Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Complex organisms rely on the fluid, nutrient, and waste transport capabilities of the cardiovascular system, whose subdivisions each have unique structure and biology to perform crucial metabolic and mechanical functions. The arterial network reacts to hemodynamic forces through constriction or dilation and also via activation of signaling pathways. Its venous counterpart is an adaptable system, with unique mechanisms for accommodating the hemodynamic stresses of the body. The lymphatic system is a distinct but closely related network that augments fluid transport and immunologic functions ( Table 3.1 ).
| Arterial Wall | Venous Wall | Lymphatic Wall | |
|---|---|---|---|
| Collagen content | Moderate | High | Mixed |
| Elastic fiber content | High | Moderate | Mixed |
| Central pressure | High | Very low | Low |
| Shear stress | High | Low | Low |
| Stretch force | High | Low | Low |
| Pulsatility | High | Low | Low |
| Compliance | Moderate | High | High |
| Oxygen tension | High | Low | Low |
| Intrinsic propulsion | None | None | Predominant |
| Valves | None | Some | Many |
Vessel wall composition changes at different locations to allow optimal function. For arteries they are mainly structured to be “resistance vessels” while properties of veins allow them to be “capacitance vessels.” Below we discuss the differences in the vessels from large arteries leaving the heart, down to capillaries and then back to large veins which return to the heart. We also discuss the lymphatic system which is a separate system that begins in interstitium and eventually returns lymph into the venous circulation.
The arterial system commences with the aorta and its branches, whose primary function is to provide a conduit for blood flow to the peripheral tissues and to smooth out the pulsations of intermittent ventricular ejection. In general, larger arteries are elastic arteries while smaller arteries are muscular arteries. The larger arteries are predominantly under control of downstream resistance vessels, and there is little evidence that vasomotor changes in large arteries occur independently of those in small resistive arteries. Changes in caliber of large arteries occur passively through changes in transmural pressure and actively through changes in vascular smooth muscle cell (SMC) contraction. Arteriolar vasomotion may induce systemic or local blood pressure changes that alter the diameter of the artery and its viscoelastic properties. Arteriolar constriction or dilation may also modify arterial SMC tone through the endothelium-dependent mechanism of high-flow dilation.
The large arteries cushion the pulsations that are characterized by the relationships between oscillatory pressure, flow, and frequency, which in turn depend on arterial diameter and elasticity. Because of their significant size, the aorta and the large arteries offer little resistance to blood flow. Large arteries store a large portion of the blood volume during systole and drain it during diastole. They are able to accommodate the stroke volume ejected with each contraction of the heart. This ability allows for propagation of the pulse and also offers dynamic resistance to the oscillatory components of pulsatile flow.
Blood pressure is highest at the origin of systemic circulation, although decreases in blood pressure are not linear with vessel diameter or distance down the vascular tree. Blood pressure decreases to 30% to 40% of aortic pressure in vessels 250 to 50 μm in diameter. Significant pressure drop occurs in the terminal arterioles, which branch into small capillaries with diameters less than 100 μm ( Fig. 3.1 ). In vessels smaller than 60 μm, no correlation has been found between central arterial pressure and microvascular pressure, suggesting that perfusion pressure is controlled directly in these blood vessels and those with smaller diameters.
