Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The term inflammatory bowel disease (IBD) encompasses two forms of chronic, idiopathic intestinal inflammation, ulcerative colitis and Crohn’s disease. Although many other inflammatory diseases affect the gut, most are distinguished by a specific identifiable causative agent or process or by the nature of the inflammatory activity. The cause of ulcerative colitis and Crohn’s disease is unknown, so these disorders are empirically defined by their typical pathologic, radiologic, clinical, endoscopic, and laboratory features. This chapter summarizes the features that usually permit an operational distinction to be made between ulcerative colitis and Crohn’s disease.
Ulcerative colitis is a diffuse inflammatory disease of unknown origin that primarily involves the colorectal mucosa but later extends to other layers of the bowel wall. The disease characteristically begins in the rectum and extends proximally to involve part or all of the colon. The diagnosis is usually made on the basis of clinical symptoms and the presence of inflamed mucosa on sigmoidoscopy and confirmed by the findings on barium enema and mucosal biopsy.
Considerable information can be gained by carefully scrutinizing the abdominal radiographs of patients with ulcerative colitis. Although attention should be focused on the colon, other abnormalities, such as renal calculi, sacroiliitis, ankylosing spondylitis, and avascular necrosis of the femoral heads, must also be excluded.
Although the extent of ulcerative colitis is generally determined by barium enema and colonoscopy, these procedures carry a higher risk in severely ill patients. The following radiographic features can be used to assess the severity and extent of the colitis: (1) the extent of formed fecal residue; (2) the appearance of the mucosal edge; (3) alterations of the haustra; (4) colonic width; and (5) mural thickness.
The distal extent of formed fecal residue provides a good indication, although not absolute, of the proximal extent of the colitis. This approach sometimes overestimates but does not underestimate the extent of disease. The following conclusions can be drawn from the extent of residue :
If no residue is visible, the patient probably has active pancolitis.
If the residue extends down into the sigmoid colon, proctitis is present (this may be indistinguishable from normal).
If residue is present only in the proximal colon, the colitis most likely extends to this level but could be more distal.
The colonic mucosal edge is normally smooth. In active colitis, the line is granular, indistinct, and somewhat fuzzy. With frank ulceration, the mucosal line is disrupted, which causes an irregular edge. With extensive ulceration, only edematous mucosal islands remain. Intramural, mottled-appearing gas shadows may be present in fulminating colitis with necrosis of the bowel wall. Linear pneumatosis suggests extremely deep ulceration or extraperitoneal perforation with gas trapped adjacent to the bowel wall.
Normal haustral clefts run in parallel, about 2 to 4 mm apart, across one-third of the diameter of the colon. Widening of the haustral clefts with loss of the parallel lines is an early manifestation of ulcerative colitis and is more obvious on radiographs than the mucosal granularity that it accompanies. The haustra may be completely absent as well. Blunting can be confused with underdistention, so it should not be diagnosed if only a small amount of gas is present within the colon.
The upper limit of normal for the diameter of the transverse colon is 5.5 cm. In chronic, “burned-out” ulcerative colitis, the colon becomes tubular and narrowed and is considerably less than 5 cm in diameter. In these patients, if the colon is more than 5 cm in diameter, the inflammation has become transmural, which indicates a fulminant colitis at risk for perforation or toxic megacolon.
In the normal colon, the distance between the line of pericolic fat and gas-filled lumen is less than 3 mm. In chronic ulcerative colitis, it increases to more than 3 mm in thickness. In a study assessing these findings, a combination of four features was pathognomonic for disease proximal to the hepatic flexure: irregularity of the mucosal edge, loss of haustral clefts, increased thickness of the colon wall, and an empty right colon. ,
The barium enema has the following roles in patients with ulcerative colitis: (1) to confirm the clinical diagnosis; (2) to assess the extent and severity of disease; (3) to differentiate ulcerative colitis from Crohn’s disease and other colitises; (4) to follow the course of disease; and (5) to detect complications. The role of barium enema has largely been supplanted by colonoscopy, multidetector computed tomography (MDCT), and magnetic resonance imaging (MRI) in patients with ulcerative colitis. The major radiographic findings on the barium enema examination in ulcerative colitis are presented in Box 41.1 ; their anatomic and pathologic origin ( Fig. 41.1 ) and significance are discussed subsequently.
