Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
For the cells of the body to function properly, they must be bathed in extracellular fluid with a relatively constant concentration of electrolytes. The total concentration of solutes in the extracellular fluid—and therefore the osmolarity—must also be precisely regulated to prevent the cells from shrinking or swelling. The osmolarity is determined by the amount of solute (mainly sodium chloride) divided by the volume of the extracellular fluid. Thus, to a large extent, extracellular fluid osmolarity and sodium chloride concentration are regulated by the amount of extracellular water. The total body water is controlled by (1) fluid intake, which is regulated by factors that determine thirst; and (2) renal water excretion, which is controlled by multiple factors that influence glomerular filtration and tubular reabsorption.
In this chapter, we discuss the following: (1) mechanisms that cause the kidneys to eliminate excess water by excreting a dilute urine; (2) mechanisms that cause the kidneys to conserve water by excreting a concentrated urine; (3) renal feedback mechanisms that control the extracellular fluid sodium concentration and osmolarity; and (4) thirst and salt appetite mechanisms that determine the intakes of water and salt, which also help control extracellular fluid volume, osmolarity, and sodium concentration.
Normal kidneys have a tremendous capability to vary the relative proportions of solutes and water in the urine in response to various challenges. When there is excess water in the body, and body fluid osmolarity is reduced, the kidneys can excrete urine with an osmolarity as low as 50 mOsm/L, a concentration that is only about one-sixth the osmolarity of normal extracellular fluid. Conversely, when there is a deficit of water in the body, and extracellular fluid osmolarity is high, the kidneys can excrete highly concentrated urine with an osmolarity of 1200 to 1400 mOsm/L. Equally important, the kidneys can excrete a large volume of dilute urine or a small volume of concentrated urine without major changes in rates of excretion of solutes such as sodium and potassium. This ability to regulate water excretion independently of solute excretion is necessary for survival, especially when fluid intake is limited.
The body has a powerful feedback system for regulating plasma osmolarity and sodium concentration that operates by altering renal excretion of water independently of solute excretion rate. A primary effector of this feedback is antidiuretic hormone (ADH), also called vasopressin .
When osmolarity of the body fluids increases above normal (i.e., the solutes in the body fluids become too concentrated), the posterior pituitary gland secretes more ADH, which increases the permeability of the distal tubules and collecting ducts to water, as discussed in Chapter 28 . This mechanism increases water reabsorption and decreases urine volume but does not markedly alter the rate of renal excretion of the solutes.
