Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An accurate and thorough understanding of the normal vascular anatomy of the spine and spinal cord is vital for the safe and appropriate performance of spinal angiography and endovascular intervention. Learning these skills is challenging because diagnostic spinal angiography is less commonly performed than cerebral angiography and is being gradually replaced by magnetic resonance angiography (MRA). In this section, we provide the reader with a concise overview of the vascular anatomy of the spinal cord and paraspinal structures as a basis for understanding spinal vascular diseases, their clinical classifications, and the approaches to treating them.
The arterial supply to the spine has a segmental distribution to the derivatives of each embryologic somite, including the paraspinal soft tissues, bone, and dura. In contrast, due to variability in the spinal cord arterial supply, the overall supply to each metamere is highly variable. The metameric arterial supply arises from a variety of parent vessels, including the vertebral arteries, aorta, iliac arteries, and others.
The spinal blood supply can be divided into a macrocirculation, which includes the vasculature of paraspinal structures up to the cord surface, and a microcirculation, which involves perforators to the cord beyond the anterior and posterior spinal arteries (ASA and PSA).
Analogous to the cerebral blood supply, the pattern of blood supply to the spinal cord is based on the pattern of its embryologic development, which occurs from the outside in at each segmental level. The segmental levels are first manifest as primitive precursors termed somites . In the first few weeks after conception, the embryo is divided into 42 to 44 somites along the rostral-caudal direction. Of these, 31 somites persist through development, corresponding to the 31 pairs of spinal nerves (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, 1 coccygeal). The derivatives of each somite and corresponding portions of the neural crest and neural tube are collectively referred to as a metamere .
The segmental arteries are numbered according to the nerve that they accompany in the neural foramen. Through their branches, they supply all the ipsilateral derivatives of their corresponding metamere: skin, muscle, bone, spinal nerve, dura, and spinal cord ( Fig. 93.1 ). In the embryonic stage of development, each segmental artery has a branch supplying the cord. By the end of fetal development, most branches have regressed, and only a few contribute significantly to spinal cord perfusion. Of the initial 62 metameric arteries (31 pairs), 4 to 8 will end up supplying the ASA (diameter, 0.2 to 0.5 mm), and 10 to 20 will supply the PSA (diameter, 0.1 to 0.4 mm). Most of the segmental arteries end up supplying the related nerves, dura, vertebral body, and paraspinous muscles. The process of regression of the spinal cord blood supply is more pronounced caudad. The dominant artery supplying the spinal cord is named the artery of Adamkiewicz (AKA).

The simplified algorithm for the vascular supply at each segmental level is: major arterial trunk (vertebral artery, aorta) → spinal/segmental artery → radiculomedullary artery (ventral radicular artery) and radiculopial artery (ventral or dorsal artery) → pial network, paired posterior or single anterior spinal arteries.
Radicular arteries originate from the following major arterial trunks :
Cervical: Vertebral arteries, ascending cervical branch of the thyrocervical trunk, deep cervical branch of the costocervical trunk, occipital and ascending pharyngeal branches of the external carotid artery
Thoracic: Branches of the costocervical trunk, internal thoracic artery of the subclavian artery, intercostal branches of the aorta
Lumbosacral-coccygeal: Lumbar branches of the aorta, median sacral artery of the aorta, lateral sacral branches of the internal iliac arteries, iliolumbar branches of the common iliac arteries
The segmental arteries form paraspinal and extradural anastomoses in the craniocaudal extension, which can be subdivided as follows :
Ventrolateral: Ascending cervical artery of the thyrocervical trunk
Pretransverse: Anterior to transverse processes, such as the vertebral or lateral sacral arteries ( Fig. 93.2 ). The latter supply the sympathetic nervous system in the thoracolumbar area.
Dorsal longitudinal: The branches to the midline insertion of the spinous process muscles, such as the deep cervical artery of the costocervical trunk
At each level, segmental arteries provide blood supply to the ventral and dorsal nerve roots through radicular arteries (see Fig. 93.1 ). Some of the radicular branches, however, supply not only the nerve root but also the spinal cord. These branches give rise to (1) the pial/coronal arterial network or the PSA and are called radiculopial arteries or (2) the anterior pial network and to the ASA and are named radiculomedullary arteries.
The dominant longitudinal blood supply to the spinal cord is provided ventrally by a single ASA and dorsally by paired PSAs. These two systems are connected via a superficial network of small pial arteries. The flow in spinal arteries can be bidirectional and depends on the size of the radiculomedullary and radiculopial arteries as well as the timing of the systolic pulse waves traveling within the aorta or vertebral arteries and being propagated into the segmental arteries at different levels.
As described earlier, radicular arteries originate from each segmental artery and supply the dorsal and ventral nerve roots after giving off branches to the paraspinous musculature, vertebral body, and dura. At the C1 level, radicular branches may be absent. Normally, radicular arteries are not seen on digital-subtracted angiograms because of the current limits of spatial resolution.
Radiculopial arteries supply the nerve roots by means of smaller branches and then run ventral to either the dorsal or the ventral nerve root to supply the pial network. Although they anastomose with pial branches of the ASA, they do not supply the ASA directly. There are more dorsal than ventral radiculopial arteries, and the dorsal radiculopial arteries are the dominant supply to the PSA. Their number varies from three to four in the cervical region, from six to nine in the thoracic region, and from zero to three in the lumbosacral region.