Mucosal granularity
Mucosal stippling
Collar button ulcers
Haustral thickening or loss
Inflammatory polyps
Confluent, contiguous, circumferential disease
Haustral loss
Luminal narrowing
Loss of rectal valves
Widened presacral space
Backwash ileitis
Postinflammatory pseudopolyps
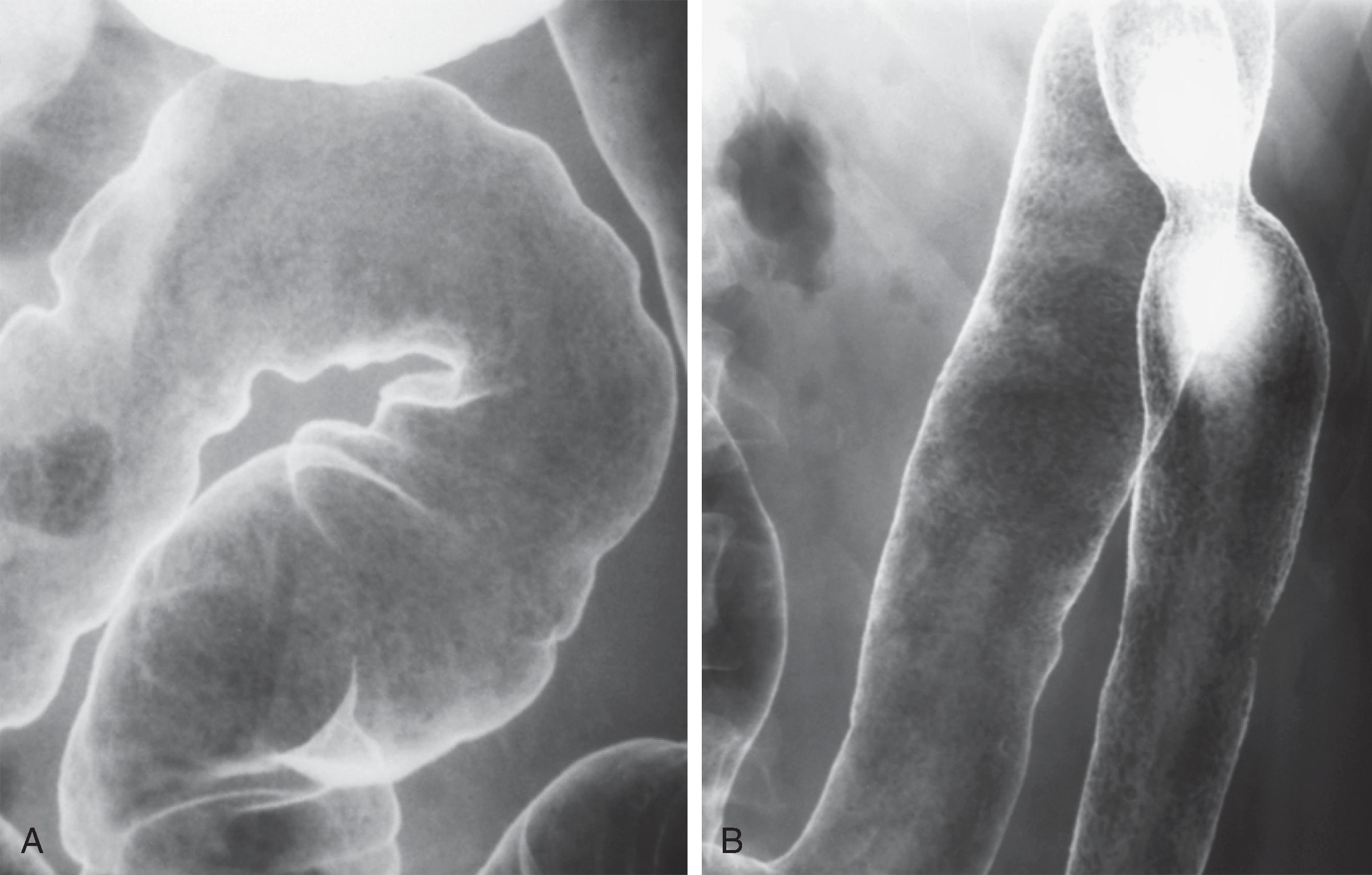
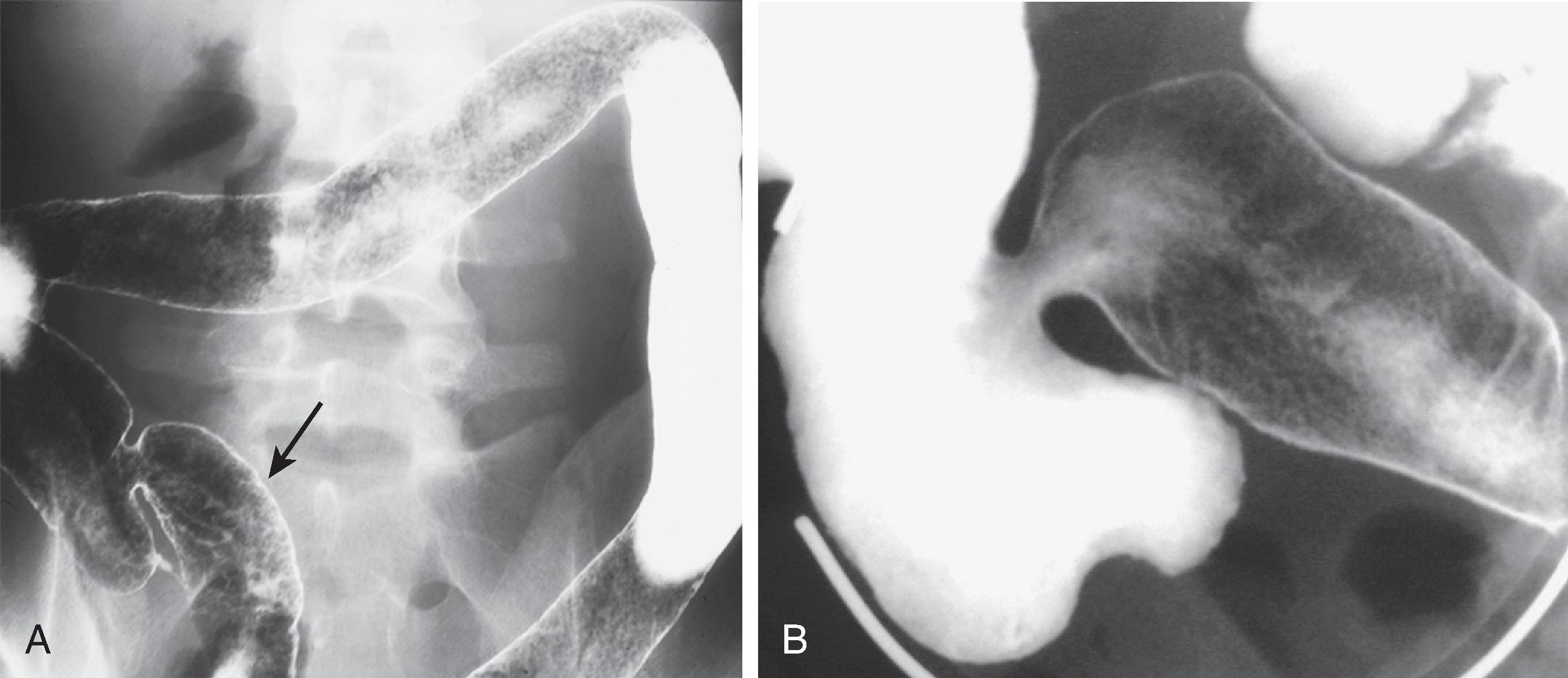
The earliest pathologic changes of ulcerative colitis are hyperemia and the accumulation of inflammatory cells in the mucosa. , These changes are manifested by a loss of normal mucosal translucency and obscuration of the submucosal pattern on endoscopy. Subtle thickening of the mucosa or haustral edema may be present radiographically, but these findings are often appreciated only in retrospect. With progressive edema and hyperemia, the mucosa develops a granular pattern ( Fig. 41.2A ). The smooth, sharp, and distinct appearance of the colonic margin seen on normal double-contrast studies is replaced by an amorphous, thickened, and indistinct mucosal line. There is a gradual transition between the normal and abnormal mucosa that extends for several centimeters. This granularity should be distinguished from the granular appearance of chronic ulcerative colitis ( Fig. 41.2B ). In chronic ulcerative colitis, the mucosal surface pattern is coarser, and there are significant changes in colonic contour.
The granular pattern develops as a result of abnormalities in the quality and quantity of mucus produced by the involved mucosa. On histologic examination, there is a reduction in the number of goblet cells, which contain less than the normal complement of mucin. , , , Normal mucus is essential to normal barium coating of the gut. Any process, whether benign or malignant, infectious or inflammatory, that alters mucin production affects mucosal coating and visualization.