When there is excess water in the body, and extracellular fluid osmolarity is reduced, secretion of ADH by the posterior pituitary decreases, thereby reducing the permeability of the distal tubule and collecting ducts to water, which causes increased amounts of more dilute urine to be excreted. Thus, the rate of ADH secretion determines, to a large extent, whether the kidney excretes dilute or concentrated urine.
When there is a large excess of water in the body, the kidney can excrete as much as 20 L/day of dilute urine, with a concentration as low as 50 mOsm/L. The kidney performs this impressive feat by continuing to reabsorb solutes without reabsorbing large amounts of water in the distal parts of the nephron, including the late distal tubule and collecting ducts.
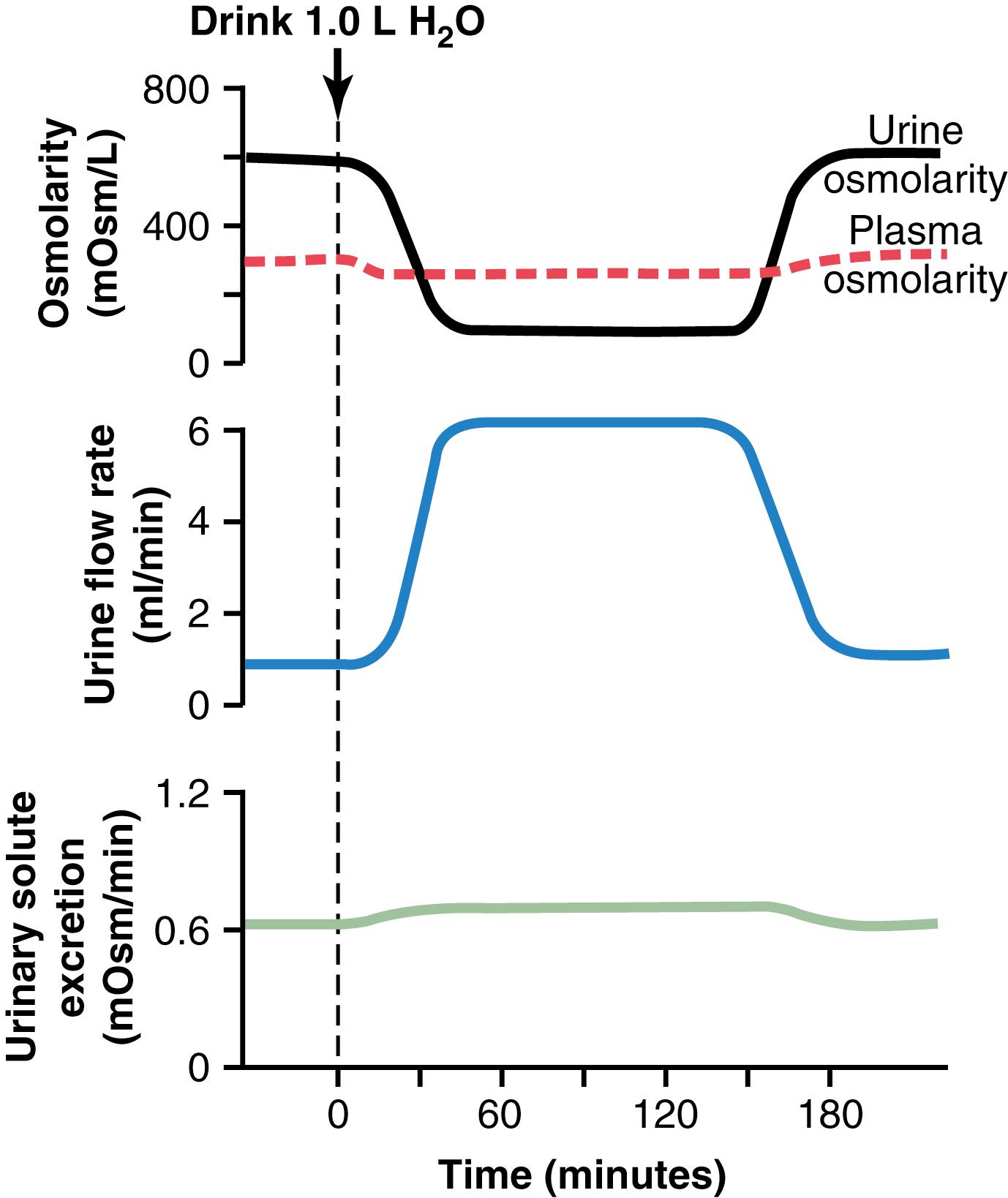
Figure 29-1 shows the approximate renal responses in a human after ingestion of 1 liter of water. Note that urine volume increased to about six times normal within 45 minutes after the water had been ingested. However, the total amount of solute excreted remained relatively constant because the urine formed became dilute, and urine osmolarity decreased from 600 to about 100 mOsm/L. Thus, after ingestion of excess water, the kidney rids the body of the excess water but does not excrete excess amounts of solutes.
When the glomerular filtrate is initially formed, its osmolarity is about the same as that of plasma (300 mOsm/L). To excrete excess water, the filtrate is diluted as it passes along the tubule by reabsorbing solutes to a greater extent than water, as shown in Figure 29-2 . This dilution, however, occurs only in certain segments of the tubular system, as described in the following sections.
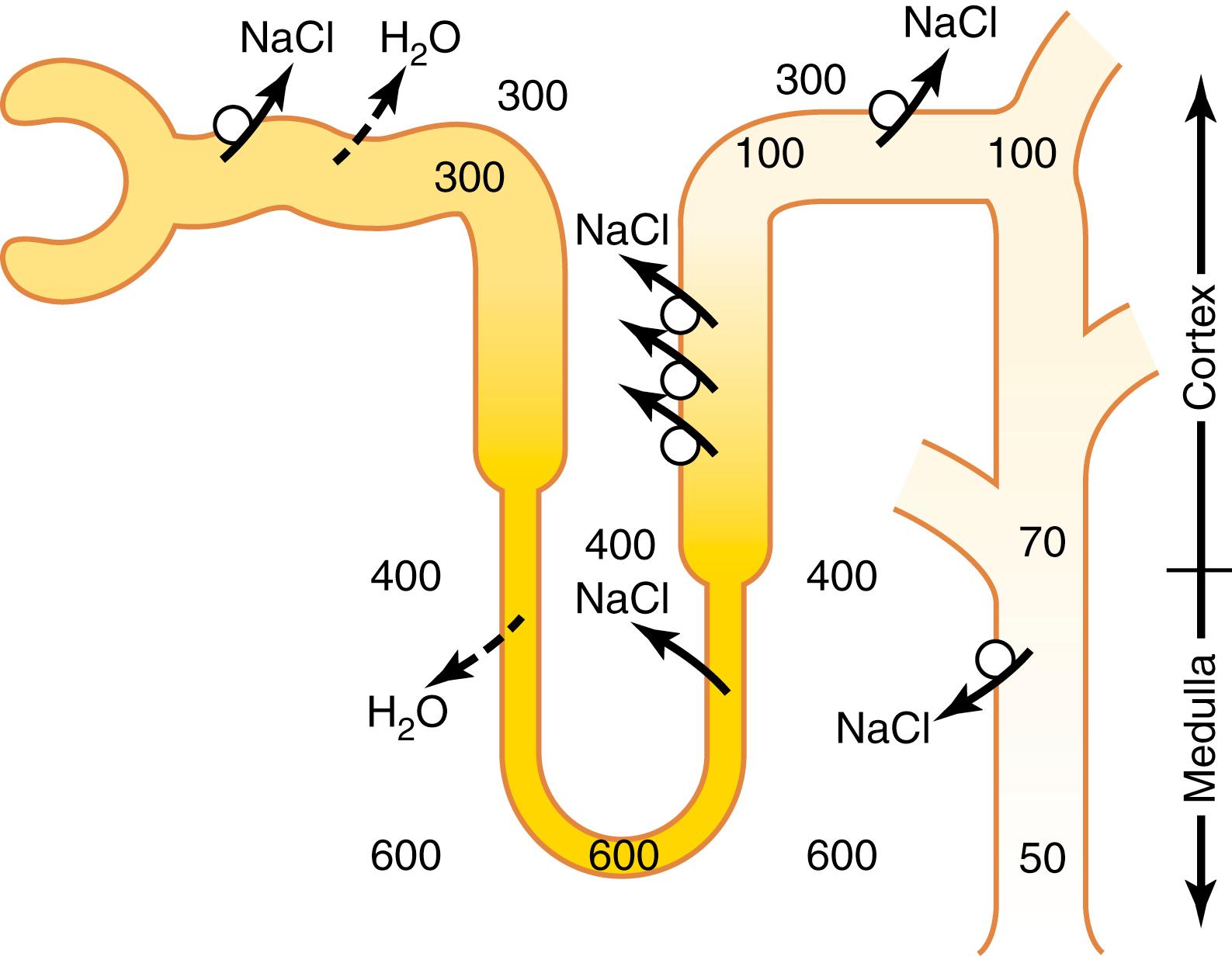
As fluid flows through the proximal tubule, solutes and water are reabsorbed in equal proportions, so little change in osmolarity occurs. Thus, the proximal tubule fluid remains isosmotic to the plasma, with an osmolarity of about 300 mOsm/L. As fluid passes down the descending loop of Henle, water is reabsorbed by osmosis, and the tubular fluid reaches equilibrium with the surrounding interstitial fluid of the renal medulla, which is very hypertonic—about two to four times the osmolarity of the original glomerular filtrate. Therefore, the tubular fluid becomes more concentrated as it flows into the inner medulla.
