Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The liver has a unique, dual blood supply in which 25% of the flow comes from the hepatic artery and 75% through the portal vein. There is an inverse relationship between these two blood supplies. If portal flow decreases, arterial flow will increase as if an impedance has been removed. In addition, there are several communications between these vessels that open in response to nervous and humoral factors: trans-sinusoidal, transvasal, and transplexal. When vascular compromise develops, the volume and direction of blood flow of an individual vessel will be altered. Multidetector computed tomography (MDCT), Doppler ultrasound, and magnetic resonance imaging (MRI) are sensitive in the diagnosis of these perfusion disorders and are discussed in this chapter.
Intraparenchymal perfusion disorders such as transient hepatic attenuation differences (THADs) and transient hepatic intensity differences (THIDs) are epiphenomena of alterations of the dual vascular supply of the liver. There is a compensatory relationship between hepatic arterial and portal venous blood supply so that arterial flow increases when portal flow decreases ( Fig. 57.1 ). This is made possible by communication among the main vessels, sinusoids, and peribiliary venules that dilate in response to autonomic nervous system and humoral factors activated by hepatic demand for oxygen and metabolites. THADs and THIDs are areas of parenchymal enhancement visible during the hepatic arterial phase after the intravenous administration of contrast media. These lesions can be classified by morphology, etiology ( Figs. 57.2 – 57.5 ), and pathogenesis.
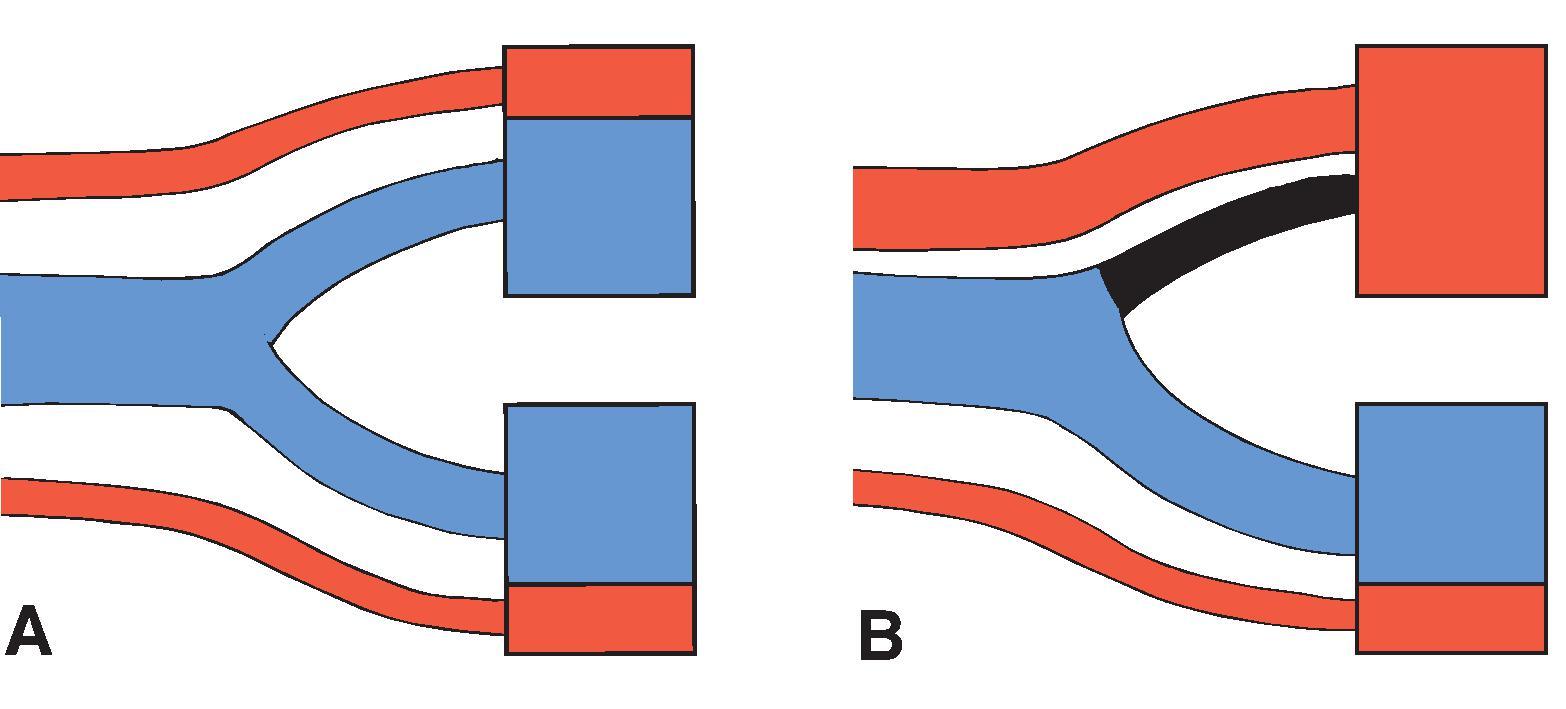
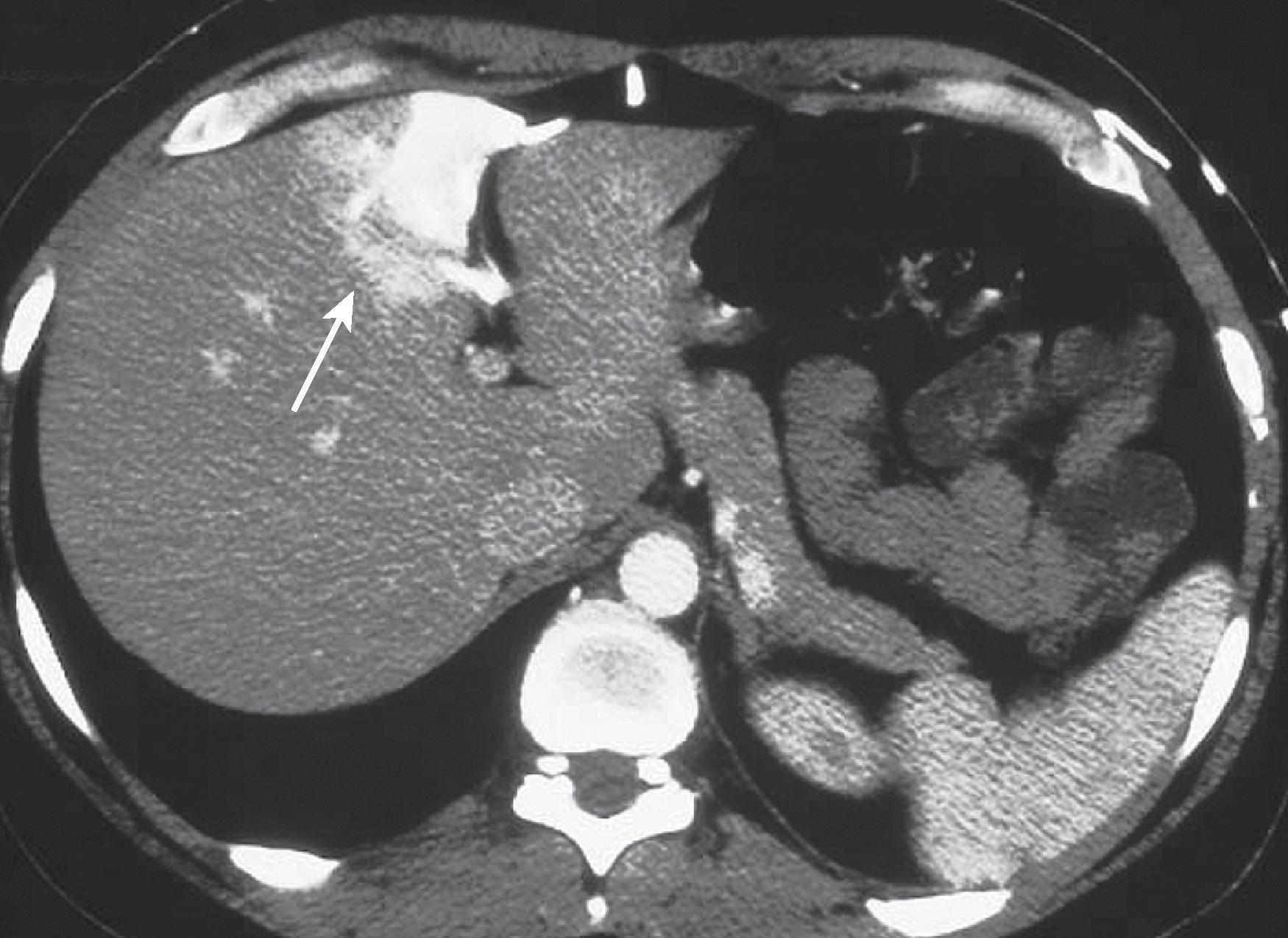
Benign and malignant masses produce two morphologic types of THADs and THIDs via four pathophysiologic mechanisms: directly, by siphoning effect of the mass (lobar multisegmental shape); indirectly, by means of portal hypoperfusion (sectorial shape) due to portal branch compression or infiltration; by thrombus, resulting in a portal branch blockade; or by flow diversion caused by an arterioportal shunt.
