Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ultrasound of the Thyroid and Parathyroid Glands: Equipment and Techniques.
The clinical utilization of ultrasound (US) continues to expand, with increasing applications in the head and neck. US remains the primary imaging tool for the thyroid gland. It is cost effective, rapid in information accrual, well-tolerated by patients, and helps patients avoid unnecessary radiation exposure. The image processing capabilities of current US machines used in tandem with high-frequency transducers can provide unmatched resolution and anatomic detail. Even small, portable systems are now able to provide meaningful imaging information. Thus US has become an indispensable tool of the clinician in the outpatient setting, and it represents an extension of the physical examination. The machines have become more affordable and portable, and there are numerous formal educational opportunities to learn the technology and its applications. In-office point of care US has many advantages for the patient; a single patient’s in-office visit may include both diagnostic and interventional US procedures, which can help maximize efficiency and convenience. Specific to the thyroid gland and its associated pathologies, US has become integral to initial assessment and workup, preoperative planning, and long-term follow-up and surveillance.
Although serum thyroglobulin (Tg) is generally considered as a sensitive marker for follicular cell derived thyroid carcinoma, it is not helpful in two clinical scenarios. First, some patients have serum antibodies directed against thyroglobulin, and this interferes with standard Tg measurement assays. Second, certain more aggressive papillary cancer histologies (poorly differentiated tall cell variant) do not generally secrete Tg, which makes it less useful to identify recurrence; in this situation, detection of locoregional recurrence may depend primarily on US. Before the mid-1990s, preoperative imaging in hyperparathyroidism was limited. Nuclear scans and US technology were both relatively low in resolution. The advent of sestamibi enabled surgeons performing parathyroidectomy to have a strong level of confidence in the prediction of the precise nature of disease and its location. The operating surgeon has a strong incentive to identify the offending enlarged parathyroid gland with complete preop US examination; the operating surgeon can look for atypical locations of the parathyroid gland, such as intrathyroidal, as well as lesions within the upper thymus, carotid sheath, para-esophagus, and undescended in the upper neck. Intrathyroidal parathyroid adenomas should be identified before surgery as a result of systematic US.
This chapter discusses the basics of US and many of its clinical applications to surgery of the thyroid and parathyroid glands. Although it is a marvelous tool that rightfully has entered the province of the clinician, it does have some limitations. In the beginning, US is time consuming. The equipment can be expensive. Some patients have thick neck tissues or abnormal anatomy, either of which can render the images difficult to interpret. When a parathyroid gland is posterior to the esophagus or low in the mediastinum, US will not be able to detect it. Certification and mentorship opportunities may be limited for some surgeons; radiologists in the hospital or community may exercise an objection to use of this modality by clinicians. In spite of these few and mostly surmountable issues, US has become an indispensable tool for the thyroid and parathyroid surgeon; US complements the surgeon’s passion for anatomy and allows a view of the relevant vascular, muscular, endocrine, lymphatic, and aerodigestive structures in real time with minimal effort.
The US system has three basic components: (1) the transducer, which delivers and receives sound energy directly to and from the tissues; (2) the console, which among other things contains the sophisticated computer software, conversion algorithms, storage, and Doppler methodology; and (3) the display, which permits the observer to view and select anatomic areas of interest. An understanding of how sound energy and tissues interact has permitted a marriage of theory and technology. Whereas artifacts with other radiologic modalities (e.g., dental restoration production of scatter and degradation of computed tomography [CT] images) present an unwanted problem, artifacts in US enhance clinical information, which can be used to advantage.
As with many technological advances in photography and computer imaging, resolution in US has reached such a level of sophistication and miniaturization that portability has become a reality. Whereas expensive large systems, by necessity, resided in radiology departments, now clinicians are able to adapt US for use in multiple offices and even in the operating room. It is easy to forget that these machines are remarkably sophisticated beneath their user-friendly interface to the clinician.
Sound waves travel relatively slowly in comparison to light and require some form of support medium. When sound waves travel through air, the relative lack of density of the transferring molecules slows transport and causes attenuation. In contrast, sound waves in fluid travel over long distances with much less degradation, depending on their frequency. The process of sono localization in many sea creatures, such as dolphins and whales, serves both to communicate with others of the same species and to receive feedback information from emitted sounds to locate food. Dolphins and other odontocetes such as toothed whales emit single high-frequency clicks for echolocation. Many other animals, including bats, owls, and elephants, emit and receive sound waves for localization and communication.