In the ascending limb of the loop of Henle, especially in the thick segment, sodium, potassium, and chloride are avidly reabsorbed. However, this portion of the tubular segment is impermeable to water, even in the presence of large amounts of ADH. Therefore, the tubular fluid becomes more dilute as it flows up the ascending loop of Henle into the early distal tubule, with the osmolarity decreasing progressively to about 100 mOsm/L by the time the fluid enters the early distal tubular segment. Thus , regardless of whether ADH is present or absent , fluid leaving the early distal tubular segment is hypo-osmotic , with an osmolarity of only about one-third the osmolarity of plasma.
As the dilute fluid in the early distal tubule passes into the late distal convoluted tubule, cortical collecting duct, and medullary collecting duct, there is additional reabsorption of sodium chloride. In the absence of ADH, this portion of the tubule is also impermeable to water, and the additional reabsorption of solutes causes the tubular fluid to become even more dilute, decreasing its osmolarity to as low as 50 mOsm/L. The failure to reabsorb water and continued reabsorption of solutes lead to a large volume of dilute urine.
To summarize, the mechanism for forming dilute urine is to continue reabsorbing solutes from the distal segments of the tubular system while reducing water reabsorption. In healthy kidneys, fluid leaving the ascending loop of Henle and early distal tubule is always dilute, regardless of the level of ADH. In the absence of ADH, the urine is further diluted in the late distal tubule and collecting ducts, and a large volume of dilute urine is excreted.
The ability of the kidney to form concentrated urine is essential for survival of mammals that live on land, including humans. Water is continuously lost from the body through various routes, including the lungs by evaporation into the expired air, the gastrointestinal tract by way of the feces, the skin through evaporation and perspiration, and the kidneys through excretion of urine. Fluid intake is required to match this loss, but the ability of the kidneys to form a small volume of concentrated urine minimizes the fluid intake required to maintain homeostasis, a function that is especially important when water is in short supply.
When there is a water deficit in the body, the kidneys form concentrated urine by continuing to excrete solutes while increasing water reabsorption and decreasing the urine volume. The human kidney can produce a maximal urine concentration of 1200 to 1400 mOsm/L, four to five times the osmolarity of plasma.
Some desert animals, such as the Australian hopping mouse, can concentrate urine to as high as 10,000 mOsm/L. This ability allows the mouse to survive in the desert without drinking water; sufficient water can be obtained through the ingested food and water produced in the body by metabolism of the food. Animals adapted to freshwater environments usually have minimal urine-concentrating ability. Beavers, for example, can concentrate the urine only to about 500 mOsm/L.