Lobar multisegmental THADs and THIDs occur when a benign hypervascular lesion or an abscess induces an increase in the primary arterial inflow, which leads to surrounding parenchyma perfusion, the so-called siphoning effect. These THADs and THIDs do not assume a triangular shape, but a straight border may be present between the arterial phenomena from adjacent parenchyma.
Sectorial THADs and THIDs follow hepatic vessel dichotomy and appear as triangular areas that result from the strict relationship between the portal hypoperfused area and the arterial reaction. These can be seen in benign and malignant tumors as well as in abscesses due to the spread of inflammatory mediators. The THADs and THIDs can be wedge or fan shaped.
THIDs and THADs can be seen in the absence of a focal lesion as a result of three mechanisms: portal hypoperfusion due to portal branch compression or thrombosis; flow diversion by arterioportal or an anomalous blood supply; or inflammation of the bile ducts or gallbladder.
Sectorial THIDs and THADs are usually caused by portal hypoperfusion due to portal vein or hepatic vein thrombosis, long-standing biliary obstruction, or arterioportal shunt that may be congenital, traumatic, or due to cirrhosis. These THIDs and THADs can have a globular shape, especially when they are adjacent to Glisson’s capsule.
Polymorphous THADs and THIDs have four major causes: external compression by a rib or subcapsular collection; anomalous blood supply from atypical arteries, collateral venous vessels, or accessory veins, especially in segment IV of the liver; inflammation of adjacent organs, such as cholecystitis and pancreatitis that spread inflammatory mediators and reduce portal inflow because of interstitial edema; and trauma, biopsy, or radiofrequency ablation of hepatic tumors.
In patients with obstruction of the superior vena cava, the medial segment of the left lobe (segment IV) of the liver will hyperenhance because of collateral veins. The internal mammary vein connects to the left portal vein by the paraumbilical vein.
Diffuse THADs and THIDs can be seen in right-sided heart failure (see later), Budd-Chiari syndrome (see later), and biliary obstruction, leading to abnormal attenuation and signal intensity adjacent to the portal triads.
The term Budd-Chiari syndrome is applied to a diverse group of conditions associated with hepatic venous outflow obstruction, at the level of either the large hepatic veins or the suprahepatic segment of the inferior vena cava. Diagnosis of this disorder is frequently difficult because of its protean causes and clinical manifestations. Sinusoidal obstruction syndrome is a subset of the Budd-Chiari syndrome in which nonthrombotic occlusion of the small presinusoidal venules occurs. This usually develops in the setting of chemotherapy, radiation therapy, and immunotherapy.
Traditionally, the radiographic “gold standard” for the diagnosis of Budd-Chiari syndrome has been hepatic venography and cavography. However, these invasive angiographic procedures are not suitable for screening of patients with nonspecific signs and symptoms. The cross-sectional imaging modalities have proved successful in the diagnosis of this disorder, and such studies can usually obviate angiography or venography.
Because of its excellent sensitivity, high specificity, noninvasiveness, relatively low cost, availability, lack of contrast requirements, and multiplanar imaging ability, sonography can be the initial screening study for the Budd-Chiari syndrome. The sonographic features ( Fig. 57.6 ) of the Budd-Chiari syndrome are stenosis of hepatic veins (which often have thick, echogenic walls and proximal dilation), echogenic intravenous thrombi, intrahepatic collaterals or extrahepatic anastomoses, and large inferior right hepatic vein.
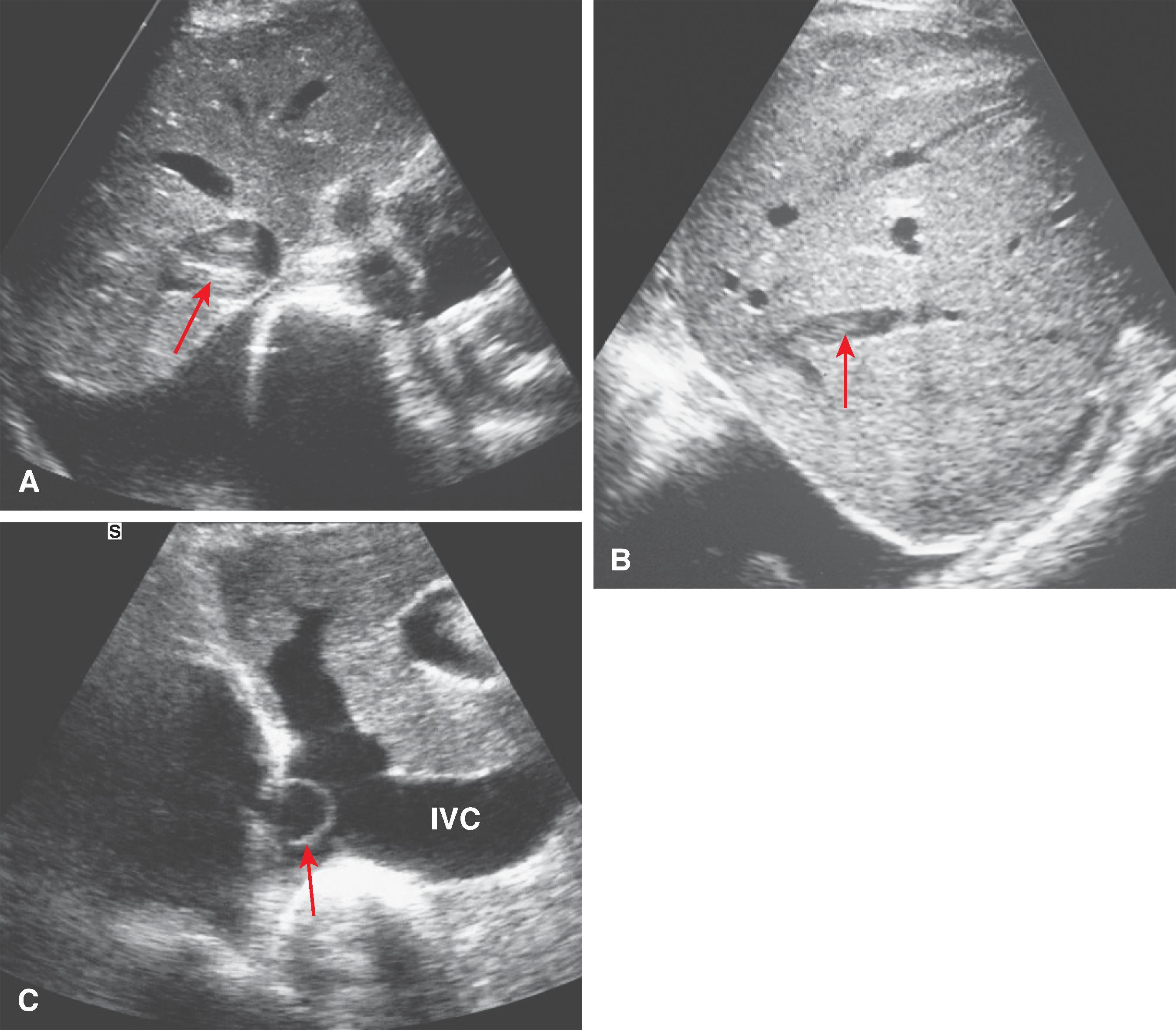
In chronic cases, the hepatic veins may not be visible. The inferior vena cava may also be narrowed by a grossly swollen liver. Ascites, abnormal hepatic shape with enlargement of the caudate lobe, and atrophy of the right lobe are present in most cases except in disease of acute onset. When both the left lobe and the caudate lobe are enlarged, confusion with cirrhosis can occur. Membranous webs are identified as echogenic or focal obliterations of the lumen.