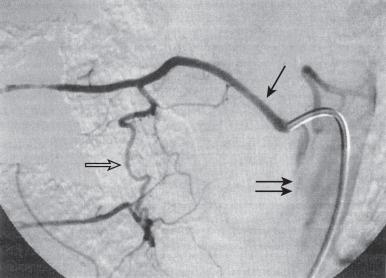
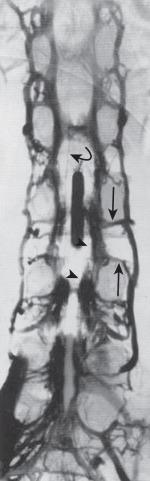
Radiculomedullary arteries are the dominant supply to the ASA. After giving off their radicular branches to the nerve roots, they run along the ventral surface of the nerve root, occasionally give off pial collateral branches, and continue to the ASA. On average, there are two to four radiculomedullary arteries in the cervical region, two to three in the thoracic region, and zero to four in the lumbosacral region. The largest radiculomedullary artery of the thoracolumbar segment is also known as the artery of Adamkiewicz (AKA; diameter, 0.55–1.2 mm). In 75% of patients, the AKA arises between T9 and T12, more commonly on the left. When its origin is above T8 or below L2, another major contributor to the ASA can be found either caudad or craniad. In 30% to 50% of cases, it also contributes significantly to the PSA. Generally, a pair of arteries arise in the cervical region from the intradural segment of each vertebral artery that fuse to one Y-shaped ASA. The typical hairpin anastomosis between the radiculomedullary arteries and the ASA is found angiographically at the lower thoracic and lumbar levels ( Fig. 93.3 ). There is only one ASA, which continues from its Y-shaped origin to the artery of the terminal filum (diameter, 0.5–0.8 mm). The ASA is located in the subpial space in the ventral sulcus of the spinal cord, dorsal to the vein; it may be partly absent or not visible angiographically, especially at the thoracic level. Because of a lack of fusion during embryologic development, a short nonfused cervical segment may be present.
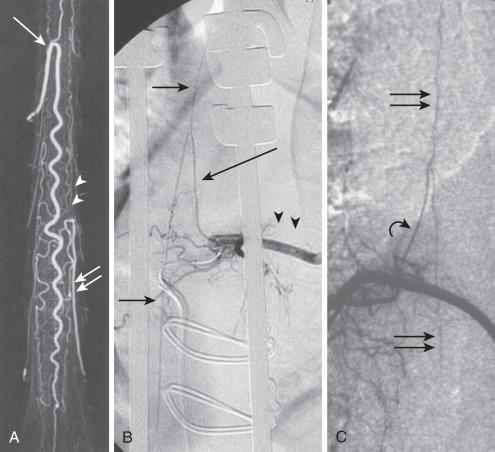
Identifying these small normal spinal arterial vessels has been a significant technical challenge in the development of noninvasive spinal imaging. Recent studies using newer techniques for contrast-enhanced MRA have shown success rates of 82.4% to 100% for detection of the ASA. Identification of the ASA remains a challenge. Sheehy et al. reported detection rates of 96% (48 of 50 patients) for the cervical segment of the ASA. Other studies of contrast-enhanced MRA at higher field strength have successfully depicted abnormally dilated ASAs in the setting of spinal vascular malformations, but reliable detection of the normal thoracolumbar ASA remains elusive.
The circulation distal to the subpial ASA, the pial network, and the PSA can be divided into centrifugal (from the center of the cord out toward the surface) and centripetal (from the cord surface toward the center of the cord) systems. The centrifugal system, also known as the sulcocommissural system, consists of 200 to 400 sulcocommissural arteries, which are located within the ventral sulcus of the spinal cord and originate from the ASA ( Fig. 93.4 ). These arteries penetrate the sulcus similarly to brain perforators and enter the central gray matter, where they branch into radially oriented small arteries that run toward the white matter. Each sulcocommissural artery usually supplies one-half of the cord. The sulcocommissural system supplies most of the spinal cord gray matter and the ventral half of the white matter. Before entering the cord substance, each sulcocommissural artery anastomoses craniad and caudad with neighboring sulcocommissural arteries (see Fig. 93.4 ). Complex, longitudinally oriented anastomoses are also seen within the white and gray matter. Whereas the sulcocommissural arteries initially run horizontally, they take an ascending course with the growth and disproportionate elongation of the spinal column. The spinal cord territory supplied by the ASA versus the PSAs is comparatively as large as the proportion of a cerebral hemisphere supplied by the internal carotid arteries versus the vertebrobasilar system. An occlusion of the sulcocommissural artery at the lumbar segment in primates can cause severe damage to the ventrolateral two-thirds of the cord at the occluded and adjacent levels.
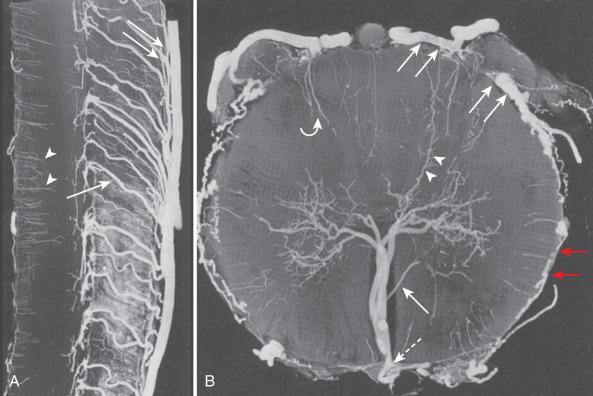
The centripetal system includes a pial network and the PSAs ( Fig. 93.5 ). At the craniocervical junction, this system receives its blood supply directly from the intradural vertebral arteries or from the posterior inferior cerebellar arteries near their origins. At all other levels, radiculopial arteries provide the blood supply to the centripetal system. Radial branches of the pial network and the PSAs extend around the circumference of the cord and anastomose with the ASA. The radial arteries and the PSAs give off perforating branches to the cord all along their courses. These short perforating branches enter the white matter in a radial fashion and extend to a portion of the gray matter. Intramedullary anastomoses with branches of the sulcocommissural arteries exist. Pial anastomoses also exist in the longitudinal axis between the anterior and posterior vascular system. These anastomoses, however, are relatively small and cannot provide adequate craniocaudal supply to the anterior spinal cord in the case of ASA occlusion. Dependent on the location and size of a pial arteriovenous fistula (anterior or posterior) or a medullary (within the spinal cord) arteriovenous malformation (AVM), the blood supply can originate from the ventral and/or the dorsal vasculature and, therefore, from the centrifugal and/or centripetal system.