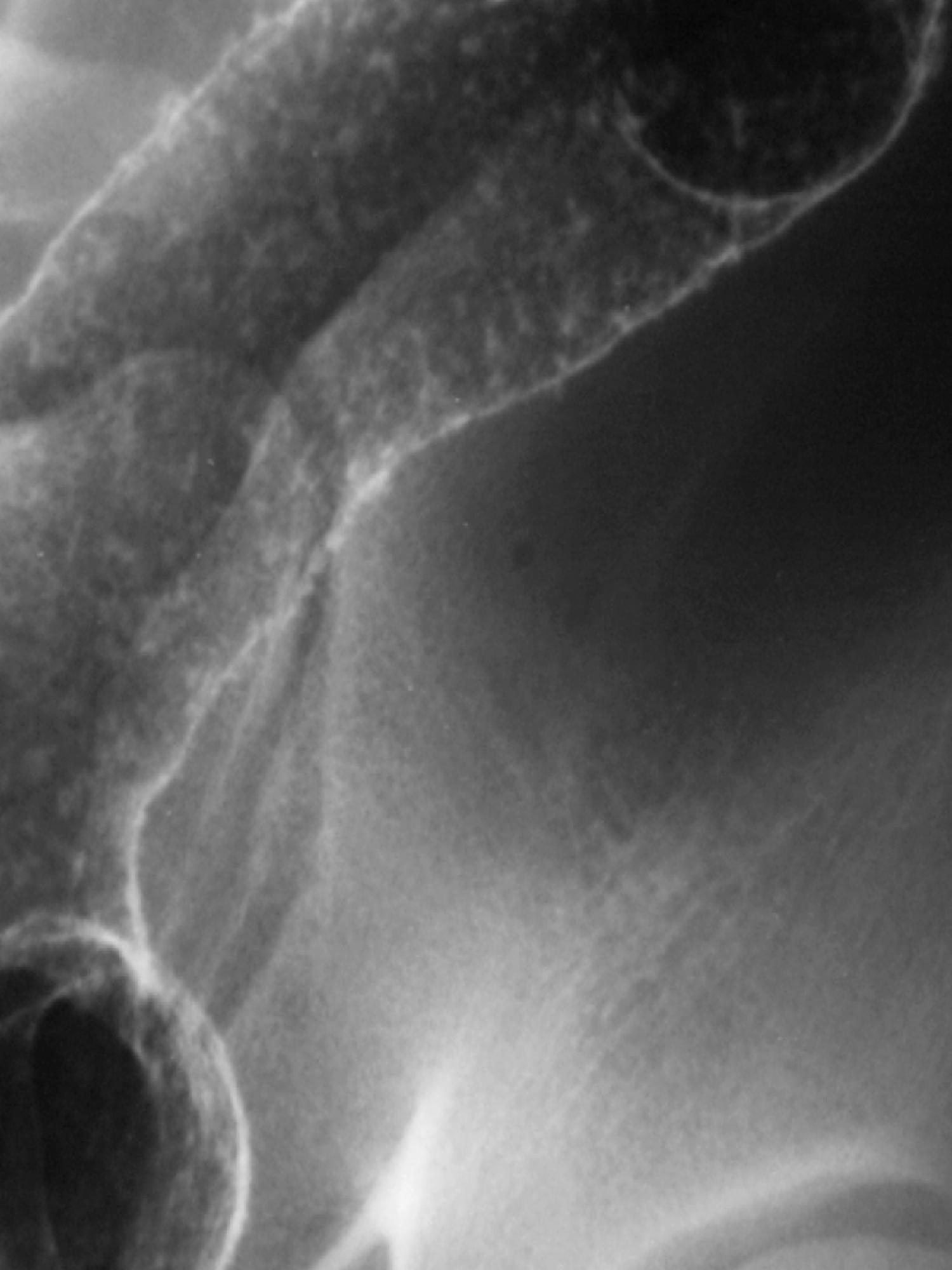
During the granular phase of ulcerative colitis, inflammatory cells accumulate at the base of the mucosal crypts. , , Cellular debris tends to block the crypts of Lieberkühn, which leads to the formation of microabscesses, better known as crypt abscesses. These crypt abscesses eventually erode into the lumen. , The ulcers deepen, and barium flecks become adherent to them, producing mucosal stippling ( Fig. 41.3 ). This stippling resembles white paint applied by dabbing a surface with the end of a fairly dry paintbrush.
