Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The most common pneumoconioses are silicosis, coal workers' pneumoconiosis, and asbestosis (see Chapters 61 and 62 ). There are numerous rare pneumoconioses related to variable occupational dust exposures. Hard metal lung disease, aluminum pneumoconiosis, talc pneumoconiosis, welders' pneumoconiosis, and chronic beryllium disease are discussed here. The high-resolution computed tomography (HRCT) findings of these uncommon pneumoconioses vary and are relatively nonspecific; however, there are predominant and characteristic findings for each type of pneumoconiosis. The most common HRCT finding of pneumoconiosis consists of centrilobular nodular and branching linear opacities, consistent with a bronchiolocentric process. Interstitial fibrosis may be manifested as reticulation, traction bronchiectasis, honeycombing, or more confluent areas of high attenuation. Similar to the other pneumoconiosis, HRCT has higher sensitivity and specificity than chest radiography in evaluating pleuroparenchymal abnormalities and is often helpful in detecting the early parenchymal changes in each type of pneumoconiosis.
Hard metal is an alloy of tungsten carbide in a matrix of cobalt to which small amounts of titanium, nickel, chromium, niobium, vanadium, or molybdenum may be added. Hard metal is heat resistant and is as indestructible as diamond. Hard metal lung disease is a rare form of occupational lung disease that can occur in workers engaged in the manufacture, use, or maintenance of tools composed of hard metal. Cobalt is generally considered the main cause of hard metal lung disease, hence also the term cobalt lung. Some experimental studies have shown that biological reactivity to a mixture of cobalt and tungsten carbide is much greater than that to cobalt alone. Hard metal lung disease is more likely to occur in poorly regulated workplaces, but because its low prevalence in exposed workers and/or the lack of correlation with the intensity or duration of exposure, the existence of a possible genetic susceptibility to hard metal disease has been suggested.
Exposure to hard metal may result in three main respiratory complications: asthma, hypersensitivity pneumonitis, and/or pulmonary fibrosis. Hard metal workers occasionally develop symptoms of asthma that are similar in their clinical manifestations to other forms of occupational asthma. The typical symptoms of cough, wheeze, dyspnea, chest tightness, and rhinitis occur after approximately 4 to 6 hours at the workplace. The clinical manifestations of hypersensitivity pneumonitis resulting from cobalt are similar to the manifestations observed in other forms of hypersensitivity pneumonitis. In the more insidious forms of hard metal disease, the symptoms are those of any other fibrotic lung diseases, with cough, gradual shortness of breath, and weight loss. Cyanosis and finger clubbing are present in the more advanced stages of the disease. The disease can be fatal and sometimes rapidly progressive.
The general and nonspecific pathologic features of hard metal interstitial lung disease include infiltration of the interstitium with lymphocytes and plasma cells, hyperplasia of the alveolar epithelium, and occasionally cellular accumulation within the alveolar lumen (desquamative interstitial pneumonia). The lesions are bronchiolocentric, and the distribution is patchy. There are occasional descriptions of granulomas in the lungs of hard metal workers with interstitial lung disease.
The most characteristic finding in hard metal lung disease is the presence of multinucleated giant cells in the interstitium and alveolar airspaces ( Fig. 63.1 ). These cells may contain large numbers of nuclei and have been described as “bizarre” or “cannibalistic” on account of the presence of inclusions, which contain apparently phagocytosed cellular material (giant cell interstitial pneumonia).
