Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There are two different clinicopathologic types of endometrial cancer. Type I endometrial cancers are caused by unopposed estrogenic stimulation, are endometrioid in histologic type, and generally have a good prognosis. Type II endometrial cancers are unrelated to estrogenic stimulation, are often nonendometrioid histologically (serous or clear cell), and have a poor prognosis.
About 5% of endometrial cancers occur in women with Lynch syndrome, which is also called hereditary nonpolyposis colon cancer (HNPCC) syndrome. This syndrome is caused by germline mutations in the DNA mismatch repair genes. Women with HNPCC syndrome have about a 40% risk of developing endometrial cancer, which is similar to their risk of developing bowel cancer.
The commonest presenting symptom of patients with endometrial cancer is postmenopausal bleeding. A transvaginal ultrasound will reveal an endometrial stripe that is wider than 4 mm, and an endometrial biopsy done in the office will usually allow histologic diagnosis. If the office biopsy is negative or shows endometrial hyperplasia, hysteroscopy and uterine curettage will be necessary to definitively exclude endometrial cancer.
Total hysterectomy and bilateral salpingo-oophorectomy is the basic treatment for stage I endometrial cancer, and this is usually performed by laparoscopic or robotic surgery. Any enlarged pelvic or paraaortic lymph nodes should be resected in all patients. Formal surgical staging, including at least pelvic lymphadenectomy, should be performed in high-risk patients, including those with serous, clear cell, or grade 3 histology, outer-half myometrial invasion, or cervical extension.
For patients with advanced disease, treatment must be individualized. The uterus, tubes, and ovaries should be removed, if possible, for palliation of bleeding and other pelvic symptoms. Some cases of advanced disease are a result of delayed diagnosis. If the patient has an advanced grade 1 or 2 tumor with positive estrogen or progesterone receptors, good responses and prolonged survival may be seen with the use of high-dose progestins or tamoxifen.
Cancer of the endometrium is the most common gynecologic malignancy in the United States. For 2013, it was estimated that there would be 45,560 new cases and 8190 deaths. It is the fourth most common malignancy found in American women after breast, colorectal, and lung cancers, and it is predominantly a disease of affluent, obese, postmenopausal women of low parity.
There are two different clinicopathologic types of endometrial carcinoma ( Table 41-1 ): an estrogen-dependent and a non–estrogen-dependent type.
| Type I | Type II |
|---|---|
| Endometrioid | Non endometrioid (serous, clear cell) |
| Mean age 63 yr | Mean age 67 yr |
| Estrogen-related | Non–estrogen-related |
| Microsatellite instability | Chromosomal instability |
| Mutations in PTEN, PIK3CA, KRAS | TP53 mutations |
| Good prognosis | Poor prognosis |
Any factor that increases exposure to unopposed estrogen increases the risk for type I endometrial cancer. If the proliferative effects of estrogen are not counteracted by a progestin, endometrial hyperplasia and possibly adenocarcinoma can result.
Obesity results in an increased extraovarian aromatization of androstenedione to estrone. Androstenedione is secreted by the adrenal glands, whereas the increased peripheral conversion occurs predominantly in fat depots, as well as in the liver, kidneys, and skeletal muscles. Granulosa-theca cell tumors of the ovary produce estrogen, and up to 15% of patients with these tumors have an associated endometrial cancer.
Unopposed estrogenic stimulation from anovulatory cycles occurs in patients who have polycystic ovarian syndrome (Stein-Leventhal syndrome) and in patients with a late menopause. In postmenopausal women taking estrogen replacement without a progestin for menopausal symptoms, the risk of cancer developing appears to be both dose-dependent and duration-dependent. This increased risk varies from 2- to 14-fold compared with nonusers. The addition of progestin in a cyclic fashion for 10 to 14 days of the month or in a continuous fashion daily throughout the month eliminates this increased risk. Women taking tamoxifen for breast cancer have a two- to threefold increased risk of endometrial cancer. Young women who use oral contraceptives have been shown to have a lower incidence of subsequent endometrial cancer.
About 5% of endometrial cancers occur in women with Lynch syndrome, which is also called the hereditary nonpolyposis colon cancer (HNPCC) syndrome. This syndrome is caused by germline mutations in the DNA mismatch repair genes. Women with the HNPCC syndrome have about a 40% risk of developing endometrial cancer, usually before the menopause. Their risk of developing bowel cancer is also about 40%.
Population screening for endometrial cancer is not feasible, because there is no simple method of cancer detection available. However, screening may be justified for high-risk women, including those with a family history of HNPCC syndrome, those with polycystic ovarian disease, and any woman with an intact uterus taking unopposed estrogen. Only about 50% of women with endometrial cancer will have malignant cells on a Papanicolaou smear.
Since the 1990s, transvaginal ultrasonography has increasingly been used for endometrial evaluation. Almost all women with endometrial hyperplasia or carcinoma will have an endometrial thickness of 5 mm or more. Tamoxifen produces a confusing ultrasonic image, which leads to frequent false-positive reports.