In patients with hepatomegaly, evaluation of the hepatic veins can be difficult because they are often compressed and may not be visible. In these patients, an abdomen distended by ascites can further interfere with sonography. Similarly, in cirrhotic livers, patent hepatic veins may be difficult to identify, which precludes placement of the duplex Doppler cursor. Visualization of hepatic webs is also a problem. Color flow Doppler examination may be helpful in these cases.
Absent or reversed flow in the hepatic veins or flat flow in the hepatic veins associated with reversed flow in the inferior vena cava as demonstrated by duplex Doppler ultrasound is diagnostic of Budd-Chiari syndrome. The flow in the portal vein may be slow or reversed.
Color Doppler sonography overcomes some of the limitations of real-time sonography by demonstrating reversed flow in the retrohepatic inferior vena cava, documenting the presence of intrahepatic venous collaterals, and confirming the absence of flow in presumably thrombosed vessels. Color flow imaging rapidly and accurately determines the status of the hepatic veins and inferior vena cava and the direction of flow. Areas of occlusion are also clearly depicted, as are areas of narrowing, tortuosity, and reversal of flow direction.
In sinusoidal obstruction syndrome, there is nonthrombotic occlusion of small hepatic veins and terminal hepatic venules, the major hepatic veins show normal hepatofugal flow direction, and the inferior vena cava is patent with flow toward the heart. Decreased, reversed, or to-and-fro flow (flow demodulation) may be seen in the main portal vein. Other Doppler criteria include decrease in spectral density, reversed flow or maximum flow in the main portal vein of 210 cm/s, portal vein congestion index (cross-sectional area of vein divided by average