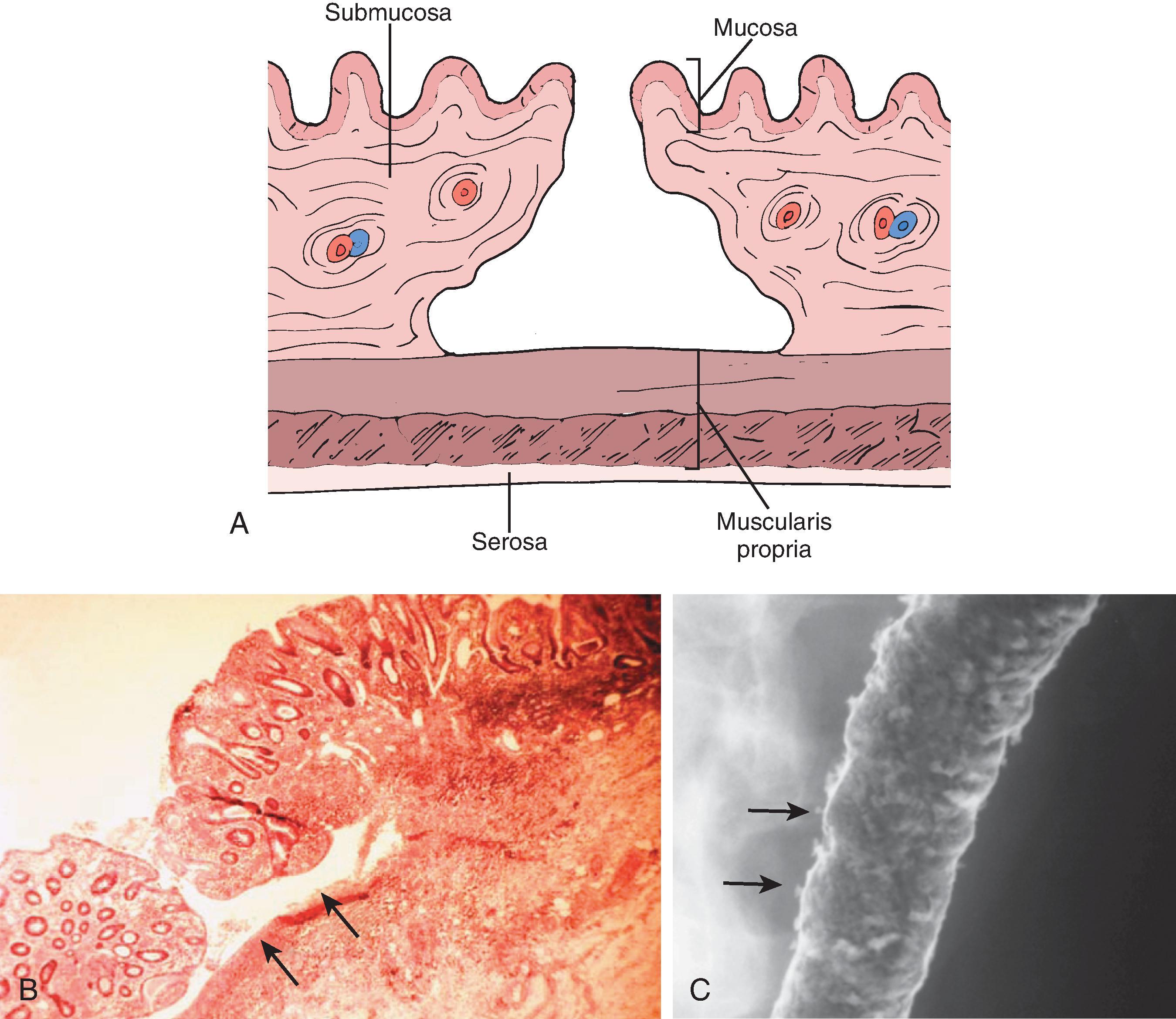
With disease progression, the ulcers of the crypt abscess breach the lamina propria and muscularis mucosae and cause undermining of the less resistant areolar tissue of the submucosa. The involved submucosa becomes necrotic, and the ulcers extend laterally, causing further undermining. This undermining is contained by the muscularis propria on the serosal side and by the muscularis mucosae on the luminal side of the colon. , The ulcers are frequently related to the taeniae. The mucosal defect is small relative to the degree of undermining and produces a flasklike, so-called collar button ulcer ( Fig. 41.4 ). As these ulcers enlarge and interconnect, the collar button ulcer configuration is lost; a network of residual islands of mucosa and inflammatory pseudopolyps is produced.
