Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fracture healing is a complex process influenced by biologic and biomechanical factors. Understanding the biology of a healing bone is critical when utilizing implants that will affect this process. Although many of the principles taught in fracture surgery have implications on a cellular level, these effects are not routinely considered by surgeons. This chapter focuses on how orthopaedic implants interact with the body's native physiology for healing fractures. A paradigm of fracture healing is presented that highlights how stability and vascularity are fundamentally linked during the fracture-repair process.
Fractures create two primary problems: mechanical dysfunction and hypoxia. Orthopaedic surgeons address mechanical dysfunction by utilizing implants to reduce strain and create an environment that promotes fracture healing. Strain, defined as a change in the length of a structure relative to its overall length after stress application, can be modulated at a fracture site through implant choice and the method of application.
Hypoxia, although less obvious, may be of greater importance. Bone is a highly vascular organ. A fracture creates not only bony discontinuity but also vascular discontinuity—leaving a large area of hypoxic tissue. After a fracture, restoration of vascular union is a prerequisite for bony union.
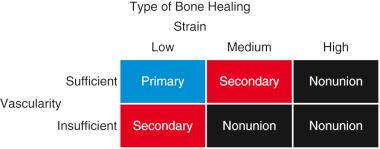
Depending on the relative amounts of strain and vascularity present, fractures heal via primary or secondary bone healing ( Fig. 4.1 ). Primary bone healing occurs in an environment of sufficient vascularity and low strain. Secondary bone healing occurs in situations with insufficient vascularity and/or low to medium strain. Identifying strain and vascularity as the main determinants of fracture healing is the first step in understanding and harnessing the processes underlying fracture repair.
Fracture healing is a highly coordinated process, characterized by teams of cells that work to address the strain and vascular discontinuity after a fracture ( Fig. 4.2 ). Depending on the microenvironment of the fracture, these teams of cells “fix” fractures using either intramembranous or endochondral ossification. Understanding the differences between these processes starts with distinguishing the major cell types: pericytes, prehypertrophic (or “spring”) chondrocytes, hypertrophic chondrocytes (the “bone bomb”), endothelial cells, osteoblasts, and osteoclasts.
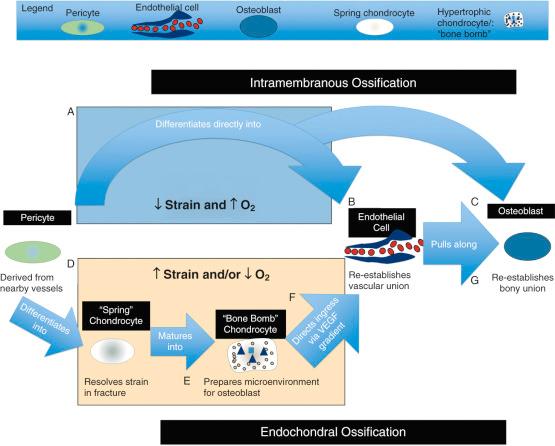
The pericyte is the “stem cell” that gives rise to the majority of new tissue required to bridge a fracture. At rest, pericytes form part of the boundary of blood vessels in bone. When a vessel is damaged as a result of fracture, pericytes are activated and begin to proliferate. Once activated, pericytes differentiate into osteoblasts or chondrocytes depending on the local microenvironment. The most significant source of pericytes for fracture healing is the vasculature of the periosteum and endosteum.
Derived from pericytes, chondrocytes thrive in the hypoxic environment of a fracture site and play a critical role in endochondral ossification and callus formation. Prehypertrophic chondrocytes, which we will term “spring” chondrocytes, function to resolve the high strain generated near the fracture by producing a biomechanical matrix. These chondrocytes proliferate in response to strain and create organized extracellular matrix proteins, formed predominately of collagen type II, until they resolve a sufficient amount of strain to stabilize the fracture site. These same spring chondrocytes are also present in healthy articular cartilage, a physiologically hypoxic environment, where they function similarly in absorbing shock and resolving strain at the joint surface.
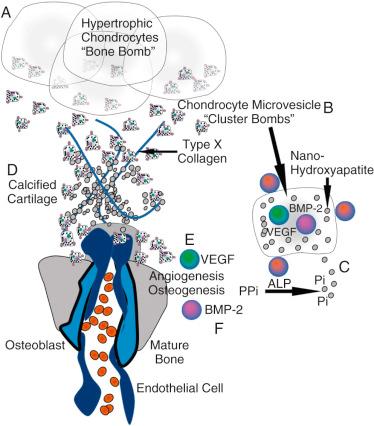
Once spring chondrocytes have sufficiently reduced interfragmentary strain, they transition to producing stores of cytokines, growth factors, and raw products for bone growth ( Fig. 4.3 ). These cells hypertrophy and progress to their second form: the hypertrophic chondrocyte, or “bone bomb,” which sets the stage for osteoblasts to arrive later and flourish. The most recent literature supports that these cells release microvesicles coated with alkaline phosphatase (ALP) to counteract pyrophosphate, the principal inhibitor of hydroxyapatite formation, and are filled with nano-hydroxyapatite, a potent seed crystal for full microhydroxyapatite. Together with type X collagen, these elements promote calcified cartilage, an important precursor of bone (see Fig. 4.3D ). In addition, these microvesicles promote angiogenesis and osteogenesis by carrying concentrated amounts of vascular endothelial growth factor (VEGF) and bone morphogenic protein-2 (BMP-2), which promote angiogenesis (see later discussion of endothelial cells) and osteogenesis (see later discussion of osteoblasts), respectively. Therefore hypertrophic chondrocytes are termed “bone bombs” because their primary biologic purpose is to promote calcified cartilage and then vascularized bone.
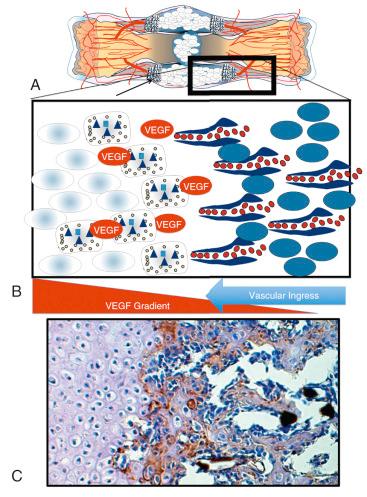
Endothelial cells manage the transition from chondroid soft tissue callus to a hard tissue callus. Endothelial cells rely on spring chondrocytes to resolve strain before their arrival because blood vessels in bone have no smooth muscle and cannot resist motion. However, once strain is resolved, endothelial cells are drawn into the soft tissue callus by VEGF, which is being produced by the hypertrophic chondrocytes.
Osteoblasts follow endothelial cells as they migrate into the soft tissue callus. As the osteoblasts enter the callus, they produce collagen type I, hydroxyapatite, BMP, and additional VEGF, encouraging further revascularization of the area. The osteoblast advances the ossification initiated by the hypertrophic chondrocyte by promoting microhydroxyapatite formation onto collagen type I (see Fig. 4.3F ). This process continues until the osteoblast has surrounded itself with hydroxyapatite and becomes an osteocyte. Fig. 4.4 shows the steps that drive this transition from a chondroid soft tissue callus to a hard callus comprised primarily of osteoblasts. This hard callus is the woven bone of an unremodeled fracture, where organization and vascularity are irregular.
With functioning blood vessels come macrophages. In an attempt to digest the large hydroxyapatite crystals made by osteoblasts, macrophages fuse and become osteoclasts. Osteoclasts create a microenvironment to break down bone and act to remodel woven bone into structurally sound units with sufficient blood supply to feed osteocytes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here