velocity), hepatic artery resistive index of 0.75 or greater, monophasic flow in the hepatic veins, and flow recorded in paraumbilical veins. Gray-scale criteria include hepatic vein diameter of less than 3 mm, portal vein diameter of more than 8 mm in children and more than 12 mm in adults, and mural thickening of the gallbladder of more than 6 mm. Nonspecific findings include hepatosplenomegaly, ascites, and visualization of paraumbilical veins.
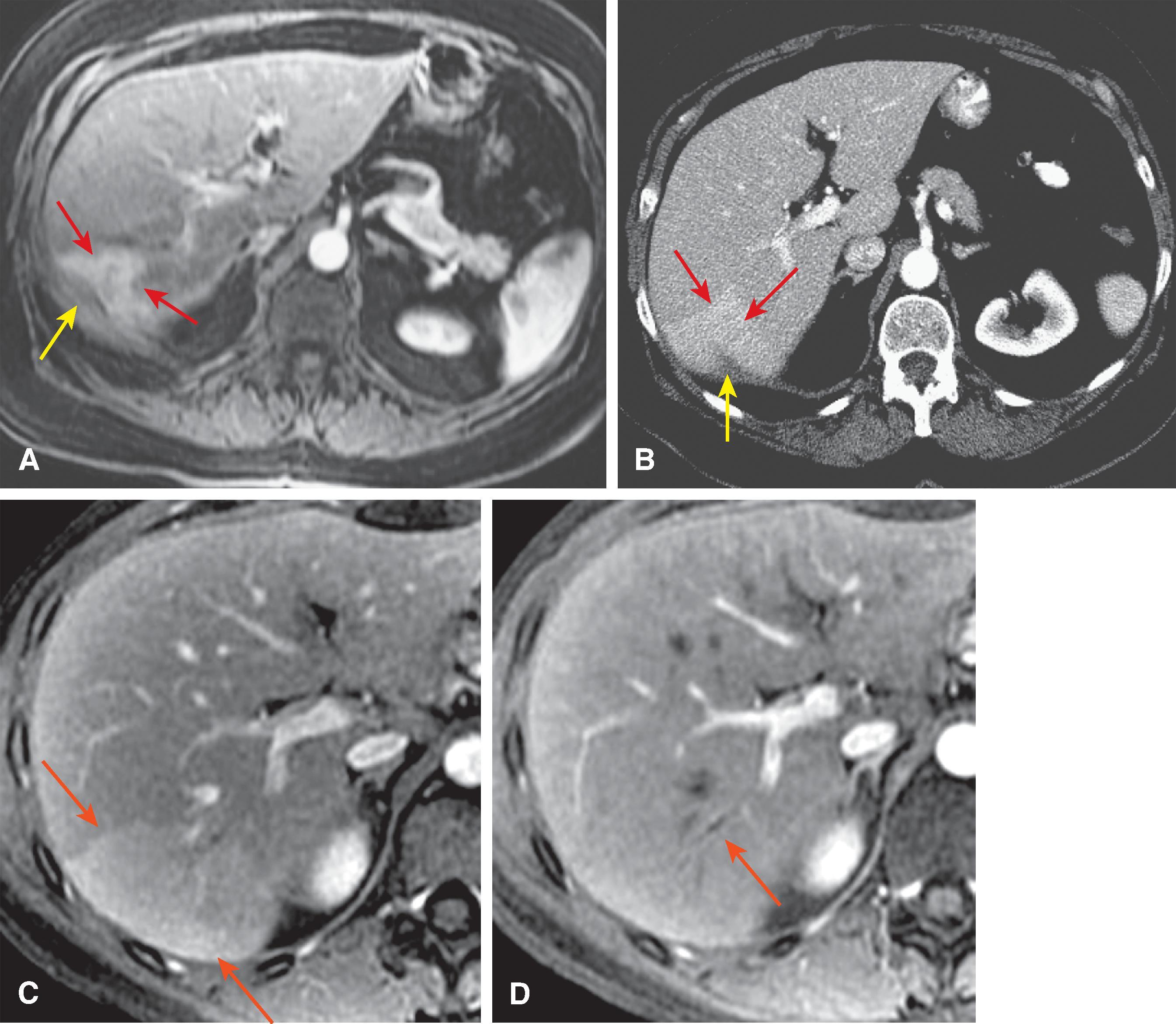
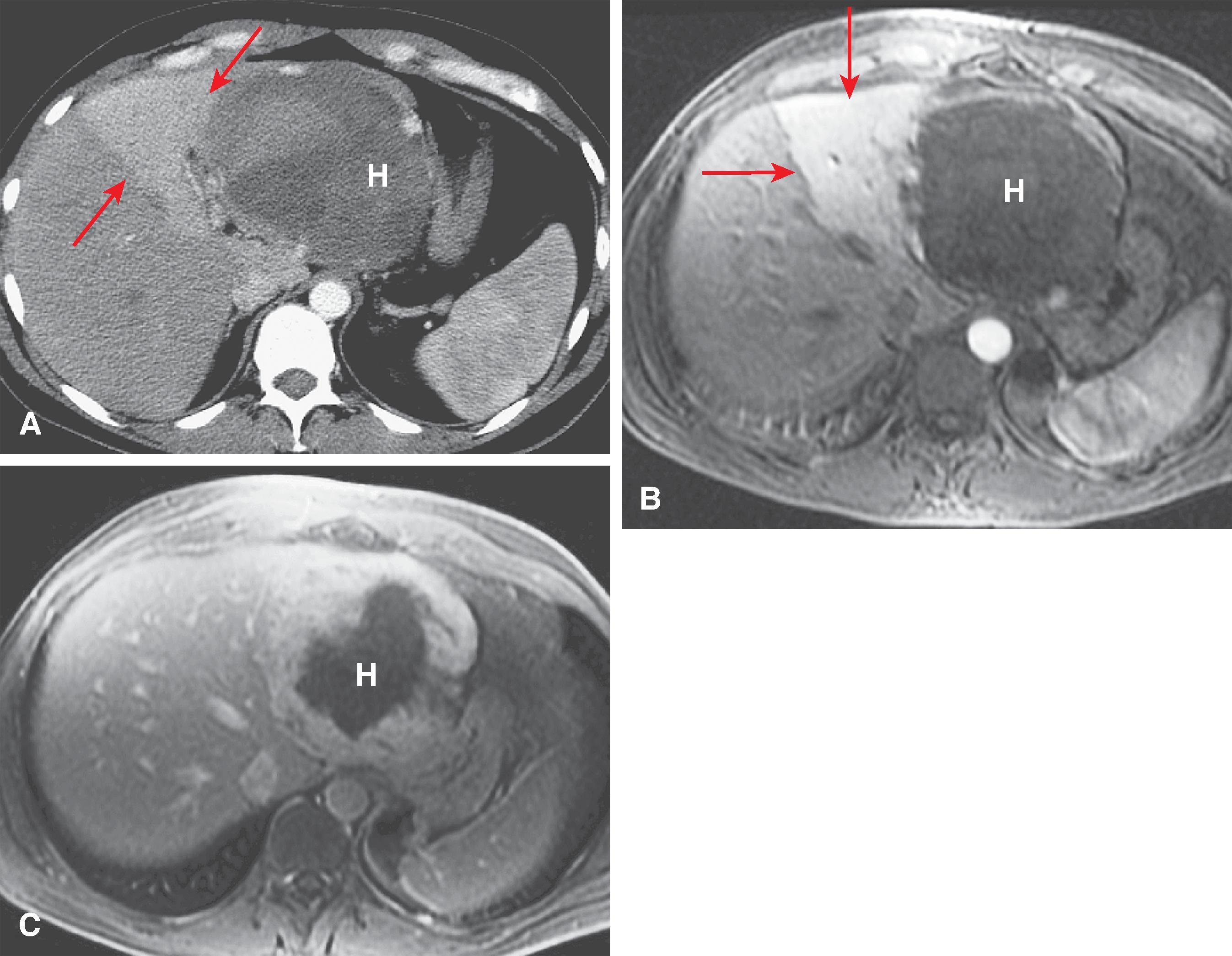
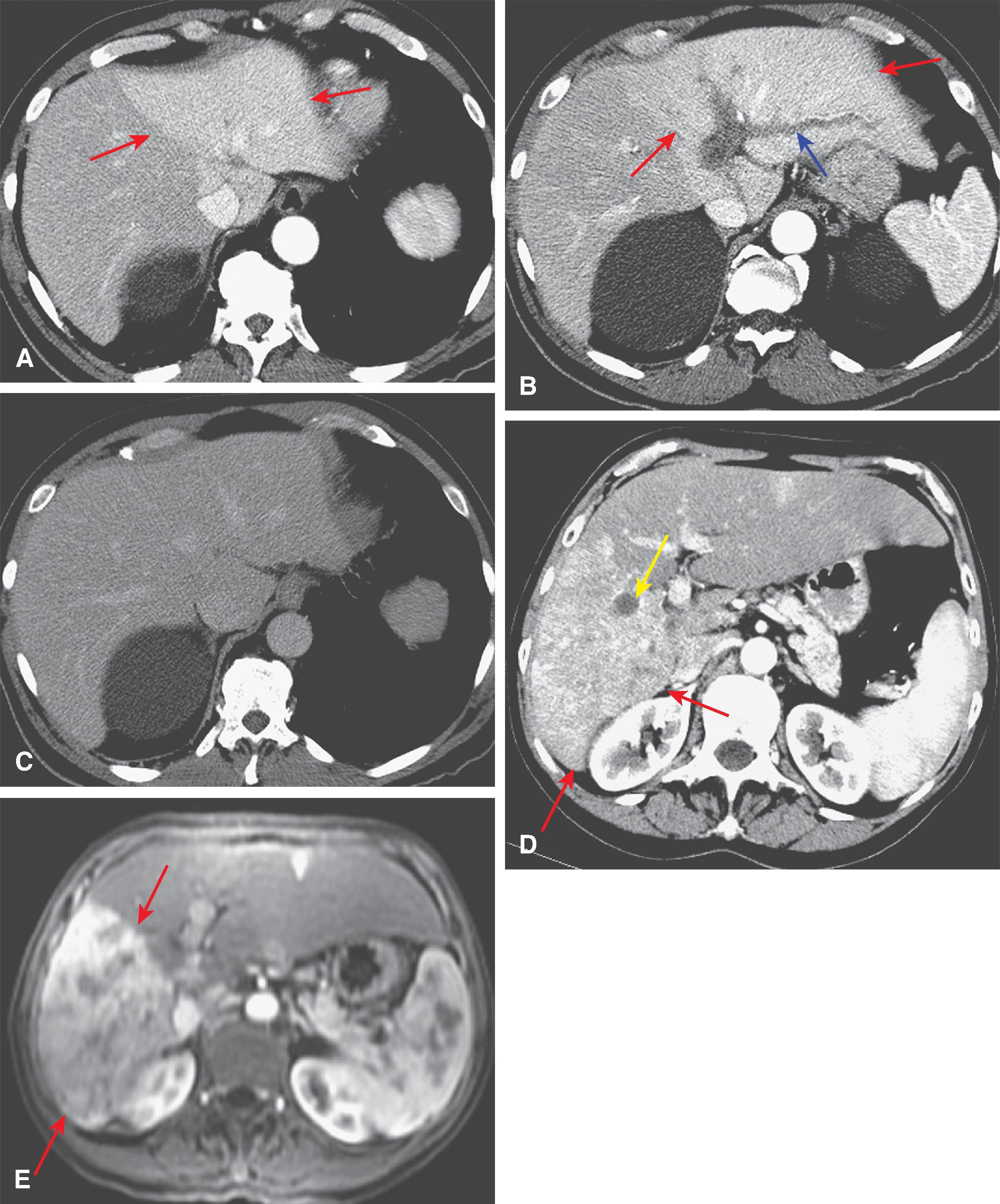
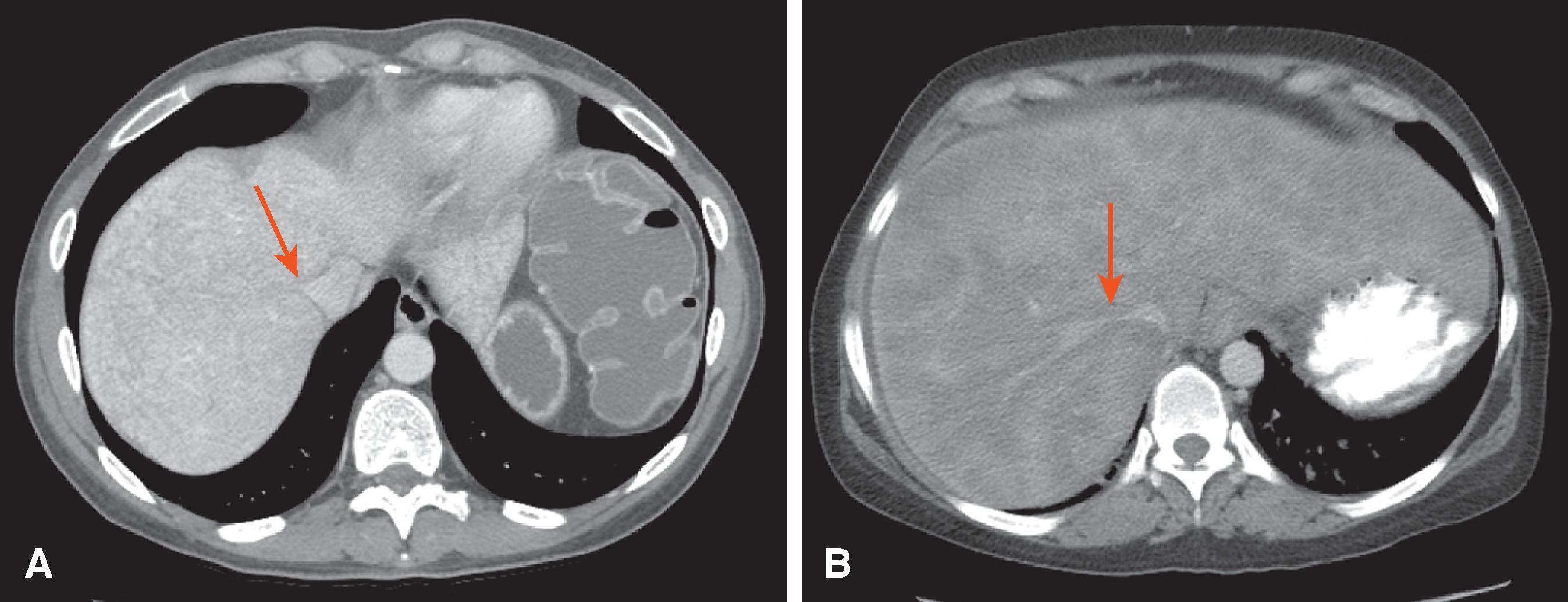
The computed tomography (CT) features of Budd-Chiari syndrome depend on the age and extent of the obstruction and the presence of coexisting changes in portal venous blood flow. , On noncontrast scans, in the acute phase of the disease, diffuse hepatic hypodensity is associated with global liver enlargement and ascites. The hepatic hypodensity is presumably due to hepatic parenchymal congestion. Hyperdense thrombus with an attenuation of 38 to 42 HU may be seen in the inferior vena cava and hepatic veins. Rarely, a calcified caval membrane is identified. After the injection of contrast material, the liver typically shows patchy enhancement; this is due to the hepatic congestion, which causes portal and sinusoidal stasis. In most patients, the central regions of the liver, including the caudate lobe and part of the left lobe, may enhance normally and appear hyperdense compared with the more peripheral parts of the liver, which show decreased enhancement. Later, a classic “flip-flop” pattern may develop ( Fig. 57.7 ), in which the contrast material from the normally enhanced central liver washes out so that this region becomes relatively hypodense compared with the peripheral zones, which are slowly accreting contrast