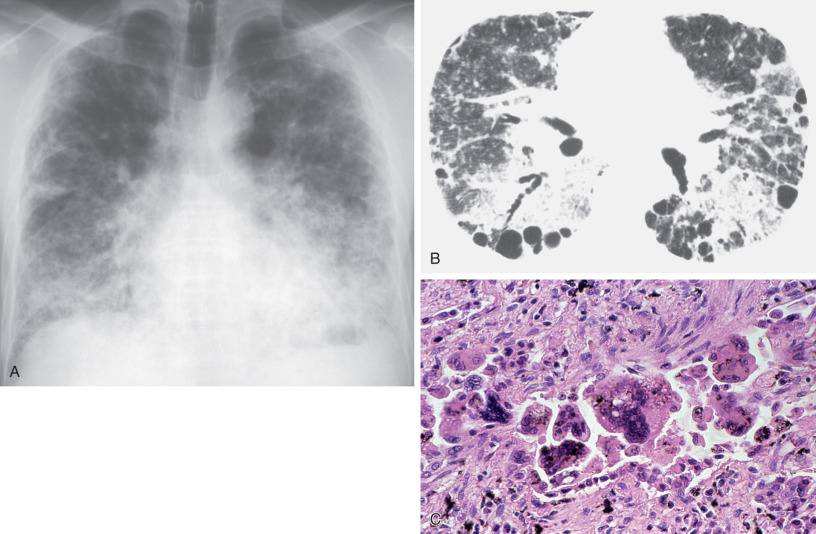
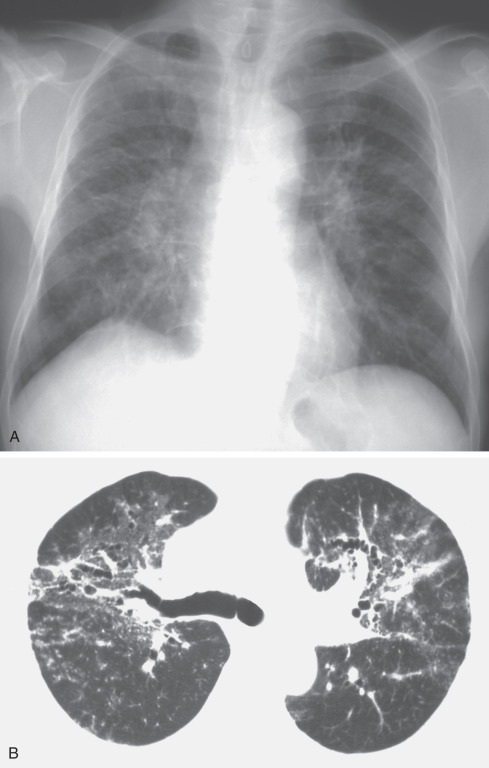
The most common radiographic abnormality consists of small irregular opacities, predominantly in the middle and lower lung zones ( Figs. 63.2 and 63.3 ; see Fig. 63.1 ). Other findings include small nodular opacities and diffuse reticulonodular or ground-glass opacities, particularly in subacute presentations. The chest radiograph may appear normal despite the presence of symptoms and functional impairment. During attacks of hard metal lung disease, a fine diffuse reticulonodular pattern can be seen.
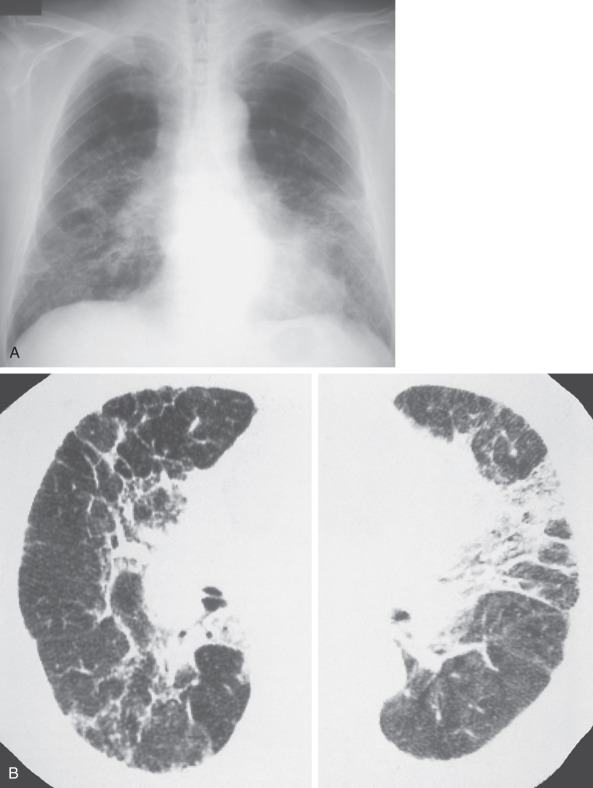
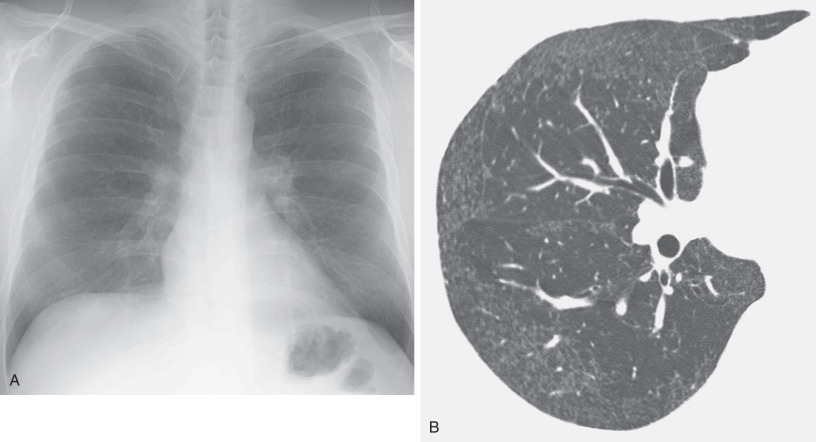
The HRCT appearance of hard metal interstitial lung disease varies and may mimic sarcoidosis, nonspecific interstitial pneumonia (NSIP), or usual interstitial pneumonia (UIP).
The findings of panlobular and multilobular ground-glass opacities or consolidation correspond histopathologically to areas of interstitial thickening caused by inflammatory cell infiltration and intraalveolar accumulation of macrophages and multinucleated giant cells. The findings of parenchymal distortion, traction bronchiectasis, and reticulation correspond histopathologically to areas of diffuse interstitial fibrosis and fibrosis around bronchovascular structures. Depending on the severity of the giant cell interstitial pneumonia, the findings may range from ground-glass opacities to dense consolidation (see Figs. 63.1, 63.2, and 63.3 ). In a fatal case of hard metal disease, which was characterized by a large amount of intraalveolar accumulation of macrophages and multinucleated giant cells within a few years of initial exposure, consolidation with lobular distribution was found.
Chest radiography is the imaging modality used in the initial assessment and follow-up of patients with hard metal lung disease. Further evaluation of the lung parenchyma can be obtained with CT. HRCT may be useful in detecting the early parenchymal changes in these patients.
