Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Typhoid fever is an acute generalized infection of the mononuclear phagocyte system, intestinal lymphoid tissue, and gallbladder caused by Salmonella enterica serovar Typhi. A broad spectrum of clinical illness can ensue, with more severe forms being characterized by persisting high fever, abdominal discomfort, malaise, and headache. In the preantibiotic era, the disease ran its course over several weeks and was accompanied by a case-fatality rate of approximately 10–20%. Before the first quarter of the 19th century, typhoid fever was not recognized as a distinct clinical entity and was often confused with other prolonged febrile syndromes, particularly typhus fever of rickettsial origin. There is considerable debate over who first clearly differentiated typhus fever from typhoid (i.e., typhus-like) fever. Medical historians have argued over who should receive credit for this clinical clarification, and it has been variously bestowed on Huxham (1782), Louis (1829), Gerhard (1837), and Schoenlein (1839). Gerhard, in Philadelphia, argued that two similar, yet distinct, febrile illnesses existed that were clearly discernible from one another on the basis of pathologic findings; one (typhoid) manifested marked intestinal lesions. Schoenlein referred to two distinct forms of typhus: “exanthematicus” and “abdominalis.” However, William Jenner is responsible for definitively dispelling controversy on the subject. , He provided precise clinical descriptions and observations of pathologic conditions from postmortem examinations that allowed a clear-cut differentiation between the two illnesses. Jenner argued that the pathologic lesions in Peyer patches and mesenteric lymph nodes were peculiar to typhoid and were never seen with typhus. In 1847, the term enteric fever was introduced in an attempt to replace “typhoid fever” and avoid confusion with “typhus.” Although this term is frequently used, it has by no means replaced the appellations typhoid fever and paratyphoid fever in common usage.
William Budd's book, Typhoid Fever: Its Nature, Mode of Spreading and Prevention, published in 1873, was a milestone in epidemiology because it clearly described the contagious nature of the disease and incriminated transmission via fecally contaminated water sources years before the causative organism was identified. Eberth (1880) visualized the causative bacilli in tissue sections from infected patients, and Gaffky (1884) grew it in pure culture. Known in earlier years as Bacillus typhosus, Eberthella typhosa, Salmonella typhi, and Salmonella typhosa, it is currently referred to as S. enterica serovar Typhi ( Salmonella Typhi). , S. enterica Paratyphi A and Paratyphi B (and uncommonly Paratyphi C) also cause enteric fever (paratyphoid fever), which is clinically indistinguishable from typhoid fever.
Inactivated (heat-killed, phenol-preserved) S . typhi was used as a parenteral vaccine as far back as 1896 by Pfeiffer and Kolle in Germany and by Wright in England. Wright administered his vaccine (three doses 2 weeks apart) to two medical officers in the Indian Army, one of whom thereafter ingested wild typhoid bacilli without developing illness.
Wright then evaluated his vaccine in 2835 volunteers in the Indian Army. Although local and generalized adverse reactions were common, his results were considered to be sufficiently encouraging for a decision to be made to vaccinate troops embarking for the Boer War in South Africa. Outcry over the frequency of adverse reactions led to a suspension of vaccination. However, on Wright's insistence, a board of inquiry was established to review data on the reactogenicity and efficacy of the vaccine. The committee concluded that the vaccine was efficacious and that its value in preventing typhoid fever exceeded the price paid in adverse reactions. By World War I, typhoid vaccination became routine in the British Army.
The transmission of typhoid fever is fostered where sanitation is primitive and water supplies are not treated, and where there exist chronic or short-term carriers of S . typhi who serve as a reservoir of infection. In such situations, human fecal material can contaminate water supplies. In most endemic areas, the peak incidence of clinically recognizable, bacteriologically confirmed typhoid fever detected by passive surveillance is typically observed among school-age children. Typhoid constitutes the main enteric disease threat faced by children in developing countries after they have survived the gauntlet of diarrheal and dysenteric infections encountered during their first 5 years of life. ,
Because the case-fatality rate in the preantibiotic era was ∼10–20%, typhoid fever was a much-feared disease. Following the discovery in 1948 that chloramphenicol (and subsequently certain other antibiotics) can successfully treat typhoid fever, dropping the case-fatality rate to well below 1%, the interest in typhoid vaccines became a weathervane for the prevalence of antibiotic-resistant strains of S . typhi . Interest in vaccines waned as long as effective and inexpensive oral antibiotics were available, only to resurge when resistant strains appeared and rendered treatment ineffectual. Beginning about 1990, strains of S . typhi that exhibited plasmid-encoded resistance to all the oral antibiotics that were the mainstays of therapy in the 1970s and 1980s (chloramphenicol, trimethoprim-sulfamethoxazole, and amoxicillin) began to disseminate throughout Asia and northeast Africa. , This emergence of multiple-drug-resistant (MDR) typhoid during the last decade of the 20th century led to an increase in the incidence of severe cases, hospitalizations, and complications, and to an increase in typhoid mortality. , In 2016 an extensively drug-resistant (XDR) strain of S . typhi appeared, associated with a large outbreak of typhoid in Hyderabad, Pakistan, leaving only one oral (azithromycin) and one parenteral (carbapenems) antibiotic as treatment options. While this portends grave implications for the treatment of typhoid, the most widely used Vi conjugate vaccine (WHO prequalified and recommended Typbar-TCV®) has been shown to be highly effective in preventing typhoid due to the XDR strain.
Typhoid fever exhibits a wide range of clinical severity. Classic full-blown cases begin with malaise, anorexia, myalgia, fever that increases in stepwise fashion to reach 39–40°C, abdominal discomfort, and headaches. A bronchitic cough is common in the early stage of illness. The fever often follows three stages. Initially, the temperature rises gradually, in stepwise fashion, by daily increments of 0.5–1°C until, after 5–7 days, a sustained temperature of 39–41°C is present. Without appropriate antimicrobial therapy, the fever remains at this level for approximately 10–14 days. With convalescence, the fever diminishes, also in stepwise fashion, over several days. During the period of sustained fever, approximately 20% of white patients manifest an exanthem (so-called rose spots) consisting of subtle, salmon-colored macules, 2–4 mm in diameter, which blanch with pressure. Rose spots are seen most often on the chest, abdomen, and back; S . typhi can be cultured from rose spots. Constipation or loose stools may occur in older children and adults, whereas diarrhea is the more common bowel pattern alteration in young children with typhoid fever.
The peripheral leukocyte count in typhoid fever is often below 4500/mm 3 , which helps in the differential diagnosis. In the human challenge model, the most consistent hematological finding was an early fall in eosinophil count, or complete absence, among those who went on to develop typhoid. Thrombocytopenia is also common; platelets dropped to <80,000/mm 3 and was observed among all volunteers participating in an experimental challenge. Liver dysfunction, as detected by mildly elevated serum transaminase values, is observed in most patients. Before the availability of fluoroquinolone antibiotics such as ciprofloxacin, approximately 10% of patients treated with earlier antimicrobials of choice, such as chloramphenicol, trimethoprim-sulfamethoxazole, or amoxicillin, manifested clinical relapses; notably, the clinical illness in relapse is much milder.
A particularly severe form of typhoid fever is occasionally encountered in which cerebral dysfunction—including obtundation, delirium, and coma—and shock are present. In the 1970s and 1980s, this severe form of typhoid was encountered not infrequently on the Indonesian island of Java. Unless steroid therapy accompanies appropriate antimicrobial therapy in patients suffering from this severe form of the disease, the case-fatality rate can exceed 20%.
Although infants may manifest severe clinical forms of typhoid fever, bacteremic S . typhi infection in children younger than 2 years of age is often remarkably mild and is not recognized clinically as enteric fever but rather as a nondescript febrile syndrome. , This was common in Santiago, Chile and Lima, Peru in the 1970s and 1980s when typhoid was hyperendemic there.
Complications may affect virtually any organ system. Two feared complications of typhoid fever, intestinal perforation and hemorrhage, are a consequence of the intestinal lesions that are prominent in the pathology of S. typhi infection. These complications occur in approximately 0.5–1.0% of cases and are more common in persons who have been ill for several weeks without proper antibiotic therapy. Other uncommon complications of typhoid fever include typhoid hepatitis, empyema, osteomyelitis, and psychosis. More rarely, arthritis, meningitis, myocarditis, and empyema of the gallbladder can occur. Approximately 2–5% of patients with typhoid fever, depending on age and sex, become chronic gallbladder carriers of the organism. , More rarely, chronic renal or bladder carriers occur, the latter in patients harboring Schistosoma haematobium or Schistosoma mansoni bladder coinfection.
Taxonomy in the genus Salmonella continues to evolve and can be confusing. Originally, speciation was based on association with distinct clinical syndromes. With the Kauffman–White serologic classification, each distinct O:H serotype was given species status, a situation that rather quickly became ponderous. In 1980, the Approved Lists described five species: Salmonella enteritidis, S. typhi, Salmonella typhimurium , Salmonella choleraesuis, and Salmonella arizonae . The most recent classification and speciation, based on DNA relatedness and molecular analysis, reduces the genus Salmonella to two species, S. enterica and Salmonella bongori . S. enterica is further subdivided into subspecies designated with Roman numerals. , , Serotypes in S. enterica subspecies I are still almost always referred to by their previous genus and species designations. Thus, for example, to avoid confusion, S. enterica serovar Typhi continues to be referred to in most international journals of microbiology, infectious diseases, epidemiology, and vaccinology as Salmonella typhi ; use of a capital letter without italics indicates that Typhi designates a serovar, not a species. Following the most current taxonomy, Salmonella typhi (abbreviated S . typhi ) is the terminology that will be used in this chapter.