Many of the applied concepts have been extracted and simplified from the most commonly referenced text by Kremkau, Diagnostic Ultrasound, Principles and Instruments . Sound is transmitted as linked sine waves with peak and trough elements, the height of which represents amplitude or loudness. A single full cycle is measured from peak to peak or trough to trough, and the number of these cycles per 1-second unit of time represents the frequency. The human ear can recognize sounds as low as 20 hertz (Hz) and as high as 20,000 Hz or 20 kilohertz (20 kHz). US is so named because its frequency emission is well above these frequency levels, in the range of millions of cycles per second or megahertz (MHz). In fact, US systems used in medicine vary from 3 to 17 MHz with advantages and disadvantages for selected emissions in low, intermediate, or high ranges. Just as a fluid wave along the surface of a body of water is generated from the physical deformation produced by a dropped pebble, US waves are transmitted to human tissues through the transducer by physical deformation. One of the key elements of the transducer is the piezoelectric crystal, which elongates and shortens in response to applied alternating electrical current. The actual physical change in these crystals imparts vibrational contact with the skin and deeper tissues to produce a transmitting wave. Special backing materials surrounding the transducer, except for the surface of the footprint that touches the skin, prevent random scatter of the wave energy. This design produces directional transmission. In a linear array transducer where the crystals are oriented such that emitted waves are perpendicular to its footprint, commonly a composite of 128 individual crystals in parallel array will generate the signal. Each crystal vibrates in synchrony with adjacent crystals such that multiple parallel sound waves of equal frequency enter the tissue.
Piezoelectric crystals exist naturally; quartz is well known for its physical vibratory properties. However, synthetically manufactured ferromagnetic crystals are generally used in diagnostic US transducers, lead zirconate titanate (PZT) being the most common. The generated sound waves from these crystals are low energy and therefore do not disrupt cell membranes or produce appreciable heat. In contrast, some US systems that have been employed in physical therapy do produce significant energy to impart deep heat for certain therapeutic benefits.
As a sound wave moves through a medium, the energy of the sound wave creates areas of compression and rarefaction of the molecules. This is displayed mathematically by the sine wave curve. The velocity of the sound wave is equal to the frequency times the wavelength. For any given medium, the velocity of the sound wave is the same regardless of the frequency. The relationship of frequency to wavelength is an inverse one; a lower frequency wave has a longer wavelength and a higher frequency wave has a shorter wavelength. Sound frequency is measured in cycles/second or hertz (Hz). US is defined at frequencies greater the 20 kilohertz (kHz; above audible sound) with medical applications of US within the 3 to 18 megahertz (MHz) range. Sound waves move through different media at different velocities and, generally, the denser the medium the higher the velocity.
As the sound waves enter the skin and deeper tissues, they meet elements of varying density, shape, and reflectivity. Most waves continue to penetrate the tissues and pass on in a linear fashion or scatter oblique to the path of the sound wave. In fact, only 1% of the transmitted sound is reflected back to the transducer.
There are three fundamental principles of sound waves: impedance, attenuation, and resolution. Acoustic impedance is the resistance of a medium to the propagation of a sound wave and is affected by the tissue density. Impedance differences between tissue interfaces results in a reflection. The greater the difference in impedance, the more the sound wave is reflected. A small impedance difference between tissue types (muscle and fat) results in a small reflection and most of the sound wave passing through; however, a large impedance difference may occur with such tissues as muscle and bone. During a large impedance difference, a large reflection and little of the sound wave will pass through as a result.
Sound waves progressively lose amplitude as they pass through tissues. This attenuation results from a combination of scattering and absorption and is dependent on the density of the tissues and depth of a targeted anatomic structure. Attenuation increases with increasing distance from the sound wave source (the transducer). The frequency of emitted sound has differing attenuation characteristics in tissue. Low frequencies in the range of 3 to 5 MHz are not attenuated readily and thus are more suited to demonstrating deeper structures. In contrast, high-frequency sound waves attenuate rapidly and have less depth of penetration. Hence, low frequency transducers are suitable for abdominal US, but higher frequency transducers (in the 10 to 15 MHz range) are ideal for visualizing the relatively superficial structures in the head and neck.
Wave emission from the transducer is not linear but has an hourglass configuration. The optimum point of tissue resolution is at the narrowest portion of this configured wave and designated the focal zone. Stated another way, the reflected waves from adjacent point tissue targets are seen as separated entities rather than combined blurred images when the focal point is properly aligned. The focal zone can be manipulated to a shallow or deeper plane within the region of interest. At the focal zone, tissue properties show the best lateral resolution . Similarly, the frequency of the transmitted wave is important. High-frequency waves produce better abilities than low-frequency waves to resolve adjacent tissue elements in the direct path of the sound wave. This property is designated axial resolution . Just as a newspaper image with relatively few points of dark ink and luminosity produces a relatively coarse image on magnification, a high megapixel photograph rendered from a digital single-lens reflex camera produces a sharper image with many more of these points per unit area. The example of the newspaper image is analogous to the lateral and axial resolution characteristics of penetrating and resolving sound waves, which combine to produce the ideal image. Where depth of penetration is the most important priority, the lower frequency waves must be utilized with some sacrifice in resolution. In US of the head and neck, where structures of interest are only a few centimeters below the skin surface, higher frequency waves with better axial resolution can be used. In summary, resolution or clarity on the monitor depends on both frequency and, to a lesser degree, alignment of the focal zone relative to the target. Selection of emitted transducer frequency and location of focal zone will depend on whether deep or superficial anatomic structures are to be studied.
