Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Clement Chu and Mark Theilmann provided helpful comments on the manuscript.
The core goal of cell-free DNA based prenatal testing (at its introduction called “NIPT”) is to provide minimally invasive, clinically accurate, and financially accessible screening for fetal chromosomal aneuploidies in the early stages of pregnancy. This goal poses certain important constraints: minimal invasiveness means the test must operate from analytes in a maternal blood sample; clinical accuracy requires that even small enrichments in placenta-derived cell-free DNA (cfDNA) must be detectable and attributable to specific genomic regions, and financial accessibility is possible only with modern, scalable genomic technologies. Next generation sequencing (NGS) satisfies each of these constraints and consequently is the chosen platform for the majority of cfDNA-based prenatal testing offerings. This chapter provides a general overview of how NGS technology works and focuses particularly on its application to cfDNA prenatal testing.
The term “next-generation sequencing” (NGS) begs the questions of what “first-generation sequencing” was and how NGS is both similar to and different from its predecessor. Sanger developed the first generation of DNA sequencing in the 1970s . His eponymous sequencing approach is an in vitro adaptation of the cellular replication machinery that cleverly leverages unextendable DNA bases. These modified bases are introduced at low concentration in a reaction minimally containing (1) a high concentration of extendable bases, (2) the single-stranded DNA template to be sequenced, (3) a short oligonucleotide primer complementary to the template onto which new bases could be synthesized, and (4) DNA polymerase enzymes that execute the extension reaction. Early Sanger sequencing experiments involved four independent reactions, each containing a single type of unextendable base (A, T, G, or C). Whenever a polymerase randomly incorporates one of the unextendable bases into the nascent DNA molecule (e.g., an unextendable G in the nascent strand incorporated opposite a C in the template), further synthesis would terminate, yielding a truncated copy of the template. Critically, since all nascent strands anchor from the same oligonucleotide primer, the position of extension termination—and hence the length of the nascent DNA strand—is a direct proxy for the base at the 3′ end of the molecule. By using gel electrophoresis to resolve the respective lengths of terminated molecules in each of the four reactions, it is possible to infer the sequence of the entire template.
Sanger sequencing became slightly more scalable with the introduction of unextendable bases that were uniquely dyed ( Fig. 1 ). Rather than achieving base-specific information by partitioning into four reactions, a capillary electrophoresis instrument coupled with a fluorescent dye detector could resolve both the relative sizes of fragments and the identity of their terminating bases . To criticize these machines as unscalable neglects one of their unmitigated triumphs: they were the workhorses that sequenced the very first human genome in the 1990s . However, with a cost in the billions of dollars and with a timeline on the order of years, genome sequencing would remain prohibitive in a clinical setting without a major technological leap.
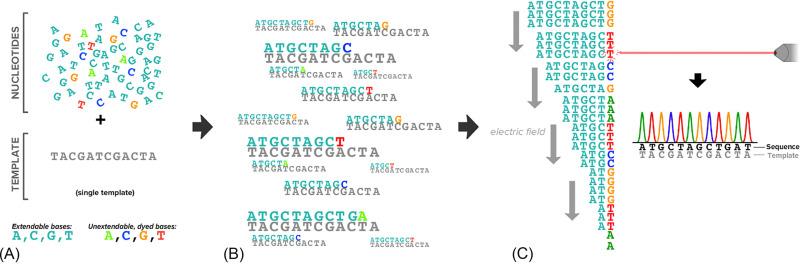
NGS revolutionized genome sequencing by overcoming many of the limitations of the Sanger technique , yet the most pervasive NGS methodology shares much in common with its predecessor. As described in further detail later, NGS also leverages extension termination and fluorescent bases, and it relies upon DNA polymerases that append a single base at a time to a nascent DNA molecule. Indeed, in many respects, an NGS experiment is comparable to performing millions or billions of Sanger reactions in parallel (hence the NGS moniker “massively parallel sequencing”). This explosive increase in throughput shattered some of the barriers (e.g., cost and turnaround time) that had largely prevented the use of genomics in routine clinical care, and it paved the way for cfDNA-based prenatal testing.
The role of an NGS device is to distill a specially prepared library of DNA molecules into a long text file of sequences, one line for each sequenced molecule. NGS sequencers perform this molecule-to-text mapping across many research and clinical contexts, spanning everything from RNA sequencing in broccoli to ribosome profiling in bacteria to DNA sequencing for NIPT in pregnant women . These applications of NGS are distinguished primarily by the steps upstream of DNA being injected into the sequencer, termed “library preparation.” Mirroring the diversity of upstream sample preparation methods is a comparably vast range of downstream analyses, one of which is the analysis method for NIPT, covered in detail in Chapter 3 . In addition to describing how an NGS machine sequences DNA, this section discusses NIPT-specific workflows upstream and downstream of sequencing.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here