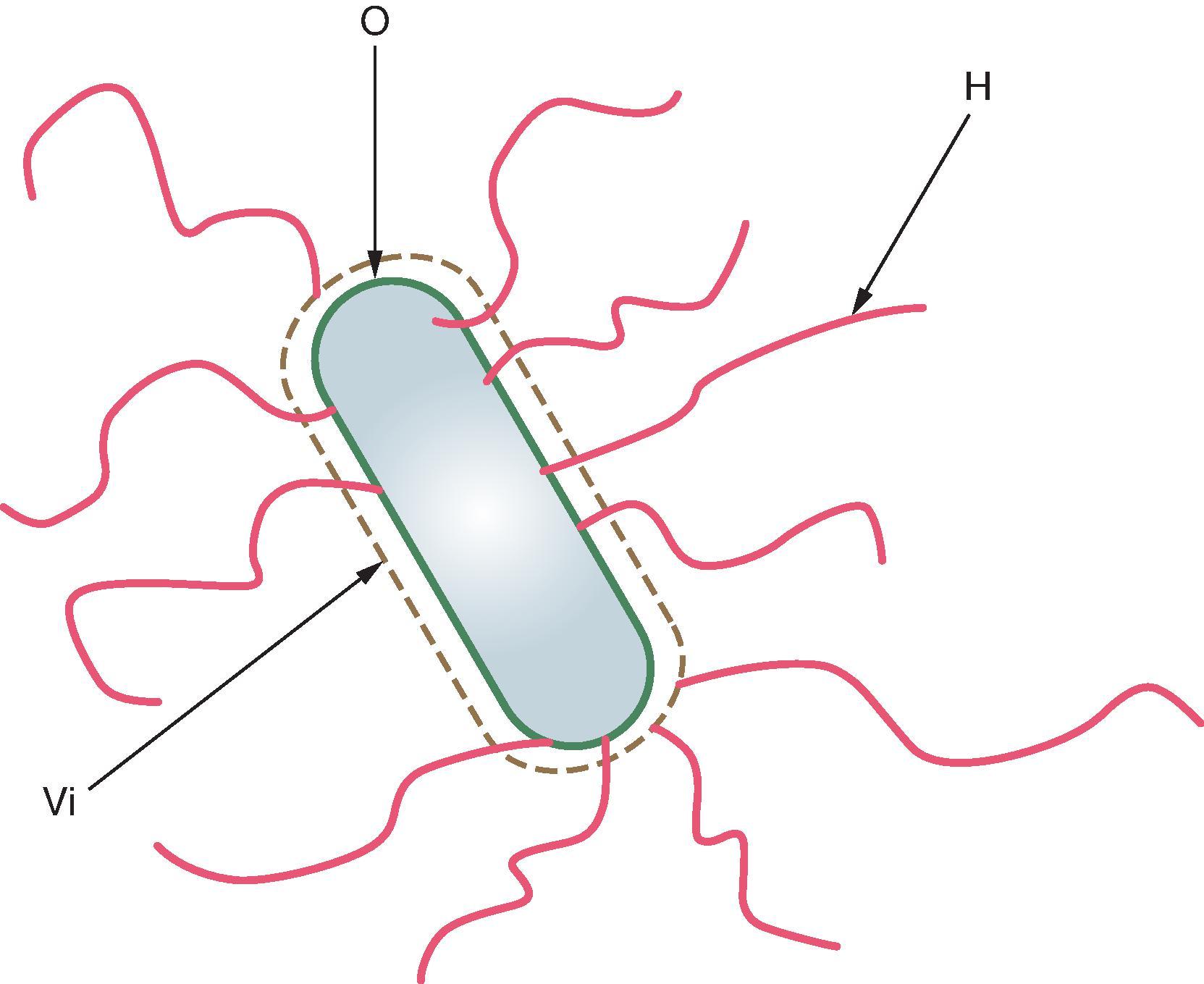
Serologically, S . typhi falls into group D Salmonella on the basis of its immunodominant lipopolysaccharide (LPS) O antigen 9. S. Typhi is motile, and its Phase 1 peritrichous flagella bear flagellar (H) antigen d, which is also encountered in approximately 80 other bioserotypes of Salmonella . Occasional isolates from Indonesia have flagella that bear other antigens (j and z66). , Strains freshly isolated from patients express on their surface a polysaccharide capsule, the Vi (for virulence) antigen. , Vi consists of a homopolymer of repeating unit (1 → 4) α-o-galacturonic acid that is 40–80% O-acetylated at the C3 position , ; the presence of Vi prevents O antibody from binding to the O antigen. There is evidence that each of the antigens O, Vi, and H, plays a protective role with certain typhoid vaccines.
At the level of the clinical microbiology laboratory, S . typhi exhibits a remarkable degree of homogeneity, in comparison with the other species of Salmonella . S. Typhi rarely exhibits biochemical or serologic variability. One exception is found in Indonesia, where a few percent of isolates bear flagellar antigen “j” or “z66” rather than “d.” Previously, only phage typing using Vi phages, pulsed-field gel electrophoresis, and ribotyping were helpful in differentiating strains from different geographic areas. Currently, high-throughput full-genome sequencing has revolutionized the ability to differentiate S . typhi strains from diverse sources. S. typhi does not ferment lactose; it produces hydrogen sulfide (H 2 S), but does not produce gas. Consequently, suspect colonies are evident on usual lactose-containing media such as Salmonella–Shigella agar or MacConkey agar as lactose-negative colonies. The biochemical pattern in triple-sugar iron agar is rather characteristic, manifested by an acid butt without gas, an alkaline slant, and obvious H 2 S production. Fresh isolates typically agglutinate with Vi but not necessarily with group D antiserum. However, if the bacteria are boiled to remove the Vi capsule that overlies the LPS O antigen, a reaction with group D antiserum is readily seen. Fig. 62.1 is a diagrammatic representation of the relationships among the O (cell wall), Vi (capsular polysaccharide), and H (flagellar) antigens of S . typhi .
When the first complete sequences of the approximately 4.8 million base-pair (bp) genomes of multiple-antibiotic–resistant S . typhi strain CT18 , and of antibiotic-sensitive strain Ty2 58 were reported, it was revealed that approximately 70–80% of the genome shares a backbone containing genes similar in sequence and order to those in Escherichia coli and other salmonellae. Intermittently along this backbone are clusters of S . enterica –specific and S . typhi –specific genes (i.e., genes not found in S. Typhimurium or other S. enterica serovars) and more than 200 pseudogenes (including several that play a role in virulence in S. typhimurium ). In addition to the 218,150-bp plasmid encoding antibiotic resistance in CT18, another 106,516-bp plasmid was found that shows common derivation with the pFra virulence plasmid of Yersinia pestis . ,
S. typhi , S . paratyphi A , and S . paratyphi B are highly invasive bacteria that pass through the intestinal mucosa of humans rapidly and efficiently to eventually reach the reticuloendothelial system, where, after an 8–14-day incubation period, they precipitate a systemic illness. S . typhi is a highly host-adapted pathogen; humans comprise the only natural host and reservoir of this infection.
Comprehension of the steps involved in the pathogenesis of typhoid fever comes from four sources: (1) clinicopathologic observations in humans, , (2) volunteer studies, , (3) studies of a chimpanzee model, and (4) analogies drawn from S. typhimurium and Salmonella Enteritidis infection in mice (the “mouse typhoid” model). , The following is a summary of the probable steps in the pathogenesis of S . typhi infection in humans.
Susceptible human hosts ingest the causative organisms in contaminated food and water. The inoculum size and the type of vehicle in which it is ingested greatly influence the attack rate for typhoid fever and also affect the incubation period. Dosages of 10 9 and 10 8 pathogenic S . typhi ingested by volunteers in 45 mL of skim milk without buffer pretreatment to neutralize gastric acid induced clinical illness in 98% and 89% of persons, respectively; dosages of 10 5 organisms caused typhoid fever in 28–55% of volunteers; in contrast, none of 14 subjects who ingested 10 3 organisms without buffer developed clinical illness.
A modern typhoid model was able to lower the inoculum of S . typhi needed to cause clinical illness and bacteremia by administering the organisms with sodium bicarbonate to neutralize gastric acid. Inocula of 10 3 or 10 4 typhoid bacilli evoked illness in 55% and 65% of volunteers, respectively. When the ingested typhoid bacilli pass through the pylorus and reach the small intestine, they rapidly penetrate the mucosal epithelium by one of two main mechanisms to arrive in the lamina propria. One mechanism of invasion involves typhoid bacilli being actively taken up by M cells, the dome-like epithelial cells that cover Peyer's patches and other organized gut lymphoid tissue. From here they enter mononuclear and dendritic cells in the underlying lymphoid tissue. In a distinct second invasive mechanism, bacilli are believed to be internalized by enterocytes, wherein they enter membrane-bound vacuoles, which pass through the cell and ultimately release the organisms at the basal portion of the cell without destroying the enterocyte. Takeuchi provided a highly descriptive electron-photomicrographic documentation of the analogous passage of S. typhimurium through intestinal mucosa. A third paracellular mode of entry also has been proposed.
On reaching the lamina propria in the nonimmune host, typhoid bacilli elicit an influx of macrophages that ingest the organisms but are generally unable to kill them. Some bacilli apparently remain in macrophages of the small intestinal lymphoid tissue. Other typhoid bacilli are drained into mesenteric lymph nodes, where further multiplication and ingestion by macrophages take place. Shortly after invasion of the intestinal mucosa, a primary bacteremia is believed to take place in which S . typhi organisms are filtered from the circulation by fixed phagocytic cells of the mononuclear phagocyte system. It is believed that the main route by which typhoid bacilli reach the bloodstream in this early stage is by lymph that drains from mesenteric nodes to eventually reach the thoracic duct and then the general blood circulation. It is conceivable that ingestion of a massive inoculum followed by widespread invasion of the intestinal mucosa could result in rapid and direct invasion of the bloodstream. As a result of this primary bacteremia, the pathogen rapidly attains an intracellular haven throughout the organs of the reticuloendothelial system (i.e., the mononuclear phagocyte system), where it resides during the incubation period (usually 8–14 days) until the onset of clinical typhoid fever.