Echogenicity is the term used to describe the appearance of an object or anatomic structure when using US. A reference point is used to define echogenicity; in the neck, normal thyroid tissue is typically referenced as the isoechoic structure. Isoechoic structures have nearly the same echogenicity as the surrounding tissue (or reference tissue). Anechoic structures have virtually no echoes with them and appear nearly black on US, which indicates through transmission of sound without reflection. Fluid filled structures, such as a thyroid cyst or the carotid artery, are described as anechoic . Hypoechoic structures appear darker, or less echogenic, than the surrounding tissue and hyperechoic structures appear brighter, or more echogenic.
The reflected sounds and their interpretation are responsible for allowing a processed image with meaningful information to form. The variations in tissues produce an acoustical mismatch, and these interfaces serve as reflectors. When there is a uniform surface from which much of the signal is reflected directly back in adjacent parallel waves, a discrete linear structure is defined. The mechanical waves are generated in packets of 1000 or more emissions per second, but then the transducer spends more time listening than transmitting. In principle, three transmitting cycles are performed and then the transducer is silent to allow the reflected waves to be received. These mechanical pulses are received by the temporarily nonvibrating piezoelectric crystals and converted back into electrical energy that the console can interpret and display as an image.
The image can be further refined by manipulation after the echoes are returned to the signal processor. Brightness can be adjusted by a turn of the gain control knob. As previously described, US waves attenuate at greater depth from surface entry. The attenuation is especially problematic when higher frequencies are employed, as in thyroid and parathyroid imaging. For example, the deeper aspects of a large goiter (with anterior-posterior dimension of 6 to 7 cm or more) will be difficult to see, because these areas are affected by attenuation. These deeper attenuated waves can be selectively recovered with time-gain compensation by increasing the gain of these waves while leaving the more superficial waves unaltered. In this way, the overall image has a more even distribution of illumination. Other methodologies such as “sono CT” change the way sound waves are delivered from the transducer. Rather than simple linear delivery of sound packets from the center of the transducer, some US units and transducer combinations have a selected option that sends sound waves in parallel from the edges of the long axis of the transducer. The waves converge at intervals somewhat similar to CT scan configurations to improve image resolution. Electronic noise is an undesirable but unavoidable element in amplification systems. Bandpass filtering can reduce the noise, which eliminates the frequencies above and below the ideal selected frequency. Harmonic imaging is a refinement that observes both the fundamental and second harmonic frequency echo reception. The fundamental frequency is filtered while permitting passage of the second harmonic, a postprocessing method that improves image quality. These are but a few examples of methods that modern US units have employed to refine image quality to a remarkable degree without any risk to the patient. In fact, even the small, basic portable machines now produce images that were simply unimaginable less than a decade ago.
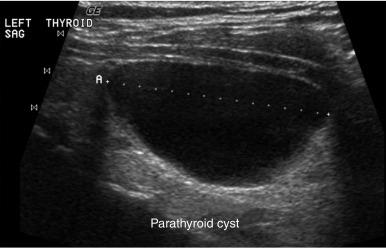
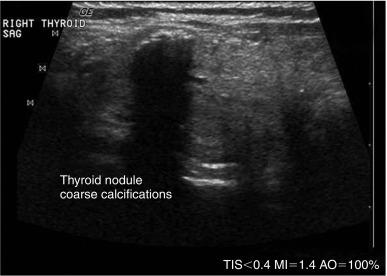
In ultrasonography, artifacts are images that appear on the display and do not represent real anatomic structures. Rather, they are shadows or enhanced representation of tissue elements that are consistent and can be informative. Pure cysts have a thin discrete capsule and are entirely fluid-filled without significant solid components. Sound enters from a superficial direction and as yet unattenuated waves easily penetrate the anterior capsule. Because the interior of the cyst is fluid without elements that reflect sound, the parallel sound waves strike the posterior capsule. Through the acoustical mismatch, the posterior capsule acts as a reflector. A large proportion of these waves penetrate this capsule and return as bright, high amplitude signals from just beyond. This produces a relatively broad reflected area that is hyperechoic to adjacent tissues and the cyst itself. This artifact is designated “posterior enhancement” and is usually diagnostic of a cyst ( Figure 13.1 ). In contrast, coarse calcifications block transmission of the sound waves to deeper tissue planes, producing a dark rectangular void deep to the densely hyperechoic structure. Known as posterior shadowing ( Figure 13.2 ), this particular artifact represents coarse calcifications often seen in portions of a multinodular goiter and to a lesser degree in some cancers. In contrast, microcalcifications ( Figure 13.3 ), generally seen in papillary carcinoma of the thyroid gland, do not produce posterior shadowing artifact as a result of their small size. These microcalcifications are small points of hyperechoic signal and are thought to represent either psammoma bodies defined histologically in papillary carcinoma of either the primary thyroid or metastatic adenopathy, or inspissated colloid. When planning fine-needle aspiration (FNA) cytology, these areas are good sampling targets under US guidance. Other artifacts may be confused with microcalcifications. One such confusion can occur with “comet tail” artifact ( Figure 13.4 ). These are hyperechoic points with a tapering core of hyperlucency extending from and deep to the circular dot. When examined more closely, the tail portion is actually a form of reverberation artifact. One accepted explanation is that areas of colloid crystallize and serve both as finite obstructions to transmission and deeper reverberation of the US waves. Ahuja has studied comet tail artifacts in a large number of thyroid conditions. Invariably, he has found that the underlying processes are benign. Examples of structures that commonly demonstrate reverberation ( Figure 13.5 ) are the anterior wall of the trachea, the anterior wall of the carotid artery, comet tails from small colloid crystals, and biopsy needles in their long axes.
