Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
More than 15% of human cancers are known today to be caused by viruses.
A hallmark of virally induced cancers is that they are associated with persistent viral infections.
Although some viruses encode oncogenes that directly contribute to the cancers they cause, other viruses are thought to result in cancer indirectly by causing chronic destruction of the target organ from which the cancer arises.
The etiologic role of viruses in cancer is established through a combination of epidemiologic and molecular evidence.
In many cases, the cancers caused by the virus represent dead-end streets for the virus—that is, the virus is no longer able to replicate in the cancer.
Virally induced cancers can be prevented through the use of effective prophylactic vaccines.
Viral genes expressed in associated cancers represent targets for therapeutic vaccines and viral-specific anticancer drugs.
Viruses cause cancers in people. More than 15% of all human cancers are thought to have a viral etiology, and this fraction is likely to grow as we investigate additional cancers for a potential viral cause and identify new human viruses. Identifying a human cancer as having a viral etiology has substantive consequences both for its treatment and its prevention. The known virally caused human cancers often express virally encoded products in the tumor cells. These viral products are potential targets for antiviral, tumor-specific therapies. Some viral infections can now be prevented with vaccines; some now can be cured with antiviral drugs; therefore it may be possible to eliminate the human cancers that require viral contributions for their development.
For example, we now can realistically look forward to the elimination of many or most cases of primary hepatocellular carcinoma (HCC) and to most cases of cervical carcinoma. This happy eventuality is thanks to the development and eventual use of vaccines effective against, respectively, hepatitis B virus (HBV) and most tumorigenic strains of human papillomaviruses (HPVs), and potent antiviral drugs active against hepatitis C virus (HCV).
The search for human tumor viruses has been propelled by a long appreciation that viruses can cause cancers in birds and rodents. Viruses were isolated as filterable extracts from avian tumors in the first decade of the last century and were shown to induce tumors in susceptible animals. Parallel findings were made in mice in the 1940s. These animal tumor viruses were in the retrovirus family and led researchers to look for retroviruses as human tumor viruses in the 1960s and 1970s. However, most of the human tumor viruses subsequently identified are in different virus families and do not conform to some of the expectations derived from the study of highly oncogenic animal retroviruses. For example, highly oncogenic tumor viruses induce tumors in animals rapidly. The inoculation of Rous sarcoma virus into the wing web of newborn chicks can induce fatal sarcomas within 2 weeks in 100% of susceptible animals. In addition, highly oncogenic tumor viruses are oncogenic because they have acquired and express potent derivatives of cellular proto-oncogenes. In fact, many of the known human proto-oncogenes were first identified as homologues of the oncogenes transduced by the highly oncogenic animal retroviruses. Known human tumor viruses, however, usually do not induce cancers rapidly; often 15 to 50 years will elapse between the primary infection and tumor development. Nor do human tumor viruses express cellularly derived oncogenes; rather, some of them have evolved to inhibit cellular tumor suppressor genes. These differences between highly oncogenic animal viruses and human tumor viruses probably have contributed to the delay in our recognition that viruses do cause cancers in people.
A second obstacle in our recognition that viruses can be tumorigenic in their human host is that we lack convincing animal models in which to test these viruses directly. For all practical purposes, all known human tumor viruses infect only people. In addition, we now know that human viruses found not to be tumorigenic in people are tumorigenic when experimentally introduced into test animals. For example, human adenovirus 12, which causes only respiratory infections in people, is highly oncogenic when inoculated into newborn hamsters. The lack of an experimentally tractable animal host for human tumor viruses has required multiple lines of evidence to affirm that a given virus can contribute to a given human cancer. In particular, epidemiologic findings have been combined with genetic and molecular analyses in cell culture to identify human tumor viruses. Experiments with mice transgenic for viral genes also have supported these identifications.
This chapter introduces the seven known viruses that cause human tumors, the tumors with which they are associated, data that support these associations, and models to explain the viral contributions to these tumors. These viruses are presented in the order of their discovery, because early findings often have provided insights for analyses of subsequently identified viruses. Finally, the chapter outlines the types of virus-specific therapies that are possible to develop and the likelihood of developing vaccines for human tumor viruses in order to limit or eliminate specific human cancers.