The maximal concentrating ability of the kidney dictates how much urine volume must be excreted each day to rid the body of metabolic waste products and electrolytes that are ingested. A normal 70-kg person must excrete about 600 milliosmoles of solute each day. If the maximal urine concentrating ability is 1200 mOsm/L, the minimal volume of urine that must be excreted, called the obligatory urine volume, can be calculated as follows:
This minimal loss of volume in the urine contributes to dehydration, along with water loss from the skin, respiratory tract, and gastrointestinal tract, when water is not available to drink.
The limited ability of the human kidney to concentrate the urine to only about 1200 mOsm/L explains why severe dehydration occurs if one attempts to drink seawater. Sodium chloride concentration in the ocean averages about 3.0% to 3.5%, with an osmolarity between about 1000 and 1200 mOsm/L. Drinking 1 liter of seawater with a concentration of 1200 mOsm/L would provide a total sodium chloride intake of 1200 milliosmoles. If the maximal urine concentrating ability is 1200 mOsm/L, the amount of urine volume needed to excrete 1200 milliosmoles would be 1.0 liter. Why then does drinking seawater cause dehydration? The answer is that the kidney must also excrete other solutes, especially urea, which contribute about 600 mOsm/L when the urine is maximally concentrated. Therefore, the maximum concentration of sodium chloride that can be excreted by the kidneys is about 600 mOsm/L. Thus, for every liter of seawater ingested, 1.5 liters of urine volume would be required to rid the body of 1200 milliosmoles of sodium chloride ingested in addition to 600 milliosmoles of other solutes, such as urea. This would result in a net fluid loss of 0.5 liter for every liter of seawater, explaining the rapid dehydration that occurs in shipwreck victims who drink seawater. However, a shipwreck victim’s pet Australian hopping mouse could drink seawater with impunity.
Urine specific gravity is often used in clinical settings to provide a rapid estimate of urine solute concentration. The more concentrated the urine, the higher the urine specific gravity. In most cases, urine specific gravity increases linearly with increasing urine osmolarity ( Figure 29-3 ). Urine specific gravity, however, is a measure of the weight of solutes in a given volume of urine and is therefore determined by the number and size of the solute molecules. In contrast, osmolarity is determined only by the number of solute molecules in a given volume.
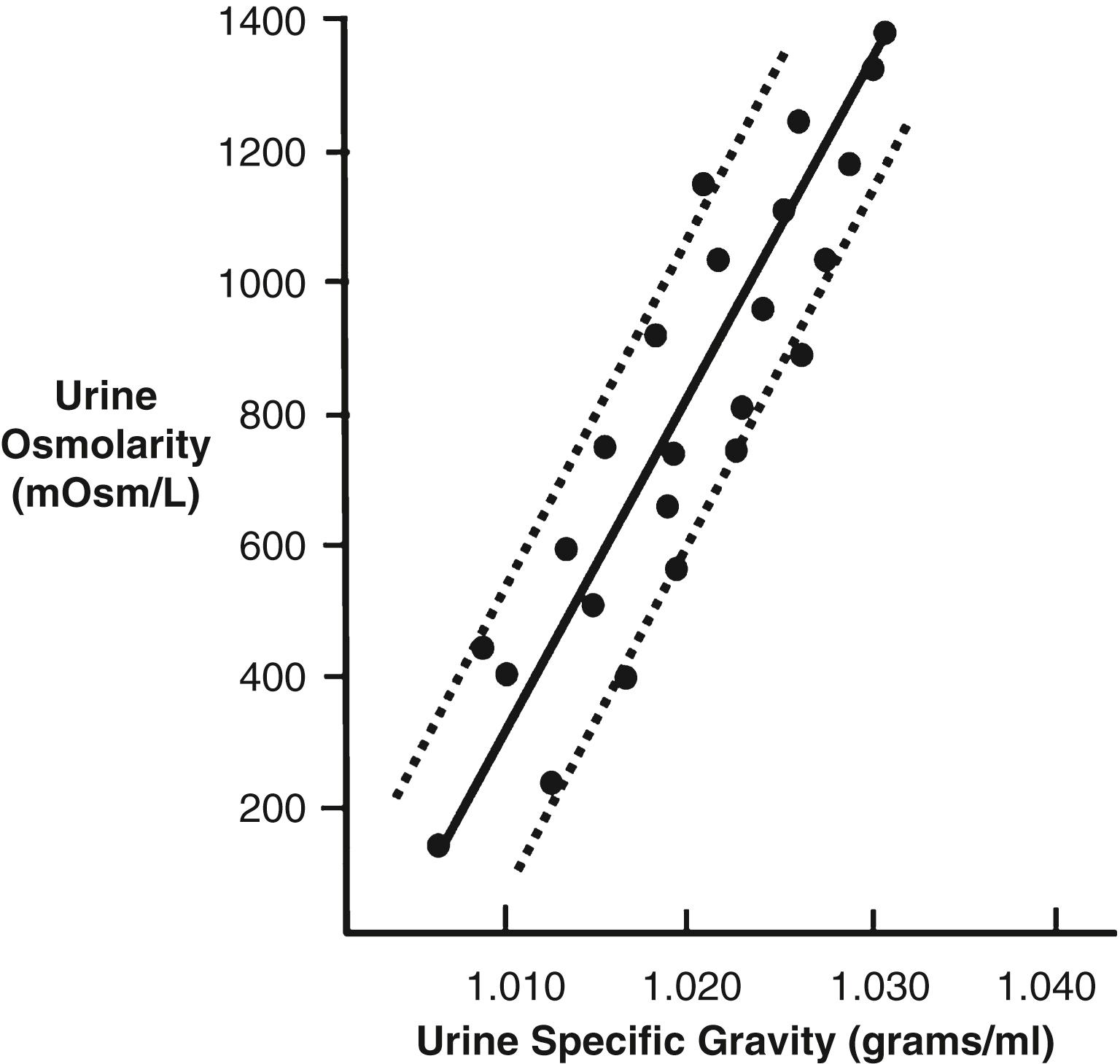
Urine specific gravity is generally expressed in grams per milliliter (g/ml) and, in humans, normally ranges from 1.002 to 1.028 g/ml, rising by 0.001 for every 35- to 40-mOsm/L increase in urine osmolarity. This relationship between specific gravity and osmolarity is altered when there are significant amounts of large molecules in the urine, such as glucose, radiocontrast media used for diagnostic purposes, or some antibiotics. In these cases, urine specific gravity measurements may falsely suggest a highly concentrated urine, despite a normal urine osmolality.
Dipsticks are available that measure approximate urine specific gravity, but most laboratories measure specific gravity with a refractometer .
