Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The urethra serves as a conduit for urine from the urinary bladder to the exterior through the external urethral meatus. In males it also serves as a conduit for semen. The epithelium of the urethra is derived from the urogenital sinus, which is formed when the endodermal cloaca divides into the rectum dorsally and the urogenital sinus ventrally, separated by the urorectal septum. In females the epithelium of the urethra is derived from the endoderm of the urogenital sinus, whereas the surrounding connective tissue and smooth muscle arise from splanchnic mesenchyme. In males the epithelium also is derived from the urogenital sinus, except in the fossa navicularis, where it is derived from ectodermal cells migrating from the glans penis. As in females, the connective tissue and smooth muscle surrounding the male urethra are derived from the splanchnic mesenchyme.
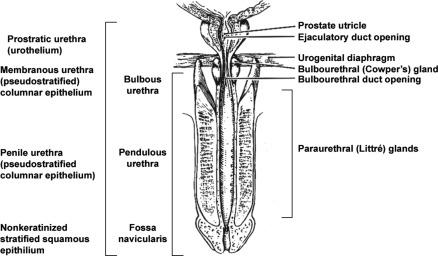
In men the urethra is 15 to 20 cm long and is divided into three anatomic segments ( Fig. 11.1 ). The prostatic urethra is approximately 3 to 4 cm long and begins at the internal orifice at the bladder neck and extends through the prostate to the prostatic apex. Most prostatic ducts open along the posterior and lateral walls of the prostatic urethra adjacent to the urethral crest, the longitudinal ridge along the dorsal wall of the prostatic urethra. In the central part of the urethral crest is an eminence called the verumontanum or colliculus seminalis. The verumontanum contains a slitlike opening that leads to an epithelial-lined sac called the prostatic utricle, a Müllerian vestige. The ejaculatory ducts empty into the urethra on either side of the prostatic utricle. The membranous urethra is the shortest segment, only 1 cm long. It extends from the prostatic apex to the bulb of the penis, traversing the musculature of the urethral sphincter and inferior fascia of the urogenital diaphragm. Cowper glands, small paired bulbomembranous urethral glands, are located on the left and right sides of the membranous urethra and secrete into it. The penile urethra is the longest segment (10 to 15 cm) and extends from the lower surface of the urogenital diaphragm to the urethral meatus in the glans penis. The orifices of the bulbomembranous urethral glands are located on the lateral surfaces of the proximal (bulbous) portion of the penile urethra. The penile urethra is surrounded by the corpus spongiosum along its length. Scattered mucus-secreting periurethral glands (Littré glands) are present at the periphery of the penile urethra except anteriorly.
The female urethra is approximately 4 cm long ( Fig. 11.2 ). At its periphery are paraurethral glands (Skene glands), which empty into the urethra through two ducts near the external urethral orifice.
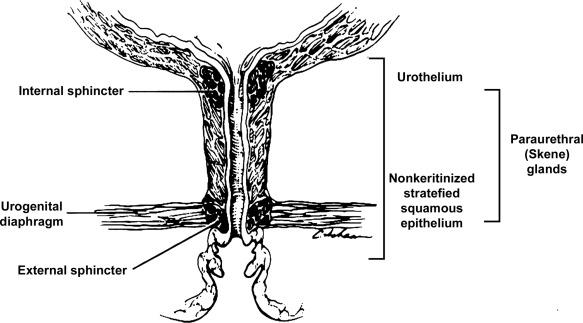
The type of epithelium lining the urethra varies along its length. In general, urothelium lines the prostatic urethra, pseudostratified columnar epithelium lines the membranous segment and most of the penile urethra, and nonkeratinized stratified squamous epithelium lines the fossa navicularis and external urethral orifice. In females the proximal third of the urethra is lined by urothelium and the distal two-thirds by nonkeratinized stratified squamous epithelium. However, it should be noted that most urethral tissues submitted for surgical pathologic examination are diseased or altered by instrumentation, both of which may cause metaplastic changes.
The lymphatic drainage of the male urethra arises from a rich mucosal network that extends the entire length of the urethra. This network is continuous proximally with that of the prostate and urinary bladder, and distally with that of the penis. The lymphatics of the prostatic and bulbomembranous segments drain to the obturator and medial external iliac lymph nodes, whereas those of the distal penile urethra drain to the superficial inguinal nodes. In females the proximal urethra drains to the external iliac, hypogastric, and obturator lymph nodes. The distal urethral lymphatics communicate freely with vulvar lymphatics and drain to the superficial inguinal nodes.