Epstein-Barr virus (EBV) was identified through the insight and advocacy of Denis Burkitt. As a young surgeon, having identified Burkitt lymphoma as a new disease entity, he analyzed the geographic and climatic distribution of this childhood lymphoma, determined that it overlapped with that of malaria, and postulated that it had an infectious etiology. At his urging and with his proffered biopsy samples, Tony Epstein and colleagues identified EBV in Burkitt lymphoma–derived cells. To do so, they developed the expertise to propagate these cells in culture. Cell lines derived from EBV-positive Burkitt lymphomas proved to be powerful tools in associating EBV with different human diseases. Different EBV-positive cell lines express different viral antigens and thereby have served as test samples for patients' expression of antibodies to EBV-encoded antigens.
The analyses of these antibodies by serologic methods led the Henles in Philadelphia to propose EBV as the cause of infectious mononucleosis. A colleague in their laboratory who had lacked antibodies to EBV-encoded antigens had developed those antibodies on presentation with infectious mononucleosis. The etiologic role for EBV in this “self-limiting lymphoproliferation” was subsequently established by careful, prospective epidemiologic studies in which serologic analysis was used to demonstrate that only immunologically naïve people were at risk of the development of infectious mononucleosis; with its development, they would first express antibodies to EBV-encoded antigens of the immunoglobulin (Ig) M class, and only later to those of the IgG class. Thus about 85% of infectious mononucleosis cases were shown to arise from a primary infection with EBV. Serologic studies also allowed the Henles to propose that nasopharyngeal carcinoma (NPC) might be caused by EBV because patients with NPC were characterized by having atypically high titers to EBV-associated antigens. However, the data that linked EBV causally to Burkitt lymphoma and NPC by the early 1970s were only “guilt by association.” Although EBV caused most infectious mononucleosis on primary infection, serologic findings also had demonstrated that children in the parts of Africa in which Burkitt lymphoma is endemic and adults in the parts of China in which NPC is prevalent all had been infected with EBV—that is, they were “EBV seropositive” long before these cancers developed.
The serologic analyses of EBV in the 1960s and 1970s illustrate a conundrum for viruses and human cancers: “How can many people be infected with a given virus, and yet how can that virus contribute to tumor development in only a few infected subjects after long periods?” This apparent paradox applies, in fact, to most cancers associated with human tumor viruses and explains a major reluctance to consider viruses as etiologic agents for human cancers. The World Health Organization (WHO), without resolving this conundrum, sponsored a prospective epidemiologic survey in Uganda to assay 42,000 youngsters serologically for evidence for or against the contribution of EBV causally to Burkitt lymphoma. The region studied had a high incidence of this cancer. Samples of blood were collected from children, their serum was stored, and for children later identified as having Burkitt lymphoma, blood samples were collected again and their titers to EBV antigens were determined. In this prospective survey, Burkitt lymphoma developed in 14 youngsters during the 5 years they were monitored, and those in whom the lymphoma developed had, before tumor development, on average a 3.4-fold higher titer of antibodies to one class of EBV antigens than did the children in whom the lymphoma did not develop. That is, for children in the portions of the world in which EBV-associated Burkitt lymphoma is endemic, a high titer of antibodies to a given set of EBV-encoded antigens represents a 30-fold risk factor for the development of Burkitt lymphoma.
Do these findings prove that EBV causes Burkitt lymphoma? No; proof in such cases for which direct experiments are not feasible ultimately comes from the accretion of supporting findings in the absence of confounding data. However, the WHO study did make it unlikely that EBV is a passenger virus that merely replicates well in tumor cells, because the antibody titers were elevated 7 to 54 months before tumor detection. Similar prospective surveys were carried out in China, which identified antibodies of the IgA class to the same set of EBV antigens as a risk factor for the development of NPC.
The association of EBV with Burkitt lymphoma and NPC and the demonstration that EBV causes the bulk of infectious mononucleosis have led researchers to consider other diseases with which EBV might be associated. During the past 20 years, EBV also has been linked to posttransplant lymphoproliferative disease (PTLD) ; oral hairy leukoplakia ; approximately one-third to one-half of cases of Hodgkin disease ; one-third of AIDS-related diffuse large B-cell lymphomas (DLBCLs) ; and one-tenth of gastric carcinomas. These linkages have been made not only through serologic findings but also by molecular genetic analyses that render the linkages more robust. The latter analyses have been made possible by the elucidation of the molecular virology of EBV in cell culture.