The basic requirements for forming a concentrated urine are (1) a high level of ADH , which increases the permeability of the distal tubules and collecting ducts to water, thereby allowing these tubular segments to avidly reabsorb water; and (2) a high osmolarity of the renal medullary interstitial fluid , which provides the osmotic gradient necessary for water reabsorption to occur in the presence of high levels of ADH.
The renal medullary interstitium surrounding the collecting ducts is normally hyperosmotic, so when ADH levels are high, water moves through the tubular membrane by osmosis into the renal interstitium; from there it is carried away by the vasa recta back into the blood. Thus, the urine-concentrating ability is limited by the level of ADH and by the degree of hyperosmolarity of the renal medulla. We discuss the factors that control ADH secretion later, but for now, what is the process whereby renal medullary interstitial fluid becomes hyperosmotic? This process involves the operation of the countercurrent multiplier mechanism .
The countercurrent multiplier mechanism depends on the special anatomical arrangement of the loops of Henle and vasa recta, the specialized peritubular capillaries of the renal medulla . In humans, about 25% of the nephrons are juxtamedullary nephrons , with loops of Henle and vasa recta that go deeply into the medulla before returning to the cortex. Some of the loops of Henle dip all the way to the tips of the renal papillae that project from the medulla into the renal pelvis. Paralleling the long loops of Henle are the vasa recta, which also loop down into the medulla before returning to the renal cortex. And finally, the collecting ducts, which carry urine through the hyperosmotic renal medulla before it is excreted, also play a critical role in the countercurrent mechanism.
The osmolarity of interstitial fluid in almost all parts of the body is about 300 mOsm/L, which is similar to the plasma osmolarity. (As discussed in Chapter 25 , the corrected osmolar activity, which accounts for intermolecular attraction, is about 282 mOsm/L.) The osmolarity of the interstitial fluid in the medulla of the kidney is much higher and may increase progressively to about 1200 to 1400 mOsm/L in the pelvic tip of the medulla. This means that the renal medullary interstitium has accumulated solutes in great excess of water. Once the high solute concentration in the medulla is achieved, it is maintained by a balanced inflow and outflow of solutes and water in the medulla.
The major factors that contribute to the buildup of solute concentration into the renal medulla are as follows:
Active transport of sodium ions and co-transport of potassium, chloride, and other ions out of the thick portion of the ascending limb of the loop of Henle into the medullary interstitium
Active transport of ions from the collecting ducts into the medullary interstitium
Facilitated diffusion of urea from the inner medullary collecting ducts into the medullary interstitium
Diffusion of only small amounts of water from the medullary tubules into the medullary interstitium—far less than the reabsorption of solutes into the medullary interstitium
The transport characteristics of the loops of Henle are summarized in Table 29-1 , along with the properties of the proximal tubules, distal tubules, cortical collecting tubules, and inner medullary collecting ducts.