material. The subcapsular portions of the liver may enhance normally because they have independent venous drainage through systemic subcapsular veins. These differences in hepatic attenuation and morphologic changes are closely related to regional disturbances in portal flow. In segments of the liver in which venous drainage is obstructed (the hepatic periphery), portal blood flow is diminished, if not reversed. Portal venous flow is normal in the central portion of the liver, where hepatic venous egress is uninterrupted. ,
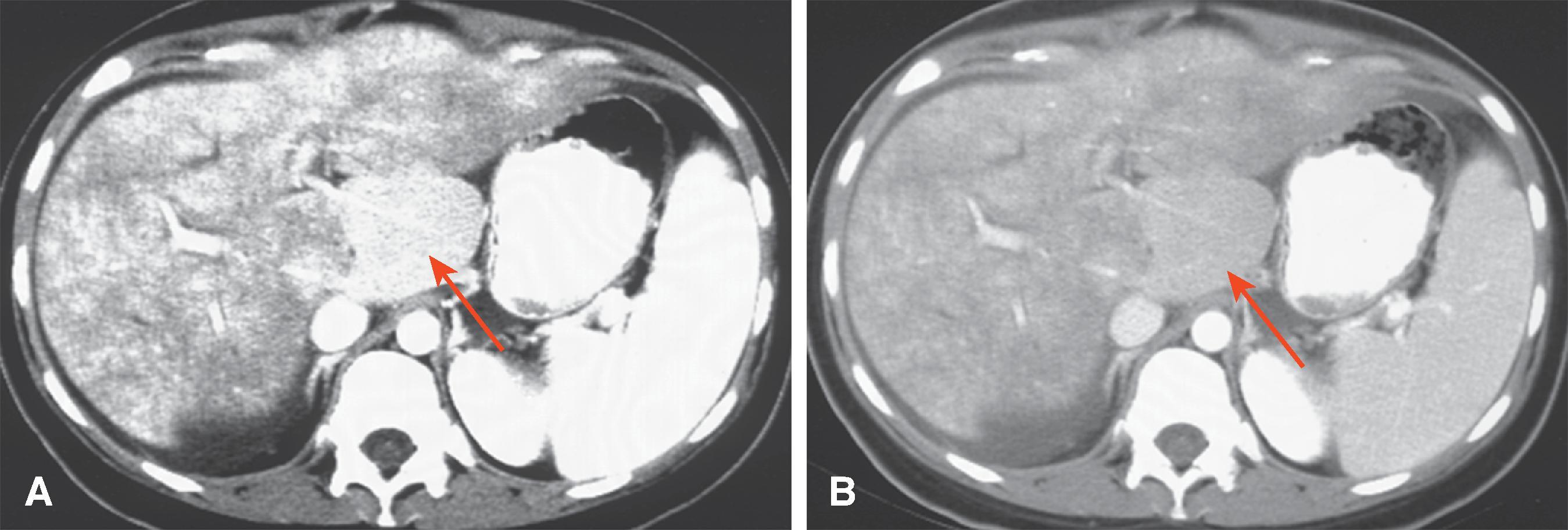
Intravascular thrombus is best seen in the acute phase of the Budd-Chiari syndrome as a low-density mass in the lumen of the hepatic veins and inferior vena cava. The wall of the thrombosed vessel may appear dense relative to the thrombus secondary to the enhancement of the vasa vasorum. The involved vessel also is often enlarged. Portal vein thrombus may be seen in 20% of cases. Compression of the inferior vena cava is also demonstrated. ,
In more chronic cases of Budd-Chiari syndrome, the thrombi and the hepatic veins are often difficult to visualize. Intravascular thrombus density, which is high for the first 3 weeks, also diminishes over time. As the obstruction becomes more chronic, the liver undergoes morphologic changes that are due to diminished or reversed portal blood flow in the involved hepatic segments. Portal venous blood carries certain hepatotropic agents, mainly insulin, and segments deprived of these agents experience nutritional ischemia and eventually atrophy. Accordingly, the caudate lobe exhibits hypertrophy, and the periphery of the liver tends to exhibit atrophy. These changes may take 2 to 4 months to appear. Unless the membranes are calcified, membranous obstruction of the inferior vena cava is more difficult to visualize.