The differential diagnosis includes all other types of interstitial pneumonias, drug-induced lung disease, sarcoidosis and other granulomatous lung disease that mimics sarcoidosis, hypersensitivity pneumonitis, and other pneumoconioses. As with any occupational disease, the cornerstone of the diagnosis is a comprehensive and detailed work history. Tungsten particles usually can be shown by energy-dispersive x-ray spectroscopy, and their presence confirms the diagnosis because tungsten is never identified as a background atmospheric contaminant in the general population. Particles of cobalt also may be found, but they are less common because cobalt is soluble in tissue fluid. A pathologic diagnosis of giant cell interstitial pneumonia is pathognomonic for hard metal lung disease. The presence of multinucleated cannibalistic giant cells in bronchoalveolar lavage (BAL) fluid also is diagnostic for hard metal lung disease, even without a surgical lung biopsy specimen. Concentrations of cobalt in the blood and urine may correlate well with levels of exposure, when measured within a few days after exposure.
Asthmatic symptoms and hypersensitivity pneumonitis usually can be easily controlled by eliminating exposure and appropriately treating the hypersensitivity reaction. In hard metal interstitial lung disease, the progression of the disease depends on the time of diagnosis and the continuation of the exposure. Apart from exposure cessation, no medical treatment has proven to be beneficial. In the early stage of disease, improvement and even complete remission may be obtained if the exposure ceases completely. The use of corticosteroids or cyclophosphamide has been reported; however, no controlled studies are available regarding the treatment of hard metal interstitial lung disease. Lung transplantation has been performed in patients with hard metal lung disease, and recurrence was observed in the transplanted lung.
