Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vitamin A (retinol) is an essential nutrient for all vertebrates. It was discovered in 1913 as an ether-soluble fraction present in certain fats and tissues, such as butter and egg yolk, that young rats required for growth and survival. Early in the history of vitamin A research, investigators demonstrated some of the fundamental properties of vitamin A, namely its indispensability for proper vision, immunity, reproduction, and epithelial cell differentiation. The chemical nature of vitamin A was elucidated in the 1930s as all- trans -retinol, which is mainly in the form of retinyl esters (RE) in tissues. β-Carotene was shown to have the same qualitative activity as retinol, and metabolic studies demonstrated that β-carotene can be converted to retinol in the intestine and thus have same biologic activity as dietary retinol or RE. In the 1940s, the acid form of vitamin A, retinoic acid, was synthesized and shown to have essentially all of the properties of retinol, except in vision. By the 1950s, the essential role of 11- cis -retinaldehyde, also known as 11- cis - retinal , in vision was demonstrated, leading to a Nobel Prize for this research.
There are two major forms of vitamin A in the human diet. The first is preformed vitamin A, comprised of retinol ( Fig. 28.1A ) and RE, which are present in foods of animal origin such as whole milk, eggs, meat, and liver. The second is provitamin A, mostly as β-carotene (see Fig. 28.1B ) but also including α-carotene and β-cryptoxanthin; these forms are present in yellow, orange, and leafy green vegetables and orange-fleshed fruits. The provitamin A carotenoids are partially converted to retinol during intestinal absorption.
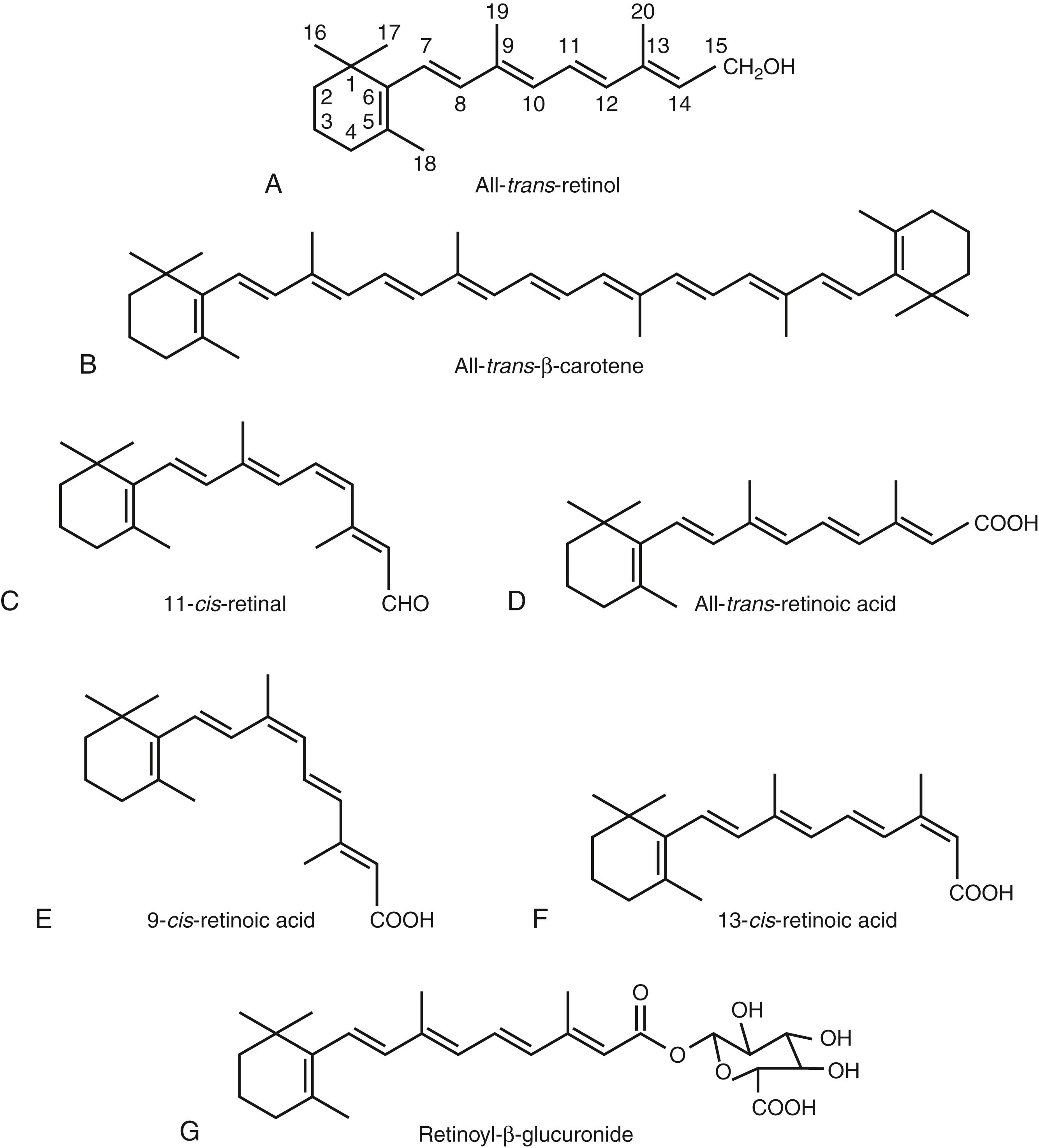