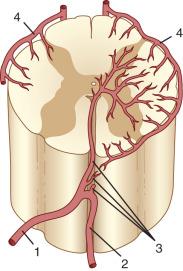
The segmental artery is centered at the level of the intervertebral disc and the corresponding nerve ( Fig. 93.6 ). It gives rise to an ascending somatic branch and a descending somatic branch. Each vertebral body is supplied by the descending somatic branches of the segmental arteries above and the ascending branches of the segmental arteries below. Because of extensive anastomoses, an injection in any one of these four segmental arteries may enhance the entire vertebral body. On angiograms in frontal projection, the arterial network on the posterior surface of the vertebral body has a characteristic hexagonal shape. Tumor blush or vascular bone metastases should not be confused with the normal angiographic blush of the vertebral body.

After passing through the capillary network, blood is transported from the deepest parts of the spinal cord toward the surface ( Fig. 93.7 ). Venous drainage of the cord can be divided into an intrinsic system, which runs in proximity to the centrifugal arterial system, and an extrinsic system, which runs in proximity to the centripetal arterial system. Unlike in the arterial blood supply, there is no dominance of the dorsal or ventral venous return. Dorsal and ventral sulcocommissural veins, which collect the venous outflow from the central gray matter, are a part of the intrinsic venous system. The venous perforators draining into the radial veins, which drain into the dorsal and ventral longitudinal collecting veins, belong to the extrinsic system. These longitudinal collecting veins finally drain into the radicular veins that run along the nerve roots and empty into the ventral epidural venous plexus. In addition to the main dorsal and ventral draining veins, there are short intersegmental lateral longitudinal veins linking adjacent radial veins. However, these lateral longitudinal venous channels are not large enough to form a functional dominant craniocaudal drainage, as do the dorsal and ventral systems.
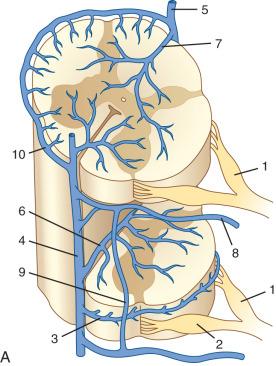
The main ventral longitudinal venous channel is known as the anterior median vein. Flow in the thoracic longitudinal channels is bidirectional. The most cranial portion drains into the cervical region and, from there, partly via the anterior medullary vein into the median anterior pontine vein and the transverse pontine vein and, finally, into the superior petrosal sinus. The most caudal part drains into the lumbar region. There can be multiple longitudinal venous channels, especially in the thoracic region and ventrally.
The radicular (radiculomedullary) veins drain either into spinal nerve venous channels in the neural foramina or into a dural venous pool. Both venous systems eventually empty into the ventral epidural venous plexus ( Fig. 93.8 ). The epidural venous system has a prominent ventral and a smaller dorsal component. The ventral epidural veins receive venous drainage from the vertebral bodies through anterior and posterior venules, the spinal cord via radiculomedullary veins, and the dura. They are also involved in some cerebrospinal fluid resorption via arachnoid granulations along the nerve root sleeves. The ventral epidural venous plexus forms a valveless, retrocorporeal hexagonal anastomotic plexus, which is continuous longitudinally. The direction of flow within this plexus is multidirectional and depends on the location of the contributing veins at each anatomic level.
The cervical portion of the plexus drains into the vertebral veins, which finally empty into the innominate veins. At the thoracic level, blood drains into the intercostal veins, which empty into the azygous and hemiazygous systems and subsequently the superior vena cava. In the lumbar segments, venous drainage involves the ascending lumbar vein on the left, the azygous and hemiazygous systems, and the left renal vein. At the sacral and coccygeal level, blood empties into sacral veins, the lateral sacral veins, and subsequently into both internal iliac veins.
Various classifications have been suggested over the past several decades for spinal vascular shunts (SVSs) based on angiographic features, pathophysiology, and neuroanatomy. However, these classifications vary, may create confusion, and can be of little value for the endovascular approach to treating most vascular abnormalities. To facilitate the understanding of spinal vascular shunts, we will address these lesions according to their location with respect to the spinal cord, including the paraspinal soft tissue ( Table 93.1 ). Vascular lesions consisting of a single direct connection between the feeding artery and the draining vein are known as arteriovenous fistulas (AVFs) or arteriovenous shunts (AVSs). However, when multiple connections are present at the precapillary level, they are called arteriovenous malformations (AVMs). The core of an AVM that appears angiographically and anatomically as a conglomeration of vessels, because of the superimposition of connections between arteries and veins and lack of spatial resolution, is defined as the nidus. Most of the spinal vascular malformations are congenital, may grow over time, and become symptomatic only in adulthood. In this chapter, we focus on AVSs, AVMs, spinal artery aneurysms, neoplastic vascular lesions, aneurysmal bone cysts, vertebral hemangiomas, and vascular metastatic disease.