Cobalt is the main cause of hard metal lung disease.
Three main respiratory effects are asthmatic reactions, hypersensitivity pneumonitis, and pulmonary fibrosis.
The most characteristic pathologic finding is the presence of “bizarre” or “cannibalistic” multinucleated giant cells, which are often also present in bronchoalveolar lavage fluid.
Common radiologic findings include the following:
Panlobular and multilobular ground-glass opacities or consolidation corresponding histopathologically to areas of intraalveolar accumulation of macrophages and multinucleated giant cells
Parenchymal distortion, traction bronchiectasis, and reticulation corresponding histopathologically to areas of interstitial fibrosis
Exposure to aluminum, alumina, and potroom fumes has been associated with diffuse interstitial fibrosis; however, the exact etiologic agent of aluminum-induced fibrosis is uncertain. Stearin-coated, mineral oil–coated, and bare aluminum metal all may be equally effective in producing fibrosis. Although exposure to aluminum metal and its oxides in the workplace is common, pneumoconioses attributable to these agents are rare, ranging from 1% to 8% of exposed employees. Work in aluminum smelters is associated with the development of asthma and chronic airflow obstruction.
Clinical symptoms in patients with pulmonary fibrosis usually consist of cough and dyspnea on exertion. Pulmonary function test results are consistent with a restrictive disease process with reduced lung volumes and decreased diffusing capacity of lung for carbon monoxide.
Histologic examination of lung tissue samples shows subpleural and interstitial fibrosis with scar emphysema and spotted granulomatous pneumonitis with giant cells ( Fig. 63.4 ). Energy-dispersed x-ray analysis shows high interstitial concentrations of aluminum. Diffuse interstitial fibrosis attributable to aluminum exposure is usually most severe in the upper lung zones, although it may be diffuse. Such a distribution is distinct from that of idiopathic pulmonary fibrosis or asbestosis. Desquamative interstitial pneumonia, granulomatous lung reaction, and pulmonary alveolar proteinosis have been described after exposure to fumes from aluminum welding.
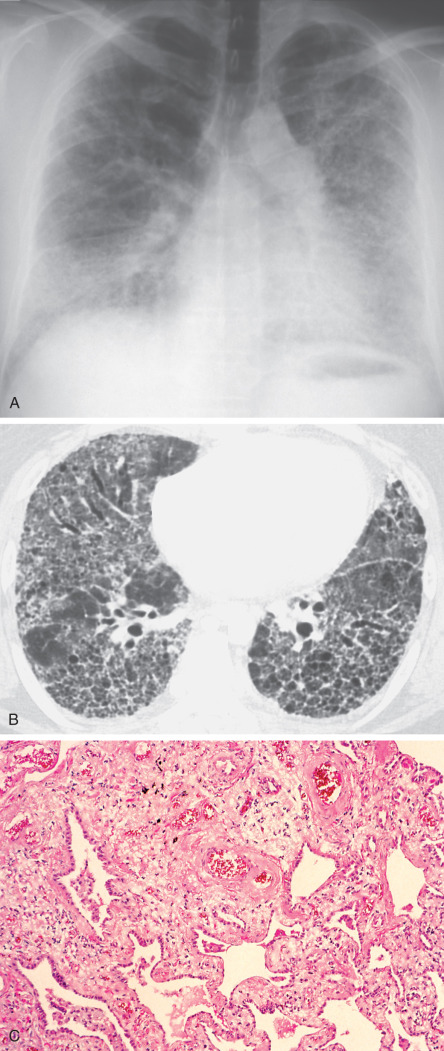
Radiographic abnormalities may become apparent after a few months or several years of exposure. Typical findings include bilateral hazy opacities or small nodular opacities more pronounced in the upper or middle lung zones and widening of the mediastinal shadow ( Fig. 63.5 ). Emphysematous changes in the basal lung and displacement of the hila upward or backward, indicating pulmonary contracture, characterize advanced cases. The reticulation and honeycombing sometimes may be diffuse (see Fig. 63.4 ). Aluminum-associated pulmonary fibrosis is associated with a high frequency of secondary spontaneous pneumothorax.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here