Vitamin A and its metabolites are referred to collectively as retinoids. Within plasma and tissues, there are 4 predominant forms of retinoids: (1) all- trans -retinol, which circulates in plasma bound to retinol-binding protein (gene name RBP4 ), by which it is delivered to most tissues for further metabolism to other forms; (2) RE, the major storage form, which is derived from the intracellular esterification of retinol with long-chain fatty acids; (3) retinal, which exists in small amounts as the metabolic intermediate all- trans -retinal, and at much higher concentrations as 11- cis -retinal (see Fig. 28.1C ) in the rod and cone cells of the retina; and (4) all- trans -retinoic acid (see Fig. 28.1D ), a quantitatively minor but physiologically important metabolite of retinol that is formed intracellularly through oxidative metabolism.
In addition to these principal forms of vitamin A, there are numerous other related molecules. However, their function is either unclear or they may be side and/or end-products of metabolic processes. 9- cis -RA (see Fig. 28.1E ) is believed to function as a ligand for the nuclear receptors of the RXR family, but its in vivo relevance is still being debated. , 13- cis -RA (see Fig. 28.1F ) does not function as a receptor ligand but is found in plasma as a retinoid metabolite, and it is used as a drug (see “The Retinoid Analogue, Isotretinoin” section). Glucuronides of retinol or retinoic acid, exemplified in Fig. 28.1G , are water-soluble compounds due to the glucuronide moiety; they are present in blood , and bile and readily excreted.
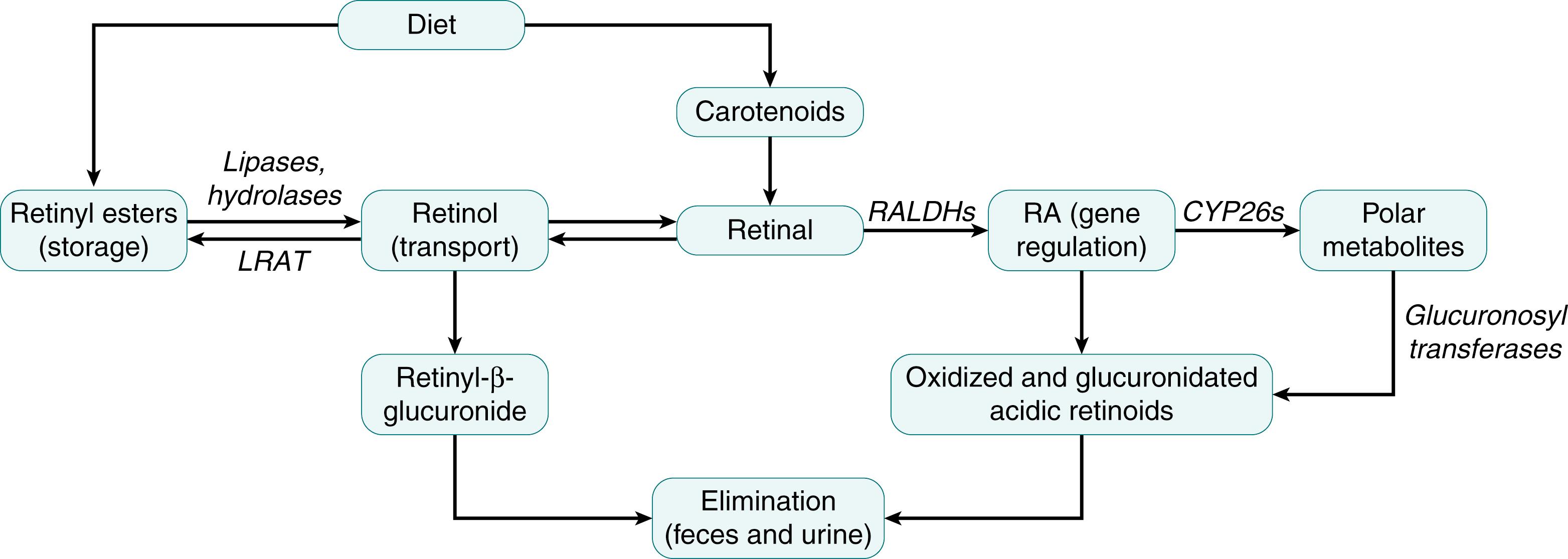
The metabolism of vitamin A is carried out by numerous proteins that function as chaperones, transport proteins, enzymes, and receptors, and together they can be considered the “machinery” for vitamin A metabolism. They also serve to assure that vitamin A is stored in the relatively inert form of RE, and to eliminate excess retinol and retinoic acid when the levels of these forms become elevated. An overview