| Anatomic Classification | Etiology | Pathophysiology | Clinical Presentation |
|---|---|---|---|
| Vascular Shunts | |||
| Paraspinal AVS (M): e.g., traumatic vertebrovertebral AVF | Congenital/acquired | Cord compression (enlarged veins) | Progressive myelopathy |
| Epidural AVS (M) | Acquired | Cord compression (enlarged veins), vascular steal, venous congestion | Progressive myelopathy |
| Dural AVS (F): single (type IA), multiple (type IB) | Acquired | Venous hypertension/cord compression; hemorrhage is rare | Progressive myelopathy, pain |
| Pial AVS (F): low flow, moderate, high flow (type IVA, IVB, and IVC) | Congenital | Cord compression (enlarged veins), hemorrhage, vascular steal, venous hypertension, venous varix | Progressive myelopathy |
| Extradural-intradural (metameric, juvenile) AVM (type III) | Congenital | Cord compression, hemorrhage, vascular steal | Pain, progressive myelopathy |
| Intramedullary AVM (type II) | Congenital | Intramedullary hemorrhage, subarachnoid hemorrhage, cord compression, vascular steal | Acute and progressive myelopathy, pain |
| Neoplastic Vascular Lesions | |||
| Cavernous angioma | Congenital | Cord compression (mass effect), repetitive bleeding | Acute and progressive myelopathy |
| Hemangioblastoma | Congenital/acquired | Asymptomatic, cord compression | Asymptomatic, myelopathy |
| Syndromes With Spinal AVM | |||
| Osler-Weber-Rendu syndrome | Hereditary (autosomal dominant) | Venous hypertension, mass effect, vascular steal, subarachnoid hemorrhage | Myelopathy, radiculopathy |
| Cobb syndrome | Congenital | Cord compression, hemorrhage, vascular steal | Pain, progressive myelopathy |
| Klippel-Trenaunay (KT) and Parkes-Weber (PW) syndromes | Congenital | Cord compression (enlarged veins, hemorrhage, vascular steal) | Progressive myelopathy |
| Vertebral Vascular Lesions | |||
| Aneurysmal bone cysts | Congenital/acquired | Hemodynamic imbalance, cord compression | Pain, progressive myelopathy |
| Vertebral hemangiomas | Incidence increases with age | Asymptomatic, cord compression | Pain, myelopathy, radiculopathy |
| Miscellaneous | |||
| Spinal artery aneurysms | Congenital/acquired | Subarachnoid hemorrhage | Subarachnoid hemorrhage symptoms |
| Hypervascular metastatic lesions | Acquired | Pathologic fractures, cord compression | Acute and progressive myelopathy |
Magnetic resonance imaging (MRI) is noninvasive, does not expose the patient to ionizing radiation, and should be the primary diagnostic tool in the evaluation of spinal vascular disease. MRI is able to delineate the spinal cord and paraspinal structures; the flow voids within vascular malformations; and the presence of edema, hemorrhage, venous congestion, and other associated processes. For patients without a known or presumptive diagnosis of spinal vascular disease, MRI remains the initial imaging modality of choice due to its ability to depict the broad range of vascular and nonvascular spinal diseases that may be the cause of a patient's neurologic symptoms. For instance, a recent study by Germans et al. found that MRI may be of utility in identifying spinal vascular malformations in patients with cerebral angiogram–negative subarachnoid hemorrhage. While extremely sensitive in detecting signal abnormalities, particularly in the T2-weighted sequences, in the spinal cord and spinal canal, a differential diagnosis for spinal cord lesions seen on MRI must always be considered. Spinal AVSs can be strongly suggested on MRI; however, conventional digital subtraction angiography (DSA) remains the gold standard for evaluation of spinal vascular diseases and is necessary for visualizing the detailed anatomy and architecture of SVSs, including arterial feeders and venous return. Angioarchitectural changes characterized on angiography may suggest aging of the lesion. Angiography will also give an estimate of the blood flow velocity within a malformation and help to guide an endovascular intervention. The addition of three-dimensional rotational angiography (3DRA) allows for better delineation of substructures of spinal AVSs, such as associated arterial or venous aneurysms, and their relationship to the shunt. It is particularly useful in delineating intramedullary from perimedullary shunts/malformations and in visualizing nidal and venous aneurysms. 3DRA helps to assess feeding arteries for planned endovascular procedures, although reduced spatial resolution and limited temporal resolution limit its value for high-flow lesions. Superselective 3DRA may overcome some of these limitations ( Fig. 93.9 ).
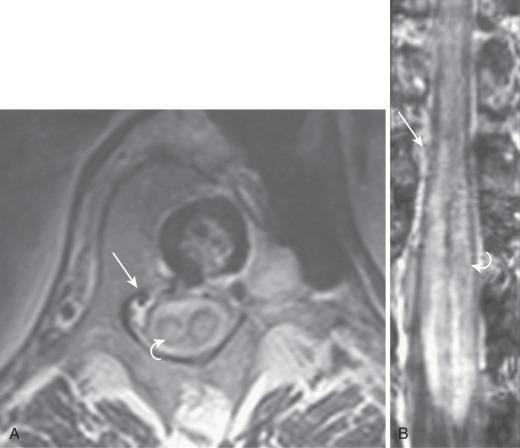
Noninvasive imaging of SVSs was initially attempted using phase contrast and time-of-flight MRI techniques. Later studies with first-pass gadolinium-enhanced MRA and computed tomography angiography (CTA) show a better definition of the arterial and venous systems of the shunt. In the most recent of these studies, Mull et al. were able to reliably distinguish between spinal dural AVF and spinal AVM in addition to identifying a large proportion of the clinically relevant vascular anatomy in each case. MRA not only allows for a proper pretherapeutic overview of the regional and lesional anatomy that will serve as a roadmap for treatment but also serves as posttherapeutic examination following endovascular treatment. Development of time-resolved spinal MR angiographic techniques with a temporal resolution of 3 to 6 seconds and spatial resolution of about 1 to 3 mm seem to provide sufficient temporal and spatial resolution to identify, localize, and follow patients suspected of having an SVS. Further improvement in spatial and temporal resolutions will allow MRA to become the premier diagnostic modality for spinal vascular disease.
With the advancement of multidetector technology, CTA has recently become a reasonable imaging modality for those patients for whom MRI is not an option, such as those with indwelling ferromagnetic material, unable to tolerate long imaging times, or for whom MRI is not available. CTA may offer several important advantages over MRA. Higher spatial resolution allows for better visualization of submillimeter-sized vessels. Several studies have demonstrated feasibility of imaging the AKA using CTA, even in children. Unlike MRI, which usually relies on suppressing surrounding tissues to optimize small vessel imaging, CTA can also be useful in the co-visualization of anatomic structures (spinal cord, bones) to improve localization of vascular lesions.