EBV is a herpesvirus; it has a double-stranded DNA of 165,000 to 170,000 base pairs and encodes more than 100 genes, with 25 of these being pre-microRNAs (miRNAs), which encode up to 50 miRNAs ( Fig. 12.1 ). As with other herpesviruses, EBV has two distinct phases to its life cycle. It can infect cells, express a small subset of its genes (see Fig. 12.1 ), and cohabit with the cell without killing it (its “latent” phase). EBV also can emerge from its latency, express all or most of its genes, amplify its DNA, assemble progeny virions, and kill its host cell by lysis (its “lytic” phase). Unlike neurotropic herpesviruses such as herpes simplex virus type 1 (HSV-1) and varicella-zoster virus, EBV in its latent phase need not be maintained in a nonproliferating host cell. Rather, it has the capacity to both initiate and maintain proliferation in at least the B lymphocytes it infects in cell culture and at early stages of primary infection in vivo . Although it is the ability of EBV to support proliferation and survival of its infected host cell that likely renders it oncogenic, the first step in its life cycle is to infect a host's B cells successfully. As with other herpesviruses, EBV has evolved a powerful mechanism to inhibit newly infected B cells from being recognized by the host's immune response. EBV encodes at least 44 miRNAs that can target a suite of cellular mRNAs for degradation. Studies using newly infected B cells and autologous T cells have shown that viral miRNAs target mRNAs encoding IL12B, resulting in suppression of type 1 helper T cell (Th1) differentiation. These viral miRNAs also control gene expression of HLA class II and three lysosomal enzymes important for proteolysis and epitope presentation to CD4+ T cells. Parallel analyses have shown that the EBV's miRNAs also inhibit recognition of infected cells by cytotoxic CD8+ T cells.
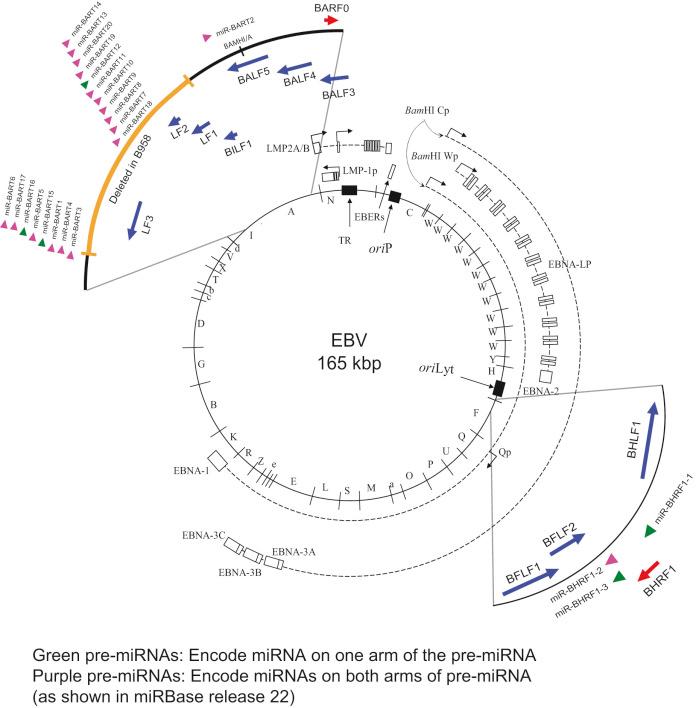
Clearly EBV's success as a pathogen reflects, in part, its ability to inhibit its host from recognizing and killing the cells that the virus infects.