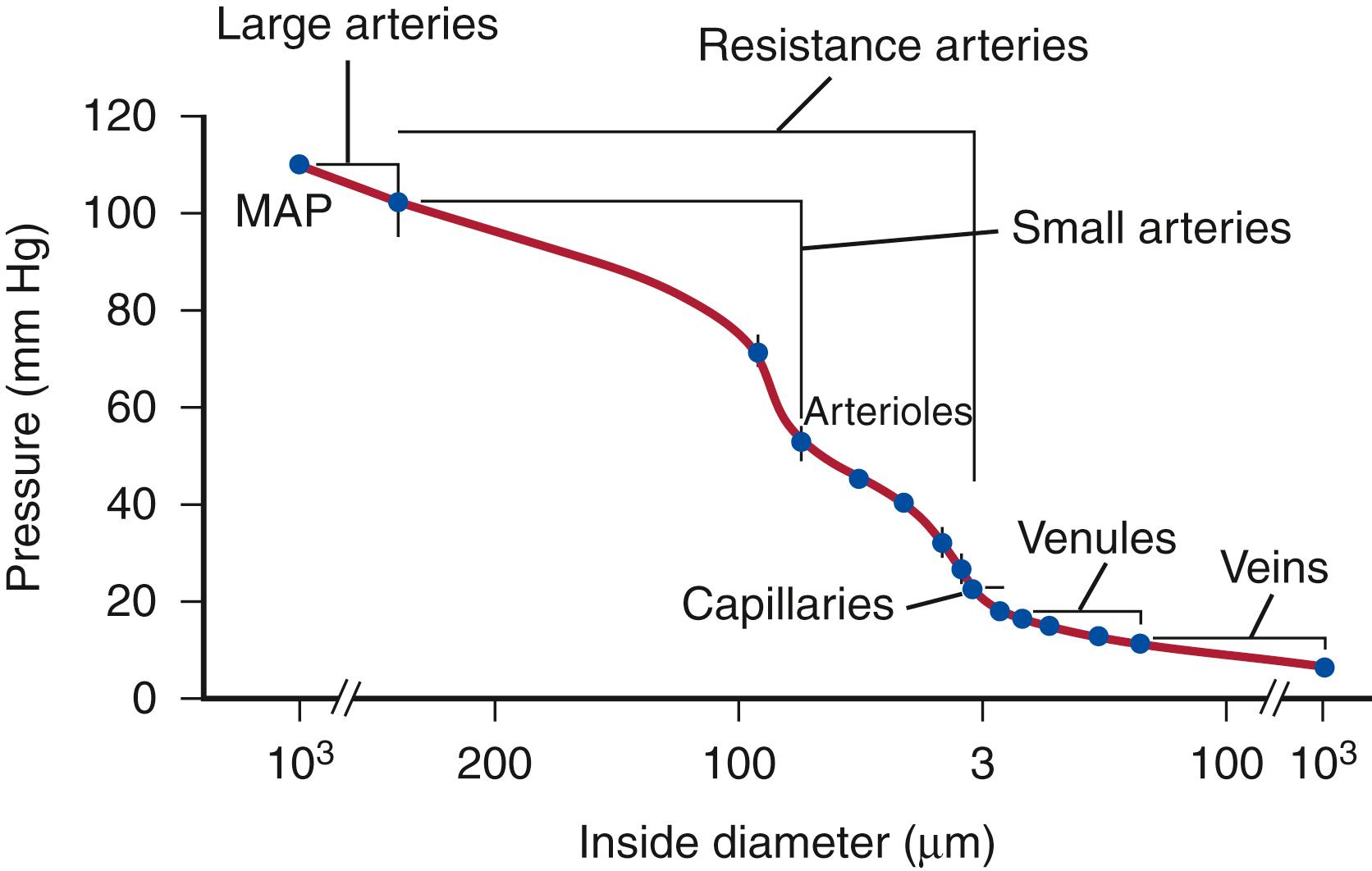
Small arteries are considered muscular arteries and are involved in vascular resistance. Initially, it was believed that resistance arteries consisted solely of arterioles with only one layer of SMC and diameters of less than 30 to 50 μm. However, approximately 50% of precapillary resistance lies proximal to the arterioles and to vessels with diameters of <100 μm. The resistance provided by a small artery is a function of the diameter–pressure relation. This compliance depends on the amount, arrangement, and characteristics of the connective tissues and SMC and on the activation level of SMC. There are approximately six layers of SMC in 300-μm vessels that decrease to a monolayer in 30- to 50-μm arterioles. The volume fraction of SMC in the media of small arteries as seen by electron micrographs is from 70% to 85%, a fraction that is greater than in larger vessels.
Arterioles are the chief source of vascular resistance and control the distribution of flow. Smooth muscle contraction and dilation is the primary source of vascular resistance. Abnormal constriction and dilation cause systemic hypertension and hypotension, respectively. The regulation of constriction and dilation is controlled by both sympathetic and parasympathetic innervation. Vasomotor responses depend on arteriolar territory, as in the case of emotional stress; emotional stress dilates arterioles of skeletal muscles through activation of cholinergic vasodilator fibers and release of epinephrine, while it constricts the splanchnic and renal arterioles.
Capillaries are responsible for the transfer of nutrient materials and waste products between blood and tissues. The blood volume of all capillaries is significantly larger than that of the aorta, causing a decrease in blood pressure and flow rate. The low rate of blood flow and large surface area facilitate the provision of nutrients and oxygen to surrounding tissue; the absorption of nutrients, waste products, and carbon dioxide; and the excretion of waste products from the body. Capillaries have a wide variety of configurations dependent on the structure of the endothelium, which is in turn related to the function of their specific vascular bed. They may be nonfenestrated continuous, fenestrated continuous, or discontinuous ( Fig. 3.2 ).