Several congenital anomalies may affect the urethra but are rarely encountered by surgical pathologists. Urethral valves are mucosal folds that project into the urethral lumen and may cause obstruction, hematuria, or inflammatory symptoms, although they are usually asymptomatic. Urethral valves are usually covered by normal urothelium but may be inflamed. The submucosa may also be inflamed and edematous. The so-called posterior urethral valves, usually seen in adult males, are associated with bladder neck hypertrophy. One study reported prenatal diagnosis in monochorionic twins.
Urethral diverticula are uncommon and often overlooked or misinterpreted. The overwhelming majority occur in women. They may be asymptomatic but can present with irritative symptoms or urinary incontinence, sometimes with localized pain. On physical examination diverticula present as a paraurethral mass that can sometimes be palpated through the vagina. It is thought that urethral diverticula may be either acquired or congenital, but there are no clear morphologic criteria to make this distinction. Most urethral diverticula in adults are acquired as sequelae of infection, trauma, calculi, obstruction, dilation, or inflammation of paraurethral glands.
Diverticula are usually lined by urothelium, although they often undergo squamous or glandular metaplasia. Nephrogenic adenoma may also arise in diverticula. The submucosa often is edematous and inflamed. Most patients with clinically apparent urethral diverticula have a major complication such as infection, stricture, lithiasis with subsequent obstruction, or carcinoma ( Fig. 11.3 ). The percentage of urethral diverticula that develop cancer is unclear, with reported incidence rates ranging from 2% to 15% of symptomatic diverticula. Carcinoma that develops in this setting is usually squamous cell carcinoma or adenocarcinoma, but may also be urothelial. Adenocarcinoma may be of the conventional type or of the clear cell type (see the section on clear cell carcinoma later in this chapter).
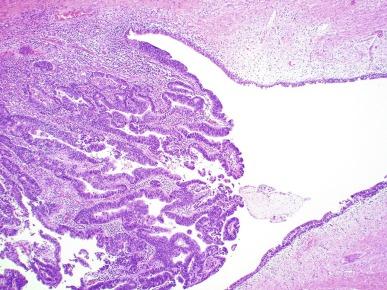
The main differential consideration for diverticulum is urethral cyst. An unusual case of endometriosis presented in a woman as a diverticulum.
Duplication of the urethra is rare and usually comes to the attention of the surgical pathologist at autopsy. The first description of a case of duplication of the urethra is attributed to Aristotle. Duplication of the urethra may be complete, extending from the bladder to the dorsum of the penis, or partial, extending from the dorsal surface or, less commonly, the ventral surface of the penis and ending blindly. Only 15% of cases of duplicated urethra, whether complete or partial, connect with the functional urethra. Most cases are asymptomatic, but the most common complication is infection. Patients may have urinary obstruction caused by compression of the functional urethra by a mass of desquamated material in the blind accessory urethra. In other cases, patients may complain of incontinence or double urinary stream.
Also known as fibroepithelial polyp, congenital urethral polyp, a rare lesion, occurs almost exclusively in males. Patients usually come to clinical attention between the ages of 3 and 9 years, but rarely they may present during infancy or adulthood. For this reason it has been suggested that congenital urethral polyp is secondary to poorly understood congenital defect in the urethral wall. Congenital urethral polyp usually arises in the prostatic urethra adjacent to the verumontanum (posterior urethral polyp). Signs and symptoms include hematuria, difficulty voiding, urinary retention, and infection. Symptoms are similar to other obstructing urethral lesions, including the urethral valve, stricture, and lithiasis.
Morphologically, congenital urethral polyp is covered by urothelium that may be inflamed, ulcerated, or exhibit squamous metaplasia. This differs from the more common prostatic urethral polyp occurring in adults, which is covered by prostatic epithelium (see the later Ectopic Prostatic Tissue and Prostatic Urethral Polyp section).
Anterior urethral polyp is rare and arises in the membranous or penile urethra. It produces the same symptoms and has the same morphology as posterior polyp. The subepithelial stroma consists of loose fibrous tissue that may be highly vascular and may contain a few fascicles of smooth muscle. If it has a long stalk, it may “telescope” into the bladder and produce bladder outlet obstruction.
Polyps in prepubertal girls and women probably arise from prolapsing urothelium that has evolved into a polyp.
Urethritis is defined morphologically as an inflammatory response within the urethra. In men they are usually asymptomatic, and the diagnosis is made by the presence of a urethral discharge and the finding of neutrophils in the urethral smear. Women are often symptomatic; the symptoms are like those of cystitis and include dysuria, urinary urgency, and urinary frequency. A urethral smear will also aid in the diagnosis in women. Urethritis may be caused by sexually transmissible agents such as Neisseria gonorrhoeae , Chlamydia trachomatis , Gardnerella vaginalis , Ureaplasma urealyticum , Mycoplasma hominis , Trichomonas vaginalis , and Candida species. In women, urethritis secondary to Neisseria , Trichomonas , or Candida rarely occurs without concomitant cervical infection.