Ongoing investigations using 320-detector-row CT scanners and more recent techniques, such as four-dimensional CT (4D-CT), offer new diagnostic methods for assessing AVS in real time. In a recent study, 4D-CT was able to detect all AVM lesions previously detected by conventional DSA, including their size, location, feeding arteries, and draining veins. However, other studies suggest that for the localization of spinal dural AVFs, 3.0 Tesla dynamic contrast-enhanced MRA may be more reliable than multidetector CTA.
In addition, exposure to ionizing radiation and the need for nephrotoxic iodinated contrast agents limit widespread application of CTA for the assessment of spinal vascular disease in the clinical setting.
In summary, advanced multidetector CT imaging and 3.0 Tesla contrast-enhanced MRA are considered, safe, rapid, and noninvasive modalities that have the potential to clearly show the extension of SVSs, feeding arteries, and fistulas. Studies have also shown the sensitivity of MRA for depicting dural AVF; defining the level of the blood supply indirectly via enlarged draining veins will help to focus and reduce the time that catheter angiography takes.
Unlike what is conventionally believed, with modern catheter techniques and when performed by a trained physician, spinal diagnostic angiography should not bear a higher rate of complications than a diagnostic angiography of the peripheral system. Infrequently, minor asymptomatic iliac or aortic dissections may be encountered in elderly patients with significant atherosclerotic disease. Frequently, angiography is used prior to a planned surgery to locate the AKA (or radicularis magna) as the major supply to the anterior spinal cord. If a vascular lesion, especially a dural AVF, is suspected, a more thorough angiography may be required. This includes angiograms of the aortic arch, descending aorta, abdominal aorta, and pelvic system. In cases of brain AVS with drainage into the spine or cervical spinal cord AVM/AVS, the vertebral arteries, thyrocervical trunk, and deep ascending cervical arteries are also studied.
If an intervention is planned, a 5-Fr or a 6-Fr guide catheter is preferred for the coaxial microcatheter placement; if the region of interest is located higher, a long femoral sheath bypasses the often tortuous aortic/iliac system. Infrequently, the guide catheter may need to be changed over an exchange wire for a stable position within the intercostal or lumbar artery. Because it is easier and less traumatic to straighten the proximal part of the segmental arteries, we prefer hydrophilic-coated exchange glide wires. A range of microcatheters, including flow-guided catheters and microwires, are available for the interventional procedures. The selection has to be tailored to the size of the vessel and the embolic material used. For diagnostic purposes, heparin is not given. For interventional procedures, heparin may be given, although rarely, to prevent inadvertent thrombosis, especially if catheters are navigated within the spinal cord vasculature. In select cases of high-flow AVSs with blood supplied from ASA or PSA, patients are administered aspirin and/or clopidogrel (Plavix) after the embolization to prevent a retrograde thrombosis of the ASA after reduction of the AVS.
Paraspinal AVSs/AVMs are rare lesions presenting with a female preponderance. They are mostly found at the thoracic or cervical level, present frequently as a fistula, and drain in enormous ectatic veins located outside the spine ( Fig. 93.10 ). The patient can present with progressive neurologic symptoms and an audible bruit. The pulsatile venous ectasia can erode the bone, enlarge the neuroforamina, invaginate into the spinal canal, and directly compress the cord, thus mimicking an extradural tumor. If paraspinal veins communicate with intradural radicular veins and the perimedullary venous plexus, the pathologic venous drainage can create venous engorgement with congestive myelopathy. Paraspinal vertebrojugular fistulas can be traumatic in origin, commonly seen after motor vehicle accidents, or iatrogenic, after placement of transjugular central lines.

MRI shows a serpiginous flow void signal corresponding to feeding artery and/or large draining veins, located outside the spine. Spinal angiography shows the supplying artery, which is usually a branch of an intercostal artery at the thoracic level or a branch from the vertebral artery when the AVM is at the cervical level. Paraspinal shunts may drain into paravertebral, epidural, or intradural venous systems. In the case of a high-flow AVM(F), distal flow within the parent artery (e.g., vertebral artery) may be absent owing to the presence of shunt and steal from the contralateral vertebral artery or cervical branches. In traumatic cases, the vertebral artery can be involved directly.
The fistulas are occluded either by placement of a covered stent within the artery or by embolization of the AVF with detachable balloons or coils, acrylate injection, Onyx, or a combination of these materials (see Fig. 93.10 ).
Also known as spinal extradural arteriovenous fistula (SEDAVF), these are rare vascular shunts anatomically distinct from the typical spinal dural arteriovenous fistula. SEDAVFs are typically supplied by radicular branches with a fistulous connection to the spinal ventral epidural (also termed extradural) venous plexus and are usually slow-flow lesions. They can be a source of neurologic morbidity as a result of venous engorgement and mass effect or venous hypertension due to recruitment of intradural perimedullary veins. Cases of subarachnoid hemorrhage (SAH) as a presentation of these lesions have also been described. Cervical SEDAVFs affect younger patients and are associated with neurofibromatosis (NF-1) and SAH. Lumbosacral lesions affect an older patient population with a male sex predilection; these patients tend to present with generally worse neurologic deficits, likely due to their tendency to have intradural venous drainage. SEDAVFs are described in two distinct subtypes: extradural AVFs with retrograde parenchymal drainage (type A) and pure extradural AVFs (type B). Lesions that drain primarily into the ventral epidural venous plexus and secondarily into the intradural/medullary venous system have been reported. Most of the reported cases are sacral, with arterial supply from the lateral sacral arteries.
