α-hydroxyl group is a critical portion of the 1,25(OH) 2 D 3 molecule responsible for high-affinity VDR binding. Calcitriol is anchored into the highly hydrophobic ligand pocket of the VDR molecule through six hydrogen bonds with the 1α-hydroxyl, the 3β-hydroxyl, and the 25-hydroxyl. However, as will be discussed below, ligand-binding affinity is not an absolute predictor of the VDR transcriptional activity conferred by the ligand.
Three different domains of the ligand-binding domain of the VDR molecule operate as dimerization surfaces for the selective association between the VDR and the RXR, which induces a VDR conformation that is essential for VDR-transactivating function. An interplay between ligand-binding and heterodimerization domains was suggested by two natural mutations (I314S and R391C) in the LBD of the VDR that confer the phenotype of vitamin D resistance by significantly impairing both VDR-RXR heterodimerization and ligand retention. Two additional domains of the LBD serve as adaptor surfaces for nuclear proteins necessary for VDR–coregulator interactions : one is the RXR-heterodimerization domain containing residue 246, which is highly conserved among nuclear receptors and forms part of the binding interface with transcriptional coactivators. Its alteration severely compromises transactivation. The second region is the activation function 2 (AF2) domain, which undergoes a dramatic conformational shift upon ligand binding ( Fig. 19.4 ) that allows the recruitment of VDR-interacting proteins including components of the transcription initiation complex, RNA polymerase II, and nuclear transcriptional coregulators that promote chromatin remodeling and control gene transcription. Removal of the AF2 domain eliminates 1,25(OH) 2 D 3 -VDR transcriptional activity with little effect on ligand binding or heterodimeric DNA binding.
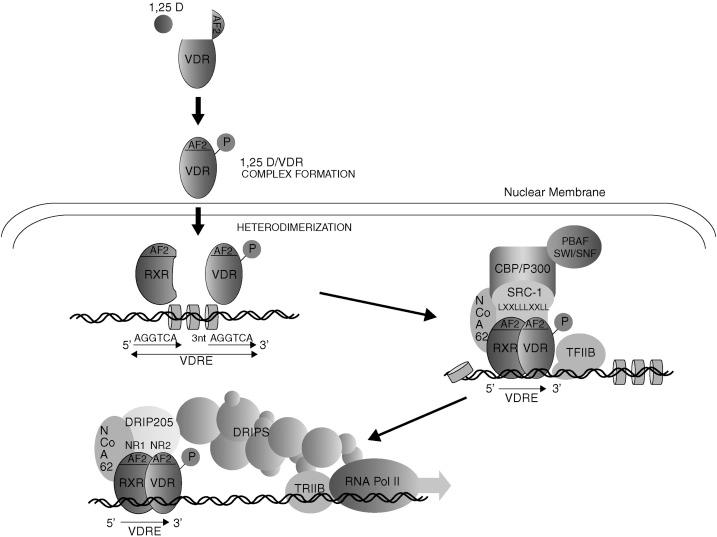
The next section presents the dynamic changes in the VDR molecule induced by calcitriol binding that are responsible for the activation of the calcitriol-bound VDR to translocate from the cytosol to the nucleus either to increase or suppress the rate of transcription of vitamin D-responsive genes by RNA-polymerase II.
This section addresses the basic molecular mechanisms involved in:
ligand/VDR complex formation
VDR heterodimerization with RXR
DNA binding
gene transactivation and transrepression
With special focus on recent findings concerning the modulation of each step under physiological conditions and in kidney disease.
