Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
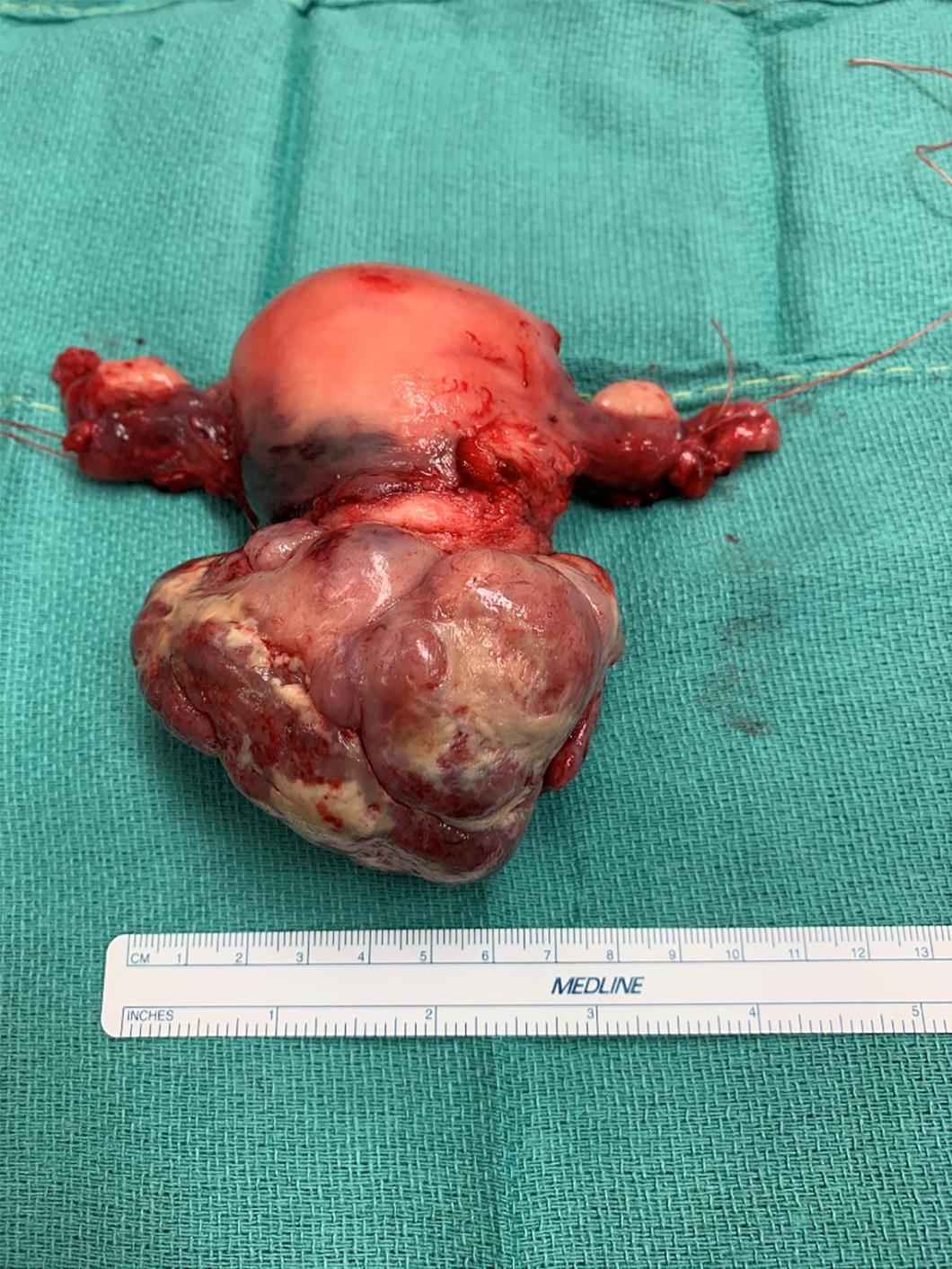
A 75-year-old African American woman presents to your clinic with complaints of new onset vaginal bleeding. She has a past medical history significant for obesity and history of breast cancer managed with tamoxifen maintenance. On pelvic exam, she is noted to have a 7 cm tumor prolapsing through the cervix into the vaginal canal ( Fig. 11.1 ). Pelvic ultrasound confirms a hyperechoic mass in the uterus with associated expansion of the endometrial canal suspicious for malignancy. Endometrial biopsy (EMB) is performed that is consistent with a diagnosis of uterine carcinosarcoma (UCS). A computed tomography (CT) of the chest, abdomen, and pelvis is ordered that demonstrates a large heterogeneous mass within the uterus and no obvious lymphadenopathy or other metastatic disease. The patient underwent a robotic assisted total hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO), and pelvic lymphadenectomy. Her final pathology was consistent with a stage II UCS. What adjuvant therapy do you recommend?