| Structure | Active NaCl Transport | Permeability | ||
|---|---|---|---|---|
| H 2 O | NaCl | Urea | ||
| Proximal tubule | ++ | ++ | + | + |
| Thin descending limb | 0 | ++ | + | + |
| Thin ascending limb | 0 | 0 | + | + |
| Thick ascending limb | ++ | 0 | 0 | 0 |
| Distal tubule | + | +ADH | 0 | 0 |
| Cortical collecting tubule | + | +ADH | 0 | 0 |
| Inner medullary collecting duct | + | +ADH | 0 | +ADH |
A major reason for the high medullary osmolarity is active transport of sodium and co-transport of potassium, chloride, and other ions from the thick ascending loop of Henle into the interstitium. This pump is capable of establishing about a 200-mOsm/L concentration gradient between the tubular lumen and interstitial fluid. Because the thick ascending limb is virtually impermeable to water, the solutes pumped out are not followed by osmotic flow of water into the interstitium. Thus, the active transport of sodium and other ions out of the thick ascending loop adds solutes in excess of water to the renal medullary interstitium. There is some passive reabsorption of sodium chloride from the thin ascending limb of Henle’s loop, which is also essentially impermeable to water, adding further to the high solute concentration of the renal medullary interstitium.
The descending limb of Henle’s loop, in contrast to the ascending limb, is very permeable to water, and the tubular fluid osmolarity quickly becomes equal to the renal medullary osmolarity. Therefore, water diffuses out of the descending limb of Henle’s loop into the interstitium, and the tubular fluid osmolarity gradually rises as it flows toward the tip of the loop of Henle.
Keeping in mind these characteristics of the loop of Henle, let us now discuss how the renal medulla becomes hyperosmotic ( ). First, assume that the loop of Henle is filled with fluid having a concentration of 300 mOsm/L, the same as that leaving the proximal tubule ( Figure 29-4 , step 1). Next, the active ion pump of the thick ascending limb on the loop of Henle reduces the concentration inside the tubule and raises the interstitial concentration; this pump establishes a 200-mOsm/L concentration gradient between the tubular fluid and interstitial fluid ( Figure 29-4 , step 2). The limit to the gradient is about 200 mOsm/L because paracellular diffusion of ions back into the tubule eventually counterbalances the transport of ions out of the lumen when the 200-mOsm/L concentration gradient is achieved.
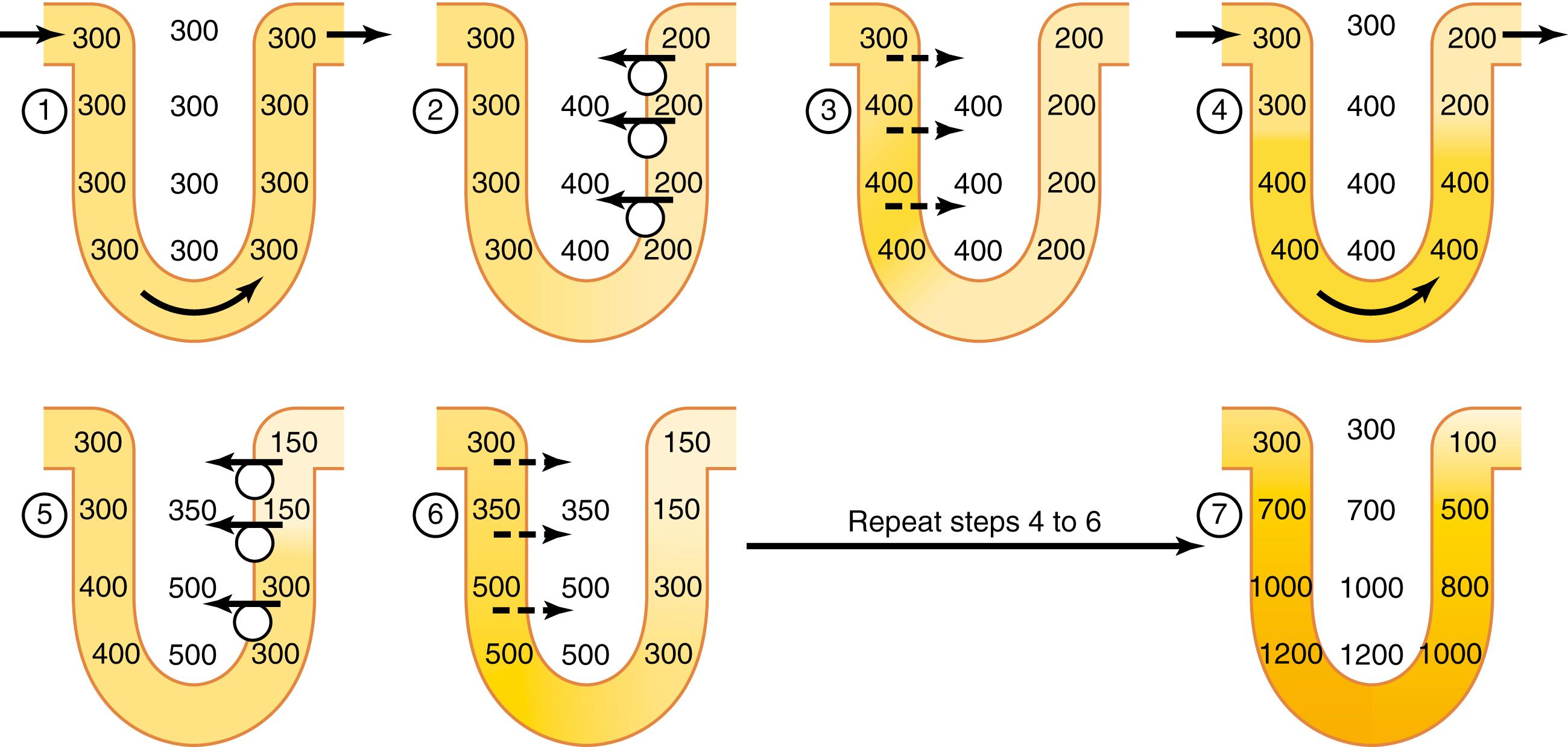
Step 3 is that the tubular fluid in the descending limb of the loop of Henle and interstitial fluid quickly reaches osmotic equilibrium due to osmosis of water out of the descending limb. The interstitial osmolarity is maintained at 400 mOsm/L because of continued transport of ions out of the thick ascending loop of Henle. Thus, by itself, active transport of sodium chloride out of the thick ascending limb is capable of establishing only a 200-mOsm/L concentration gradient, which is much less than that achieved by the countercurrent multiplier system.