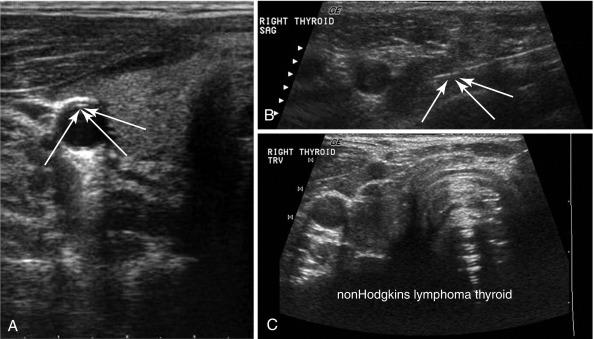
Doppler is a relevant and technically different process than grayscale US in the assessment of vascularity of anatomic and pathologic elements. In simplistic terms, the Doppler shift of sound waves occurs when waves imparted at an angle to a blood vessel strike directionally moving red blood cells and are reflected. If the waves are reflected toward the transducer, the velocity of this reflection is augmented. If the sound waves are reflected away from the transducer, signifying blood flow away from the transducer, the velocity is reduced. This velocity of red cell movement can be calculated, and directional flow given a color designation—that is, flow toward the transducer is red and flow away from it is blue by convention. These Doppler images are superimposed over the corresponding B mode display in a rapidly alternating fashion such that the eye sees a moving color video rendition of this activity. This color Doppler imaging and flow interpolation is highly relevant to the study of carotid and peripheral vascular anatomy and restriction of flow. Power Doppler is a separate technique that ignores these calculations and directional relationships. Power Doppler is more sensitive to and has better resolution for small vessels with low flow, such as those found within a lymph node or parathyroid adenoma ( Figure 13.6 ). In fact, power Doppler can often be used as a differentiating tool between these two structures in the clinical setting. Power Doppler will still reveal large vessels in the head and neck but cannot render actual flow values.
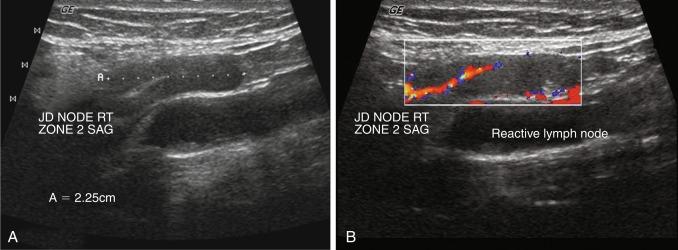
US is the first-line recommended imaging modality for the thyroid gland and thyroid nodules. Its use in thyroid disorders is widely acknowledged, and its benefits and indications continue to expand. Surgeons and endocrinologists in the office-based setting have adopted thyroid US for the evaluation and management of patients with thyroid, parathyroid, and many other head and neck disorders. Its versatility, convenience, safety profile, ability to offer dynamic real-time images, low cost compared with other radiologic modalities, and the outstanding quality of the images produced by high frequency, high-resolution US, have all contributed to its popularity. The relatively superficial location of the thyroid gland in the neck makes evaluation with US easy and practical. The images obtained from high resolution US allows for straightforward detection and description of thyroid gland pathology. Normal thyroid is uniform and homogeneous in appearance and described as isoechoic . Box 13.1 lists the principal goals of and indications for thyroid ultrasonography.
Assess palpable thyroid nodules and enlargement
Assess nonpalpable thyroid nodularity generally detected incidentally
Identify characteristics associated with malignancy
Assess the thyroid and extrathyroidal neck in patients with thyroid cancer before treatment
Monitor treated thyroid cancer patients for early evidence of recurrence
Monitor nodules, goiters, or lymph nodes in patients undergoing treatment or observation of thyroid disease
Screen high-risk patients (with familial forms of thyroid cancer, a history of radiation exposure, fluorodeoxyglucose [FDG] avidity on positron emission tomography [PET], etc.)