Using conventional MRI, it is difficult to distinguish dural from epidural shunts. The diagnosis is determined when MRA identifies the draining veins as serpentine, linear, or curved structures around the surface of the cord. Intravenous injection of gadolinium–diethylenetriamine pentaacetic acid (Gd-DTPA) enhances the dilated veins and allows a better delineation.
The ideal treatment of a type A SEDAVF is obliteration of shunts leading to the successive disappearance of the epidural venous lake as well as extradural and intradural drainers. Endovascular treatments include transvenous and transarterial embolization. Transvenous embolization may achieve a dramatic and definitive cure for this subtype of SEDAVF by means of occluding the fistula located on the venous site through epidural venous lake. One limitation, however, is the technical challenges associated with such access. The endovascular treatment of these shunts consists of a supraselective infusion of acrylate or Onyx with obliteration of the most proximal part of the draining vein and the feeding artery or a surgical obliteration of the arteriovenous connection.
Although considered an alternative option, transarterial embolization is limited, as SEDAVFs usually have more feeders than a classical spinal dural AVF. Transarterial embolization risks incomplete obliteration of the draining veins, which could lead to further recruitment of feeders with recanalization of the lesion.
Microsurgical obliteration with sole drainer occlusion can lead to complete obliteration of the epidural venous lake and provide satisfactory outcomes in cases of a type A SEDAVF with a single draining vein. When facing a case with multiple intradural draining veins or recurrent SEDAVF, complete shunt occlusion by coagulating the epidural venous lake or a multimodal approach with endovascular procedures may be a feasible option.
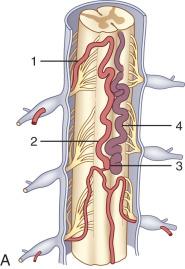
Also known as dorsal intradural AVF or type I spinal AVM, this type represents the most common of spinal vascular lesions, accounting for about 70% of all arteriovenous shunts of the spine, and should be in the differential diagnosis in an adult presenting with gradually worsening myelopathy. Most authors have classified this lesion as type A if fed by a single arterial feeder and type B if fed by two or more feeders. The most common location for these malformations is between T4 and L3, with the peak incidence occurring between T7 and T12. Although rare, possibly owing to the helpful effect of gravity on venous drainage above the level of the right atrium, dural shunts may occur above the level of the heart. When present, cervical spinal dural AVSs, particularly high cervical shunts, have unique angioarchitectural characteristics different from those seen in the other spinal regions. Cervical spinal dural AVSs, similar to other locations, seem to be more prevalent in men (male/female ratio, ~ 2 : 1), with an average age in the mid-fifties, and with predominant arterial supply arising from the vertebral artery. Hemorrhagic presentation of a thoracolumbar dural AVS is considered extremely rare. However, previous reports have shown that cervical spinal dural AVSs may be more frequently associated with SAH. It is also important to emphasize that spinal AVSs at any level can manifest with congestive myelopathy of the conus; thus, a complete spinal angiogram is necessary. Spinal AVSs also may rarely be located in the sacral region and have a higher tendency of recurrence compared to their thoracolumbar counterparts.
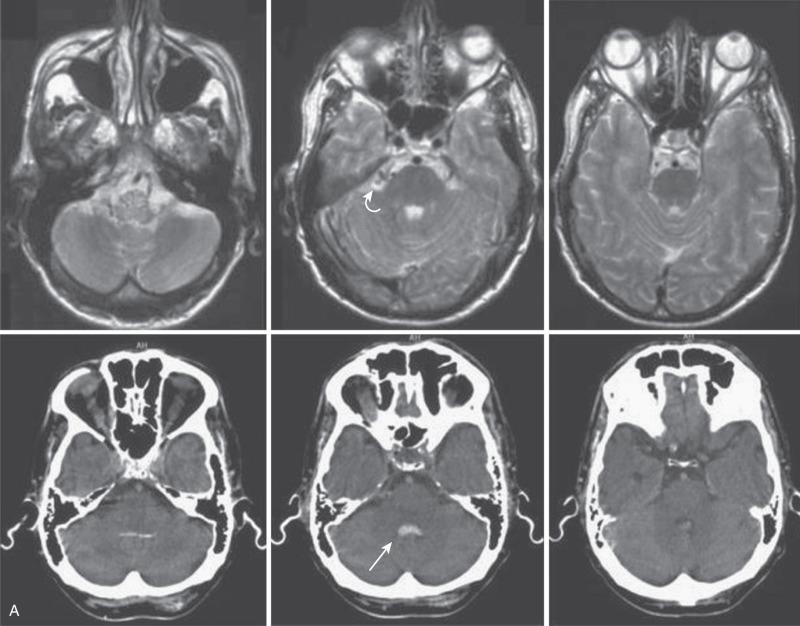
In general, spinal dural AVSs are composed of tiny arterial connections between the dural branch of a radicular artery (only rarely of a radiculomedullary artery) at the level of the proximal nerve root and a radiculomedullary vein ( Fig. 93.11 ). Branches of adjacent radicular arteries may be involved in blood supply because of an extensive intradural collateral network. The arterialized radiculomedullary vein then transmits the increased flow and pressure to the valveless coronal venous plexus and longitudinal spinal veins. Subsequently, the radiculomedullary vein is enlarged and tortuous. The mean intraluminal venous pressure is increased to 74% of the systemic arterial pressure. The normal venous pressure in the coronal venous plexus is 23 mm Hg and approximately twice that of the epidural venous plexus, which is necessary for venous drainage. In one series, the mean venous pressure in the coronal venous plexus was measured at 40 mm Hg. Because the venous hypertension affects the normal venous return and extends into venules, it finally causes a venous infarction of the spinal cord. The progressive myelopathy often leads to paraplegia and bowel, bladder, and sexual dysfunction, with gradual worsening over months to a few years. Most of the patients become severely disabled within 3.5 years. The majority of patients (79–85%) are men, and 86% of patients are 41 years of age or older at presentation. The mean age at presentation is 55 years old; however, patients as young as 1 month of age have been reported as a result of hereditary hemorrhagic telangiectasia. The most common presentation is progressive paraparesis, with sensory changes and complaints of back and leg pain. The progression is usually continuous, but it can also present in a stepwise fashion or a waxing-waning course with gradual progression. In 10% to 20% of patients, the presentation is an acute exacerbation. The symptoms can be exacerbated by any physical activity that increases intraabdominal pressure and thus central venous pressure, as well as by an upright posture (impaired venous drainage due to hydrostatic pressure). A brain dural AVS of the posterior fossa (e.g., tentorial dural AVS) can mimic a spinal dural AVS if the venous drainage takes the pontomedullary path into the spinal cord venous system ( Fig. 93.12 ). These dural brain AVSs have been classified as type V. Thus, a diagnostic cerebral workup of the posterior circulation and both internal and external carotid arteries is a part of the spinal angiography. The most common misdiagnosis for these lesions is transverse myelitis, ischemic spinal cord infarction, disc disease, and spinal cord tumors.