Step 4 is the additional flow of fluid into the loop of Henle from the proximal tubule, which causes the hyperosmotic fluid previously formed in the descending limb to flow into the ascending limb. Once this fluid is in the ascending limb, additional ions are pumped into the interstitium and water remains in the tubular fluid until a 200-mOsm/L osmotic gradient is established, and the interstitial fluid osmolarity rises to 500 mOsm/L (step 5). Then, once again, fluid in the descending limb reaches equilibrium with the hyperosmotic medullary interstitial fluid (step 6) and, as the hyperosmotic tubular fluid from the descending limb of the loop of Henle flows into the ascending limb, still more solute is continuously pumped out of the tubules and deposited into the medullary interstitium.
These steps are repeated over and over, with the net effect of adding more and more solute to the medulla in excess of water. With sufficient time, this process gradually traps solutes in the medulla and multiplies the concentration gradient established by the active pumping of ions out of the thick ascending loop of Henle , eventually raising the interstitial fluid osmolarity to 1200 to 1400 mOsm/L, as shown in step 7 .
Thus, the repetitive reabsorption of sodium chloride by the thick ascending loop of Henle and continued inflow of new sodium chloride from the proximal tubule into the loop of Henle is called the countercurrent multiplier . The sodium chloride reabsorbed from the ascending loop of Henle keeps adding to the newly arrived sodium chloride, thus “multiplying” its concentration in the medullary interstitium.
When the tubular fluid leaves the loop of Henle and flows into the distal convoluted tubule in the renal cortex, the fluid is dilute, with an osmolarity of only about 100 to 140 mOsm/L ( Figure 29-5 ). The early distal tubule further dilutes the tubular fluid because this segment, like the ascending loop of Henle, actively transports sodium chloride out of the tubule but is relatively impermeable to water.
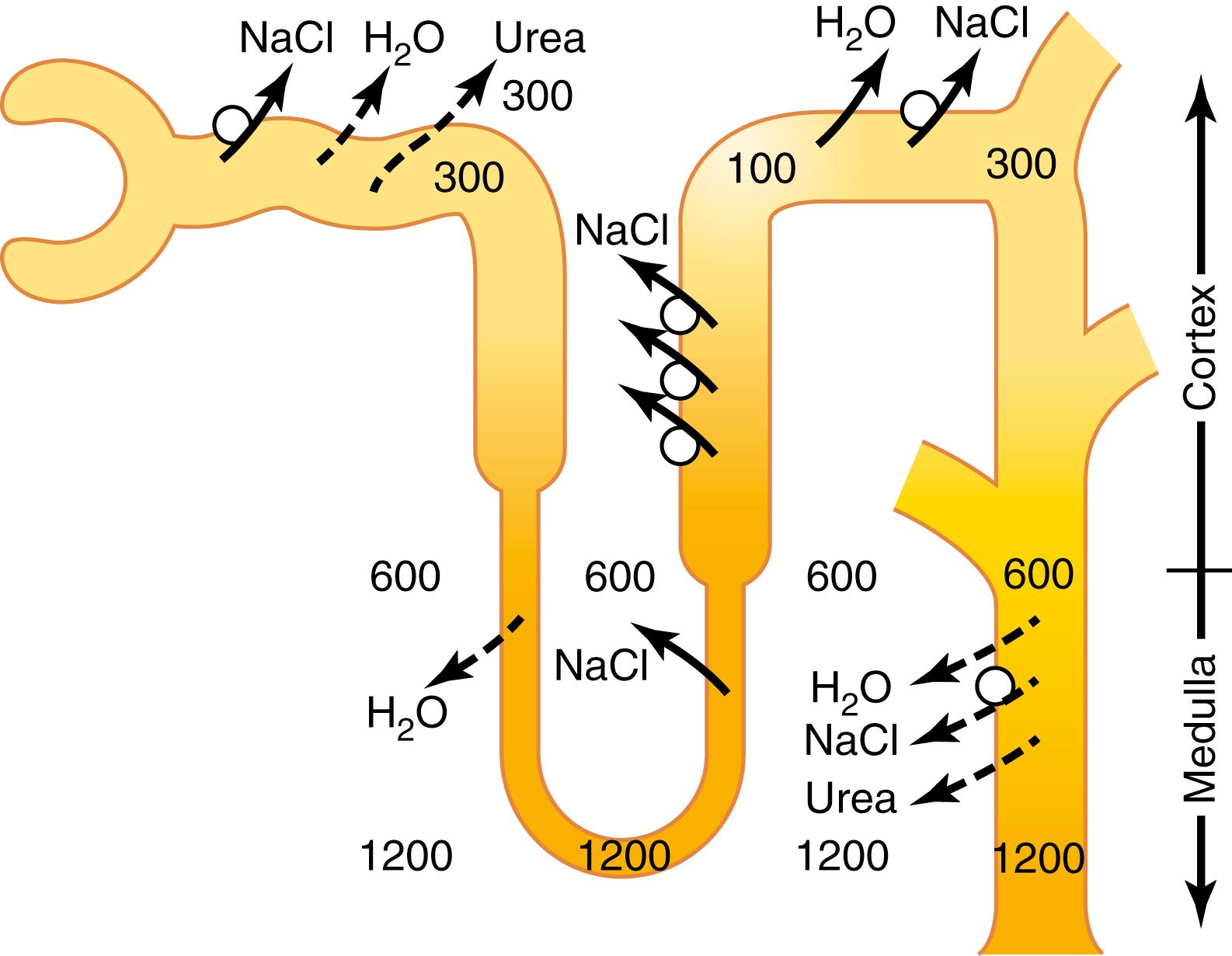
As fluid flows into the cortical collecting tubule, the amount of water reabsorbed is critically dependent on the plasma concentration of ADH. In the absence of ADH, this segment is almost impermeable to water and fails to reabsorb water but continues to reabsorb solutes and further dilutes the urine. When there is a high concentration of ADH, the cortical collecting tubule becomes highly permeable to water, so large amounts of water are now reabsorbed from the tubule into the cortex interstitium, where it is swept away by the rapidly flowing peritubular capillaries. Because large amounts of water are reabsorbed into the cortex , rather than into the renal medulla , this helps preserve the high medullary interstitial fluid osmolarity .