Screen for thyroid lesions in patients with other diseases in the neck, such as hyperparathyroidism, who are undergoing treatment planning
Guide fine-needle aspiration (FNA) biopsy and other interventions
In past decades, palpable thyroid nodules were surgically excised to establish a pathologic diagnosis. Advances in two techniques, namely US and FNA cytology, and ultimately the combination of these techniques, served to revolutionize the treatment of thyroid nodules and to reduce the incidence of unnecessary surgery for nodules that are predictably benign. Continued improvements in US technology have further improved the ability to identify and distinguish malignant features of thyroid lesions. These same improvements have led to an enhanced ability to localize pathologic parathyroid glands, which will be discussed later in this chapter.
The thyroid gland is ideally suited for US evaluation because of the gland’s superficial position and easy accessibility, its distinctive echotexture, and the fact that different thyroid disorders tend to demonstrate characteristic sonographic findings. Still, the value of the thyroid US examination depends on the skill, the experience, and the motivation of the examiner, as well as on the quality of the equipment used. Thyroid US provides greater anatomic detail than CT, magnetic resonance imaging (MRI), or radionuclide studies.
Thyroid US is recommended for all patients with suspected thyroid nodules, including patients with palpable abnormalities, nodular goiter, and thyroid lesions incidentally found by other imaging studies. Appropriate initial management uses US to characterize any and all thyroid nodules to determine which ones potentially need further evaluation, if at all. A goal of characterizing by US is to recognize patterns that may distinguish benign from malignant nodules and determine which ones warrant further investigation with FNA biopsy.
Routine screening thyroid US is not recommended for the general population because of the high incidence of thyroid nodules that are not clinically significant. Palpable thyroid nodules occur in approximately 7% of the general adult population, but the incidence of nonpalpable thyroid nodules visible by US is up 70%.
US can help confirm the presence of a thyroid nodule; objectively characterize the nodule’s size, location, and appearance; evaluate for benign or suspicious features; and evaluate for the presence of other thyroid nodules or cervical lymphadenopathy. Although certain US characteristics of thyroid nodules are associated with malignancy, FNA cytology remains the gold standard for diagnosis. Despite its utility, the diagnostic role of FNA is limited by an overall 3% false-negative rate and a 10% nondiagnostic rate.
The use of US guidance improves the overall sensitivity, specificity, and accuracy of FNA compared with palpation-guided FNA. US-guided FNA appears to be most valuable in patients with nonpalpable nodules, small palpable nodules, posterior nodules, multiple nodules, partially cystic nodules, or concomitant glandular disease. US-guided FNA is also beneficial for sampling specific areas of a nodule, such as the solid part of a mixed solid-cystic nodule. Cesur et al. found the rates of inadequate FNA samples to be significantly improved in palpable nodules 1 to 1.5 cm using US- versus palpation-guided FNA (37.6% versus 24.4%, p = 0.009), but not for palpable nodules 1.6 cm or larger. US-guided FNA is also specifically recommended when repeating FNA for a nodule with an initial nondiagnostic cytology result.
US of the thyroid is a painless and relatively quick procedure that does not require any preparation such as hormone withdrawal, low-iodine diet, or fasting. The examination is best performed with the patient in a supine position with the neck extended, although it can be performed on the seated patient. Images are obtained in the transverse and sagittal planes, noting first the overall dimensions of each thyroid lobe. A linear-array, high-frequency (7.5 MHz or greater) transducer with color Doppler capability is recommended. By convention, the left end of the transducer is directed to the patient’s right side for the transverse plane and in a cranial direction for the sagittal plane. The respective images show the patient’s right side toward the left side of the screen on transverse view and the cranial aspect toward the left side on sagittal view. Attention is given to the echogenicity and vascularity of the tissue as well as the presence of any discrete nodules.
A normal thyroid lobe measures approximately 1.5 to 2 cm in the transverse dimension, 4 to 5 cm in the sagittal plane, and 2 cm in the anterior-posterior dimension. If the sagittal length is greater than the width of the US transducer and its borders extend beyond the image, the length can be approximated by using the dual-screen option when available and “splicing” together the superior and inferior portions of the lobe ( Figure 13.7 ). The thyroid isthmus has an average length of 1.2 to 1.5 cm in the sagittal plane, and the pyramidal lobe is seldom visible. Normal thyroid parenchyma echoes are fine, uniform, and slightly hyperechoic compared with the surrounding muscles because of the gland’s iodine content.
Methods exist for estimating thyroid volume by applying a mathematical formula to measurements obtained from US. However, prospective studies have shown high interobserver variability in estimating thyroid nodule volume and poor correlation between predicted and actual thyroid volumes. Nevertheless, some use thyroid volume to facilitate iodine-131 ( 131 I) dosimetry in patients with thyrotoxicosis.