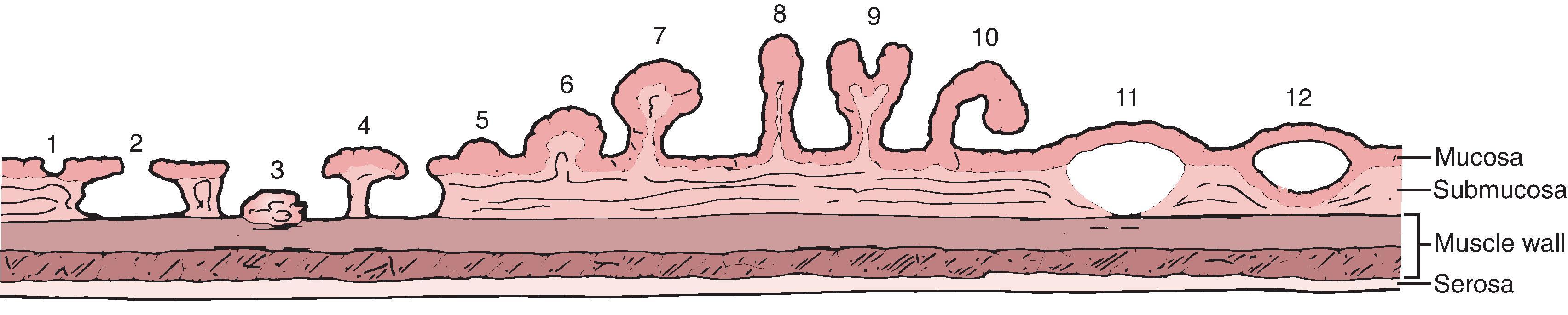
A variety of different mucosal protrusions may be seen on barium studies in patients with IBD. , Their appearance and significance depend on the stage of disease and pathologic origin.
In patients with severe ulcerative colitis, there is extensive mucosal and submucosal ulceration in which only small islands of mucosa and submucosa may survive. The inflamed edematous mucosa protrudes above the surrounding areas of ulceration, which gives a polypoid appearance ( Fig. 41.5 ). Because these represent merely the remnants of preexisting mucosa and submucosa rather than new growths, they are termed pseudopolyps. Inflammatory pseudopolyps are the natural progression of collar button ulcers: the ulcers extend and interconnect so that the pseudopolyp rather than the ulcers becomes the major radiographic finding. Inflammatory pseudopolyps usually occur in ulcerative colitis but may also be seen in Crohn’s disease. The cobblestoning pattern seen in Crohn’s disease is another type of pseudopolyp, in which larger islands of preserved mucosa are surrounded by linear and transverse ulcerations.
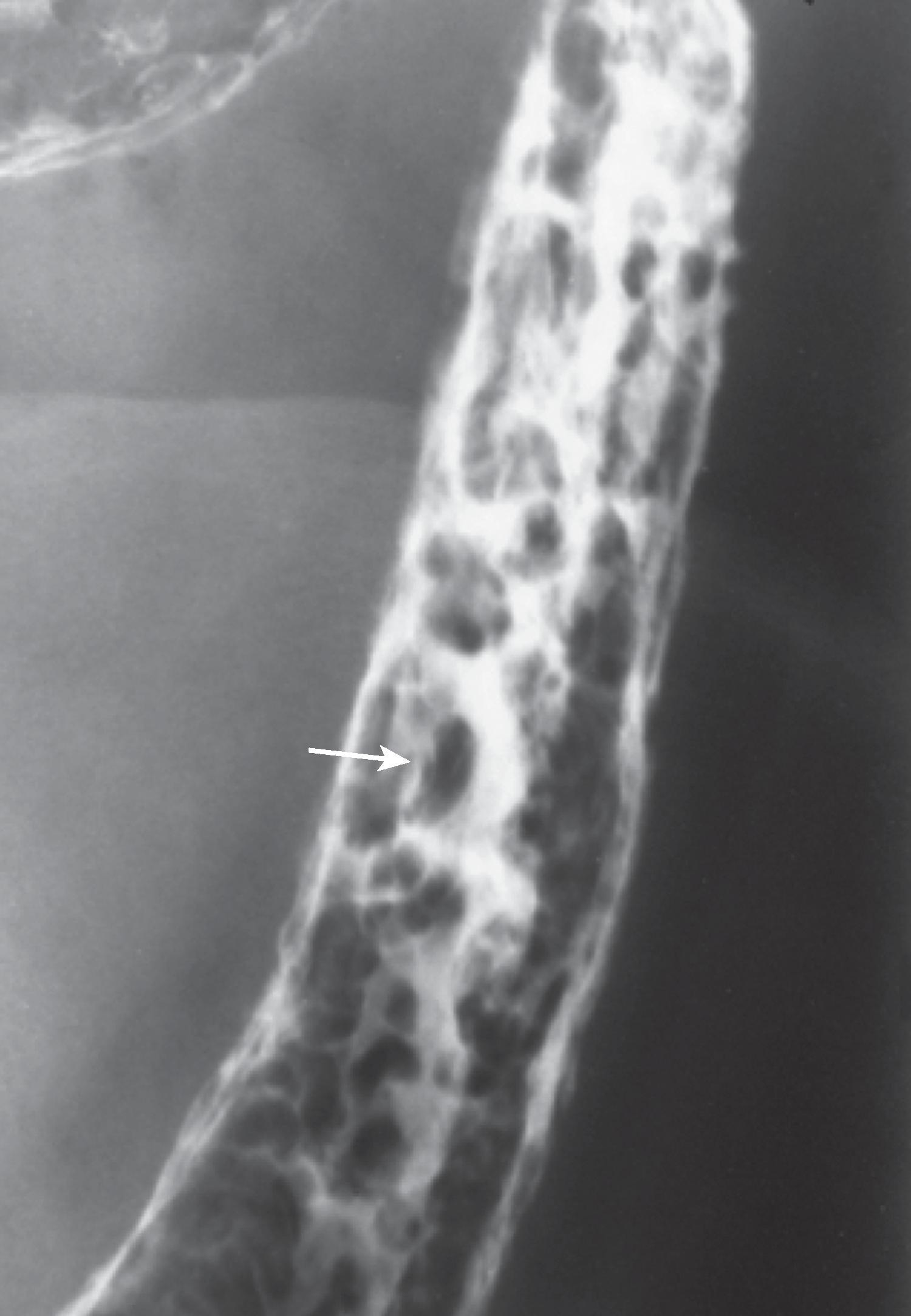
When IBD goes into remission, the denuded mucosa heals and is covered by granulation tissue that has a granular appearance similar to that observed in the early stages of ulcerative colitis. , , , In some patients, the regenerated mucosa has a tendency to overgrow ( Fig. 41.6 ). This overgrowth often results in polypoid lesions that may be small and rounded, long and filiform, or proliferate into a bushlike structure simulating a villous adenoma. Because the regenerating mucosa is cytologically normal, it is not a true neoplasm but a