Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Adenomas of the small intestine are relatively uncommon, compared with adenomas of the large intestine. Around 80% arise in the periampullary duodenum. Duodenal adenomas occur over a wide age range (mean 60 years), with an equal sex distribution. Patients with familial adenomatous polyposis (FAP) have a markedly increased incidence of duodenal and ampullary adenomas (nearly 100%), which are often detected by endoscopic screening after prophylactic colectomy. The mean age of patients with FAP with such adenomas is two decades younger than that of patients with sporadic lesions. Patients with MUTYH -associated polyposis also have an increased risk of duodenal adenomas. Small duodenal adenomas are asymptomatic, whereas large adenomas may present with occult gastrointestinal (GI) bleeding or, rarely, obstruction. Ampullary adenomas may present with abdominal pain and biliary obstruction.
Duodenal adenomas are usually sessile polyps or plaquelike lesions, most less than 1 cm in size. Ampullary adenomas often show a delicate, feathery appearance correlating with villous architecture. Small adenomas in patients with FAP may be subtle or endoscopically inapparent.
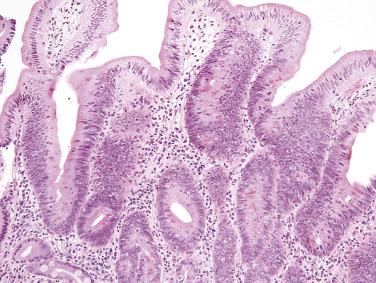
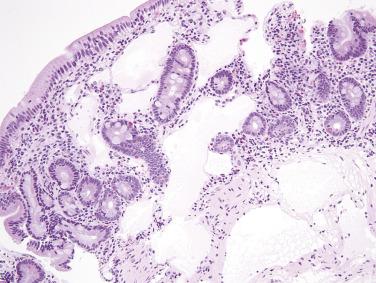
Similar to adenomas of the large intestine, duodenal and ampullary adenomas may be classified as tubular, tubulovillous, or villous (see later discussion on large intestine). Small duodenal adenomas are more often tubular, with hyperchromatic, elongated nuclei showing pseudostratification extending to the mucosal surface ( Fig. 9.1 ). Paneth cells are often prominent, which can be a helpful diagnostic clue, especially for the distinction between adenoma and an inflammatory polyp (polypoid duodenitis). Villous adenomas predominate in the ampulla. Pyloric gland adenomas (similar to those arising in the stomach) may also be identified in the duodenum; such lesions are composed of closely apposed small glands lined by cuboidal cells with palely eosinophilic, ground-glass cytoplasm.
Adenocarcinomas of the small intestine are rare. As with adenomas, the most common site is the periampullary duodenum; jejunal and ileal adenocarcinomas are particularly rare. A slight male predominance is seen, and the peak incidence is in the seventh decade. Significant risk factors for adenocarcinoma of the small intestine include Crohn disease, celiac disease, Lynch syndrome, and FAP. Patients with Peutz-Jeghers syndrome, juvenile polyposis, and MUTYH -associated polyposis are also at increased risk. Adenocarcinomas rarely develop in ileostomies and ileal pouches after colorectal surgery. Small intestine adenocarcinomas are usually asymptomatic in early stages; most patients therefore present with advanced tumors when bowel obstruction occurs. The most common symptoms include abdominal pain, weight loss, vomiting, and occult GI bleeding. Duodenal adenocarcinomas have a worse prognosis than distal small intestinal tumors.
Duodenal adenocarcinomas are usually relatively circumscribed with a polypoid appearance and central ulceration, whereas jejunal and ileal tumors most often show circumferential involvement with extensive ulceration and an annular appearance. Ileal adenocarcinomas may mimic Crohn disease. Multifocality and lack of a mucosal component should raise the possibility of metastasis from elsewhere.
Adenocarcinomas of the small intestine resemble colorectal adenocarcinomas and often contain a mucinous component. Although they are more often poorly differentiated than their large intestinal counterparts, primary signet ring–cell carcinomas of the small intestine are rare and should be distinguished from gastric metastasis.