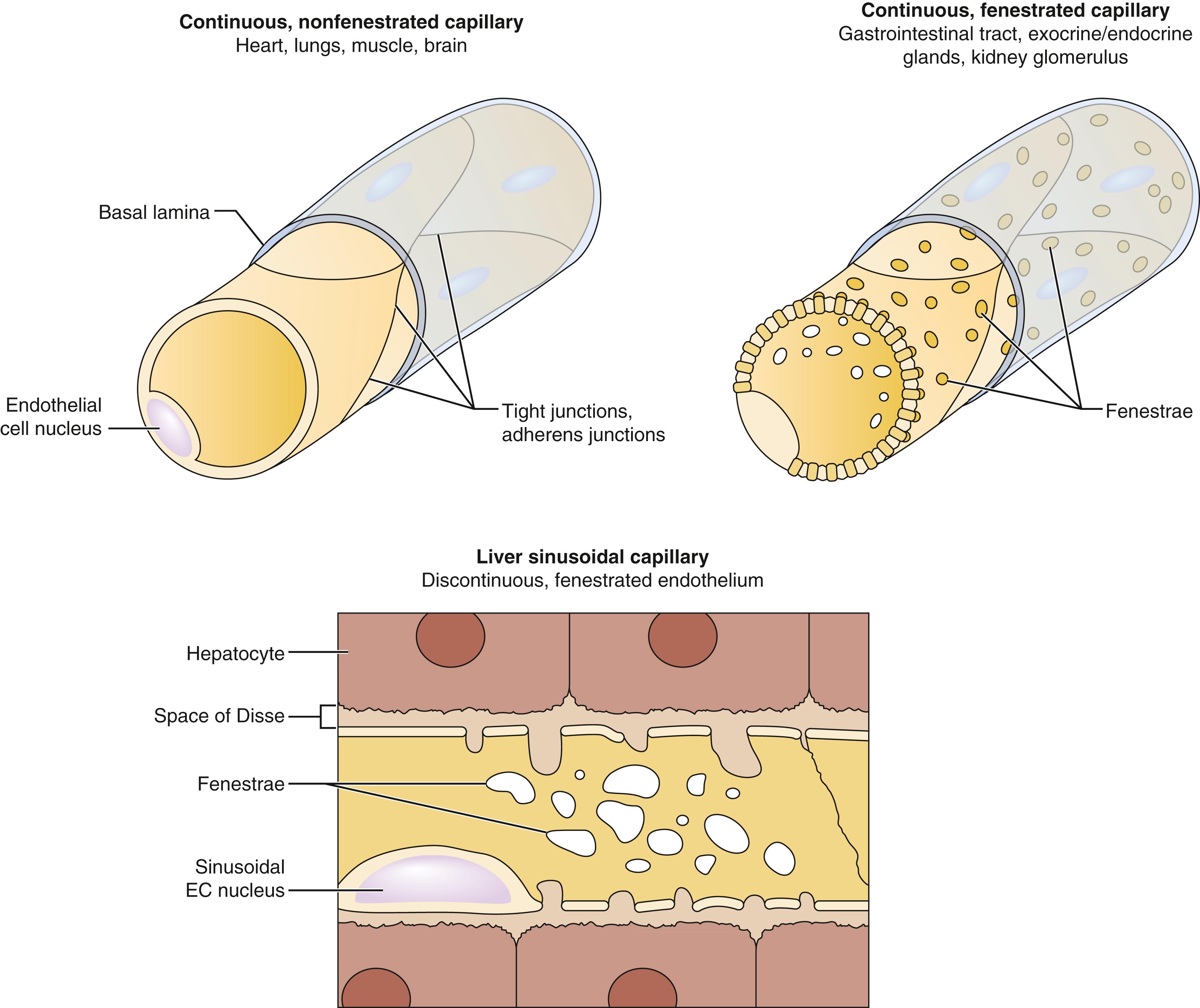
Nonfenestrated continuous endothelium functions as a selective filter and exists in capillaries all throughout the body. Fenestrated endothelium occurs in locations of increased filtration or transendothelial transport, including the capillaries of exocrine and endocrine glands, gastric and intestinal mucosa, choroid plexus, glomeruli, and renal tubules. The majority of fenestrae have a 5- to 6-nm nonmembranous diaphragm across their openings, although fenestral density varies in differing vascular beds and increases with greater need to absorb or secrete. Discontinuous endothelium possesses larger fenestrations, ranging from 100 to 200 nm in diameter. These fenestrations lack a diaphragm and contain gaps or pores within individual cells. Such capillaries form large irregularly shaped vessels, such as in hepatic sinusoids.