Clinical illness is accompanied by a fairly sustained but low level (∼1–10 CFU/mL) secondary bacteremia. In their report of experimental S . typhi challenge studies in volunteers, Hornick and colleagues described one volunteer who began a 7-day course of oral chloramphenicol only 1 day after ingesting pathogenic S . typhi , and who developed clinical typhoid fever 9 days after the antibiotic was discontinued. This report provides evidence that the typhoid bacilli have attained their intracellular haven within 24 hours after ingestion.
The Vi antigen is a virulence property. , , Felix and Pitt, , who originally described and named the antigen, showed that Vi antigen enhanced the pathogenicity of S . typhi for mice. Virtually all strains freshly isolated from patients possess this Vi polysaccharide capsule. Epidemiologic observations and studies in volunteers support the contention that S . typhi strains that possess Vi are more virulent than strains lacking this polysaccharide. , Nevertheless, Vi-negative strains have caused typhoid fever when fed to volunteers.
S . typhi and S . paratyphi A encode a S . typhi toxin that was hypothesized to be integral to the pathogenesis and severity of typhoid fever and potentially to portend a new generation of typhoid vaccines. , An isogenic deletion mutant of the virulent Quailes strain of S . typhi was constructed, and volunteer challenge studies were undertaken in which randomly allocated subjects ingested the parent Quailes or the derivative deleted of the toxin. No strain-discernable difference was observed in the clinical picture or in the bacteriological or immunologic response of recipients of the toxin-deleted versus the wild-type strain. Some would argue that this result was not unexpected, since sensu stricto S . paratyphi B , which does not encode typhoid toxin, causes enteric fever that is clinically indistinguishable from S . typhi disease and similarly can result in chronic carriers.
Based on current understanding of the pathogenesis of typhoid fever, it is obvious that disparate types of vaccines that elicit differing immune responses may each prevent typhoid illness by interfering with the pathogen at distinct points in its pathogenesis. For example, live oral S . typhi vaccine strain Ty21a, which does not express Vi capsular polysaccharide, but stimulates sIgA antibodies against other surface antigens, appears to block invasion at the mucosal surface. Ty21a also elicits powerful cell-mediated immune responses, including cytokine-producing CD4 + cells and cytotoxic CD8 + cells, that attack the bacteria after it has gained its intracellular niche within cells of the mononuclear phagocyte system in the spleen, liver and bone marrow. In contrast, parenterally administered purified Vi polysaccharide vaccine and Vi-protein conjugate vaccines protect by stimulating serum antibodies that are believed to attack the typhoid bacilli mainly during the primary bacteremia, likely by exhibiting opsonophagocytic mechanisms; these anti-Vi antibodies may also transude onto the mucosal surface and bind S . typhi prior to mucosal invasion. Thus, disparate licensed typhoid vaccines stimulate divergent immune responses that impede the pathogen at distinct steps in its pathogenesis.
During the primary bacteremia that follows ingestion of typhoid bacilli and seeds the mononuclear phagocyte system, organisms also reach the gallbladder, an organ for which S . typhi has a remarkable predilection. After intravenous inoculation, S . typhi rapidly appears in the gallbladder of rabbits. , S. typhi can be readily cultured from bile or from bile-stained duodenal fluid in patients with acute typhoid fever. In approximately 2–5% of patients, the gallbladder infection becomes chronic. , The proclivity to become a chronic carrier is greater in female patients and increases with age at the time of acute S . typhi infection, thereby resembling the epidemiology of gallbladder disease. The infection tends to become chronic in persons who have a preexisting pathologic gallbladder condition at the time of acute S . typhi infection.
Humans are the sole reservoir of S . typhi infection as well as the only natural host. The infection is typically transmitted when susceptible hosts ingest food or water that has been contaminated by fecal matter. In contrast, transmission from person to person by direct contact is exceedingly uncommon. The one rare exception is reports of typhoid as a sexually transmitted infection among men who had sex with men (including oral–anal contact) in a high-income country setting.
Typhoid fever represents the quintessential infectious disease for which transmission is related to levels of sanitation and quality of water supply. Typhoid fever can abound wherever sanitation and food hygiene are primitive. The highest incidence usually occurs where water supplies serving large populations are contaminated by human fecal matter. This situation existed at the end of the 19th century in most large cities in the United States and Western Europe, where piped water supplies were available but the water was usually untreated. The water sources (usually rivers) were also the repository for the discharged sewage of the cities. In this manner, the transmission of typhoid fever was amplified. With the introduction of water treatment at the turn of the 20th century, including sand filtration and chlorination, the incidence of typhoid fever plummeted precipitously in the large cities of the United States, despite the continued existence in those cities of many chronic carriers of S. typhi. Typhoid fever remains endemic in most areas of the world without the resources to implement improved water and sanitation infrastructure, and consequently where fecal contamination of water sources still occurs. This includes regions in many countries in Africa, Asia, Latin America, and Oceania.
Confirmation of the diagnosis of typhoid fever requires recovery of S. typhi from a suitable clinical specimen. Because of practicality and relative ease of access, multiple blood cultures should be obtained from patients in whom the diagnosis is suspected clinically. The rate of recovery of S . typhi in blood cultures depends on many factors, including the volume cultured, the ratio of the volume of blood to the volume of culture broth in which it is inoculated (ideally, the ratio should be at least 1 : 8), the inclusion of anticomplementary substances in the medium (such as sodium polyanethol-sulfonate, or bile), and whether the patient has already received antibiotics to which the S . typhi is sensitive. With three 5-mL blood cultures, S. typhi can be recovered from the blood in approximately 60–70% of suspected cases.
The gold standard of bacteriologic confirmation of typhoid fever is the bone marrow culture, which is positive in 85–96% of cases, even when the patient has received antibiotics. , In the 1980s, great interest was shown in the use of duodenal string devices to obtain bile-stained duodenal fluid for culture. In the 1970s–1990s, when a commercial source of duodenal strings was widely available, the combination of one duodenal string and two blood cultures provided a sensitivity of bacteriologic confirmation equal to that achieved with bone marrow cultures but without the invasiveness of the latter. Stool cultures led to recovery of the organism in only 26–65% of cases.
Serodiagnosis of typhoid fever has been attempted since the late 19th century, when Widal and Sicard (1896), among others, , showed that the serum from patients with typhoid fever agglutinated typhoid bacilli. The Widal test, still practiced today in many areas, involves the search for agglutinins in the patient's serum and may be performed with antigen in tubes or on slides; the former is generally more accurate. By careful choice of antigen, both O and H antibodies can be selectively measured. By use of S. typhi strain 0901, which lacks flagellar and Vi antigens, S. typhi O antibody can be selectively measured. To detect antibodies against the appropriate H antigen (d), a strain such as Salmonella Virginia is selected that possesses the identical Phase 1 flagellar antigen (d) but shares no O somatic antigens with S. typhi . Most patients with typhoid fever have elevated levels of O and H antibody at the time of onset of clinical illness. , Anderson emphasized the importance and usefulness of H titers in serodiagnosis of typhoid fever. However, others reported that the prevalence of H antibodies in adults living in endemic areas is too high for the test to be useful in that age group. Nevertheless, it can be helpful in diagnosing children younger than 10 years of age in endemic areas and persons of any age from nonendemic areas. A history of inoculation with parenteral killed whole-cell vaccines invalidated the Widal test. One report showed optimistic results for use of the slide test for O agglutinins of S . typhi , even for adults in endemic areas. Several relatively simple commercial serodiagnostic kits exist for detecting antibodies to S . typhi O antigen and to a 50-kDa outer membrane protein antigen but their sensitivity and specificity limits their utility.
Serologic tests to measure Vi antibody using highly purified Vi antigen have been developed and utilized, including a commercial assay. Although measuring the titer of serum Vi antibody is practical for the detection of chronic S . typhi carriers, most of whom have highly elevated titers of Vi antibody, , , it is of little help in diagnosing acute typhoid fever because only a minority of patients with acute infection manifest detectable Vi antibody.
Many attempts have been made to develop tests that detect S . typhi antigens or nucleic acid in blood, urine, or body fluids, thereby providing a rapid diagnostic test for typhoid fever. These tests have failed to warrant the enthusiasm of the initial reports. The immunoassays are based on the detection of the O, Vi, or other antigens of S . typhi in blood or urine using coagglutination, enzyme-linked immunosorbent assay (ELISA), or countercurrent immunoelectrophoresis. The polymerase chain reaction methods attempt to amplify S . typhi genes. These assays aim to be more sensitive, practical, economical, and rapid than bacteriologic culture, yet comparably specific. So far, no assay has adequately accomplished these objectives, and a satisfactory test that might replace bacteriologic culture remains a laudable but elusive goal.
The ultimate goal is to have a simple, highly sensitive, specific, practical and rapid diagnostic test to identify patients with typhoid and paratyphoid fever such that specific antibiotic therapy can be initiated in a clinically ill patient who has sought care in a health care facility where there is no blood culture capability. , One iteration of such a test would be suitable for use in hospitals and large health centers that have clinical laboratories in endemic areas in LMIC. A simpler iteration of the test would allow use by primary health care workers in the field or in dispensaries without laboratories. Since nucleic acid-based polymerase chain reaction (PCR) assays have been successfully used to develop diagnostic tests for other infections, multiple groups have tried to develop analogous assays for diagnosing enteric fever. Primers to amplify specific Salmonella genes by quantitative polymerase chain reaction (qPCR) to identify S . typhi and differentiate it from S . paratyphi A , S . paratyphi B , S . paratyphi C , and from the most prevalent invasive nontyphoidal Salmonella serovars including S . typhimurium , S . enteritidis , and S . dublin have been described. , Disappointingly, heretofore it has not been possible to translate this molecular information to a practical format that allows the direct diagnosis from a small sample of blood without a preliminary enrichment in culture. The reason is that during the secondary bacteremia that accompanies clinical typhoid fever the patient's blood contains only ∼1–10 CFU/mL; so the amount of template for the molecular diagnostic amplification reaction is too low prior to culture of the sample. A preliminary culture of blood for a few hours increases the number of Salmonella per ml such that detection by PCR becomes successful. , Two other molecular techniques that have shown promise are microwave-accelerated metal-enhanced fluorescence (MAMEF), and LAMP, a form of isothermal nucleic acid amplification.