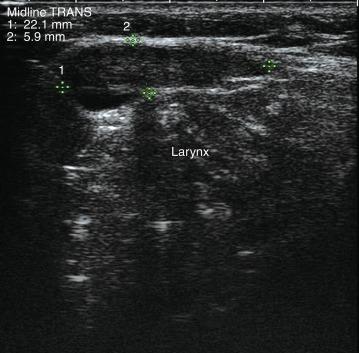
A complete US examination of the thyroid gland should also include examination of the regional lymphatics, noting any abnormally enlarged or atypical lymph nodes. Lymphatic metastases of thyroid carcinoma most often occur in the pretracheal and paratracheal lymph nodes or along the lateral neck jugulodigastric chain. Absence of a cervical thyroid gland in an untreated patient or presence of a thyroglossal duct cyst ( Figure 13.8 ) should also prompt US inspection of the midline neck from the base of the tongue to the sternum; this may assess for possible lingual or undescended thyroid. Laryngeal function can often be evaluated in the process.
US is a powerful tool in the diagnosis and management of thyroid nodules. All patients with a confirmed or suspected thyroid nodule should have a formal US of the entire neck, including the thyroid gland. Many distinctive US characteristics of malignant thyroid nodules have been identified ( Table 13.1 ). Although these US characteristics offer high sensitivity, no single criterion offers sufficient specificity to differentiate benign from malignant lesions. When characteristics are combined into sonographic patterns, specificity improves as well as the ability to stratify nodules based on risk of malignancy. One large prospective, observational study comparing US and FNA results with surgical pathology found that performing FNA on nodules with one of three US criteria—microcalcifications, blurred margins, or hypoechoic pattern—missed only 2% of cancers. Kim et al. prospectively analyzed 155 incidentally discovered, nonpalpable, solid thyroid nodules and found a mean number of 2.6 suspicious US characteristics per malignant nodule and an overall sensitivity and specificity of 94% and 66%, respectively.
| Margins | Blurred, ill-defined |
| Halo/rim | Absent, avascular |
| Shape | Irregular, spherical, tall |
| Echo structure | Solid |
| Echogenicity | Hypoechoic (especially markedly so) |
| Calcifications | Microcalcifications, internal |
| Vascular pattern | Intranodular, hypervascular |
| Elastography | Decreased elasticity |
| Lymph nodes | Abnormal lymphadenopathy |
Particular US features of thyroid nodules and their ability to suggest benign versus malignant lesions include the following.
Nodule size does not predict malignancy, because nodules of all sizes harbor a similar risk of malignancy. The risk of malignancy for palpable thyroid nodules is approximately 10%, and a similar incidence of malignancy is found in nodules smaller than 1 cm. Thyroid cancers less than 1 cm in size tend to behave in an indolent manner, and therefore suspicious subcentimeter lesions that are not treated should be followed with periodic US surveillance with the option for further evaluation by FNA if growth is observed. FNA biopsy of subcentimeter nodules can be considered if there is high risk of malignancy (family history of thyroid cancer, history of external beam or ionizing radiation, history of thyroid cancer, or positron emission tomography [PET] positive nodules); if there is a suggestion of extrathyroidal spread on US imaging; or if there is suspicious concomitant lymphadenopathy, in which case FNA of the lymph node should be performed.
Benign lesions are often associated with a hypoechoic circumferential vascular “halo” ( Figure 13.9 ) thought to represent compressed vascularity within thyroid parenchyma adjacent to a nodule. Neoplasms may display a partial or absent halo, and its presence or absence has been found to be suggestive but not diagnostic. For malignant lesions, the halo is typically avascular and represents a true capsule that may by invaded by neoplastic cells. The interface between a nodule and the surrounding thyroid parenchyma is typically well-defined; however, the borders of a malignant nodule can be irregular or infiltrative. Extrathyroidal extension, with part of the nodule extending beyond the thyroid capsule into the surrounding soft tissue of the neck (i.e., strap muscles), should be noted on US and is highly suggestive of malignancy. The mobility of the nodule with respect to surrounding structures can be assessed by dynamic palpation during US. Fixation suggests malignant invasion of the surrounding tissue.
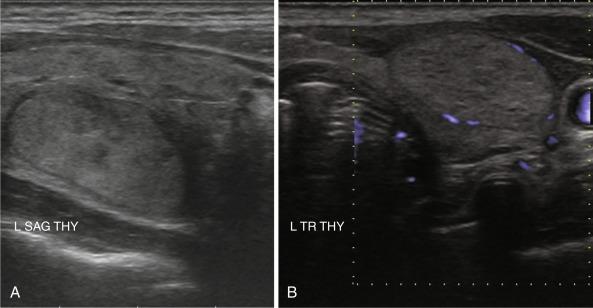
Nodule shape has been implicated as a prognostic factor. Nodules that are more tall than wide on transverse view (i.e., greater anteroposterior diameter than transverse diameter [ Figure 13.10 ]) are more likely to harbor cancer. This characteristic has been found to predict malignancy in breast US as well. However, one retrospective analysis also found that thyroid nodules with a more spherical shape had a higher incidence of malignancy. Irregular shape and microlobulated borders ( Figure 13.11 ) have also been implicated in malignancy.