pseudopolyp. Because this occurs during mucosal healing, it is termed a postinflammatory pseudopolyp . These polyps represent the aftermath of a severe attack of colitis and may be the only sign of previous disease. Mucosal bridges are also postinflammatory pseudopolyps, in which a bridge of mucosa survives between islands of mucosa surrounded by ulceration. With remission, the underside of the mucosal bridge and underlying ulcer reepithelialize. ,
Postinflammatory pseudopolyps can also be seen after ischemia, after severe infection, and in Crohn’s disease. Filiform polyps have also been described in the esophagus, stomach, and small bowel in patients with Crohn’s disease.
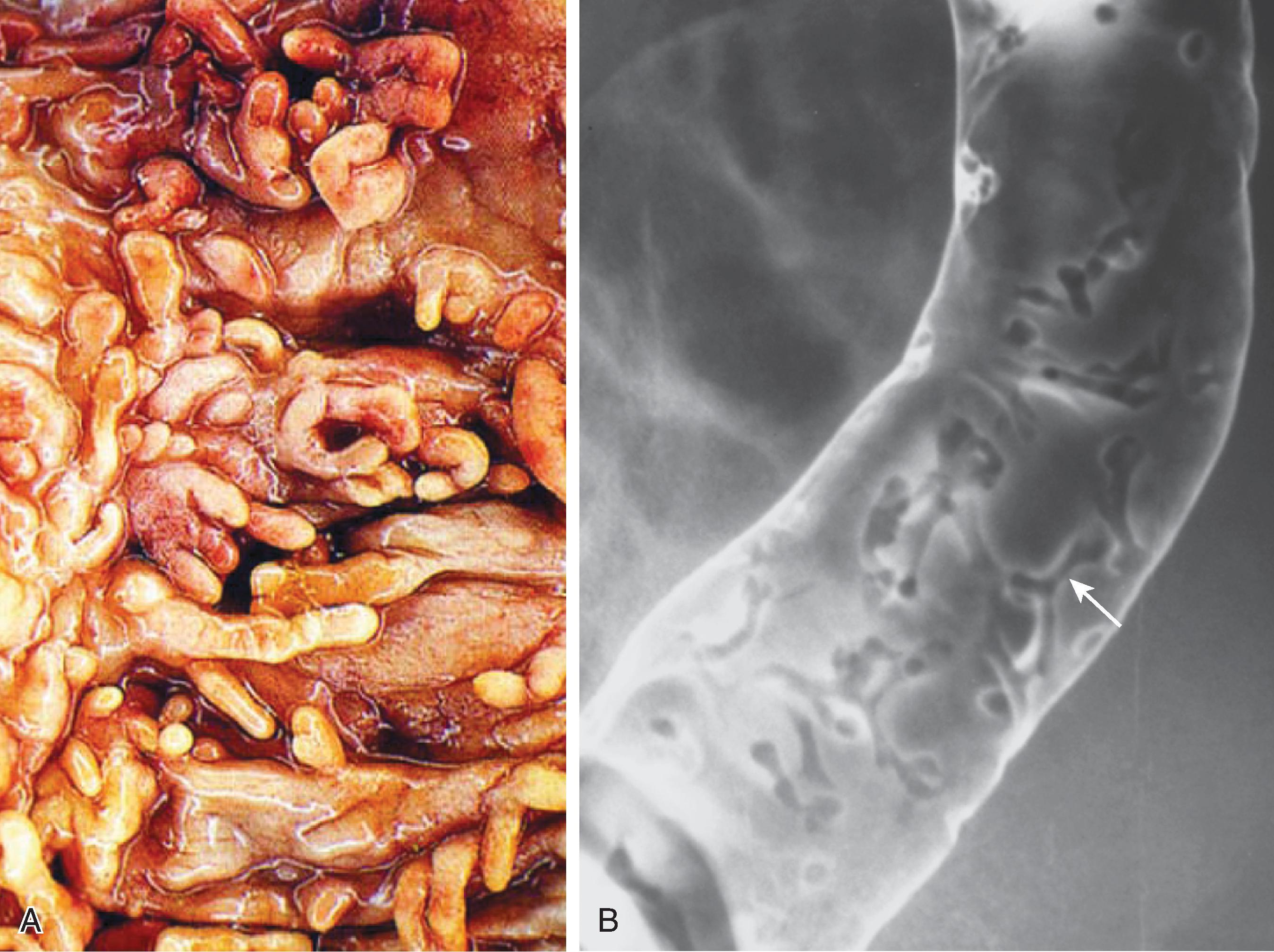
In 10% to 40% of patients with chronic ulcerative pancolitis, the distal 5 to 25 cm of ileum is inflamed ( Fig. 41.7 ). This ileitis occurs only in the presence of pancolitis and usually resolves 1 to 2 weeks after colectomy. , Although small ulcerations may be present, this is not a primary inflammation of the ileum. The distal ileum can be used to form an ileostomy or pouch. The pathogenesis of this disorder is uncertain but may relate to the reflux of colonic contents into the small bowel, hence the term reflux or backwash ileitis.
Barium studies reveal a chronic pancolitis associated with a patulous and fixed ileocecal valve that easily refluxes and persistent dilation of the terminal ileum. The normal fold pattern is absent, and the mucosa is granular.
In the course of ulcerative colitis, the haustral folds undergo two major changes: (1) early in the disease, they are edematous and thickened; (2) with chronic disease, they become blunted or may be completely lost. , This evolution of haustral changes can be understood by a brief review of haustral formation.