The hallmark report of Woodward and colleagues in the late 1940s first showed that the antibiotic chloramphenicol could drastically ameliorate the severity of clinical illness and drop the case-fatality rate of typhoid to less than 1% (with early initiation of therapy). Several reviews summarize treatment of typhoid prior to 2016. Prior to 2015, with some exceptions, most S . typhi strains globally were sensitive to oral ciprofloxacin and to parenteral ceftriaxone, and these antibiotics were highly effective. Oral azithromycin was also effective. In low- and middle-income country (LMIC) settings, where cost of therapy is a critical factor, amoxicillin and trimethoprim-sulfamethoxazole also remained useful oral drugs if prevalent strains were sensitive.
The ability to treat typhoid fever in endemic areas and in travelers changed abruptly in 2016 with the emergence of extensively drug-resistant (XDR) S . typhi causing epidemic disease in Sindh province of Pakistan. Whereas MDR S . typhi encoding resistance to fluoroquinolones, ampicillin, chloramphenicol, and trimethoprim/sulfamethoxazole were highly prevalent in Pakistan in the period 2009–2014, these strains were susceptible to parenteral ceftriaxone. Thus, the abrupt appearance of XDR typhoid cases bearing those resistances plus solid resistance to ceftriaxone constituted a public health emergency. Klemm et al. determined that the Sindh province epidemic strain carried: a chromosomally integrated transposon encoding resistances to chloramphenicol, amoxicillin/ampicillin, and TMP-SMZ; a chromosomal mutation in gyrA that confers intermediate resistance to ciprofloxacin; an IncY plasmid that encodes broad quinolone resistance ( qnrS ) and resistance to extended-spectrum beta-lactamase that results in resistance to ceftriaxone; additionally, this IncY plasmid also has a VirB Tra locus that allows self-transmission of the plasmid to S . typhi and S . paratyphi A strains of other lineages. The clinical consequence of this armamentarium of resistances to antimicrobials is that the Sindh strain was susceptible to only a single oral antibiotic, azithromycin, and to only a single class of parenteral antibiotics, carbapenems (that are extremely expensive for LMICs). This raised the specter that if such XDR strains of S . typhi also acquired resistance to azithromycin and carbapenems, they would be untreatable and typhoid fever in LMICs would become a public health threat like it was in the preantibiotic era. The XDR S . typhi has spread to other countries in South Asia and importations have occurred in Europe and North America. S . typhi strains resistant to azithromycin have appeared in Bangladesh due to a point mutation in the gene encoding the efflux pump protein AcrB whereby at residue 717 a glutamine replaces the arginine residue. This identical mutation has also been identified in a S . typhi strain in Pakistan, which, fortunately, is not an XDR strain. Surveillance is ongoing to monitor for the possible emergence of XDR S . typhi in Pakistan that include resistance to azithromycin.
Before the appearance of fluoroquinolone antibiotics, to achieve a credible cure rate in the treatment of chronic biliary carriers of S . typhi , it was necessary to perform cholecystectomy followed by several weeks of intravenous ampicillin or oral amoxicillin plus probenecid. When ciprofloxacin and norfloxacin became available, it was found that 4 weeks of oral therapy without surgical removal of the gallbladder could successfully treat 75–90% of chronic carriers despite the presence of gallstones. The fact that these oral bactericidal antibiotics are highly concentrated in bile led to these encouraging clinical results.
In the United States and other high-income countries, approximately 80% of cases of typhoid fever have a history of travel to endemic areas , ; the vast majority of these ill travelers have never received typhoid vaccine or were not recently vaccinated. Whereas typhoid fever has long been a notifiable infectious disease in the United States, enteric fever caused by S . paratyphi A and B and invasive infections caused by S . paratyphi C were notified only within the general salmonellosis category with nontyphoidal Salmonella serovars. To improve monitoring of the burden of invasive S . paratyphi as well as typhoid fever cases in the United States (in part recognizing the emergence of multiple drug-resistant S . paratyphi A in South Asia), and to improve surveillance for antimicrobial resistance among enteric fever serovars, in 2008 the Centers for Disease Control and Prevention created the National Typhoid and Paratyphoid Surveillance (NTPFS) system and coordinated it with the National Antibiotic Resistance Monitoring System (NARMS). The relationship with NARMS was intended to assure that all state public health laboratories send all isolates of S . paratyphi A and S . paratyphi C to CDC, as well as sending S. typhi isolates. State laboratories were not requested to send S . paratyphi B isolates because the uncommon D-tartrate nonfermenting sensu stricto S . paratyphi B pathovar strains that cause paratyphoid fever are difficult to differentiate from the strains of the pathovar that do ferment D-tartrate (and that were previously referred to as S . java ) and that cause gastroenteritis. The molecular or biochemical methods to differentiate between these pathovars are not readily available in all contributing laboratories. Moreover, sensu stricto S . paratyphi B that cause enteric fever are currently very uncommon worldwide.
The first 5-year report of the NTPFS-NARMS surveillance coordination documented the value of this upgraded surveillance. Over the period 2008–2012, NTFPS received notification of 2341 enteric fever cases (1872 Typhi and 469 Paratyphi A), for an annual average of 374 typhoid and 94 paratyphoid A cases. The NTFPS ascertained that 86% of the typhoid cases and 92% of the Paratyphi A cases were foreign travel-associated. In total, 1465 cases reported travel to a single country, UN region, or subregion. Travelers to three countries accounted for 81% of these 1465 travel-associated typhoid cases and 93% of the paratyphoid A cases, including India (61% and 73%, respectively), Bangladesh (12% and 12%), and Pakistan (8% and 8%). Several years earlier, CDC had calculated risks of typhoid based on cases per million travelers to particular countries ; India had the highest incidence (89 typhoid/10 6 travelers), which peaked in 2003 at 122/10 6 travelers.
NTFPS determined that in the period from 2008 to 2012 there were 524 domestically acquired U.S. typhoid fever cases and 36 Paratyphi A cases. An asymptomatic S . typhi carrier was believed to be responsible for 32 of the 524 domestically acquired typhoid cases.
To further document the importance of international travel making typhoid fever a potential threat even in high-income countries, within a few months of the recognition of the outbreak of typhoid due to XDR S . typhi that began in November 2016 in Hyderabad, Pakistan, XDR cases were confirmed in Europe, , the United States, Canada, United Kingdom, Australia, and Taiwan. , Nevertheless, secondary spread from travel-associated cases is rare in high-income settings, reinforcing the point that transmission of these pathogens is impeded by provision of clean water and adequate sanitation accompanied by appropriate personal hygiene practices.
In endemic areas, different epidemiologic patterns of typhoid may be observed depending on the type of surveillance (active, passive, mixed), socioeconomic level, and the main modes of transmission. Thus, notable differences in age-specific incidence and in seasonality have been reported based on these factors. Using passive surveillance that detects patients sufficiently ill to seek healthcare, an age-specific incidence of typhoid fever may be seen where there is a low incidence in infants younger than 1 year of age, a low or moderate incidence in preschool-age children 2–4 years of age, a peak incidence in school-age children (5–19 years), and a low incidence in adults older than 35 years. The relatively low incidence in infants in some surveillance sites relates in part to decreased exposure to vehicles of transmission, and the clinical consequence of S . typhi infection in the infant host often being mild or atypical illness, even in the face of bacteremic infection. , Evidence based on the systematic collection of blood cultures from febrile children (mainly detected during active household surveillance visits or when children seek care at ambulatory healthcare facilities) suggests that in some countries in Asia, bacteremic S . typhi infection in children 1–4 years of age is common. Moreover, in some areas of South and Southeast Asia, high incidences of typhoid fever in preschool children have been recorded through passive surveillance. , It is assumed that in developing countries with primitive conditions of human waste disposal and widespread contamination of water supplies, water represents the most common vehicle of transmission, and that the number of organisms ingested is usually small. Thus, multiple subclinical and mild infections are believed to occur for each full-blown clinical case. In contrast, in more-developed countries with good sanitation, typhoid is transmitted when chronic carriers contaminate food vehicles through a breakdown in proper practices of personal and food hygiene. In these foodborne transmissions the inocula ingested are presumed to be large, and high attack rates ensue; under these conditions, fewer subclinical cases occur.
It is difficult to quantify the magnitude of the typhoid fever problem worldwide because the clinical picture is confused with that of many other febrile infections (including paratyphoid), and the capacity for routine bacteriologic confirmation is absent in most areas of the less-developed world. , Nevertheless, the periodic estimates reported by the Institute of Health Metrics and Evaluation (IHME) through their Global Burden of Disease surveillance provide helpful guideposts. IHME estimated that in 2017 there occurred 14.3 million cases of enteric (typhoid and paratyphoid) fever (95% uncertainty interval [UI] 12.5–16.3) worldwide, of which 76.3% of these cases, that is, ∼10.9 million (9.3–12.6), were estimated to be due to S . typhi and 3.4 million (2.7–4.2) due to S . paratyphi . This represents a substantial 44.6% decline from the 25.9 million estimated worldwide cases of enteric fever reported in 1990. Age-standardized incidence rates declined from 439.2 (376.7–507.7) per 100,000 person-years in 1990, to 197.8 (172.0–226.2) per 100,000 person-years in 2017. Using an estimated global case fatality risk of 0.95% (0.54–1.53) in 2017, it was estimated that 135,900 deaths (76,900–218,900) from typhoid (116,000) and paratyphoid (19,900) fever occurred across the world in 2017; this represents a 41.0% (33.6–48.3) decline from the estimated 230,500 deaths (131,200–372,600) in 1990.