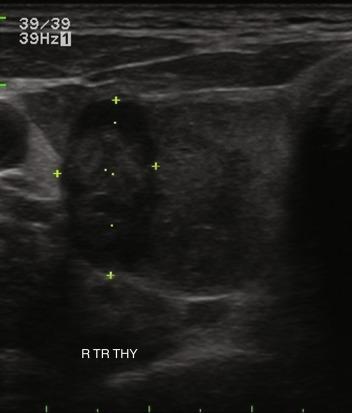
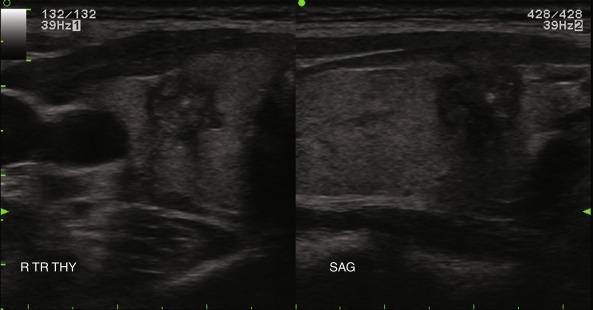
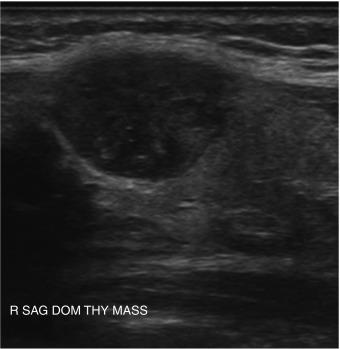
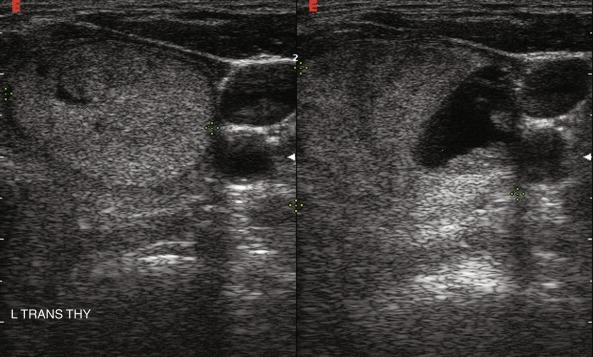
Nodule composition varies from purely cystic to completely solid. Many thyroid nodules have cystic components, such as cystic degeneration of a follicular adenoma ( Figure 13.12 ) or are found in the setting of multinodular goiter. Malignancy has been more closely associated with solid nodules than with cystic or mixed nodules. Purely cystic nodules are unlikely to be malignant, and those with a spongiform appearance ( Figure 13.13 ), defined as an aggregation of multiple microcystic components in more than 50% of the nodal volume, are more than 99% specific for benign histology.
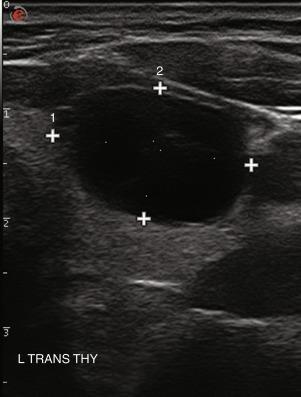
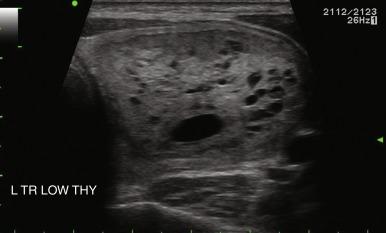
The echogenicity of a thyroid nodule should be compared with that of surrounding thyroid tissue. In diffuse thyroid disorders, such as thyroiditis, the entire abnormal gland may be hypoechoic, so assessment of any nodule must be relative to the normal thyroid gland, not the surrounding abnormal tissue. Most benign adenomas or adenomatous nodules are slightly hyperechoic to slightly hypoechoic compared with normal thyroid tissue ( Figure 13.14 ), whereas malignant nodules are frequently markedly hypoechoic ( Figure 13.15 ) or more hypoechoic than adjacent muscle.