of the vitamin A biochemical pathways discussed below is shown in Fig. 28.2 . Not all reactions occur in all cells and the transport of metabolites through plasma provides a means of intertissue communication. An overview of the metabolic interrelationships among vitamin A during its metabolism in the intestine, liver, and other organs is illustrated in Fig. 28.3 .
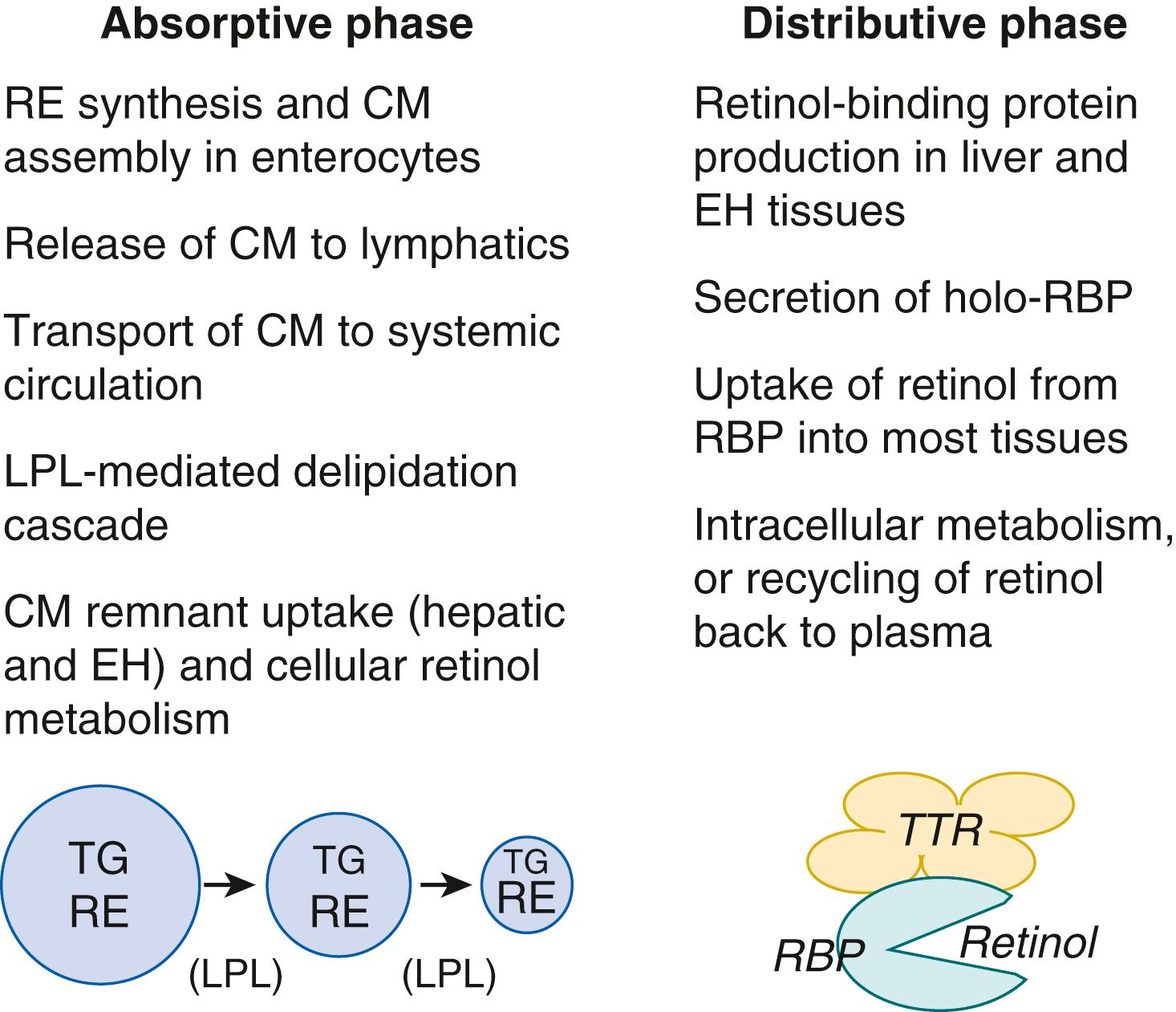
In the digestive process, RE, the primary form of vitamin A in foods, must first be solubilized and hydrolyzed to liberate unesterified retinol. Micelle formation is required to solubilize the RE, and therefore the diet must contain adequate dietary fat. Hydrolysis is catalyzed by pancreatic lipases present in the intestinal lumen and by RE hydrolases associated with the brush border of enterocytes. Once the micelle containing the released retinol traverses the unstirred water layer and reaches the apical surface of the enterocyte, retinol is transported across the apical brush border membrane. Most retinol is absorbed in the upper small intestine. Overall, the absorption efficiency of retinol is high, as approximately 70% to 90% of a dose of vitamin A was absorbed in adult rats and nearly 100% in neonatal rats. However, the absorption efficiency can be compromised in diseases such as cystic fibrosis and other lipid malabsorption syndromes. In the enterocyte, most of the retinol becomes bound to a relatively abundant cytosolic protein, cellular retinol-binding protein (CRBP) type 2. The concentration of CRBP2 is lower in the small intestine of immature fetuses relative to that in the mature newborn and adult rat. Retinol is then transferred to the enzyme lecithin:retinol acyltransferase (LRAT), which esterifies the retinol. LRAT is present in the membranes of intestinal enterocytes as well as in other vitamin A-storing tissues. The esters formed are mostly retinyl palmitate, stearate, oleate, and