A more granular view of the incidence of typhoid in areas where the disease is a public health problem can be garnered by reviewing data from vaccine field trials carried out from 1980 to 2021 in Latin America, Asia, and Africa. The incidence of typhoid fever that occurred in the control subjects during the periods of surveillance provided helpful data, albeit from a biased set of geographic sites where typhoid was known to exist and bacteriologic infrastructure was available to confirm cases. Incidence data from the control groups of multiple field trials of typhoid vaccines are shown in several of the chapter tables, collectively providing information of typhoid burden in multiple countries from 1980 to 2020. Mogasali et al. have reviewed the surveillance methods and incidence data from population-based studies of typhoid in the period 1990–2013. Levine similarly reviewed burden data form vaccine field trials and population-based surveillance studies covering the period of the 1950s through 2008.
Seroepidemiologic studies that measure the prevalence of S . typhi H antibody have also been useful in quantifying the prevalence of typhoid fever in different geographic areas. , However, this seroepidemiologic technique has not been widely applied.
The emergence in South Asia of paratyphoid fever caused by multiple-antibiotic-resistant S . paratyphi A poses a challenge for both indigenous populations , and travelers to this region from industrialized countries, because currently there are no licensed paratyphoid vaccines, and licensed oral Ty21a and parenteral Vi-based typhoid vaccines do not offer cross-protection against S . paratyphi A .
Three populations are at particularly high risk of developing typhoid fever and benefit from immunoprophylaxis with safe, effective, inexpensive, and practical vaccines. These include children and adults in endemic areas, , travelers and military personnel from industrialized countries who visit endemic areas in less-developed countries, and clinical microbiology technicians.
Seroepidemiologic studies in Peru and Chile in the 1970s (when typhoid was highly endemic in those countries) showed that by 15–19 years of age, 50–80% of teenagers had serologic evidence of past infection with S . typhi . , In endemic areas, typhoid fever is a major cause of absenteeism from school and from employment. Direct expenditures for hospitalization and medication further raise the public health costs of this disease. , For areas in which it is unlikely that improved sanitation and treated water supplies will become a reality in the near future, a safe vaccine that confers long-term protection (e.g., Ty21a or Vi conjugate ) would be particularly beneficial in relation to its cost, because an initial investment in immunization would provide many years of protection. In many countries where typhoid fever exhibits its peak incidence in school-age children, a “captive” population, a school immunization program could target the high-risk population of schoolchildren. For some urban slums and other populations in South Asia where the typhoid fever incidence is often high in preschool children, the increasing use of a second measles-containing vaccine in the second year of life offers an additional opportunity to administer a dose of Vi conjugate along with MCV2. , Travelers from industrialized countries who visit less-developed countries in which typhoid fever is endemic are at particular risk of developing the disease. , ,
Travelers are probably at special risk in endemic areas because they do not have the background immunity that much of the indigenous population has acquired as a consequence of multiple subclinical infections. For U.S. travelers, the Indian subcontinent (India, Pakistan, and Bangladesh), Mexico, Philippines, and Haiti are the areas of highest risk. Since 1990, isolates from Asia and northeast Africa have shown increasing resistance to many clinically relevant antibiotics, including chloramphenicol, amoxicillin, and trimethoprim-sulfamethoxazole. , , , Partial resistance to ciprofloxacin and other fluoroquinolones also became prevalent in Southeast Asia and other regions during this period. , However, the emergence in Sindh Province, Pakistan, of XDR strains of S . typhi amenable to treatment only with oral azithromycin or parenteral carbapenems poses the possibility of typhoid fever becoming untreatable in the near future if resistance to those antibiotics is also acquired. ,
Microbiology technicians in laboratories, particularly clinical laboratories, also constitute a high-risk group for the development of typhoid fever. The Centers for Disease Control and Prevention (CDC) reported that 11.2% of the sporadic (i.e., not outbreak-associated) cases of typhoid fever in the United States in a 33-month period (January 1, 1977 through September 30, 1979) occurred in laboratory technicians. In the course of their work, these people process stool or blood cultures containing S. typhi before it is recognized that the pathogen is present.
Humans are the sole reservoir of S . typhi infection as well as the only natural host. The infection is transmitted when susceptible hosts ingest food or water vehicles that have been contaminated by fecal matter containing S . typhi . In contrast, transmission from person to person by direct contact is exceedingly uncommon. As a consequence of these epidemiologic features, typhoid fever represents the quintessential infectious disease for which transmission is related to levels of sanitation and quality of water supply. Typhoid fever can abound wherever sanitation and food hygiene are primitive. The highest incidence usually occurs where water supplies serving large populations are contaminated by fecal matter. This situation existed at the end of the 19th century in most large cities in the United States and Western Europe, where piped water supplies were available but the water was usually untreated. The water sources (usually rivers) were also where the cities discharged their untreated sewage. In this manner, the transmission of typhoid fever was amplified, causing the disease to be highly endemic in large cities throughout the United States and Europe. With the introduction of water treatment at the turn of the 20th century, including sand filtration and chlorination, the incidence of typhoid fever plummeted precipitously in the large cities of the United States, despite the continued existence in those cities of many chronic carriers of S. typhi . Typhoid fever remains endemic in most of the less-developed areas of the world, where fecal contamination of water sources still occurs. This includes regions in many countries in Africa, Asia, and some in Latin America and Oceania.
Another source of amplified transmission is the use of untreated sewage to irrigate vegetable and fruit crops which are subsequently eaten uncooked. Bringing sewage-contaminated vegetables into the kitchen or food preparation areas introduces the possibility of cross contaminating other foods with S . typhi . , If practiced on a large-scale, irrigation with untreated sewage can sustain high rates of typhoid transmission even where the population has access to potable water. , ,
Data quantifying the incidence of bacteriologically confirmed typhoid fever are available from control (usually placebo recipient) groups participating in large-scale field trials of typhoid vaccines, or from populations in sites being prepared for field trials. It may be argued that such populations represent hot spots of typhoid and that the incidence rates from such areas cannot be readily extrapolated to other developing regions. However, it is in such hotspots that introduction of safe, effective, typhoid vaccines is sorely needed. In sites being prepared for field trial evaluations of typhoid vaccines and from field trials carried out in the late 1970s through 2020 in South America (Chile), Africa (South Africa, Malawi), and Asia (Nepal, Indonesia, India, Pakistan, Bangladesh), annual incidence rates of 198–1280 cases per 10 5 person-years of observation (PYO) were recorded. , , ,
Passive protection by means of antiserum or immunoglobulin is not used to prevent typhoid or paratyphoid fever.
Parenteral and oral inactivated whole bacterial cell vaccines were administered to humans beginning in the very late 19th century and within the first 15 years of the 20th century. These included heat-inactivated, phenol-preserved, typhoid bacilli vaccines that were widely used by the military in Great Britain, Europe, and the United States. Over the ensuing decades through the late 1960s, various alternative vaccines were evaluated that included other forms of inactivated whole-cell vaccines and subunit vaccines administered parenterally, orally or via aerosol, and early attenuated strains of S . typhi given as live oral vaccines. The specific vaccines and clinical results are reviewed in the chapters on typhoid vaccine in the first two editions of this book. ,
One of these historic types of typhoid vaccine, the acetone-inactivated and dried whole-cell parenteral vaccine, conferred a moderately high level of efficacy in well-designed field trials, , albeit frequently accompanied by reactogenicity. Protection endured for up to 7 years, and the immune responses to the H and Vi antigens elicited by this vaccine helped pave the way for modern parenteral typhoid vaccines.
This type of vaccine was prepared because evidence showed that acetone inactivation preserved the Vi antigen (thereby increasing potency in active mouse protection assays) and improved the stability of the vaccine on long-term storage. Although the manufacturing process required to prepare acetone-inactivated, dried vaccine was more demanding than for making heat-phenolized vaccine, there were some high-income country manufacturers of this vaccine until the 1990s, including Wyeth, which made a lyophilized formulation used by the U.S. military.
Parenteral heat-inactivated, phenol-preserved, whole-cell typhoid vaccines were also used from the end of the 19th century, , , , through the early years of the 21st century. These vaccines were relatively easy to prepare and standardize, conferred a lower level of protection than the acetone-inactivated vaccine, and were similarly reactogenic.
In the 1960s and 1970s, several large-scale field trials and experimental challenge studies in volunteers assessed the efficacy of oral inactivated whole-cell vaccines. While well tolerated, oral killed whole-cell vaccines conferred only modest protection, if at all.
In their chronological order of development and licensure, there are currently three types of modern, licensed typhoid vaccines: Ty21a live oral vaccine; unconjugated purified Vi polysaccharide parenteral vaccine; and Vi polysaccharide-conjugate parenteral vaccines ( Table 62.1 ).
| Type of Vaccine | Product Name a | Producer | Site of Manufacture |
|---|---|---|---|
| Parenteral purified Vi polysaccharide | Typhim Vi b | Sanofi Pasteur | France |
| Typherix | GlaxoSmithKline | Belgium | |
| TypBar | Bharat Biotech | India | |
| Shantyph | Shanta Biotech | India | |
| Bio Typh | BioMed | India | |
| Zerotyph inj | Boryung | South Korea | |
| Typhevac inj | Shanghai Institute of Biological Products | China | |
| Live oral Ty21a | Vivotif | Emergent Travel Health, Inc. | Switzerland |
| Zerotyph caps | Boryung | South Korea | |
| Parenteral Vi conjugate a | z b | Bharat Biotech International | India |
| TyphiBEV b | BiologicalE | India | |
| PedaTyph | Bio-Med | India | |
| ZyVac-TCV | Cadila Healthcare (Zydus Cadila) | India |
a These vaccines were licensed in the country of manufacture as of August 1, 2021.
b These vaccines had WHO prequalification as of August 1, 2021.