The most common symptom of endometrial cancer is abnormal vaginal bleeding, which is present in 90% of patients. Postmenopausal bleeding is always abnormal and must be investigated. The most common conditions associated with postmenopausal bleeding are listed in Table 41-2 . In the premenopausal or perimenopausal patient, diagnosis is often delayed because frequent or heavy bleeding is usually thought to be dysfunctional in nature.
| Factor | Approximate Percentage |
|---|---|
| Exogenous estrogens | 30 |
| Atrophic endometritis, vaginitis | 30 |
| Endometrial cancer | 15 |
| Endometrial or cervical polyps | 10 |
| Endometrial hyperplasia | 5 |
| Miscellaneous (e.g., cervical cancer, uterine sarcoma, urethral caruncle, trauma) | 10 |
As menopause approaches, menstruation often becomes lighter and less frequent. If the bleeding becomes heavier or more frequent, it should be investigated.
A general physical examination may reveal obesity, hypertension, and the stigmata of diabetes mellitus. Evidence of metastatic disease is unusual at initial presentation, but the chest should be examined for any effusion and the abdomen carefully palpated and percussed to exclude ascites, hepatomegaly, or evidence of upper abdominal masses.
On pelvic examination, the external genitalia are usually normal. The vagina and cervix are also usually normal, but they should be inspected and palpated carefully for evidence of involvement. A patulous cervical os or a firm, expanded cervix may indicate extension of disease from the corpus to the cervix. The uterus may be of normal size or enlarged, depending on the extent of the disease and the presence or absence of other uterine conditions, such as adenomyosis or fibroids. The adnexa should be palpated carefully for evidence of extrauterine metastases or an ovarian neoplasm. A granulosa cell tumor or an endometrioid ovarian carcinoma may occasionally coexist with endometrial cancer.
Any woman who presents with postmenopausal bleeding should undergo transvaginal ultrasonography. If the endometrial thickness is greater than 4 mm, endometrial evaluation is necessary. An outpatient endometrial biopsy is usually feasible with a sampling device such as a Pipelle, GynoSampler, or Vabra aspirator. Outpatient endometrial biopsy has a diagnostic accuracy of about 95%. If the endometrial biopsy reveals endometrial cancer, definitive treatment can be arranged. If the endometrial biopsy is negative for cancer or reveals endometrial hyperplasia, a hysteroscopy and fractional dilation and curettage should be performed with the patient under general anesthesia. Specimens from the endometrium and endocervix should be submitted separately for histologic evaluation to determine whether the tumor has extended to the endocervix.
In a premenopausal patient with high-risk factors and abnormal uterine bleeding, the endometrium must be sampled. If there are no high-risk factors present, failure to respond to medical management or a suspicious transvaginal ultrasound is also an indication for hysteroscopy and uterine curettage.
In 1988, the International Federation of Gynecology and Obstetrics (FIGO) changed from a clinical to a surgical staging system for endometrial cancer. This surgical staging system was further revised in 2009. The latest FIGO staging system is shown in Table 41-3 .
| Stage | Description |
|---|---|
| I * | Tumor confined to the corpus uteri |
| IA * | No or less than half myometrial invasion |
| IB * | Invasion equal to or more than half of the myometrium |
| II * | Tumor invades cervical stroma, but does not extend beyond the uterus † |
| III * | Local and/or regional spread of the tumor |
| IIIA * | Tumor invades the serosa of the corpus uteri and/or adnexa ‡ |
| IIIB * | Vaginal and/or parametrial involvement ‡ |
| IIIC * | Metastases to pelvic and/or paraaortic lymph nodes ‡ |
| IIIC1 * | Positive pelvic nodes |
| IIIC2 * | Positive paraaortic lymph nodes with or without positive pelvic lymph nodes |
| IV * | Tumor invades bladder and/or bowel mucosa, and/or distant metastases |
| IVA * | Tumor invasion of bladder and/or bowel mucosa |
| IVB * | Distant metastases, including intraabdominal metastases and/or inguinal lymph nodes |
* Either Grade 1, Grade 2, or Grade 3.
† Endocervical glandular involvement should be considered only as stage I and no longer as stage II.
‡ Positive cytology has to be reported separately without changing the stage.
In addition to a thorough physical examination, blood studies should include a complete blood count; determinations of hepatic enzymes, serum electrolytes, blood urea nitrogen, and serum creatinine; and a coagulation profile. A routine urinalysis should be performed. It is usual to perform computed tomography (CT) of the chest, pelvis, and abdomen, particularly in high-risk cases, to detect any enlarged lymph nodes, liver or lung metastases, hydronephrosis, or adrenal masses. Magnetic resonance imaging is useful for differentiating superficial from deep myometrial invasion or detection of cervical involvement. It may be useful for triaging patients to a gynecologic oncologist.