On MDCT, large regenerative nodules robustly and homogeneously enhance during the arterial dominant phase and remain slightly hyperdense on the portal venous dominant phase images as well.
MRI is a useful screening modality for Budd-Chiari syndrome by virtue of its ability to display directly the portal veins, hepatic veins, and inferior vena cava in numerous planes. A number of specific MRI abnormalities are associated with this syndrome ( Figs. 57.8 – 57.12 ): complete absence of hepatic veins or striking attenuation of their caliber, demonstration of thrombus or absent vascular flow in hepatic veins, comma-shaped intrahepatic collateral vessels, and marked constriction of the inferior vena cava. Less specific findings include enlargement of the caudate lobe, ascites, and inhomogeneity of the hepatic parenchyma. Vascular patency can confidently be confirmed when the vessel has no signal.
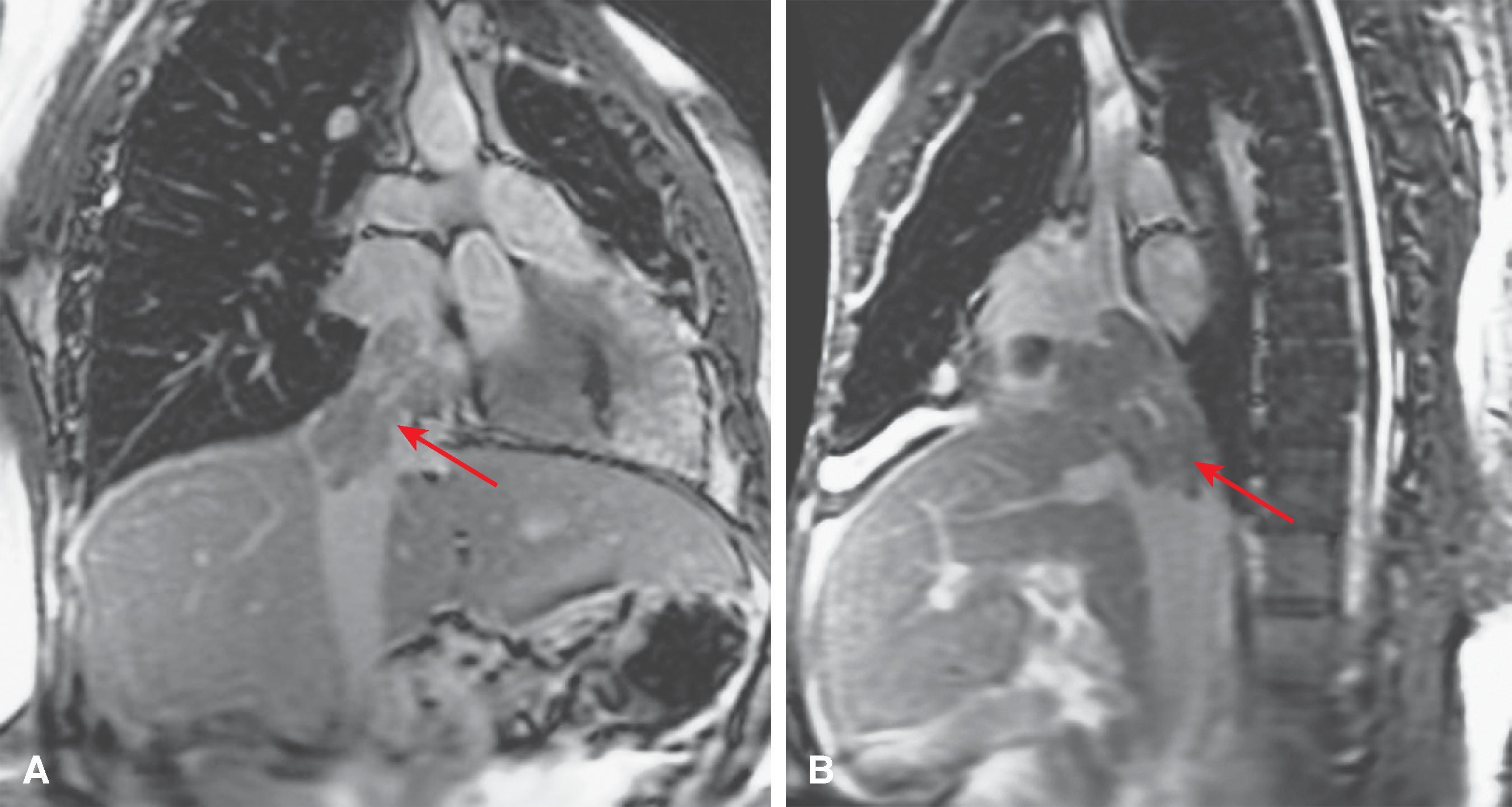
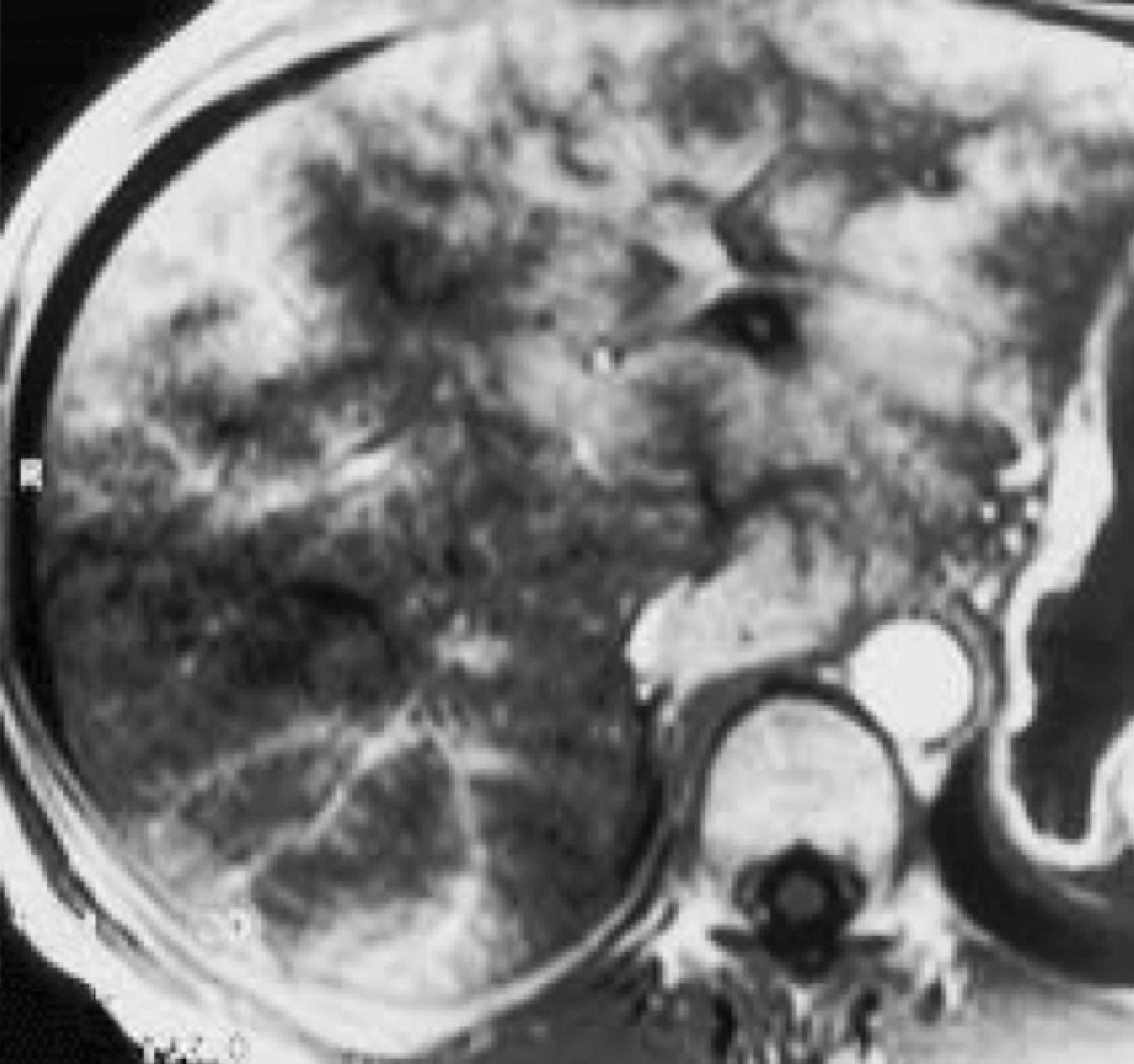
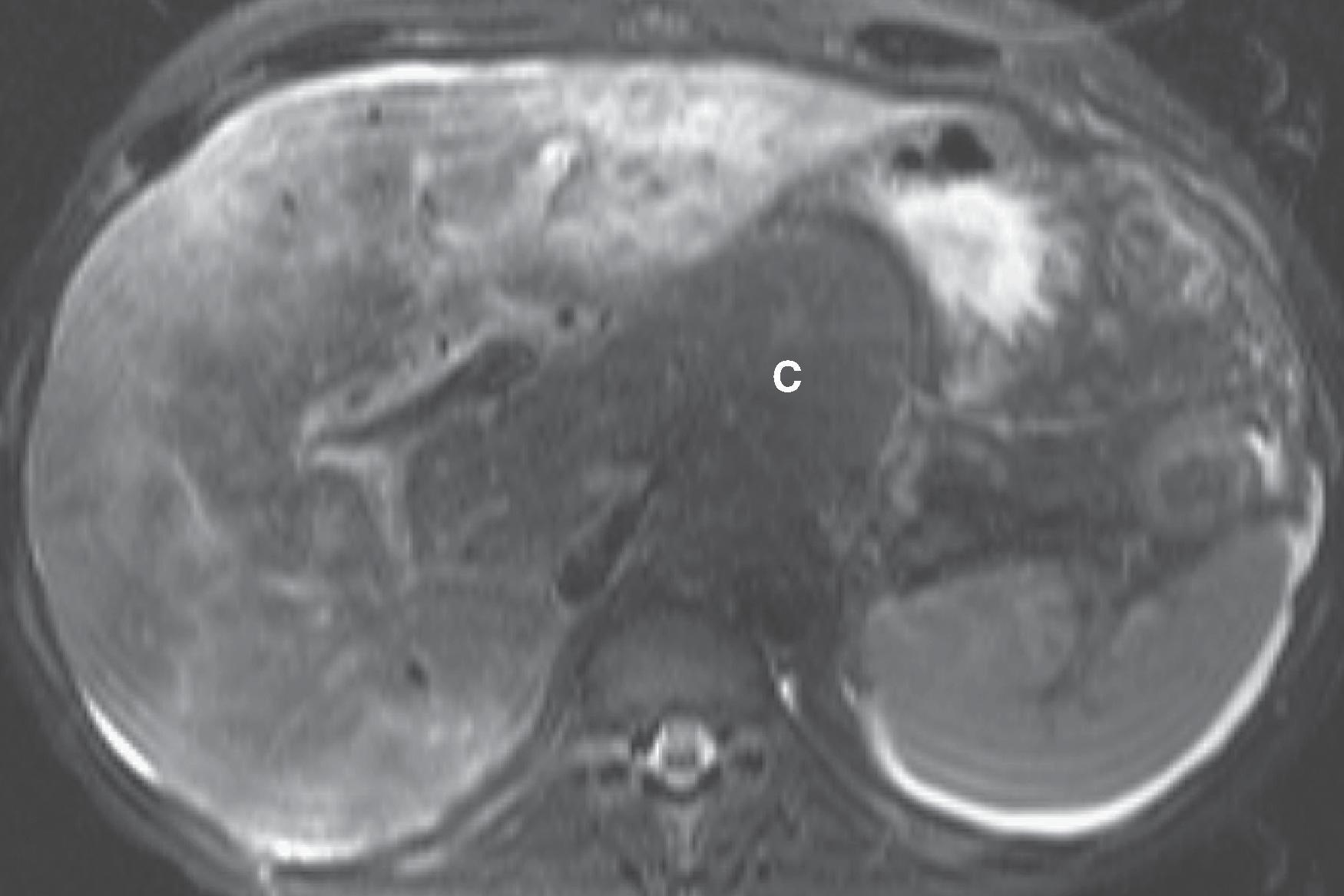
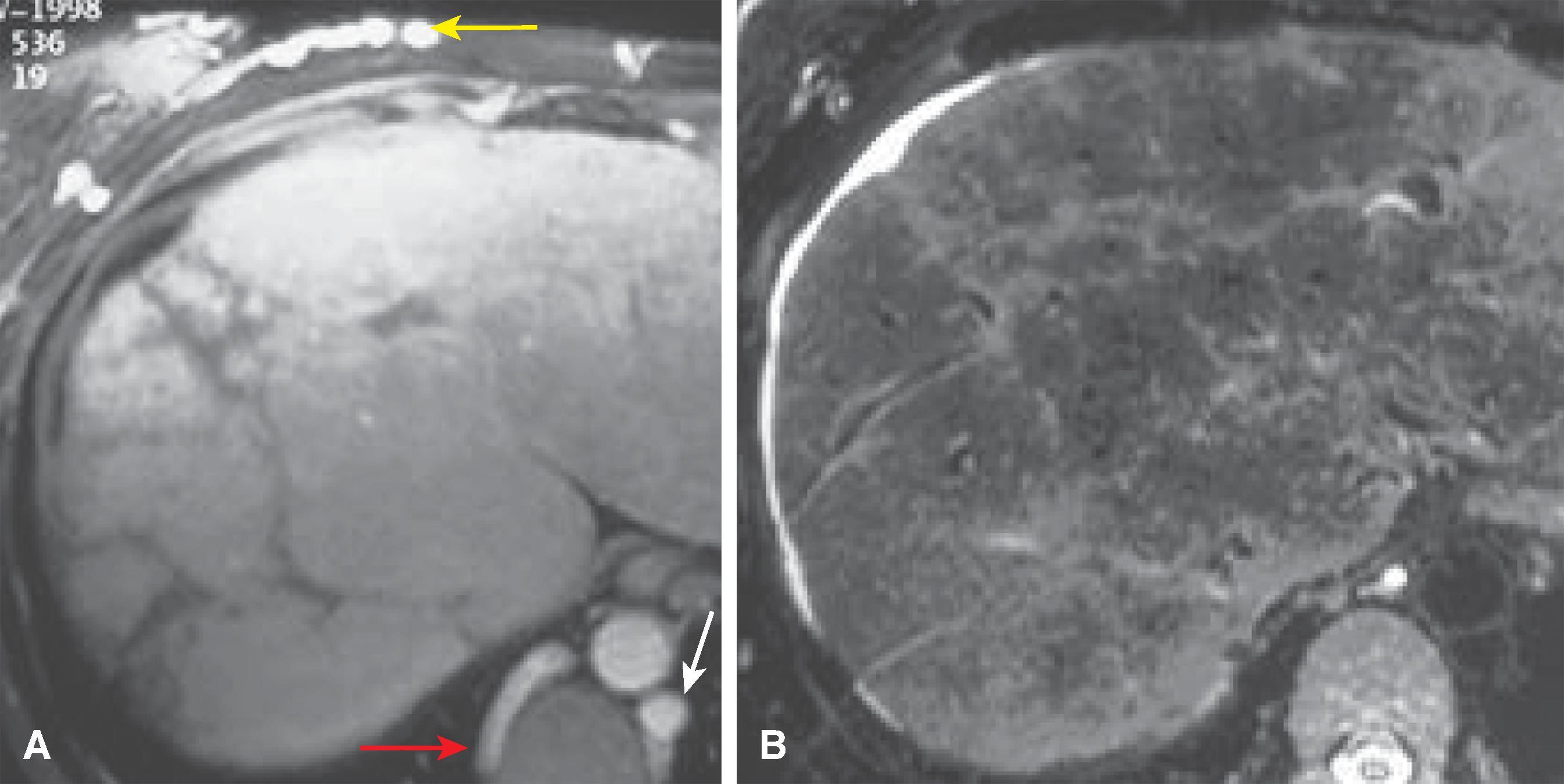
The multiplanar capabilities of MRI are of great value in depicting tumor thrombus in patients with Budd-Chiari syndrome secondary to neoplasm. Bland and tumor thrombus can be distinguished by the administration of gadolinium. Tumor thrombus may show contrast enhancement.