EBV efficiently induces and maintains proliferation of infected B cells. In genetic experiments in which two viral genes, EBNA2 and LMP1 (see Fig. 12.1 ), are expressed conditionally within the context of the virus, each gene product when assayed alone needs to function for infected B cells to continue to proliferate. These observations are particularly telling because they help to explain the multistep evolution of Burkitt lymphoma. Many other genetic analyses have shown that four additional viral genes— EBNA1, EBNA3a , EBNA3b, and EBNA3c (see Fig. 12.1 )—affect some facet of cell proliferation or survival. EBNA1 is essential for the viral genome to be maintained as a plasmid in cells. Its inhibition leads to the loss of the viral DNA from tumor cells and to their death. EBNA3a acts at the stage of initiation of proliferation. EBNA3b is a tumor suppressor. EBNA3a and EBNA3c inhibit p16 INK4 and p14 ARF through complex, distinct binding to promoter elements A seventh viral gene, LMP2a, can substitute for the signaling provided by a functional B-cell receptor (BCR) to support the survival of murine and human B cells that lack functional BCRs. This activity of LMP2a likely underlies one contribution of EBV to the tumor cells in Hodgkin disease that fail to express functional BCRs. Two additional genes of EBV are expressed as nontranslated, small RNAs, termed “EBERs.” They contribute to the efficiency with which EBV induces and maintains proliferation of B lymphocytes infected in culture ; they also induce type I interferon and mediate other activities that potentially contribute to tumor phenotypes. The EBERs usually are highly expressed in EBV-positive tumor cells and thus provide a convenient, sensitive assay for identifying these tumor cells.
All of the transforming genes of EBV that are expressed as proteins are recognized by the host's T-cell response. EBNA1, which can be the only viral protein detected in some EBV-positive tumors, is recognized in an atypical response involving CD4+ T cells and its endogenous major histocompatibility complex class II processing. The host's cytotoxic response is sufficiently robust that patients recovering from infectious mononucleosis lack B cells that detectably express RNAs that encode these transforming genes. The surviving EBV-infected B cells are in distinct, differentiated states in which they no longer proliferate and detectably express only LMP2a (see Fig. 12.1 ), a viral gene product not directly required for cellular proliferation.
A failure of this robust immune response to EBV's transforming proteins contributes to PTLD. The infected proliferating B cells in these immunosuppressed patients are of donor origin and express all of EBV's transforming proteins, as well as the EBERs. Two types of successful treatments for PTLD demonstrate the critical role of the patient's immune response in failing to limit this “iatrogenic” tumor. First, if immunosuppression can be reduced for the patient such that the transplant is still tolerated, PTLD may regress. Second, several investigative groups have amplified the donor's T cells that are cytotoxic for EBV's transforming proteins before bone marrow transplantation. Treatment of patients with PTLD who have these syngenic, specific T-killer cells has cured the disease. These encouraging findings underscore the important role of the immune response in limiting survival of EBV-infected cells. The need to immunosuppress recipients of solid organ transplants increases their risk of developing PTLD. A controversy has arisen about the efficacy of treating recipients with drugs that inhibit EBV's lytic cycle to prevent PTLD. If the virus that causes PTLD is derived from its release following transplantation, then blocking that release could diminish the incidence of this lymphoproliferation. A large retrospective study of patients at high risk, those who are EBV seronegative and receive organs from seropositive donors, has shown that prophylaxis with drugs that target viral DNA synthesis during the lytic cycle (acyclovir, ganciclovir, valacyclovir, and valganciclovir) do not decrease the incidence of PTLD, which ranges from 1% to 8%. It is therefore essential to monitor such high-risk recipients for the potential development of PTLD after transplantation.
Two startling features of tumor cells freshly isolated from Burkitt lymphoma biopsy specimens help to explain the evolution of this tumor when considered in the context of EBV's transforming genes and the immune response to them. These tumor cells express only EBNA1 among the required viral transforming proteins, along with the EBERs and miRNAs, yet they proliferate. They also display a chromosomal translocation between one of three human Ig loci and the c- myc proto-oncogene. These translocations are likely fostered by expression of the activation-induced cytidine deaminase gene, which is essential for Ig class switching. The juxtaposition of an Ig locus to c- myc drives expression of the proto-oncogene in these B cells as it does in murine plasmacytomas that display similar translocations. These observations can be arranged to provide a satisfying though necessarily speculative model for the genesis of Burkitt lymphoma. First, EBV infects young children living in regions of central Africa in which malaria is endemic. Malaria is a T-cell immunosuppressive disease that decreases the children's ability to limit proliferation of infected cells and increases their viral loads. Although the youngsters with severe EBV infections have increased antibody titers to viral antigens, they have more proliferating B cells and are at increased risk for the chromosomal translocations, fostered by the recombination mechanism, that occur in B cells and use signals at Ig loci. A rare Ig/c -myc translocation provides the cell some undefined selective advantages. In addition, multiple viral genes including LMP1, EBNA2, EBNA3a, and EBNA3c are shut off and the cell proliferates, acquires additional mutations (often mutations inactivating p53), and evolves rapidly into a Burkitt lymphoma.