Attenuated live oral S . typhi vaccine strain Ty21a was derived in the prerecombinant DNA era by treating wild-type strain Ty2 with the mutagenic agent nitrosoguanidine and selecting a mutant that exhibited complete absence of activity of the enzyme uridine diphosphate (UDP)-galactose-4-epimerase and substantial (∼80%) reduction in activity of two other enzymes, galactokinase and galactose-1-phosphate uridyl transferase. A further mutant was selected that also lacked the ability to express Vi antigen. Ty21a is the only licensed live oral typhoid vaccine; currently, the only commercial formulation available contains the vaccine within enteric-coated capsules.
To purify Vi, the polysaccharide capsule of S . typhi , hexadecyltrimethylammonium bromide is added to concentrated culture supernatant after which differential centrifugation and precipitation are performed. The potency of the purified polysaccharide is determined by molecular size and O-acetyl content; phenol, 0.25%, is added as a preservative. Each 0.5 mL dose is contains 25 mcg of purified Vi polysaccharide.
Currently there are four licensed Vi conjugate products, with several others expected to be licensed in 2022. The licensed Vi conjugates include: (i) Typbar-TCV ® (Vi linked to tetanus toxoid); (ii) Zyvac TCV ™ (Vi linked to tetanus toxoid) ; (iii) PedaTyph ™ (Vi linked to tetanus toxoid) ; (iv) TyphiBev ™ (Vi linked to CRM 197 ).
In the 1970s, 1980s, and 1990s, successive technologies were utilized including chemical mutagenesis, repetitive passage and selection for streptomycin-dependent strains, nonrecombinant bacterial genetic methods, and, finally, recombinant DNA technology, to develop attenuated strains of S . typhi to serve as well tolerated, protective live oral vaccines. , Details about these strains and their clinical evaluation are described in earlier editions of this book. Out of this extensive experience, one strain, Ty21a, emerged as a licensed live oral vaccine.
Ty21a was derived from wild-type strain Ty2 in the prerecombinant DNA era by treatment with the mutagenic agent nitrosoguanidine, selection of a mutant that exhibited complete absence of activity of uridine diphosphate (UDP)-galactose-4-epimerase, a reduction of ∼80% in activity of galactokinase and galactose-1-phosphate uridyl transferase, and further selection for inability to express Vi antigen. This strain was designated Ty21a.
For many years, it was thought that the galE and Vi mutations together accounted for the impressive safety of Ty21a. It is now recognized that the galE and Vi mutations in Ty21a do not by themselves explain the attenuation of this strain. Other mutations induced by the non-specific chemical mutagenesis also contribute to the safety of this oral vaccine strain. In addition, a mutation in rpoS ( katF ), which encodes an RNA polymerase sigma factor and is inherited from the wild type parent strain, also contributes to attenuation ; this rpoS mutation diminishes the ability of the bacteria to survive under stress, including nutrient deprivation.
Early attempts in the 1950s to prepare Vi antigen for use as a parenteral purified Vi polysaccharide vaccine unwittingly denatured the polysaccharide. , Landy and coworkers , isolated Vi antigen from Citrobacter freundii (previous designations of this bacterium include Paracolon ballerup , Bethesda ballerup, and Escherichia coli 5396/38) and S . typhi for use as a parenteral vaccine. Organisms grown on solid agar were inactivated with acetone, after which the dried, acetone-killed bacteria were submitted to multiple extractions with saline, ethanol, and acetic acid to separate Vi from protein, LPS, and nucleic acid. This early attempt to purify Vi denatured the polysaccharide, resulting in a complete loss of O -acetyl and a diminution of N -acetyl moieties. , , Landy's denatured Vi vaccine was never evaluated for efficacy in field trials. However, in experimental challenge studies in volunteers, a single 25-µg parenteral dose conferred modest (25% vaccine efficacy), insignificant, protection.
To purify Vi under nondenaturing conditions, Wong and colleagues and Robbins and Robbins treated S . typhi with hexadecyltrimethylammonium bromide. This extraction method resulted in nondenatured purified Vi that preserved the O - and N -acetyl moieties. Fig. 62.2 shows the chemical structure of Vi polysaccharide.
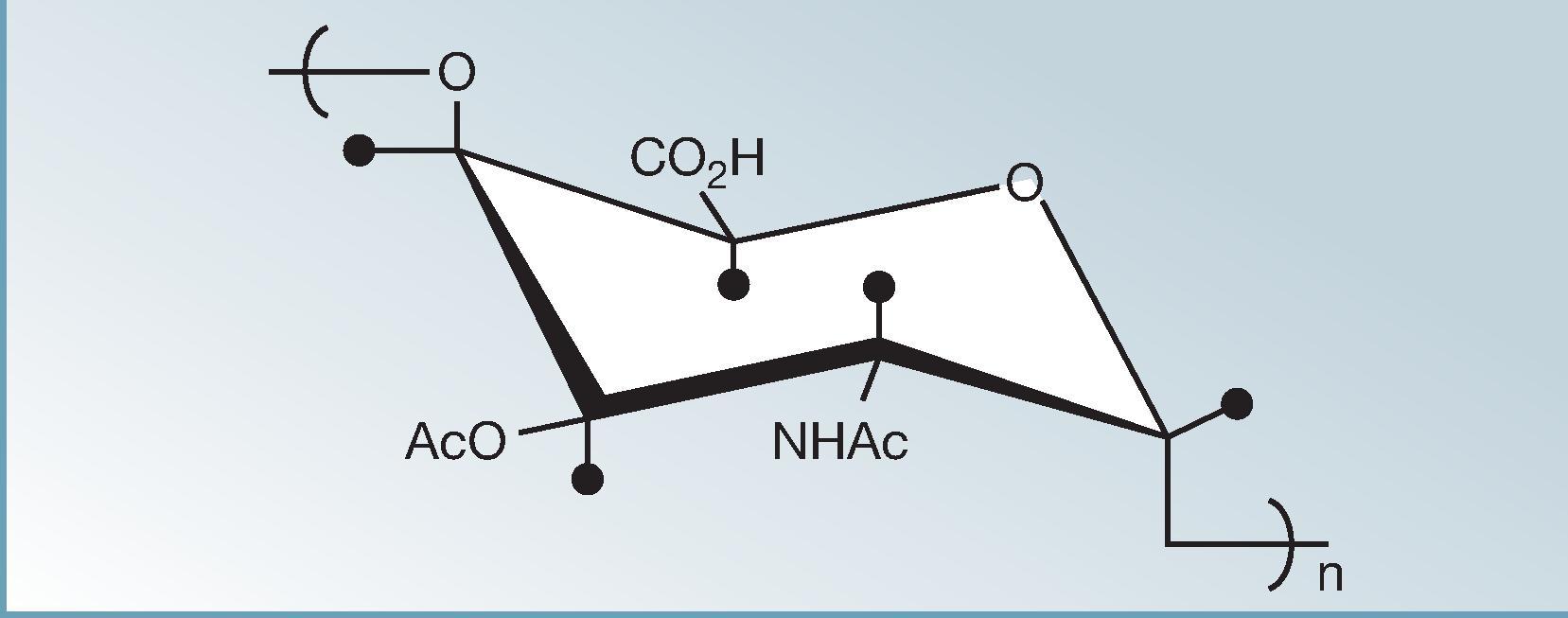
Vi polysaccharide, like other purified polysaccharides, acts like a T-lymphocyte-independent antigen that does not confer immunologic memory, is poorly immunogenic in infants, stimulates relatively short-lived serum antibody responses in persons of any age, and titers cannot be boosted by administering additional doses of Vi vaccine. Shousun Szu and John B. Robbins at the National Institute of Child Health and Human Development in the U.S. pioneered studies to create a well-tolerated, immunogenic, and efficacious Vi conjugate vaccine. They showed that when Vi is conjugated to certain carrier proteins, such as tetanus toxoid, cholera toxin, cholera toxin B subunit, or recombinant exotoxin A of Pseudomonas aeruginosa, it behaves like a T-lymphocyte-dependent antigen. , In animal models, subsequent inoculations with their Vi conjugate vaccines boosted the serum Vi antibody titer. Clinical studies carried out in adults, school-age children, and preschool-age children in Vietnam compared the immunogenicity of two formulations of Vi-rEPA conjugate prepared using two different conjugation methods. The conjugate option prepared by treating the carrier protein with the homobifunctional linker adipic acid dihydrazide (ADH) and binding to Vi in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) elicited significantly higher titers of serum IgG Vi antibody than the other Vi conjugate (and higher than Vi polysaccharide alone), yet was well tolerated. The superior conjugate showed a high rate of seroconversion in all ages after a single injection (containing 24 µg of Vi and 21.5 µg of rEPA) but a gradation in GMT that decreased progressively from adults to toddlers. The group of subjects given a second dose of this conjugate 6 weeks later exhibited a clear booster response in IgG Vi antibody titers.