As the tubular fluid flows along the medullary collecting ducts, there is further water reabsorption from the tubular fluid into the interstitium, but the total amount of water is relatively small compared with that added to the cortex interstitium. The reabsorbed water is carried away by the vasa recta into the venous blood. When high levels of ADH are present, the collecting ducts become permeable to water, so the fluid at the end of the collecting ducts has essentially the same osmolarity as the interstitial fluid of the renal medulla—about 1200 mOsm/L (see Figure 29-4 ). Thus, by reabsorbing as much water as possible, the kidneys form highly concentrated urine, excreting normal amounts of solutes in the urine while adding water back to the extracellular fluid and compensating for deficits of body water.
Urea contributes about 40% to 50% of the osmolarity (500–600 mOsm/L) of the renal medullary interstitium when the kidney is forming a maximally concentrated urine. Unlike sodium chloride, urea is passively reabsorbed from the tubule. When there is a water deficit and blood concentration of ADH is high, large amounts of urea are passively reabsorbed from the inner medullary collecting ducts into the interstitium.
The mechanism for reabsorption of urea into the renal medulla is as follows. As water flows up the ascending loop of Henle and into the distal and cortical collecting tubules, little urea is reabsorbed because these segments are impermeable to urea (see Table 29-1 ). In the presence of high concentrations of ADH, water is reabsorbed rapidly from the cortical collecting tubule, and the urea concentration increases rapidly because urea is not very permeant in this part of the tubule.
As the tubular fluid flows into the inner medullary collecting ducts, still more water reabsorption takes place, resulting in an even higher concentration of urea in the fluid. This high concentration of urea in the tubular fluid of the inner medullary collecting duct causes urea to diffuse out of the tubule into the renal interstitial fluid. This diffusion is greatly facilitated by specific urea transporters, UT-A1 and UT-A3 . These urea transporters are activated by ADH, increasing transport of urea out of the inner medullary collecting duct even more when ADH levels are elevated. The simultaneous movement of water and urea out of the inner medullary collecting ducts maintains a high concentration of urea in the tubular fluid and, eventually, in the urine, even though urea is being reabsorbed.
The fundamental role of urea in contributing to urine-concentrating ability is evidenced by the fact that people who ingest a high-protein diet, yielding large amounts of urea as a nitrogenous waste product, can concentrate their urine much better than people whose protein intake and urea production are low. Malnutrition is associated with a low urea concentration in the medullary interstitium and considerable impairment of urine-concentrating ability.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here