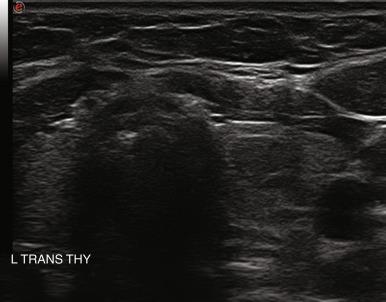
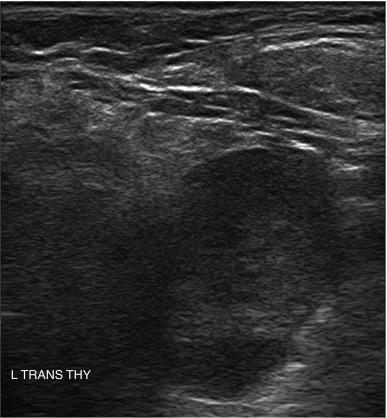
The presence of calcifications has variable significance. Peripheral calcification, also referred to as “eggshell calcification,” has traditionally been considered a benign feature representing previous hemorrhage and degenerative change. However, a disruption in a rim calcification with soft tissue extrusion is suggestive of malignancy. Coarse calcifications (measuring > 1 mm) demonstrate posterior acoustic shadowing because the sound waves cannot penetrate the calcium deposit and are reflected back to the transducer. Calcifications alone are not associated with cancer, but they are more worrisome for potential malignancy when seen in the center of a solid hypoechoic nodule (see Figure 13.2 ). Microcalcifications (< 1 mm hyperechoic lucencies) are even more strongly associated with an increased risk of malignancy. A reported 45% to 60% of malignant nodules demonstrate microcalcifications, as opposed to 7% to 14% of benign nodules. Approximately 60% of patients with microcalcifications have malignant disease. Microcalcifications in malignant nodules are often attributed to psammoma bodies in papillary thyroid carcinoma (PTC) ( Figure 13.16 ), but are also frequently seen in medullary thyroid carcinoma (MTC). Although suggestive of malignancy, the overall specificity of microcalcifications for thyroid carcinoma has been reported to range from 71% to 94% with a sensitivity of 35% to 72%. Therefore microcalcifications should not be solely relied upon to differentiate benign from malignant lesions.
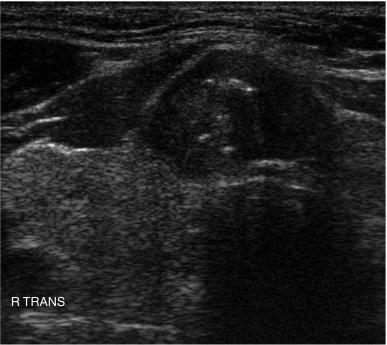
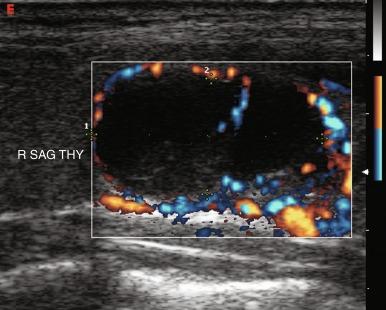
The vascular pattern around or within a nodule may correlate with the probability of malignancy. Chammas et al. classified thyroid nodules according to the pattern of vascularity seen with power Doppler into five types: absent blood flow, perinodular flow only, perinodular flow as great or greater than central blood flow, mainly central nodular flow, and central flow only. Nodules with exclusively central blood flow or central blood flow greater than perinodular flow had a higher incidence of malignancy ( Figure 13.17 ). Follicular carcinomas also tend to show a moderate increase in central vascularity by power Doppler compared with follicular adenomas that favor peripheral flow ( Figure 13.18 ). In general, increased vascularity in the interior of a thyroid nodule suggests malignancy but should not be considered a pathognomonic feature. The evidence correlating vascular pattern with malignancy is inconsistent, such that it is no longer included in the list of criteria used for risk stratification of thyroid nodules.
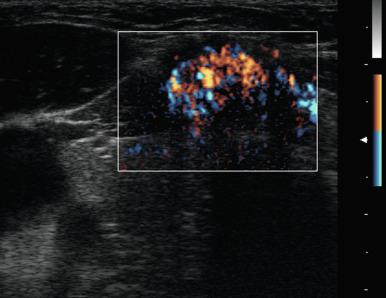
The presence of contact between a nodule and the adjacent thyroid capsule is defined when there is no intervening thyroid tissue between the thyroid nodule and the capsule. Capsular contact of greater than 25% by US has been found to be a useful marker for predicting extrathyroidal extension of cancer ( Figure 13.19 ).
Elastography, which uses pressure applied with the US transducer to measure tissue stiffness, appears to hold promise in differentiating benign from malignant lesions and may help guide surveillance and selection of nodules for FNA. Elastography is discussed in greater detail later.
Overall, the most common types of nodules are benign hyperplastic (colloid) nodules and benign follicular adenomas ( Figure 13.20 ). Colloid nodules consist of colloid and benign follicular cells and are associated with small, hyperechoic, internal lucencies on US, known as the “comet tail” sign within small cystic spaces (see Figure 13.4 ). Follicular adenomas are the most common type of thyroid neoplasm and are, with few exceptions, not considered a forerunner of carcinoma. They are typically round, well-encapsulated lesions with a clear margin or “halo” that distinguishes them from the surrounding normal thyroid tissue. Spontaneous or traumatic hemorrhage may occur into the nodule ( Figure 13.21 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here