Szu and colleagues , pursued a stepwise program to develop an optimal Vi conjugate vaccine that culminated in a candidate for clinical trials consisting of Vi polysaccharide conjugated to recombinant P. aeruginosa exotoxin A (Vi-rEPA). After demonstration of the safety and immunogenicity of this conjugate, the efficacy conferred by a two-dose (6 weeks apart) immunization schedule of the vaccine was evaluated in a randomized, controlled field trial in Vietnam in 11,091 children 2–5 years of age. The conjugate vaccine used in the Phase 3 field trial of efficacy in Vietnam contained 22.5 µg of Vi and 22 µg of exoprotein A per 0.5-mL dose. In total, 5525 children received two doses of Vi-rEPA and 5566 received placebo. During 27 months of follow-up, active surveillance was carried out to detect cases of typhoid fever by visiting children weekly. Subjects who had a temperature of ≥37.5°C for at least 3 days were referred to a health center, where 5 mL of blood were drawn for bacteriologic culture. After the code was broken, an additional 19 months of follow-up were instituted by means of passive surveillance ( Table 62.2 ). Over the full 46 months of follow-up (27 months using active and 19 months using passive surveillance methods), overall vaccine efficacy was 89.0% (95% CI, 76.0–96.9%) ( Table 62.2 ). , Subsequently, the prototype Vi-rEPA conjugate demonstrated satisfactory immunogenicity when young Vietnamese infants were immunized according to a four-dose EPI schedule (priming immunization with three doses at 2, 4, and 6 months of age followed by a reinforcing dose at age 12 months), and there was no significant interference with other EPI vaccines. The pioneering preclinical and clinical research with Vi-rEPA paved the way for development of a series of Vi conjugates that became licensed vaccines in India, with two attaining WHO prequalification ( Table 62.1 ). , One of the new Vi conjugates, Typbar TCV® (Vi-TT) stimulates long-lived serum Vi antibody responses, and high-level protection following administration of just a single dose to infants, toddlers, or older children ( Table 62.2 ).
| Country | TCV Product | No. Doses | Control Product | Ages | Study Period (Subject Follow-Up) | Total NumberVaccinated | Blood Culture-Confirmed Typhoid Fever Cases | Cases/100,000 Person-Yrs | |||||||||
| TCV | Control | TCV | Control | TCV | Control | Vaccine Efficacy (95% CI) | |||||||||||
| Individual-Randomized Trials | |||||||||||||||||
| Vietnam | Vi-rEPA | 2 | Saline placebo | 2–5 years | 27 mos a | 5525 | 5566 | 4 | 47 | 32 | 375 | 91.5% (77.1–96.6) | |||||
| Vietnam | Vi-rEPA | 2 | Saline placebo | 2–5 years | 19 mos b | 5525 | 5566 | 3 | 17 | 34 | 193 | 82.4%(22.3–99.1) | |||||
| Vietnam | Vi-rEPA | 2 | Saline placebo | 2–5 years | 46 mos c | 5525 | 5566 | 7 | 64 | 38 | 342 | 88.9(76.0–96.9) | |||||
| Nepal , | Vi-TT (Typbar TCV®) | 1 | Men-AfriVac | 9 mos–<16 yrs | 24 mos d | 10,005 | 10,014 | 13 | 62 | 72 | 342 | 79.0%(61.9, 88.5) | |||||
| Malawi | Vi-TT (Typbar TCV®) | 1 | Men-AfriVac | 9 mos–12 yrs | 18 mos e | 13,945 | 13,937 | 10 f | 12 g | 61 f | 62 g | 40 f | 47 g | 242 f | 243 g | 84% f (68–92) | 81% g (64–90) |
| Malawi | 9 mos–4 yrs | 5044 | 5158 | 5 f | 5 g | 20 f | 20 g | 55 f | 55 g | 216 f | 20 g | 74% f (32–90) f | 74% g (32–90) | ||||
| Malawi | 5yrs–12 yrs | 8901 | 8779 | 5 f | 7 g | 41 f | 42 g | 31 f | 43 g | 257 f | 41 g | 88% f (70–95) | 84% g (64–93) | ||||
| Cluster-Randomized Trials | |||||||||||||||||
| Country | TCV Product | No. Doses | Control Vaccine | Ages | Study Period (Subject Follow-Up) | Total NumberVaccinated | Blood Culture-Confirmed Typhoid Fever Cases | Cases/100,000 Person-Yr | |||||||||
| TCV | Control | TCV | Control | TCV | Control | Total Effectiveness (CI) | |||||||||||
| India | Vi-TT(PedaTyph) | 2 | None | 6 mos–12 yrs | 12 months h | 905 in 12 schools | 860 in 12 schools | 0 | 11 | 0 | 1280 | 100%(97.6–100) i | |||||
| Bangladesh | Vi-TT (Typbar TCV®) | 1 | Live JE | 9 mos–15 yrs | Up to 25 months (mean 17.1 mos) | 30,882 in 75 clusters | 30,685 in 75 clusters | 29 | 192 | 96 | 635 | 85% i (76–91) | |||||
| 9–23 mos | 4 | 23 | 143 | 820 | 81%(39–94) i | ||||||||||||
| 2–4 yrs | 12 | 62 | 194 | 967 | 80%(62–89) | ||||||||||||
| 5–15 yrs | 13 | 107 | 61 | 509 | 88%(78–93) i | ||||||||||||
| Postintroduction Effectiveness Study Nested Within a Mass Vaccination Campaign | |||||||||||||||||
| Country | TCV Product | No. of Doses | Study Design | Ages | Study Period (Subject Follow-Up) | Total NumberVaccinated | Blood Culture-Confirmed Typhoid Fever, n | Cases/100,000 Person-Yr | |||||||||
| TCV | Control | TCV | Control | TCV | Control | Vaccine Efficacy (95% CI) | |||||||||||
| Pakistan | Vi-TT | 1 | Prospective cohort | 6 mos–10 yrs | 12 months | 13,436 | 9971 | 47 | 728 | 350 | 7301 | 94.9% (93.2–96.3) | |||||
a Blood-culture confirmed typhoid fever after 27 months of follow-up using active surveillance. Per protocol analysis.
b Blood-culture confirmed typhoid fever after 19 months of follow-up using passive surveillance. Intention-to-treat analysis.
c Blood-culture confirmed typhoid fever after 46 months of combined follow-up including 27 months of active and 19 months of passive surveillance.
d Blood-culture confirmed typhoid fever after 2 years of follow-up. Per protocol analysis.
e Blood-culture confirmed typhoid fever after 18–24 months of follow-up.
f Per protocol analysis, excludes any typhoid cases in first 2 weeks after vaccination.
g Vaccine efficacy for intention-to-treat analysis (95% CI): 9 mos–12 yrs, 81% (64–90); 9 mos–4 yrs, 74% (32–90); 5—12 yrs, 84% (64–93).
h Open label, randomized by school. 765 received two doses.
i 97.5% confidence interval. TCV, typhoid conjugate vaccine; Vi-rEPA, Vi conjugated to recombinant exoprotein A of Pseudomonas aeruginosa ; mos., months; Typbar TCV®, WHO prequalified Vi-tetanus toxoid conjugate vaccine manufactured by Bharat Biotech International (Hyderabad, India); PedaTyph, Vi-tetanus toxoid conjugate vaccine manufactured by BioMed (Ghaziabad, India); MenAfriVac, meningococcal group A conjugate vaccine; JE, live attenuated Japanese encephalitis vaccine from the Chengdu Institute of Biological Products, Chengdu, China; CI, 95% confidence interval, except for the Bangladesh trial, which used 97.5% CI.
The global landscape status of licensed Vi conjugate vaccines and some of their salient features are shown in Table 62.3 , summarizing the considerable progress made since 2017. Four Vi polysaccharide conjugate vaccines have been licensed by the national regulatory authority of India, two of which have been prequalified by WHO, including Typbar-TCV™ (Bharat Biotech International, Vi linked to tetanus toxoid) , , and TyphiBEV™ (Biological E, Vi linked to CRM 197 ). Two other Vi conjugates licensed in India do not have WHO Prequalification (PedaTyph [BioMed, Vi linked to CRM 197 ] and ZyVAC-TCV™ [Cadilla Healthcare, Vi linked to TT]).
| Product | Developer (Manufacturer) | Source of Vi CPS | Carrier Protein | Conjugation Strategy | Amount of Conjugated Vi in Each Dose | National Regulatory Authority (If Licensed) | WHO Prequalification |
| Typbar TCV® | Bharat Biotech International, Hyderabad, India | S . Typhi | Tetanus toxoid (TT) | TT is modified by attaching the homobifunctional linker adipic acid dihydrazide (ADH). Vi is then conjugated to TT-ADH using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) coupling chemistry | 25 µg | Licensed by the Drug Controller General of India (DCGI), Central Drugs Standard Control Organization (CDSCO) | December 2017 |
| PedaTyph | BioMed, | S . Typhi | Tetanus toxoid | Has not been described | 5 µg | Licensed by the DCGI, CDSCO | No |
| TyphiBEV | Biological E, Hyderabad, India, and GSK Vaccines Institute of Global Health, Siena, Italy | Citrobacter freundii | CRM 197 | Carboxylic groups of the Vi polysaccharide purified from Citrobacter freundii are activated with N -(3-dimethylaminopropyl)- N '-ethylcarbodiimide hydrochloride (EDAC) and N -hydroxysuccinimide (NHS) to generate NHS active esters. The activated Vi is randomly linked to CRM197, previously derivatized with ADH as a spacer. | 25 µg | Licensed by the DCGI, CDSCO | January 2021 |
| ZyVAC TCV | Cadila Healthcare | S . Typhi | Tetanus toxoid | TT is modified by attaching ADH linker. Vi is then conjugated to TT-ADH using EDC coupling chemistry | 25 µg | Licensed by the DCGI, CDSCO | No |
| Vi conjugate vaccines not licensed as of August 1, 2021 but in advanced clinical development | |||||||
| Vi-DT | SKBioscience and the International Vaccine Institute (Korea) | S. Typhi | Diphtheria toxoid (DT) | DT is modified by attaching the homobifunctional linker adipic acid dihydrazide (ADH). Vi is then linked to DT-ADH using EDC coupling chemistry | Application expected to be submitted to the Ministry of Food and Drug Safety (MFDS) of Korea in 2021 | ||
| Vi-DT | Bio Farma (Indonesia) | S . Typhi | Diphtheria toxoid (DT) | DT is modified by attaching the homobifunctional linker adipic acid dihydrazide (ADH). Vi is then linked to DT-ADH using EDC coupling chemistry | 25 µg | ||
| Vi-TT | Eubiologics (Korea) | S . Typhi | CRM 197 | CRM 197 with attached ADH linker is activated with EDC and conjugated to Vi. | 25 µg | Application expected to be submitted to the Ministry of Food and Drug Safety (MFDS) of Korea in 2021 | |
| Vi-rEPA | NIH (Lanzhou Institute of Biological Products, Lanzhou, China) | S . Typhi | Recombinant exotoxin A of Pseudomonas aeruginosa | rEPA is modified by attaching the homobifunctional linker ADH. Vi is then linked to rEPA-ADH using EDC coupling chemistry | 25 µg | Regulatory status not known | |
Several other Vi conjugates manufactured elsewhere in Asia are believed to be near licensure by their national regulatory authorities. These include Vi conjugated to diphtheria toxoid (Vi-DT) manufactured by SK-Bio of the Republic of Korea (after conjugate technology transfer from the International Vaccine Institute), , a similar Vi-DT manufactured by Bio Farma in Indonesia, and another conjugate manufactured in Korea (Eubiologics Vi-CRM 197 conjugate) ( Table 62.3 ).