In comparison with colorectal adenocarcinomas, small intestinal adenocarcinomas more often express cytokeratin 7 (CK7) (30%–50% of tumors) and less often CK20 (only around 60%). CDX-2 is usually positive but often with a more heterogeneous staining pattern, including areas with only weak staining or without demonstrable expression.
In the most recent (2017) World Health Organization (WHO) classification of tumors of endocrine organs, a uniform nomenclature and grading system for (neuro)endocrine neoplasms of the pancreas was introduced ; this system is currently also applied to GI tract tumors ( Table 9.1 ). Neuroendocrine tumors (NETs) (formerly widely known as carcinoid tumors) are well-differentiated neoplasms composed of cells with features similar to those of their normal counterparts, usually including strong expression of chromogranin and synaptophysin and various peptide hormones according to site, and generally showing only mild nuclear atypia. NETs are separated into three grades (G1, G2, and G3) according to mitotic count and Ki67 proliferation index (see Table 9.1 ). Neuroendocrine carcinoma (NEC) is reserved for poorly differentiated (high-grade) neuroendocrine neoplasms with marked nuclear atypia and a high Ki67 proliferation index (usually >60%). This category includes small cell carcinoma and large cell NEC. This classification should also be applied to metastatic lesions when possible (e.g., metastatic NET should not be designated carcinoma —this should be reserved only for high-grade, poorly differentiated neoplasms, and grading should be attempted if the biopsy specimen contains sufficient tissue).
| Classification/Grade | Mitotic Index (per 10 hpf) | Ki67 Proliferation Index |
|---|---|---|
| Well-differentiated neuroendocrine neoplasms (neuroendocrine tumors) | ||
| Neuroendocrine tumor G1 | <2 | <3% |
| Neuroendocrine tumor G2 | 2–20 | 3%–20% |
| Neuroendocrine tumor G3 | >20 | >20% |
| Poorly differentiated neuroendocrine neoplasms (neuroendocrine carcinomas) | ||
| Neuroendocrine carcinoma | >20 | >20% * |
* The Ki67 proliferation index for neuroendocrine carcinomas is usually >60%.
The small intestine is the most common site for NETs within the GI tract, accounting for nearly 50% of all GI NETs. Ileal primary tumors are much more frequent than jejunal or duodenal tumors. Small intestinal NETs occur over a wide age range with a peak incidence in the sixth decade. Patients with jejunoileal serotonin-producing (enterochromaffin cell) NETs usually first present with vague abdominal complaints, which may progress to intestinal obstruction. A small subset of patients (5%–10%), predominantly those with distal ileal tumors associated with liver metastasis, manifest the carcinoid syndrome, characterized by diarrhea, flushing, and fibrosis of the endocardium and right-sided heart valves. Patients with duodenal NETs usually have no symptoms, although some present with obstruction or jaundice. Gastrin-producing duodenal NETs (gastrinomas), which account for the majority of duodenal NETs, are associated with Zollinger-Ellison syndrome (severe peptic ulcer disease resulting from hypergastrinemia and uncontrolled secretion of gastric acid) in a large proportion of cases. Gastrinomas associated with multiple endocrine neoplasia type 1 (MEN-1) are nearly exclusive to the duodenum. Duodenal somatostatin-producing NETs are usually asymptomatic, unlike their pancreatic counterparts. Patients with neurofibromatosis type 1 (NF1) have a markedly increased risk of development of somatostatin-producing NETs of the duodenum and periampullary region and may also have gangliocytic paragangliomas (see Chapter 28 ).