While endometrioid carcinomas are the most common gynecologic malignancy in developed countries, a diagnosis of UCS, a subtype of endometrial cancer, is rare. UCS historically known as malignant mixed Mullerian tumor or malignant mixed mesodermal tumor (MMMT), is now considered one of the high-grade histologies of uterine cancer. In the past, carcinosarcomas were treated and studied as sarcomas. Table 11.1 outlines uterine cancer subtypes and their frequencies. The worldwide annual incidence of UCS ranges between 0.5 and 3.3 cases per 100,000 women with the age-adjusted incidence rate rising from 1.0 per 100,000 in 2000 to 1.4 per 100,000 in 2016. UCS accounts for only 5% of all uterine tumors, yet it is responsible for 15% of all deaths from uterine corpus malignancies. For all stages, the median overall survival is less than 2 years, and the 5-year overall survival rate is 33.4%. When comparing survival by stage of disease, even women with stage I disease have poor survival outcomes. The 5-year survival rates for stages I/II, III, and IV disease are 59%, 22%, and 9%, respectively. In comparison, the 5-year survival rate for stage I high-grade uterine endometrioid cancer is over 80%. Women with stage IV UCS have a median overall survival of only several months.
| Histology | Percentage of total cases | Cases/year in US a |
|---|---|---|
| Endometrioid–Grade 1/2/3 | 75%–80% | 49,900–53,000 |
| Serous | 10% | 6657 |
| Clear cell | < 5% | 3300 |
| Carcinosarcoma | < 5% | 3300 |
The pathogenesis of UCS is controversial but the currently accepted theory is that UCS originates from the metaplastic transformation of a single cell. While UCS was previously included and studied in the sarcoma category, it is now categorized as a high-grade dedifferentiated carcinoma. UCS is comprised of both carcinomatous (epithelial) and mesenchymal (stromal) components. The epithelial components are graded as either low grade or high grade and are most likely to metastasize and be the source of recurrence as opposed to the sarcomatous component. The stromal component can be graded as homologous or heterologous. Examples of homologous sarcomas include leiomyosarcoma, fibrosarcoma, and endometrial stromal sarcoma; examples of heterologous sarcomas include rhabdomyosarcoma, chondrosarcoma, and osteosarcoma. The distinction between homologous and heterologous is based on whether the stromal component is comprised of cell types native to the uterus.
Age is a significant risk factor in the development of UCS. Carcinosarcomas are more commonly found in older women, with usual age of onset in the seventh decade of life. Black women are also noted to be at increased risk of developing UCS. A 2003 SEER analysis showed that black women had significantly higher incidence rates than white non-Hispanic women (RR 2.33 [1.99–2.72]). This was concordant with a 2004 SEER study that found the age-adjusted incidence rate of carcinosarcoma to be twofold greater in black women as compared to white women.