Postcapillary venules are the smallest of venous vessels and form from the confluence of several capillaries. Their dimensions have ranges of 50 to 650 μm in length and 10 to 50 μm in diameter. Similar in structure to large capillaries, they have an endothelium and pericytes but no SMC layer. The attenuated endothelium is 0.2 to 0.4 μm wide. The endothelium is loosely organized and leaky with only a few intercellular junctions linking cells. A thin basal lamina is present. Given these characteristics and their slow flow, they are the main sites for white blood cell diapedesis and tissue exudate from circulation to interstitial tissue during inflammation. , Postcapillary venules drain into collecting venules, which are larger but structurally similar with more pericytes. These continue to drain into muscular venules, which have one or two layers of SMC.
Veins form from the confluence of multiple venules. Typical small and medium-sized veins are 1 to 9 mm in diameter and contain valves. Compliance is extremely high for veins in comparison with arteries. A relaxed vein can increase in volume by up to 200% for a small increase in transmural pressure from 0 to 10 mm Hg. This increase reflects a change in geometry, with a low-pressure vein having an ellipsoidal cross-section that becomes circular with a small pressure increase, greatly enlarging the cross-sectional area. The adventitia, usually the thickest layer, consists primarily of longitudinally oriented collagen fibers. It may be continuous with the surrounding collagen of the supporting tissue.
Venous valves are an important structural distinction found in veins that are not present in arteries; venous valves are characteristic of small and medium-sized veins more than 2 mm in diameter. Composed of local semilunar infoldings of wall intima, the valves project into the lumen in the direction of blood flow and prevent backflow as the blood returns to the heart against gravity. A reverse velocity of 30 cm/s appears required for valve closure. Structurally, valves are composed of a mixed collagen–elastic fiber core of connective tissue covered by thin endothelium, making them stronger and more elastic than the vein wall. They are typically found distal to the confluence of venous branches forming larger veins. The valve sinus is wider than the lumen above and below the cusps. Generally, valves assist in the caudal-to-cephalad flow of blood, but also ensure the flow from the superficial veins to the deep system. A notable exception to this is in the feet, where flow is from the deep muscles in the sole to the superficial veins in the dorsum of the foot. Valves disappear more proximally in the venous system and are absent in the common iliac veins and inferior vena cava, as well as the veins of the head and neck.
The exact starting point for the development of venous diseases such as chronic venous insufficiency and varicose veins is not completely clear; however, there is little doubt that venous valve incompetence plays a role. A reduction in the number of valves per unit length has been seen in chronic venous insufficiency. The normal architecture of valve leaflets is also changed in chronic venous insufficiency, with significant infiltration by monocytes and macrophages. Valves exposed to abnormally high pressures experience increased MMP-2 and MMP-9 levels, favoring the accumulation of ECM; remodeling with reductions in leaflet height, width, and total disappearance also occurs.
The superior and inferior venae cavae are the largest veins and deliver deoxygenated blood into the right atrium of the heart. At the entrance to the heart, venae cavae and pulmonary veins have extensive vasa vasorum and can have a small amount of cardiac tissue in the adventitia. Other large systemic veins, such as the portal, pulmonary, azygos, splenic, and mesenteric veins, have similar features. The innermost layers have a distinct layer of fibroelastic tissue and a thin layer of circumferentially oriented SMC. A thick adventitia is most notable for the numerous bundles of longitudinally oriented collagen and smooth muscle fibers. Elastic fibers may be scattered throughout all the layers.
The lymphatic system is a unidirectional flow network originating in the interstitial space throughout the body, draining fluid from the ECM into initial lymphatics, which in turn contribute to gradually larger precollecting lymphatics, collecting lymphatics, trunks, and ducts.
The lymphatic system starts in connective tissue spaces at blind-ended vessels called initial lymphatics. These are most abundant in the connective tissue of the skin; beneath the mucous membranes of the respiratory, gastrointestinal, and genitourinary tracts; and in the connective tissue of the liver. Skin on the lower extremities has a denser network of lymphatics than other areas. The capacity for transport is also higher in the lower extremities to compensate for increased interstitial fluid filtration due to gravity.