About 75% of endometrial cancers are pure adenocarcinomas. When squamous elements are present, the tumor is called an adenocarcinoma with squamous differentiation. Such tumors are graded on the glandular component of the lesion. Less often, clear cell, squamous, or serous carcinomas occur, and all carry a worse prognosis.
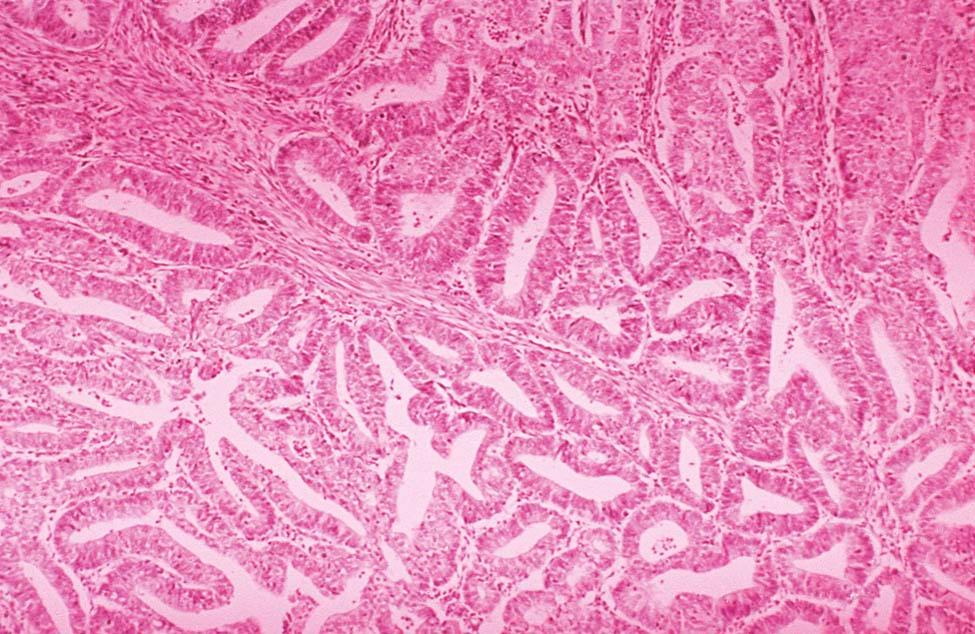
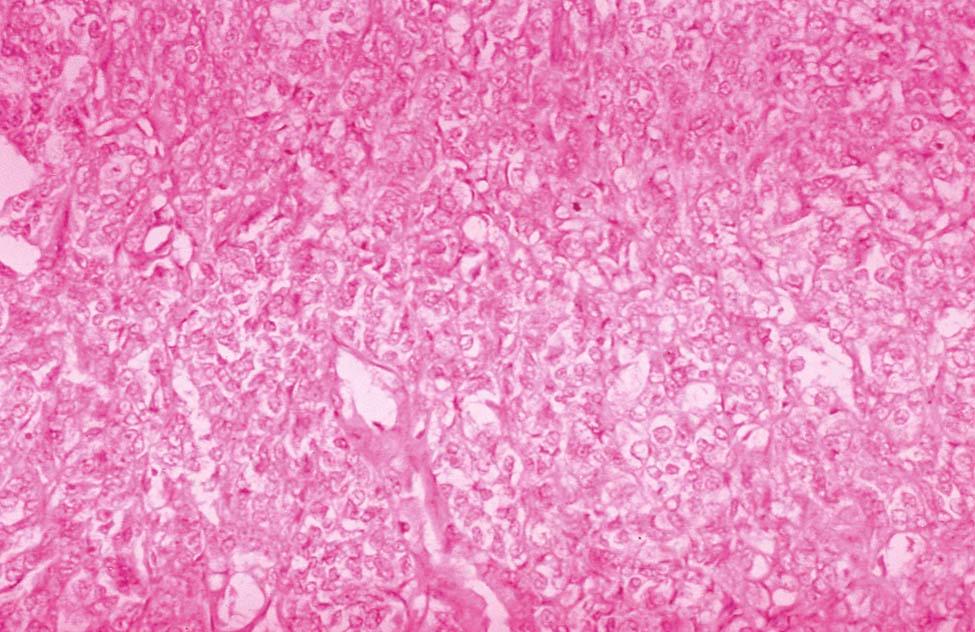
Invasive adenocarcinoma of the endometrium demonstrates proliferative glandular formation with minimal or no intervening stroma. Tumor grade is determined by both the degree of abnormality of the glandular architecture and the degree of nuclear atypia. A lesion that is well differentiated (grade 1) forms a glandular pattern similar to normal endometrial glands ( Figure 41-1 ). A moderately well-differentiated lesion (grade 2) has glandular structures admixed with papillary, and occasionally solid, areas of tumor. In a poorly differentiated lesion (grade 3), the glandular structures have become predominantly solid with a relative paucity of identifiable endometrial glands ( Figure 41-2 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here