![FIG. 93.11, Dural arteriovenous fistula (AVF; intradural AVF, type I spinal arteriovenous malformation [AVM]). (A and B) A single location and single arterial feeder (type IA) and multiple dural arterial supply (type IB): 1, dural branches of radicular artery supplying AVM within the dura; 2, arterialized radiculomedullary vein; 3, dilated posterior median vein. (C) Intraoperative image of a dural AVF after opening of the dura shows an arterialized and congested posterior median vein ( arrow ) and a markedly swollen spinal cord. (D) Contrast medium–enhanced T1-weighted magnetic resonance (MR) image shows a nonspecific, diffuse, flame-shaped enhancement and swelling of the spinal cord ( curved arrow ) and dilated posterior median vein ( arrow ). (E) Three-dimensional time-of-flight MR angiogram in the coronal plane shows congested radiculomedullary vein ( single arrow ) and dorsal median vein ( double arrows ). (F) A microcatheter has been navigated through an intercostal artery ( arrowheads ), and superselective angiography of the radicular artery ( long arrow ) delineates the dural AVF ( curved arrow ), the retrograde draining and congested radiculomedullary vein ( double arrows ), and the congested dorsal median vein ( large arrow ). (G) After embolization, a plain spine radiograph shows N -butyl-cyanoacrylate cast within the radicular artery ( arrow ), the point of fistulization ( curved arrow ), and the radiculomedullary vein ( double arrows ). (H) Control angiography of the intercostal artery ( arrow ) shows a complete dural AVF obliteration. FIG. 93.11, Dural arteriovenous fistula (AVF; intradural AVF, type I spinal arteriovenous malformation [AVM]). (A and B) A single location and single arterial feeder (type IA) and multiple dural arterial supply (type IB): 1, dural branches of radicular artery supplying AVM within the dura; 2, arterialized radiculomedullary vein; 3, dilated posterior median vein. (C) Intraoperative image of a dural AVF after opening of the dura shows an arterialized and congested posterior median vein ( arrow ) and a markedly swollen spinal cord. (D) Contrast medium–enhanced T1-weighted magnetic resonance (MR) image shows a nonspecific, diffuse, flame-shaped enhancement and swelling of the spinal cord ( curved arrow ) and dilated posterior median vein ( arrow ). (E) Three-dimensional time-of-flight MR angiogram in the coronal plane shows congested radiculomedullary vein ( single arrow ) and dorsal median vein ( double arrows ). (F) A microcatheter has been navigated through an intercostal artery ( arrowheads ), and superselective angiography of the radicular artery ( long arrow ) delineates the dural AVF ( curved arrow ), the retrograde draining and congested radiculomedullary vein ( double arrows ), and the congested dorsal median vein ( large arrow ). (G) After embolization, a plain spine radiograph shows N -butyl-cyanoacrylate cast within the radicular artery ( arrow ), the point of fistulization ( curved arrow ), and the radiculomedullary vein ( double arrows ). (H) Control angiography of the intercostal artery ( arrow ) shows a complete dural AVF obliteration.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/VascularAnatomyoftheSpineImagingandEndovascularTreatmentofSpinalVascularDiseases/10_3s20B9780323393973000933.jpg)
MRI identifies draining vessels as serpentine areas of flow void within the spinal canal on T2-weighted images. In axial T1- and T2-weighted MR images, dilated coronal veins with a low signal located around the spinal cord are visible. Enlarged radiculomedullary veins on dynamic contrast medium–enhanced MRA help to guide catheter angiography and help to reduce the contrast load for the patient. The spinal cord can appear enlarged and hypointense on T1-weighted images; the flame-shaped spinal cord edema that spares the cord periphery can be well appreciated on T2-weighted images. Because it represents venous infarction, contrast medium enhancement within edematous changes is considered prognostically bad for recovery of neurologic function. Spinal angiography shows a direct shunting of contrast agent from the radiculomeningeal artery supply into the extensive and tortuous draining radiculomedullary and perimedullary venous systems. The draining vessels are the coronal venous plexus situated along the dorsal surface of the spinal cord. Angiography also helps to delineate the ASA and guide the embolization. AVS feeding vessels can originate anywhere from vertebral artery to lateral sacral arteries ( Fig. 93.13 ).
Last, normal cerebrospinal fluid pulsations typically dorsal to the cord on T2-weighted images may mimic a dural malformation.
A cure can be achieved by opening the dura at the affected level and surgically disconnecting the radiculomedullary vein from the dural arterial supply. Microsurgical treatment can be performed with exceedingly high obliteration rates and long-term postoperative improvement of preexisting deficits in the majority of cases. Despite recent advances in endovascular techniques and materials, there is a subgroup of patients for which surgery remains the best treatment option.