linoleate, , which are very hydrophobic. The newly formed RE are packaged into the lipid core of nascent chylomicrons as they are assembled in the enterocytes. Chylomicron vitamin A content directly reflects the vitamin A content of the recent meal, or dose of supplemental vitamin A, that is being absorbed, and therefore it can be highly variable, from a trace to larger amounts, although still low in comparison to dietary fat.
Carotenoids are generally far less bioavailable than preformed vitamin A. Their bioavailability depends significantly on the type of food in which they are present. β-Carotene in milk, butter, and dietary supplements is relatively soluble and absorbed with higher efficiency than β-carotene, or other provitamin A carotenoids, that are present within fibrous foods, such as green leafy vegetables. In these foods, they are bound within membranes and must first be liberated from the food matrix through the intestinal digestive process before the carotenoids can be solubilized into micelles and absorbed. The absorption process utilizes a scavenger receptor class B type I transporter in the brush border membrane. Once within the enterocytes, β-carotene may undergo cleavage by the enzyme known as β-carotene oxygenase-1 (BCO-1), which cleaves β-carotene into retinal. The retinal is then enzymatically reduced to retinol and esterified by LRAT to RE. Although this is the major route for the intestinal metabolism of β-carotene in humans, some lesser portion of the newly absorbed β-carotene becomes incorporated directly into the chylomicron core and is absorbed intact. Chylomicrons containing RE and/or β-carotene are secreted into the lymphatic vessels. Additionally, small amounts of retinol or β-carotene may also be oxidized within the intestine, presumably in enterocytes, to form retinoic acid. Retinoic acid is secreted into the portal circulation, bound to albumin.