Two recent findings affect this speculative model for Burkitt lymphoma and have implications for the other EBV-associated cancers as well. Studies of the replication of EBV's plasmid genome have revealed unexpectedly that only 84% of the viral DNAs on average are synthesized each cell cycle. This defect in DNA synthesis occurs in all cell types tested and indicates that EBV DNA must be lost from proliferating cells. Proliferating populations of cells can remain EBV positive only if the daughter cells that retain EBV genomes are provided with selective advantages, such that they outgrow their sisters that lose EBV. All EBV-associated tumors maintain EBV DNA as plasmids in most of the tumor cells; therefore EBV must provide all of its associated tumors one or more selective advantages. This profound insight means that EBV provides lymphomas such as AIDS-related DLBCL with selective advantages, even though the EBV-negative and EBV-positive forms of the disease appear similar, or that EBV provides selective advantages to NPC tumors in vivo even though these tumors typically lose EBV on explantation into cell culture. EBV, when found as a plasmid in tumor cells, cannot merely be a passenger.
This insight has been tested with two forms of Burkitt lymphomas and PTLD. Tumor cells were engineered to force the loss of their EBV plasmids. All of these tumors died by apoptosis on the loss of EBV. Viral miRNAs rescued the Burkitt lymphomas that expressed the fewest viral genes. These observations underscore the role of EBV in maintaining its associated tumors and the importance of the miRNAs it encodes in oncogenesis.
Treatments for Burkitt lymphoma have evolved to favor high-dose, short-term chemotherapies that can yield greater than 90% 5-year survival rates for children with localized disease. This high rate has not been achieved, however, in some centers in Africa, where the rate of event-free survival over 12 months has been found to be only 33% for patients at all stages of the disease.
All EBV-associated cancers contain EBV DNA and express EBNA1, EBERs, and all or a subset of EBV's miRNAs (see Fig. 12.1 ). We know less about the genesis of these other tumors, but findings with NPC provide evidence for an unexpected contribution of EBV to its etiology. Chan and colleagues have shown that EBV infects cells that already can be distinguished as being “preneoplastic” in the evolution of NPC. This finding might lead one to think that EBV is merely a passenger in this tumor. However, the fact that 100% of NPC tumors are infected with EBV and that most tumor cells retain viral plasmid genomes means that EBV must provide these tumor cells with selective advantages so that the cells with EBV outgrow those that inevitably lose the plasmid. It is not known what this selective advantage is, but it is reasonable to hypothesize that EBV could provide these cells with proliferative or survival signals, as it does with Burkitt lymphoma cells. Viral gene expression in NPC biopsies correlates with decreased expression of host cell genes involved in antigen display, which likely facilitates the growth of these cells in vivo as well. Some of EBV's miRNAs are particularly well expressed in biopsies of NPCs relative to their levels in Burkitt lymphomas and cells infected in vitro . The viral miRNAs are thus likely candidates for providing NPC tumor cells with selective advantages that act in vivo .
The many clinical and basic scientific studies of EBV and its associated cancers can be extracted to yield some lessons for tumor viruses in general. First, the viral genomic nucleic acid remains in tumor cells and expresses one or more viral genes. Second, the virus contributes information to infected cells, which provides them with a selective advantage both in evolving toward and sustaining tumor cells. However, this information is not sufficient for tumor formation. Additional, multiple, rare events must occur in the infected cells for them to evolve into tumors. These additional essential events explain both why the associated tumors develop in only a fraction of the people infected with a given tumor and why the tumors usually develop only after long delays. That tumor viruses are retained in their associated tumors and help sustain these tumors indicates that targeting the transforming genes of tumor viruses is a rational approach to the treatment of these tumors therapeutically.