The degree of enhancement of the liver parenchyma supplied by a thrombosed hepatic vein depends on the acuteness and duration of the thrombosis. Involved parenchyma enhances less than surrounding liver in acute thrombosis. In chronic thrombosis, enhancement is more variable and may be increased. In patients with acute Budd-Chiari syndrome, the congested liver may have a higher water content and longer T2 relaxation time than the spared caudate lobe. Also, the peripheral liver enhances less than the central liver after intravenous administration of gadolinium because of increased parenchymal pressure with resultant diminished blood supply from both the hepatic artery and the portal vein.
Large regenerative nodules are bright on T1-weighted MR images and show the same enhancement pattern after the intravenous administration of gadopentetate dimeglumine (Gd-DTPA). They are predominantly isointense or hypointense relative to the liver on T2-weighted images.
On contrast-enhanced CT and MRI studies, multiple enhancing nodules are identified that are usually 5 to 7 mm in diameter ( Fig. 57.13 ).
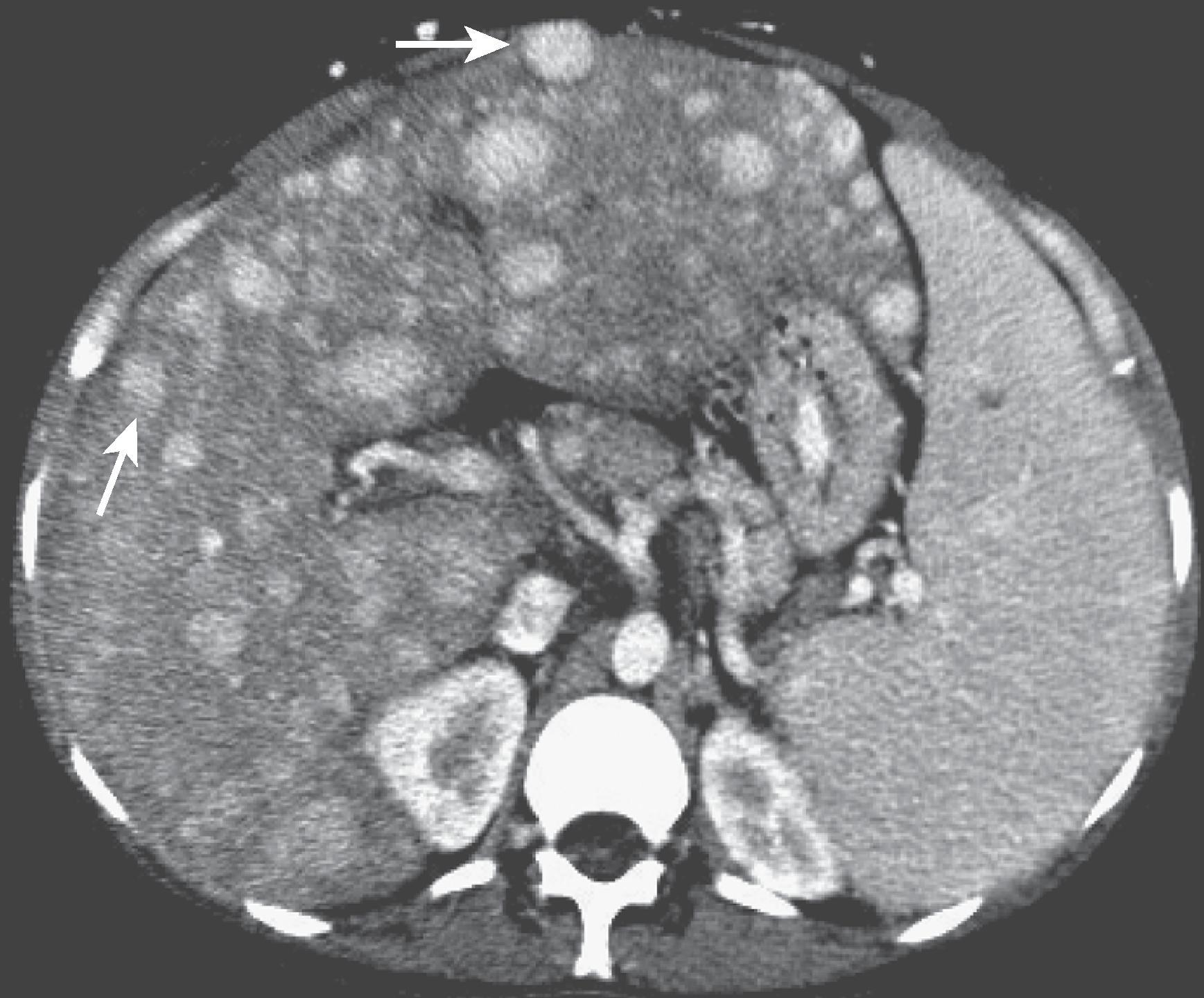
Passive hepatic congestion is caused by stasis of blood within the liver parenchyma as a result of compromise of hepatic venous drainage. It is a common complication of congestive heart failure and constrictive pericarditis, wherein elevated central venous pressure is directly transmitted from the right atrium to the hepatic veins because of their close anatomic relationship. The liver becomes tensely swollen as the hepatic sinusoids engorge and dilate with blood. These changes may be transient, and full recovery follows once the patient’s congestive heart failure is corrected. In chronic right atrial failure, cardiac cirrhosis may ensue. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here