In tissues that express lipoprotein lipase (LPL), most of the chylomicron triglyceride is digested, leaving the majority of RE in the chylomicron remnant. However, in some extrahepatic tissues, such as adipose tissue, skeletal muscle, heart, lungs, and kidneys, LPL may also be able to hydrolyze RE. Additionally, during lactation the mammary gland takes up significant amounts of chylomicron RE, in proportion to the RE content of the chylomicron. The ability of these tissues to take up chylomicron RE postprandially may account for their accumulation of vitamin A in a dietary dose-dependent manner. Nevertheless, in adults, the majority of chylomicron RE, as well as the β-carotene in chylomicrons, remain within the chylomicron remnant and are cleared into the liver. Because the processing of chylomicron triglycerides takes place very rapidly, the half-life of chylomicron remnant RE in plasma is very short, on the order of minutes, and thus dietary RE circulate in postprandial plasma very transiently. As compared to adult animals, extrahepatic tissues in neonatal rats , and piglets play a significantly greater role in the clearance of chylomicron RE from plasma, especially after supplementation with vitamin A.
Studies in adult humans have shown that a fraction of the absorbed β-carotene may undergo a slow conversion to retinol over many days after the main peak of β-carotene absorption. This suggests that tissues besides the intestine (most likely the liver) can generate vitamin A. Although the BCO-1 gene is expressed mainly in the intestine, its mRNA is also present in other tissues although at lower levels, consistent with a possible capacity for extrahepatic metabolism of β-carotene to retinol after intestinal absorption. Various single nucleotide polymorphisms (SNPs) in the human BCO-1 gene have been implicated in alterations in the efficiency of carotene cleavage. Interestingly, the distribution of BCO1 SNPs differs among ethnic groups, and, although still unproven, this may correlate with the variability in β-carotene conversion to vitamin A that has been observed among human subjects.
RE contained within chylomicron remnants is cleared into the liver, specifically hepatocytes, which it enters via receptor-mediated endocytosis, where it is rapidly hydrolyzed. In both the adult and neonate, the liver accumulates vitamin A as RE when vitamin A intake exceeds the body’s requirements. Under conditions of vitamin A sufficiency, most of the released retinol is transferred from hepatocytes to hepatic stellate cells, where retinol is bound to CRBP type I and reesterified by LRAT and then stored as RE within cytoplasmic lipid droplets. Storage also serves as a detoxification mechanism, removing excess “free” retinol. When peripheral tissues require retinol, these stored esters are hydrolyzed, and retinol is mobilized back to hepatocytes. Hepatocytes are also the major site of synthesis of RBP. The newly released retinol combines with apo-RBP to form the holo-RBP complex that is released from the liver into the circulation. In the condition of vitamin A deficiency, apo-RBP is still synthesized, but it accumulates in the liver without retinol bound to it. If vitamin A is ingested or injected, the holo-RBP complex is then rapidly released from liver to plasma.
Due to the short half-life of chylomicrons in plasma, RE is present in plasma transiently after meals. In contrast, retinol bound to RBP as holo-RBP is present continuously. In the fasted state, holo-RBP constitutes nearly all (>95%) of total plasma retinol. RBP is a 21-kDa protein with a single binding site for all- trans -retinol, and the concentrations of both in plasma are highly correlated. Although RBP is synthesized mostly in hepatocytes, RBP4 mRNA is present in measurable amounts in several extrahepatic tissues, including the kidney. Therefore these tissues might also contribute RBP, or holo-RBP, to plasma. Within plasma, holo-RBP circulates in association with another plasma protein, transthyretin (TTR, previously known as prealbumin ). The complex of holo-RBP and a tetrameric TTR protein has a molecular mass of approximately 55 kDa. The increased size of holo-RBP-TTR, as compared to holo-RBP alone, is believed to limit the filtration rate of the complex in the kidney and thereby to reduce the loss of holo-RBP in urine. In contrast, apo-RBP has a reduced affinity for TTR, and consequently, it is rapidly filtered and excreted in the urine.