Alternatively, an endovascular intervention in experienced hands is very safe and effective. Endovascular therapy entails the infusion of N -butyl cyanoacrylate (NBCA) into the radiculomedullary vein after selective microcatheterization of the feeding artery. Complete obliteration has been reported in up to 90% of patients, but with recurrence rates of up to 23%. In pediatric patients, endovascular treatment (even partial but targeted) also appears to be a safe and stable therapeutic alternative in the management of spinal dural AVSs. The availability of newer acrylate and liquid embolic agents and more experienced physicians has diminished the recurrence rate significantly. The consensus among interventional neuroradiologists at this time is that the successful treatment of these malformations consists of acrylate penetration of the fistula and the proximal radiculomedullary draining vein without entering the spinal cord draining system (see Fig. 93.11 ). The protocol used in some centers is an endovascular approach as the first line of treatment because of its noninvasive nature, low complication rate, and the ability to obtain immediate angiographic control and confirmation of obliteration. Factors determining the success of endovascular treatments among patients with spinal dural AVFs seem to include the presence of antegrade flow toward the draining vein and injection of NBCA glue less than 30%; these factors were associated with higher chance of draining vein penetration and, therefore, successful endovascular spinal dural AVF obliteration as reported in a recent single-center experience. If the acrylate should not penetrate the radiculomedullary veins, the patient requires a surgical disconnection; the radiopaque acrylate mixture then can be used as a landmark for fluoroscopic-guided intraoperative localization. Alternatives for liquid embolic agents include Onyx; however, it has been associated with a higher recurrence rate, and possibly because of its deeper penetration into smaller vessels potentially connecting to the ASA or PSAs, considered less suitable for the endovascular embolization of spinal dural AVFs.
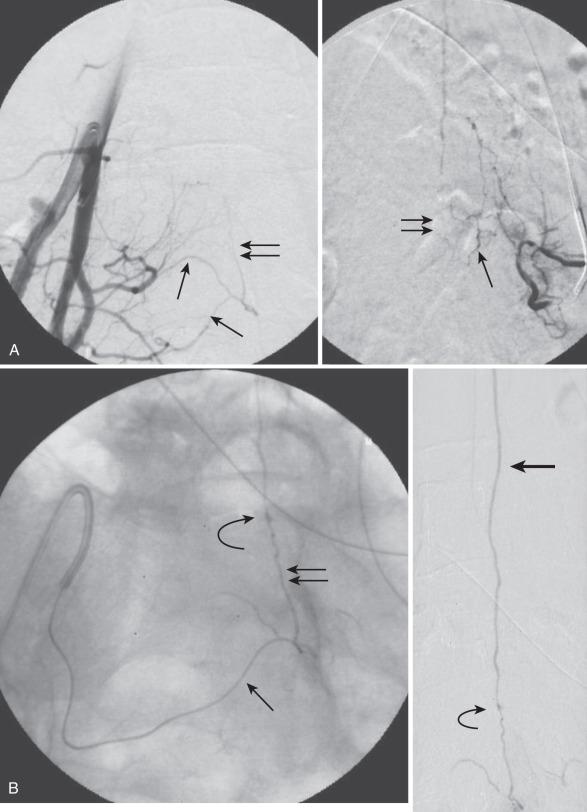
Also called ventral intradural AVF, perimedullary fistula, or type IV spinal AVM, a pial AVS is a direct fistula between the ASA and the coronal venous plexus ( Fig. 93.14 ). Radiculopial supply may also be involved. Based on the size of the AVF and the blood flow, three different subtypes (A, B, and C) have been described.
Subtype A (Merland subtype I) is a small shunt with slow flow, with moderate venous hypertension. There is no enlargement of the ASA and only minimal dilatation of the ascending draining vein. The location of the fistula can be difficult to find, but it is characterized generally by the point at which a vessel caliber change between the smaller artery and the larger ectatic vein is seen. Owing to the change from a higher arterial impedance to a lower venous one, a change in blood flow pattern and velocity can be observed on high-speed angiograms. The ASA is the only feeder, and the AVF is typically located along the anterior aspect of the conus medullaris or proximal filum terminale.
Subtype B (Merland subtype II) is a moderate-sized (intermediate) shunt with moderate enlargement of the feeding artery(ies) and the draining veins. The location of the fistula is marked by venous ectasia. This is a high flow rate shunt; associated flow-related arterial and venous aneurysms may be present. There are several abnormally dilated feeding arteries, composed of the ASA and one or two arteries from the dorsolateral pial network (PSA), all of which converge on the fistula. These are typically located at the level of the conus. Blood returns via tortuous and dilated ascending perimedullary veins.
Subtype C (Merland subtype III) is a giant fistula with very large arterial feeder(s) from the ASA and dorsolateral pial network (PSA) converging into the fistula and draining directly into a giant venous ectasia, often embedded within the substance of the cord. These fistulas are rare, although, in at least one large series, they were the largest subtype of ventral intradural AVF. The location of the fistula is more difficult to ascertain because of the giant, ectatic draining vein. The giant, ectatic draining vein usually drains into the local metameric efferent veins, which are also dilated. These lesions are typically located at the thoracic or cervical levels.
The clinical signs and symptoms may be due to vascular steal, especially with higher flow, venous hypertension, and mass effect with venous enlargement and aneurysm formation, as well as hemorrhage. More than 90% of patients present with neurologic deficits. They almost always appear before age 40 years and often present during the first decade, with mean age at diagnosis being between 11.5 and 13.5 years. SAH is the presenting sign in approximately 40% of patients, but according to some authors only subtype C fistulas present as hemorrhage. Occasionally, hematomyelia has also been reported. Paraparesis or paraplegia is the most common sign, with progressive deterioration over time. Radiculomyelopathy or radiculopathy can also be present, presumably owing to mass effect from dilated venous structures. These lesions can be seen anywhere along the spine. When the fistula is located ventrolaterally or posterolaterally, a significant involvement of the dorsolateral pial network (PSA) is present.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here