At birth, colonic haustra are usually absent, which explains why it is difficult to differentiate colonic from small bowel gas in neonates. As the colon grows, the circular muscle of the muscularis propria outgrows the longitudinal muscle, the taeniae coli. This differential growth rate causes the taeniae to shorten the colon in an accordion-like fashion, with production of saccular haustra that usually are first seen by the age of 3 years.
Haustra are fixed anatomic landmarks in the proximal colon because the circular muscle is fused to the taeniae. In the distal colon, haustra are created by active contraction of the taeniae. Consequently, the colon can normally be devoid of haustra distal to the midtransverse colon; loss of haustra in the proximal colon is always abnormal. ,
In early ulcerative colitis, the haustra are deformed and thickened because of edema and may produce a corrugate outline to the colon, which has been called the indenture sign. , In chronic ulcerative colitis, the haustra are often lost for two reasons, alterations in the muscle tone of the taeniae , and the fact that the colon is shortened. In this disease, the taeniae coli become relaxed for some unknown reason. This relaxation is associated with abolition of the haustral pattern. With healing, the haustra may reappear as they gain tone. A second major factor is also at work in chronic ulcerative colitis—massive hypertrophy and fixed contraction of the muscularis mucosae, which causes foreshortening of the colon. Thus, the normal accordion-like array of colon on the relatively shorter taeniae is lost.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here