The enteric-coated capsule formulation of Ty21a consists of gelatin capsules coated with hydroxypropylcellulose-phthalate lacquer (27–33 mg per capsule) to render the capsules resistant to acid. Each coated capsule contains 2.0–10.0 × 10 9 colony-forming units (CFU) of Ty21a, 5–50 × 10 9 nonviable Ty21a, 3.3–34.2 mg of sucrose, 0.2–2.4 mg of ascorbic acid, 0.3–3.0 mg of an amino acid mixture, up to 180–200 mg of lactose, and 3.6–4.0 mg of magnesium stearate (U.S. specifications). The sucrose, ascorbic acid, and the amino acid mixture are used as stabilizers for the lyophilization process. The lyophilized bacteria are mixed with lactose to achieve a defined potency of the lyophilate. Magnesium stearate is added to the lyophilized bacteria for a defined fluidity of the lyophilate.
Typhim Vi® (Sanofi Pasteur) is described as an example to illustrate the sort of constituents found within a dose of a typical purified Vi polysaccharide vaccine. S. typhi strain Ty2 is grown in a semisynthetic medium. Casein-derived raw materials are used early in manufacturing during the fermentation process. Vi polysaccharide is extracted from concentrated culture supernatant by addition of hexadecyltrimethylammonium bromide, and the product is purified by differential centrifugation and precipitation. Each 0.5 mL dose may contain residual amounts of formaldehyde (≤100 mcg) used for the inactivation of the bacterial culture. The potency of the purified polysaccharide is assessed by molecular size and O-acetyl content. Phenol, 0.25%, is added as a preservative. The vaccine contains residual polydimethylsiloxane or fatty-acid ester-based antifoam. The vaccine is a clear, colorless solution. Each 0.5 mL dose is formulated to contain 25 mcg of purified Vi polysaccharide in a colorless isotonic phosphate buffered saline (pH 7 ± 0.3) and 0.5 mL of Sterile Water for Injection.
Typbar TCV® (Bharat Biotech International) is given as an example of a licensed, WHO prequalified Vi conjugate. One dose (0.5 mL) of Typbar TCV® contains 25 µg of Vi polysaccharide from S . typhi linked to tetanus toxoid protein in isotonic saline. Multidose (2.5 mL) vials contain five doses of Typbar TCV® in isotonic saline with 5 mg of 2-phenoxyethanol preservative.
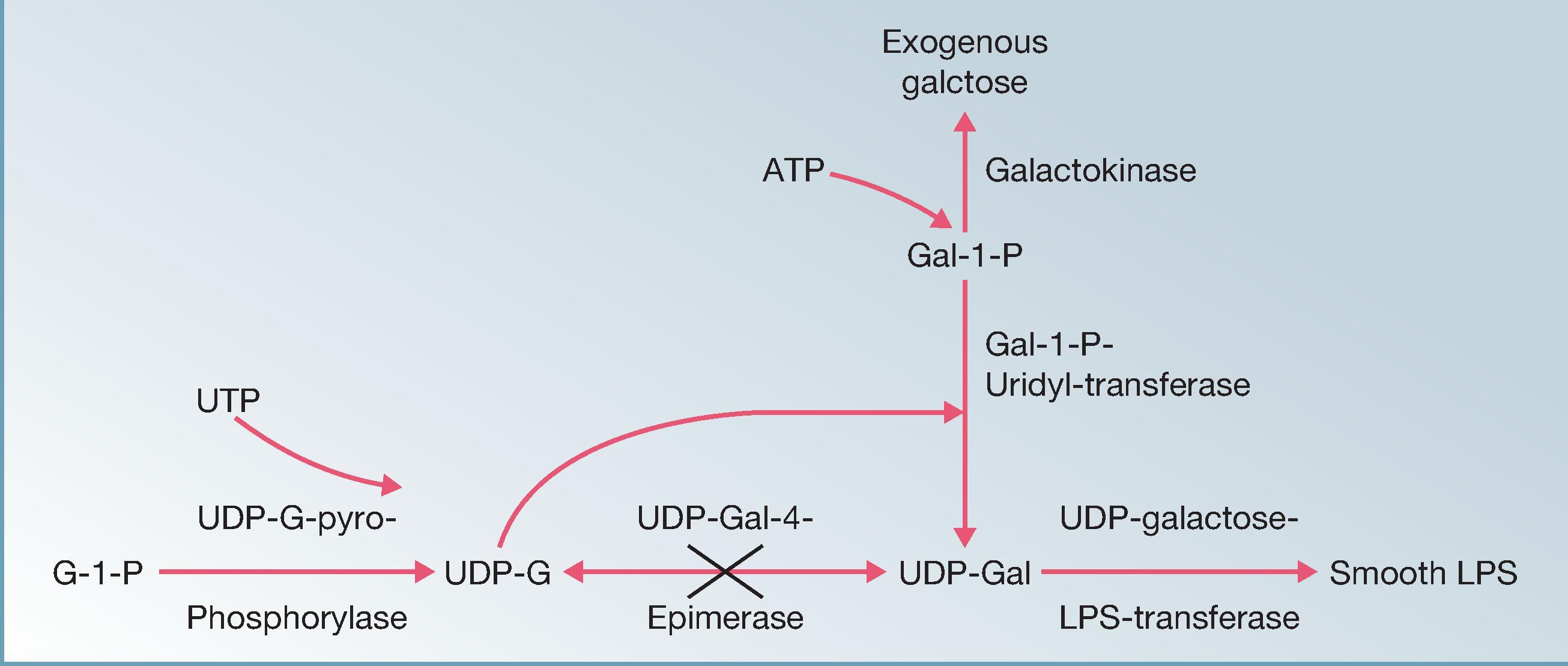
Galactose residues are an important component of the smooth LPS O antigen in wild-type S . typhi . The enzyme encoded by galE , UDP-galactose-4-epimerase, isomerizes UDP-glucose to UDP-galactose, and vice versa ( Fig. 62.3 ). UDP-galactose provides galactose residues that can be incorporated into the smooth LPS O antigen of S . typhi . When grown in the absence of galactose, Ty21a does not express smooth O antigen because it has no source of UDP-galactose; in this state it is not immunogenic. In contrast, when Ty21a is grown in the presence of exogenous galactose, the two other Leloir pathway (galactose metabolism) enzymes ( Fig. 62.3 ) allow this monosaccharide to be assimilated to UDP-galactose and used to synthesize smooth O antigen. However, because of the lack of epimerase, Ty21a accumulates galactose-1-phosphate and UDP-galactose when grown in the presence of exogenous galactose ( Fig. 62.3 ). If the concentration of galactose reaches a certain level, accumulation of these intermediate products leads to bacterial death by lysis. An appropriate concentration of galactose is included in the medium in an early step of manufacture to ensure expression of smooth O antigen.
Scale-up of production of Vi to an industrial manufacturing process was initially worked out by scientists in the laboratory of John B. Robbins at the National Institute of Child Health and Human Development and Sanofi Pasteur. S. typhi strain Ty2 is cultured in 1000-L fermenters, after which the bacteria are fixed with formaldehyde and Vi polysaccharide is extracted from supernates using cetrimonium bromide. The Vi is further purified, dried, dissolved in a buffer solution, and filter sterilized. After appropriate controls of this material, it is filled into single-dose syringes.
Typbar TCV®— To manufacture Typbar TCV®, Bharat Biotech International uses Vi polysaccharide purified as it is for Typbar™, their unconjugated Vi vaccine. The carrier protein, tetanus toxoid (TT), is modified by attaching the homobifunctional linker adipic acid dihydrazide (ADH). Vi is then linked to TT-ADH using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) coupling chemistry.
TyphiBEV —Biological E chemically links Vi purified from Citrobacter freundii to nontoxic mutant diphtheria toxin CRM 197 . The carboxylic groups of the Vi polysaccharide chain are activated with N -(3-dimethylaminopropyl)- N '-ethylcarbodiimide hydrochloride (EDAC) and N -hydroxysuccinimide (NHS) to generate NHS active esters. Subsequently, the activated Vi is randomly linked to CRM 197 , previously derivatized with ADH as a spacer.
ZyVac-TCV— Cadila Health Care also attaches ADH to TT and then links TT-ADH to Vi, using EDC coupling.
PedaTyph— For PedaTyph no detailed description is available for the specific method of purification of the Vi polysaccharide component or of its conjugation to TT. The manufacturer website states that they developed a “unique process of conjugation.”
Ty21a is currently manufactured by two companies: Emergent Biosolutions, Berne, Switzerland (Vivotif®) and Boryung Pharmaceutical Co., Ltd., of Seoul, Korea (Zerotyph®). Under license, Ty21a is also distributed by a number of other vaccine distributors.
There are presently two licensed manufacturers of purified Vi among industrialized countries, Sanofi Pasteur Vaccines (Typhim Vi, available in the United States and many European and other countries) and GSK Biologics (Typherix, available in many European and other countries). In addition, several vaccine manufacturers in Asia (China, India, Vietnam), , Russia, and Cuba make Vi products for local and regional consumption.
Typbar TCV® is manufactured by Bharat Biotech International of Hyderabad, India, PedaTyph vaccine is manufactured by Bio-Med Private Limited, located in Ghaziabad, India. , TyphiBEV is manufactured by Biological E of Hyderabad, India, and ZyVAC-TCV is manufactured by Cadila Health Care (Zydus) of Ahmedabad, India.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here