Reiter syndrome is characterized by the triad of urethritis, conjunctivitis, and arthritis. The cause is uncertain, but it is usually preceded by an enteric or venereal infection. The syndrome occurs predominantly in men between the ages of 18 and 40 years, but women occasionally are affected. Urethritis is the most common initial symptom. Other urologic manifestations of Reiter syndrome include prostatitis and hemorrhagic cystitis. In the acute phase the mucosa appears congested and may contain shallow ulcers. Symptoms commonly subside within 2 to 4 weeks but recur at irregular intervals in 50% to 75% of cases. It is important to recognize that not all involved organ systems may be symptomatic at the same time, so this syndrome should always be included in the differential diagnosis of urethritis in young adults.
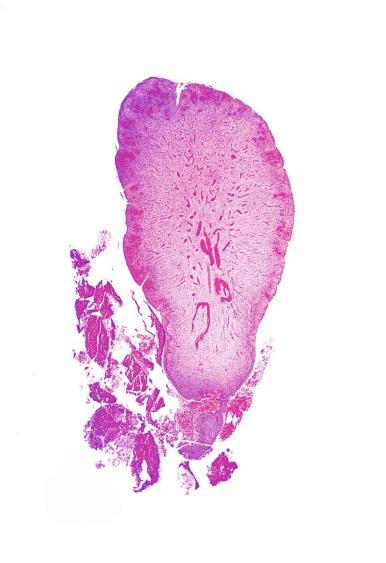
Urethral caruncle is a pedunculated or sessile polypoid lesion in women located in the distal urethra near the meatus. Grossly, it has a fleshy, pink-red appearance and bleeds readily ( Fig. 11.4 ). Patients may be asymptomatic, although commonly they experience dysuria, urinary frequency, or obstructive symptoms. Three histologic subgroups are described: papillomatous, angiomatous, and granulomatous. This separation is based on the most prominent component (surface epithelial, vascular, and inflammatory, respectively), but this distinction has no apparent clinical relevance. The surface epithelium may be urothelial or squamous and is invariably inflamed ( Fig. 11.5 ); caruncle covered by metaplastic columnar epithelium has been reported. The epithelium may be hyperplastic and constitute the bulk of the lesion. The underlying stroma is richly vascular and inflamed, occasionally containing glandular elements thought to be derived from Skene glands.
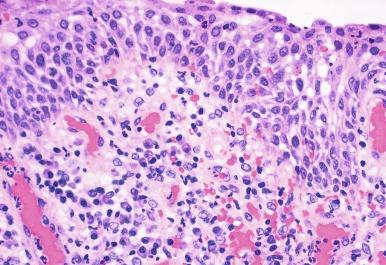
Rarely the stroma of urethral caruncle may contain atypical mesenchymal cells, actually reactive myofibroblasts, mimicking sarcoma (pseudosarcomatous fibromyxoid tumor) ( Fig. 11.6 ). The mixed inflammatory infiltrate and rich vascularity, in combination with the clinical setting, should establish the correct diagnosis. Pseudosarcomatous fibromyxoid tumor may appear spontaneously or follow a pelvic surgical procedure by weeks or months (postoperative spindle cell nodule) and present not only as a polypoid lesion but also as a paraurethral mass. Like other pseudosarcomatous lesions involving the urothelial tract, these atypical but reactive myofibroblasts may display cytokeratin, actin, and IgG-4. Epithelial membrane antigen is invariably negative.
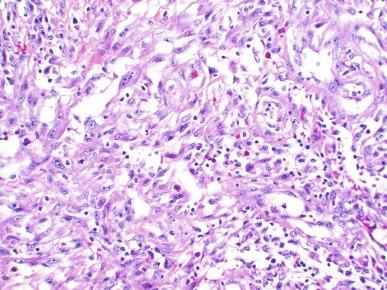
Polypoid urethritis is the urethral counterpart of polypoid cystitis, although an association with indwelling catheter has not been noted with urethral lesions. Polypoid urethritis is a nonneoplastic inflammatory lesion that usually resolves spontaneously after removal of the inflammatory stimulus. It is commonly found in the prostatic urethra near the verumontanum, appearing as single or multiple polypoid or papillary growths. Morphologically it is characterized by abundant edematous stroma containing distended blood vessels and a chronic inflammatory infiltrate ( Fig. 11.7 ). The overlying urothelium may be ulcerated or exhibit metaplastic and proliferative changes such as squamous metaplasia, Brunn nests, or urethritis cystica.