HBV was identified by virtue of being recognized as an antigen in the sera of donors detected by antibodies in sera of other infected donors. Thoughtful analyses by Blumberg correlated the presence of the antigen with hepatitis, a correlation strengthened by the seroconversion of a laboratory member who contracted hepatitis. By the late 1960s, blood donated to blood banks was screened for the antigen, positive samples were removed, and only negative samples were used for transfusions. This early insightful intervention led to a significant reduction in transfusion-associated hepatitis. Blumberg also demonstrated a striking association between HBV antibodies to its antigens, and HCC. These early findings have been built on to demonstrate that HBV does cause HCC, which is either the fifth or sixth most common cancer in people today. Of the approximately 500,000 new cases of HCC in the world each year, HBV is estimated to cause between 50% and 70% of them. Most of the rest of these cases are attributable to HCV, a member of the Flaviviridae family.
Two types of data have established HBV's causal role in HCC. A prospective survey of 22,707 male civil servants in Taiwan was initiated at the end of 1975. Of these subjects, 3454 were found to be positive for HBV's surface antigen (HBsAg), indicating that they were chronically infected with HBV. The entire group of 22,707 members was observed on average for 8.9 years. By the end of 1986, HCC had developed in 152 of the 3454 HBsAg-positive men, whereas it had developed in only 9 of the 19,253 HBsAg-negative men. The relative risk of the HBsAg-positive cohort for the development of HCC was therefore 100 times greater than that for the HBsAg-negative group. This prospective epidemiologic study provides robust data that the presence of HBV is strongly associated with the development of HCC.
The second type of data demonstrating that HBV can cause HCC has been derived by removing HBV from a population and determining whether the incidence of HCC declines. Taiwan began vaccinating children in 1984, first with a plasma-derived antigen and eventually with a recombinant antigen. Between 1984 and 1994, the incidence of infection as monitored by the presence of HBsAg had dropped from 9.8% to 1.3% among children 12 years or younger. Similar findings have been made in the Gambia, where children vaccinated during their first year develop into only 10% as many chronically infected 9-year-olds as do unvaccinated children. HCC is a cancer that peaks between 50 and 60 years of age and rarely occurs in children from 6 to 14 years of age. The incidence of HCC in this latter population in Taiwan dropped from 0.64 per 100,000 per year when averaged from 1981 to 1990 to 0.36 per 100,000 per year when averaged between 1990 and 1994, a finding that is statistically significant ( P < .01). This decline presumably reflects the eightfold reduction of chronic HBV infection in children at risk for the development of HCC. Removal of a virus from a population with a subsequent decrease in an associated cancer in that population provides compelling evidence that the virus contributes causally to the cancer. We can expect that a decline of HCC in the vaccinated adult population will be more striking in decades to come.
Although it is clear that HBV causes HCC in people, it is not clear how it does so. Researchers now invoke two distinct contributions, direct or indirect, to explain HBV's oncogenesis. HBV encodes one gene, pX ( Fig. 12.2 ), which can affect viral and cellular transcription and has been proposed to contribute directly to oncogenesis. HCC in general evolves in patients with marked liver cirrhosis. HBV can contribute to that cirrhosis by providing targets for T-cell killing and thereby could contribute indirectly to oncogenesis.
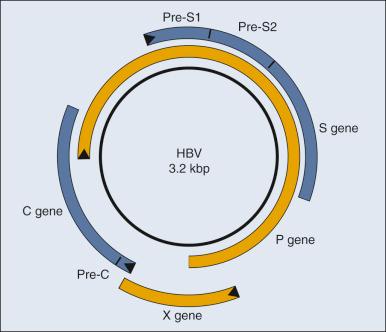
Molecular virology studies of HBV have illuminated the viral life cycle but have yet to identify its mode of oncogenesis. HBV is a small, enveloped virus with a double-stranded DNA genome, one strand of which is incomplete. The complete viral duplex DNA is 3.2 kilobase pairs (kbp) in length (see Fig. 12.2 ), serves as a template for transcription by RNA polymerase II, and is replicated via reverse transcription of a greater than full-length RNA transcript of approximately 3.4 kb. All members of the Hepadnaviridae family preferentially infect hepatocytes. This tropism is apparently mediated by a cellular receptor expressed in hepatocytes and by viral transcription that is controlled in part by cellular transcription factors principally expressed in hepatocytes. Unlike most DNA viruses, HBV undergoes its complete life cycle to yield progeny virions, which exit from hepatocytes via secretory pathways without killing the host cell. This anomalous behavior of hepadnaviruses means that, in the absence of an exogenous function of the host, an infected hepatocyte could survive, carry out its normal functions, and release large amounts of infectious HBV for long periods. Accordingly, some chronically infected people have large amounts of HBV in their sera.