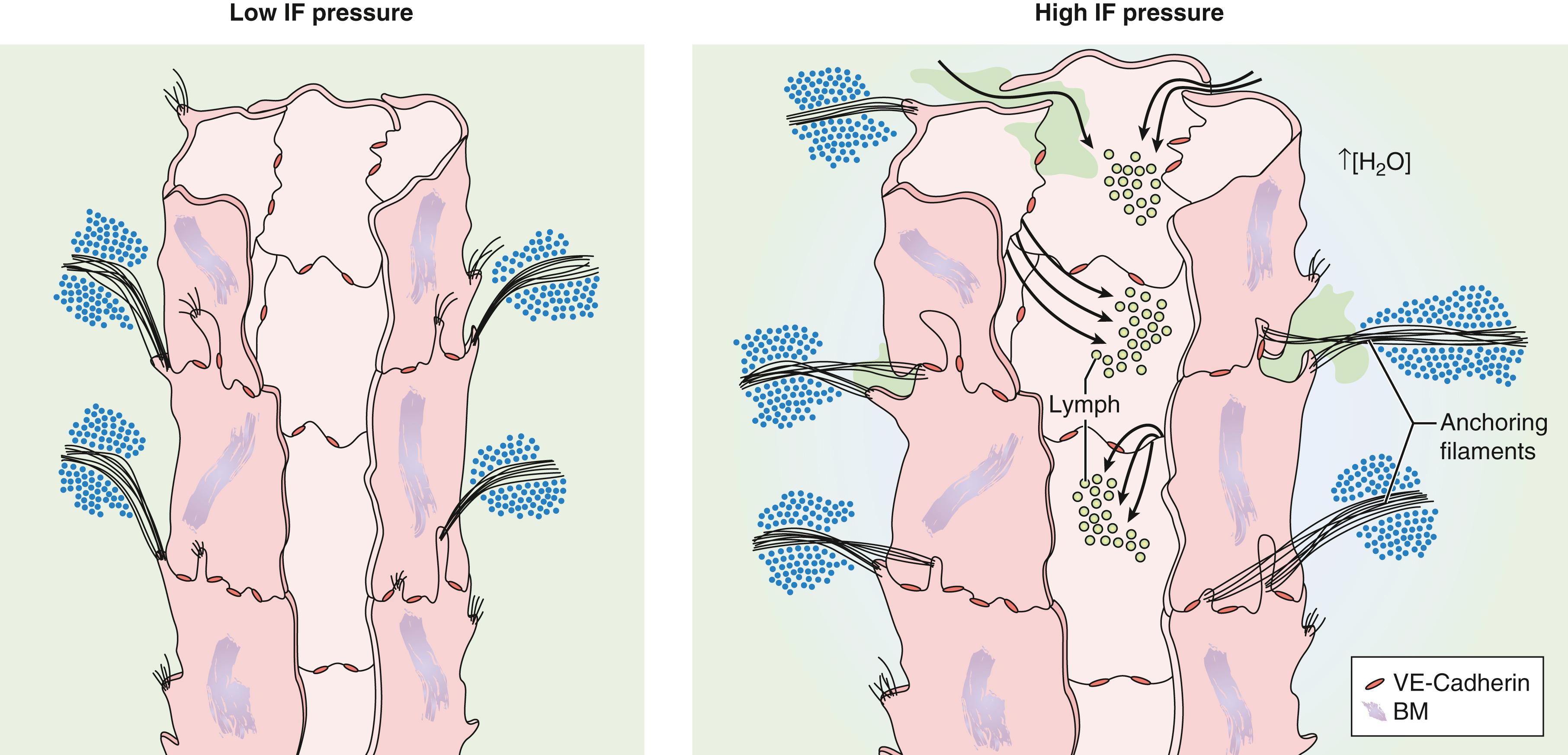
The influx of lymph is enabled by endothelial microvalves. When the vessels are collapsed, the endothelial cells (EC) have overlapping extensions of cell membrane interconnected by discontinuous buttonlike junctions. Gaps are made possible by anchoring filaments, 6 to 10 nm in diameter, that anchor the EC externally to the surrounding connective tissue. , When the interstitial fluid pressure increases and the surrounding matrix expands, the EC anchored to this matrix are prevented from collapsing and allow fluid to flow through the resultant gaps between them. As the interstitial fluid decreases and internal pressure rises, the EC come together, overlap, and the gaps close ( Fig. 3.3 ). In this way, the unidirectional flow of lymph into the lymphatics is ensured.
Precollecting lymphatics are segments of vessels connecting the initial lymphatics to collecting vessels. Like vein walls, lymphatic walls project bicuspid, one-way valves (termed secondary valves) into the vessel lumen. Unlike in larger collecting lymphatics, these valves are irregularly spaced and sometimes constitute only a single leaflet. At this level, SMC also start to appear around the vessels in one or more layers. These smooth muscle layers may contract and begin the forward propulsion of lymph through the system. However, many areas still are without any muscular layer or basal lamina and can act as a primary valve. Therefore, precollecting lymphatics fulfill a dual function in absorbing more lymph and also propelling it forward. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here