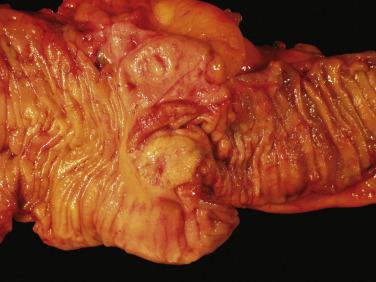
Jejunoileal NETs present as mucosal or submucosal nodules with a yellow cut surface after formalin fixation ( Fig. 9.2 ). Most tumors are between 1 and 3 cm. Around 30% of patients present with multiple primary tumors. Infiltration through the bowel wall into the mesentery, associated with a marked desmoplastic reaction leading to luminal narrowing and stricture formation, is common. Mesenteric and lymph node metastases are often larger than the primary tumor. Duodenal NETs are usually less than 2 cm. MEN-1–associated gastrinomas are often multiple and very small (<5 mm) and may be difficult to identify grossly.
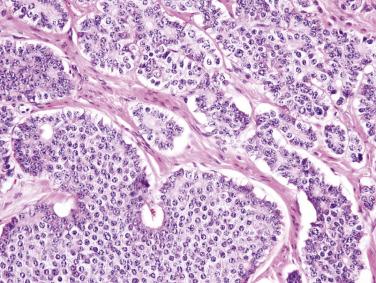
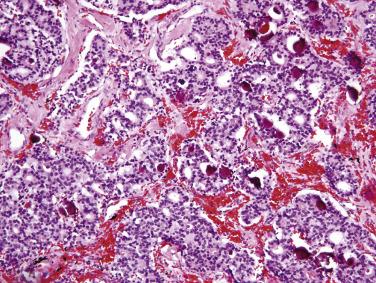
Jejunoileal serotonin-producing NETs are composed of uniform rounded cells arranged predominantly in nests, often with focal glandlike structures ( Fig. 9.3 ). The tumor cells typically contain round nuclei with stippled chromatin, indistinct nucleoli, and small amounts of cytoplasm with indistinct cell borders. The cytoplasm may be clear or contain brightly eosinophilic granules. Occasional cells may show marked pleomorphism, which is of no prognostic significance. Tumor cells infiltrating through the muscularis propria are often arranged in delicate cords. Histologic features do not predict clinical behavior. Most tumors that invade beyond the submucosa are clinically malignant, although they typically pursue a relatively indolent clinical course. Gastrin-producing duodenal NETs typically show a trabecular and cordlike architecture. Somatostatin-producing NETs show a predominantly glandular, cribriform architecture, with uniform cells having small, hyperchromatic nuclei and palely eosinophilic cytoplasm. In around one-third of such tumors, psammoma bodies are observed within the glands ( Fig. 9.4 ). Psammoma bodies are not specific for somatostatin-producing NETs and may sometimes be seen in gastrinomas and other NETs. Gangliocytic paragangliomas are composed of an admixture of spindle-shaped Schwann cells, epithelioid neuroendocrine cells with uniform round nuclei and amphophilic cytoplasm arranged in sheets and cords, and large ganglion cells with vesicular chromatin and eosinophilic cytoplasm ( Fig. 9.5 ).
Small intestinal NETs are usually strongly positive for chromogranin and synaptophysin. Nearly all jejunoileal NETs show diffuse nuclear staining for CDX-2, whereas thyroid transcription factor 1 (TTF-1), polyclonal PAX-8 (PAX-6), and ISL-1 are negative. Duodenal NETs are often positive for both polyclonal PAX-8 (PAX-6) and CDX-2. In gangliocytic paraganglioma, the spindle cells are strongly positive for S-100 protein, whereas the neuroendocrine cells express broad-spectrum keratins, chromogranin, and synaptophysin.
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal neoplasm of the small intestine. Although originally classified as smooth muscle tumors, GISTs are now known to arise from interstitial cells of Cajal or precursors of such cells. The term gastrointestinal autonomic nerve tumor (GANT), which formerly applied to GISTs with particular ultrastructural features, is obsolete. Around 30% of all GISTs arise in the jejunum and ileum, whereas 5% arise in the duodenum. Proper diagnosis is critical, both for prognostication and for targeted therapy with tyrosine kinase inhibitors. Nearly all small intestinal GISTs are positive for KIT (CD117).