Mammalian hepadnaviruses encode pX, which is not found in the avian species; only the mammalian members are known to cause HCC in their hosts. This correlation has focused interest on pX as being likely to contribute to the oncogenesis of mammalian hepadnaviruses. It is difficult to gauge pX's potential role in the oncogenesis of HBV; the literature includes much information about it, yet no ready synthesis of this information explains such a role. Most HCC tumor biopsies retain viral sequences encoding pX, but few express the protein detectably. It has been proposed that pX associates with p53 and inhibits its activation of apoptosis ; however, pX is not detected in HCC biopsy specimens, and between 30% and 90% of such biopsy specimens have mutations in p53. The viral protein pX in studies in cell culture can bind one subunit of RNA polymerase II, as well as transcription factor IIB. It also associates with Smad4, an integral member of the transforming growth factor–β (TGF-β) signaling pathway, to foster this pathway's signaling. How these different transcriptional activities of pX might contribute to the evolution of HCC is unclear. A study has reported that pX can induce markers for “stemness” in hepatocytes by activating β-catenin and epigenetic upregulation of miR-181, leading to the hypothesis that pX may contribute to the induction of cancer stem cells in the context of HCC.
HBV is often integrated in HCC tumors, and it has been proposed that integration of the viral DNA could affect transcription of nearby cellular genes. This suggestion has been strengthened by the recognition that the woodchuck member of the Hepadnaviridae family contributes to HCC via insertional mutagenesis. However, HBV has not been found to integrate at sites that can be interpreted to affect its oncogenesis. In addition, HBV DNAs cloned from HCC biopsy specimens have been tested and found not to score as enhancer sequences in hepatoma cells in culture.
The hypothesis that HBV contributes to the development of HCC indirectly by inducing rounds of cirrhosis and subsequent liver regeneration is appealing. HBV infection can be acute or chronic; it appears that acute infection correlates with a robust cytotoxic T-cell response to all viral antigens, whereas chronic infection correlates with a weak T-cell response. These cytotoxic responses do lead to the death of hepatocytes; however, experiments in mice transgenic for HBV genes and in infected chimpanzees indicate that a potent noncytotoxic mechanism also exists for limiting viral expression in infected hepatocytes. In these experiments, immune cells release γ-interferon, which by some means inhibits viral gene expression and promotes loss of viral DNA from infected cells. The administration of interleukin (IL)-18 limits viral replication efficiently in a transgenic mouse model by inducing production of both type 1 and type 2 interferons. Chronic but not acute infection correlates with the eventual development of HCC. How these two modes of eliminating infected cells would be balanced to yield long-term or chronic infection, the resulting cirrhosis, and the concomitant hepatocellular regeneration required for the accumulation of mutations predisposing to HCC is not known.
A role for cytotoxic T cells in the evolution of HCC has been modeled in mice transgenic for HBV surface proteins (see Fig. 12.2 ). These mice are tolerant to these viral antigens, but when the mice are reconstituted with syngeneic, nontransgenic bone marrow and subsequently challenged with syngeneic, immune, nontransgenic splenocytes, cirrhosis develops, and they maintain cytotoxic T cells specific for HBsAg. These animals have long-term liver damage, and by 18 to 20 months of age, HCC develops.
Although vaccination against HBV is effective at preventing infection and subsequent disease, there are still approximately 250 million people in the world chronically infected with it and at risk for developing cirrhosis and HCC. This risk translates into approximately 700,000 people dying each year, so 15% to 25% of the chronically infected will eventually die from it. Current treatments for the chronically infected include long-term use of pegylated interferon-α and nucleoside analogues such as tenofovir.
These treatments are not cures, and reduce detection of viral antigens in only 10% of patients. Important to note, they do not eliminate the viral DNA resident in hepatocyte nuclei, which sustains the chronic infection. Much necessary work is now focused on developing effective treatments for eliminating HBV from infected people.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here