Additional risk factors for UCS parallel risk factors for endometrial adenocarcinoma, including nulliparity, obesity, and use of exogenous estrogen. Multiple small series demonstrated the relationship between tamoxifen exposure and development of UCS with median time from initiation of tamoxifen to diagnosis of UCS averaging around 9 years. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial, the risk of endometrial cancer in those women 50 or older on tamoxifen was higher than those taking placebo after 7 years of follow up (RR 5.33 [2.47–13.17]). The incidence of endometrial cancer in the 13,388 women studied was 15.64 vs 4.68/1000 woman-years in those on tamoxifen vs placebo. Most commonly, these cancers are low grade endometrioid cancers and stage 1. Tamoxifen-related carcinosarcomas are estimated to comprise approximately 8% of all carcinosarcomas. Prior pelvic radiation is also associated with the development of UCS. In one retrospective study examining the diagnosis of endometrial cancer following pelvic radiotherapy for cervical cancer, 35% of women were diagnosed with UCS which was a significant increase from the baseline sporadic rate of 6%. Prognostic factors in UCS include those similar to all malignancies such as age, stage, and presence of lymphovascular space invasion. In carcinosarcoma, the carcinoma component of serous or clear cell and the sarcoma component of rhabdomyosarcoma portend a worse prognosis. Predictors of improved survival in UCS include age < 40, Asian race followed by white, followed by African American, the utilization of postoperative radiotherapy, undergoing lymphadenectomy, and early stage of disease. Table 11.2 demonstrates prognostic factors in uterine carcinosarcoma.
| Factor | Improved survival | Worsened survival |
|---|---|---|
| Stage | I/II (59% at 5 years) | III/IV (22 and 9% at 5 years) |
| Lymphovascular space Invasion | Negative | Positive |
| Age | < 40 years | > 40 years |
| Race | Asian/Caucasian | African-American |
| Carcinoma component | Serous, clear cell | |
| Sarcoma component | Rhabdomyosarcomatous | |
| CA125 elevation | Postoperative elevation |
Tumors are typically polypoid, pedunculated and soft, though color and consistency are variable secondary to various quantity of necrosis and hemorrhage. Tumors that are significantly polypoid may protrude through the cervix and may appear as a primary cervical neoplasm. The median size is 5.6 cm with a range from 2.5 to 20 cm.
UCS, also known as MMMT, are malignant neoplasms composed of both a malignant carcinomatous component and a malignant sarcomatous component. These neoplasms are in contrast with adenosarcoma that while having epithelial and mesenchymal elements, only the latter component is malignant.
The carcinoma and sarcoma areas are intermixed, with components that may appear well differentiated to highly anaplastic. Just over half of all UCS are predominantly composed of the sarcomatous component.
The carcinoma component may include one or more of the following histotypes: endometrioid, serous, undifferentiated or dedifferentiated carcinoma, or clear cell carcinoma ( Fig. 11.2 ). Of these the most common epithelial component is serous carcinoma. Rarely, the epithelial component can be a mesonephric-like carcinoma. The sarcomatous component may be composed of homologous and/or heterologous components. Homologous components are those in which the mesenchymal differentiation is that of native uterine corpus mesenchymal elements such as a leiomyosarcoma, fibrosarcoma, endometrial stromal sarcoma or high-grade undifferentiated sarcoma. Heterologous components are those in which the differentiation of the sarcoma is not native to the uterine corpus, including rhabdomyosarcoma, chondrosarcoma, liposarcoma, or osteosarcoma ( Figs. 11.3 A, B, and 11.4 ). While carcinosarcomas are overall considered high grade, the presence of the heterologous sarcomatous components needs to be noted, as these have an even worse prognosis, particularly if rhabdomyosarcoma. Rarely somatically derived germ cell tumors such as yolk sac tumor may be present. UCS are usually deeply myoinvasive; however, some tumors may be confined to a polyp. The metastases are usually composed of only the carcinomatous component (90%), sometimes of only the sarcomatous component, or can be mixed.