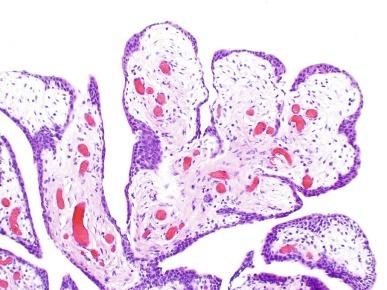
Polypoid urethritis does not usually recur after resection unless the cause of the irritation persists. At the time of urethroscopy, it may be confused with papillary urothelial tumor, although experienced urologists will recognize it as a benign, reactive, or low-grade lesion and rarely confuse it with high-grade, aggressive neoplasm.
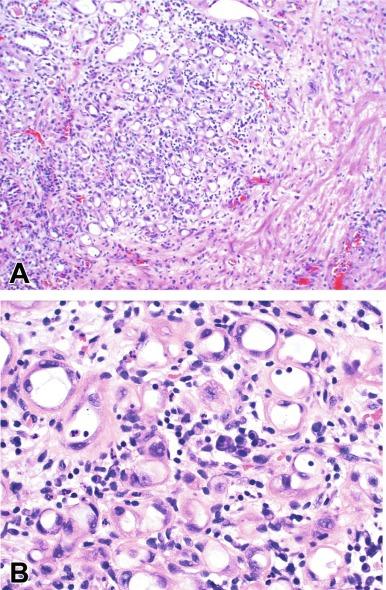
Similar to Brunn nests and urethritis cystica, in most cases, nephrogenic adenoma is a reactive, proliferative lesion that may occur anywhere along the urothelial tract as a consequence of local irritation. It is most common in the urinary bladder, but occasionally arises in the urethra. Nephrogenic adenoma is thought to arise through metaplasia of the urothelium in response to an inflammatory stimulus or local injury, and some investigators prefer the term nephrogenic metaplasia. However, Mazal et al. described cases of nephrogenic adenoma in patients who had undergone renal transplant from donors of the opposite sex and demonstrated that the lesions were of donor origin. Thus it is likely that some lesions represent implantations from shed renal tubules. Often this lesion is an incidental finding at surgery for other reasons. The most common symptom is hematuria. It appears grossly as flattened, erythematous areas or as discrete papillae. Microscopically the latter architecture consists of complex papillary structures covered by cuboidal epithelium with basophilic or eosinophilic cytoplasm that may be vacuolated. The nuclei are round to oval, hyperchromatic, centrally located, and may contain small nucleoli. Mitotic figures are uncommon. The same epithelium may form discrete tubules in the underlying stroma. These have distinct lumina that are usually empty but may contain deeply eosinophilic secretions or pale basophilic material ( Fig. 11.8 A and B). These tubules are thought to arise through a process of invagination from the surface epithelium, much like Brunn nests. Each is surrounded by a distinct basement membrane. Infrequently, cuboidal cells are present in the stroma singly or in small groups lacking a visible lumen, or they may have a signet ring cell appearance. The luminal secretions may be periodic acid–Schiff positive, diastase resistant, or mucicarminophilic, but intracytoplasmic mucin is less frequent. In a study of 26 cases of nephrogenic adenoma involving the prostatic urethra, Allan and Epstein found that 77% of cases extended into smooth muscle, which is not surprising given the anatomy of this site. The lesion often appears infiltrative and may be confused with adenocarcinoma, especially in cases lacking a papillary component and composed primarily of tubules in the stroma. The surrounding stroma may be edematous and inflamed, but there is no desmoplastic reaction to the epithelial cells. Allan and Epstein reported focal immunoreactivity for prostate-specific antigen and prostatic acid phosphatase in 36% and 55% of cases, respectively, although others have not confirmed this finding. However, strong PAX2/PAX8 and cytokeratin 7 positivity, combined with attention to the cytomorphologic features, should be sufficient to arrive at the correct diagnosis.
No convincing evidence has been reported that nephrogenic adenoma is a preneoplastic condition, although rare cases coincidentally coexist with or precede the development of carcinoma. Nevertheless, it is possible to have common predisposing conditions and consequently experience development of nephrogenic adenoma independently. For example, nephrogenic adenoma and adenocarcinoma have been reported in association with urethral diverticulum. Like other proliferative lesions of the urothelium, nephrogenic adenoma may recur after resection if the inflammatory stimulus is not removed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here