Retinoic acid, the acidic form of vitamin A, circulates in plasma at generally low concentrations (5 to 20 nM). , The concentration of retinoic acid does not correlate well with plasma retinol, but it is increased in plasma after a high-vitamin A meal such as animal liver. In addition, minor quantities of other retinoid compounds are present in plasma, including cis -isomers of retinoic acid and small amounts of water-soluble metabolites such as the glucuronide adducts of retinol, retinoic acid, and their oxidation products.
The concentration of retinol in plasma is quite stable and regulated within a relatively narrow range, so long as there is adequate vitamin A for its secretion as holo-RBP from liver. Conversely, plasma retinol, and RBP with which it is closely correlated, decreases when hepatic stores of RE are too low. The figure of 20 μg total retinol/g liver (equal to 70 nmol/g) is generally considered a value below which hepatic secretion of holo-RBP is impaired. In adult animals and humans, plasma retinol concentrations above 1.05 μM are generally accepted as indicating vitamin A adequacy; levels between 0.7 and 1.05 μmol/L as indicating vitamin A-marginal status; and levels less than 0.7 μmol/L as indicating vitamin A deficiency. Whether these same numerical criteria are also appropriate in newborn and infants, however, is unknown. In general, serum retinol levels are lower in infants than in children, even when vitamin A intakes are adequate, suggesting that this is a physiologically determined level that may be appropriate for age. Serum retinol concentrations tend to increase with age, reaching a plateau after puberty that is slightly higher in boys than girls. Plasma retinol is usually lower in preterm infants than in full-term infants. , Retinol concentrations in plasma and serum are nearly identical, and either can be used for analysis.
RBP is a negative acute phase protein whose rate of synthesis in the liver decreases in states of infection or inflammation. Thus, a reduction in RBP concentration, and therefore also of retinol secretion as holo-RBP, may reflect inflammation rather than a deficiency of vitamin A, although the retinol concentrations cannot be distinguished. For example, plasma retinol may be low, and urinary retinol loss increased, in children with diarrheal disease. After inflammation is resolved, and if vitamin A storage is adequate, plasma retinol can return to previous normal values as RBP synthesis increases again. Other rare causes of low serum retinol are transport defects due to mutations in the genes for RBP4 or TTR . Average plasma retinol concentrations tend to be lower in low-income countries than in developed countries, but whether this is due to differences in nutrition, higher rates of inflammation, or other causes is not certain. The use and interpretation of serum retinol distributions to evaluate the impact of vitamin A supplementation in public health programs is discussed in Palmer et al.
In the fasting state, β-carotene is transported in low- and high-density lipoproteins. Its concentration varies with carotenoid intake and may also co-vary with plasma lipid and lipoprotein concentrations.
A plasma membrane–spanning receptor protein, Stimulated by Retinoic Acid 6 (STRA6), has been shown to bind the holo-RBP-TTR complex. STRA6 is expressed in several types of cells; it appears to be necessary for the normal uptake of retinol by retinal pigment epithelium cells. It may also play a role in other tissues, such as lung and spleen, in which its expression is up-regulated by retinoic acid. When STRA6 protein as expressed in cultured cells, it catalyzes retinol release from holo-RBP, retinol loading into apo-RBP, and retinol exchange between retinol- binding proteins, and therefore it transports retinol both into and out of the cell. , , In transfected cells, the coexpression of STRA6, CRBP, and LRAT facilitated the uptake and retention of retinol by the cell, likely by binding the internalized retinol and trapping it intracellularly as RE.
Recycling of retinol between plasma, liver, and peripheral tissues has been demonstrated in studies of retinol kinetics analyzed by mathematical modeling. In adult humans, the average retinol molecule recirculated between plasma and tissues greater than 3 times before undergoing irreversible degradation. The recycling number was even higher in rats, with 12 to 13 passages before irreversible retinol disposal, and it was higher still in neonatal rats. These data suggest that retinol circulates in a highly dynamic state, with rapid bidirectional transfer between plasma and tissue compartments. Although the liver expresses very little STRA6, another structurally similar transmembrane protein, referred to as RBPR2, has been described in liver, where it may be suited to taking up plasma retinol as it recycles from peripheral tissues. The kidney also plays a role in retinol recycling and conservation through the reuptake of holo-RBP from the renal filtrate.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here