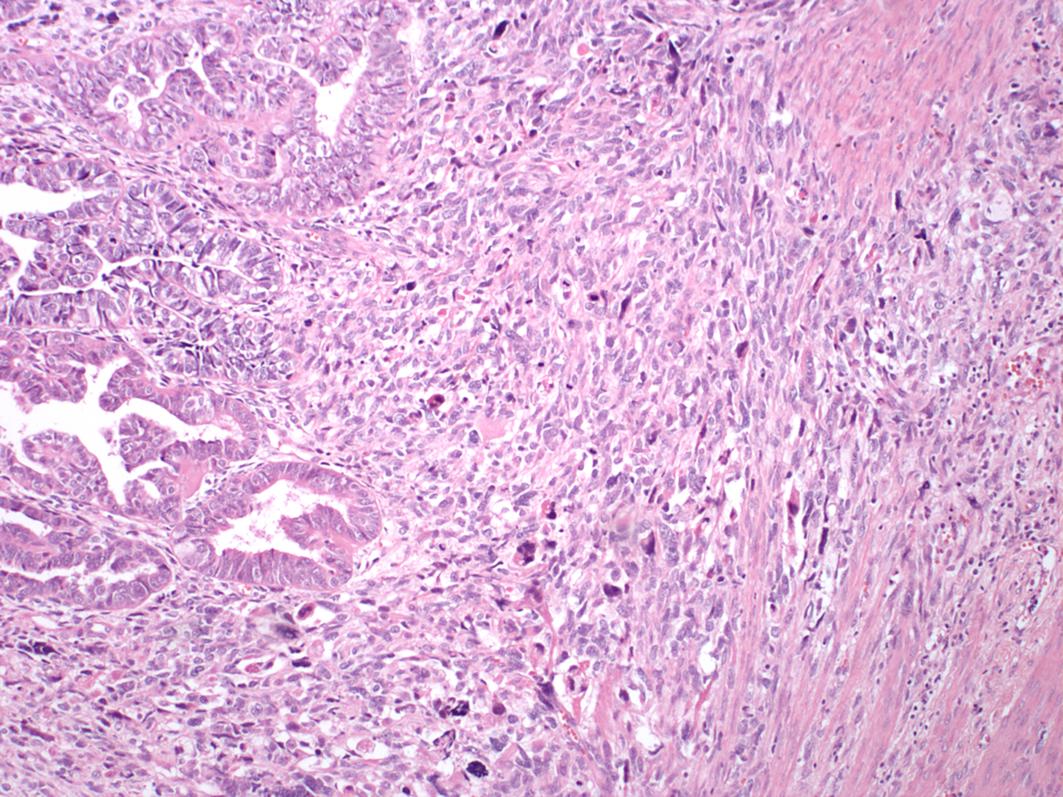
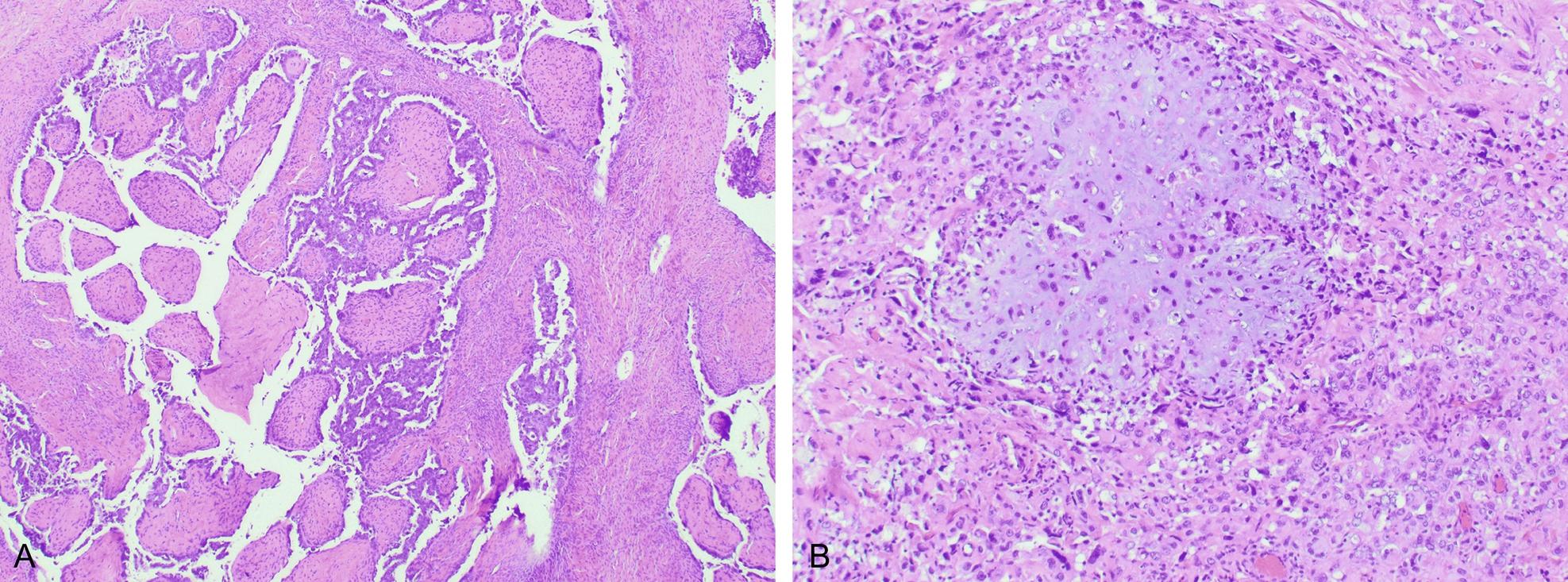
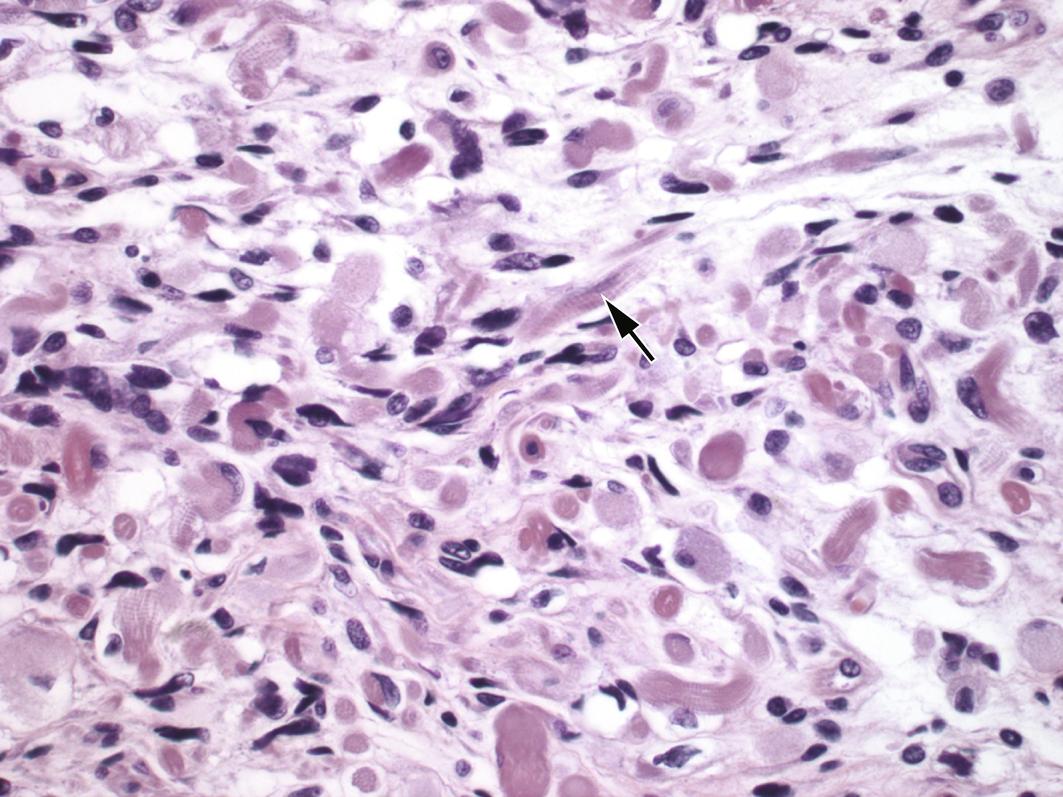
The epithelial component is positive for keratin markers while the sarcomatous component is usually negative. The epithelial component is usually positive for PAX-8, and variably positive for hormone receptors, depending on the subtype. Both epithelial (if serous carcinoma) and sarcoma components can show aberrant expression of p53.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here