Intracellular levels of both ligand and VDR determine the magnitude of ligand/VDR complex formation in a vitamin D target cell. As indicated previously, both calcitriol and its precursor, 25-hydroxyvitamin D, can bind and activate the VDR, although with distinct affinities. How do these lipophilic molecules enter target cells from the circulation? The calcitriol synthesized in the kidney by mitochondrial 1α-hydroxylase and the 25-hydroxyvitamin D produced by the liver are transported in the blood by carrier proteins. Vitamin D-binding protein (DBP) is the main carrier. However, calcitriol also binds albumin and lipoproteins. Recently, the prior dogma that the free form of calcitriol and other steroid hormones trigger biological responses after entering target cells by simple diffusion through the plasma membrane has been challenged. The uptake of 25-hydroxyvitamin D by renal proximal tubular cells occurs through megalin-mediated endocytosis, thus raising the possibility that calcitriol entrance to target cells could also involve megalin-mediated endocytosis, as recently demonstrated for sex steroids in reproductive tissues. Megalin is expressed in several epithelial cell types including parathyroid cells and osteoblasts, whose responses to calcitriol are critical for mineral and skeletal homeostasis. In kidney disease, a reduction in parathyroid or osteoblast megalin levels, similar to that demonstrated in renal proximal tubular cells after 5/6 nephrectomy in rats, could contribute to the resistance to calcitriol therapy in these patients. The expression of 1α-hydroxylase in parathyroid cells and osteoblasts also suggests that endogenously produced 1,25(OH) 2 D 3 can bind and activate the VDR. 1,25(OH) 2 D 3 production, however, is contingent upon the adequacy of both circulating levels of 25-hydroxyvitamin D and cellular uptake, the latter susceptible to the same limitations in kidney disease as those outlined for calcitriol entrance. In fact, impaired uptake of 25-hydroxyvitamin D was demonstrated in peripheral blood mononuclear cells from hemodialysis patients compared to that in monocytes from normal individuals. Differential cellular uptake of the less calcemic calcitriol analogs compared to calcitriol could contribute to their tissue-specific potency. The LDL receptor was shown to mediate calcitriol uptake by human fibroblasts, supporting the existence of an active component in the cellular uptake of vitamin D metabolites. However, the more rapid uptake of the 22-oxa-calcitriol analog that binds DBP poorly supports either the “free” hypothesis or a facilitated endocytosis of the alternative carrier.
The concentration of ligand available for VDR binding is also dependent on the rate of its inactivation within the cell. 24-hydroxylase (CYP24A1) is the enzyme responsible for 25-hydroxyvitamin D and calcitriol degradation. CYP24A1 is induced by calcitriol in every vitamin D-responsive tissue through a classical VDR-mediated mechanism. Calcitriol induction of its own degradation operates as a fine local tuning for cellular activation of the VDR and also as a highly efficient mechanism to maintain serum calcitriol levels within the narrow limits required for normal calcium homeostasis, despite the large seasonal variations in serum 25-hydroxyvitamin D levels. Reduced 1,25(OH) 2 D 3 clearance and signs of vitamin D intoxication in the 24-hydroxylase null mice support a critical role for ligand inactivation in the in situ control of the VDR responses to vitamin D. Conversely, calcitriol induction of its own degradation is a limitation for therapy and suggests an advantage of local calcitriol production over exogenous administration.
25-hydroxyvitamin D, calcitriol, and less calcemic calcitriol analogs differ markedly in their VDR-binding affinity. However, once inside the cell, cell-specific variations in the expression of intracellular vitamin D-binding proteins (IDBPs) that mediate the delivery of the ligand to and from the VDR modulate ligand-VDR association/dissociation rates. This influences both the half-life of the VDR molecule, which is protected from proteosomal degradation through ligand binding and the degree of VDR activation. Physical interactions of liganded VDR with SUG1, a component of the proteosome complex, targets the VDR for ubiquitination and subsequent proteolysis, thus providing an additional in situ control of transcriptional responses to 1,25(OH) 2 D 3 . In fact, reduced recruitment of SUG1 to the VDR by 1,25(OH) 2 D 3 analogs in vitro accounts for their higher transactivating potency. It is unclear at present what directs the liganded VDR to the proteosome or to the transcriptional preinitiation complex. Differential expression of parathyroid IDBPs may contribute to VDR-binding saturation for the calcitriol analog 22-oxacalcitriol at much lower concentration of the ligand compared to other tissues with similar VDR content.
Upon ligand binding, the repositioning of helix 12 in the AF2 carboxy terminus of the VDR ligand-binding domain imparts a major conformational change in the three-dimensional structure of the VDR that acts as a switch to activate the VDR. Despite the prediction that the structure of the ligand would induce unique conformational changes in the VDR molecule, thereby altering its biological function, crystallographic analysis could not attribute the unusual actions of vitamin D analogs in vivo and in vitro to their ability to induce a differential VDR conformation. This ligand-induced activation step appears to be required for the recruitment by the VDR of motor proteins that rapidly translocate cytoplasmic VDR along microtubules to the nuclear membrane where importin facilitates nuclear entrance. In human peripheral blood monocytes, disruption of microtubular integrity is sufficient to abolish 1,25(OH) 2 D 3 induction of 24-hydroxylase gene transcription.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here