Small intestinal GISTs affect adults with a peak in the seventh decade and an equal sex distribution. Most patients present with vague abdominal symptoms, pain, anemia, or GI bleeding; bowel obstruction and tumor rupture may also occur. Small tumors may be discovered incidentally by radiology or at surgery. Minute incidental GISTs (micro-GISTs or GIST tumorlets) of the small intestine are much less common than those of the stomach. Patients with NF1 have a high risk of developing multiple GISTs (usually clinically benign) at this site.
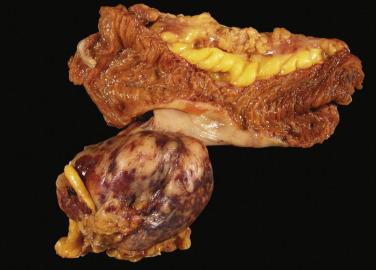
Small intestinal GISTs range from small mural nodules to large exophytic, pedunculated masses ( Fig. 9.6 ). The average size is around 5 cm for duodenal tumors and 7 cm for jejunal and ileal tumors. Large tumors may cause adhesions between multiple loops of bowel. They are generally well circumscribed with either a fibrous or soft and fleshy cut surface. Cystic degeneration and intratumoral hemorrhage are common in large tumors.
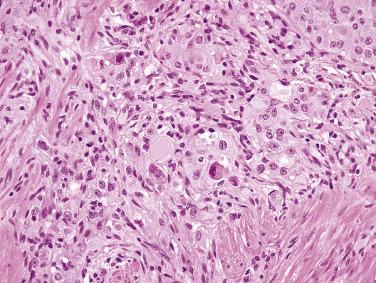
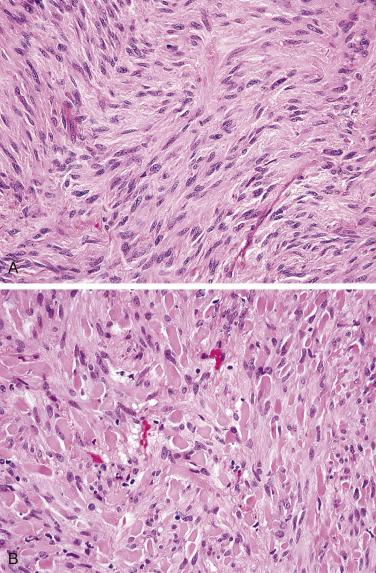
Small intestinal GISTs are nearly always composed of spindle cells, arranged in sheets, short fascicles, or a vaguely storiform architecture. The spindle cells contain remarkably uniform, ovoid to elongated nuclei with fine chromatin, inconspicuous nucleoli, and fibrillary, palely eosinophilic or basophilic cytoplasm ( Fig. 9.7A ). Nuclear palisading may be observed. Pleomorphism is not a feature of small intestinal GISTs and should suggest an alternative diagnosis. The mitotic rate is usually low. Skeinoid fibers (extracellular globules of collagen) are a distinctive feature of GISTs at this anatomic site (see Fig. 9.7B ). Unlike GISTs of the stomach, epithelioid cytomorphology in the small intestine is usually associated with a high mitotic rate and malignant behavior.
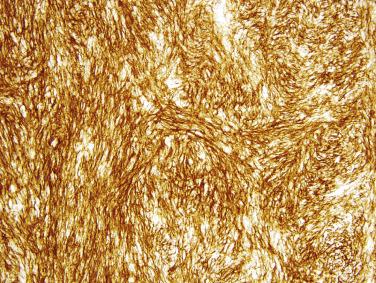
Small intestinal GISTs are more consistently positive for KIT (around 98% of cases) than gastric GISTs, usually with a strong and diffuse cytoplasmic staining pattern ( Fig. 9.8 ). DOG1 (anoctamin 1 [ANO1]) shows similar sensitivity and specificity as KIT for small intestinal GIST. KIT and DOG1 are highly specific for GIST among mesenchymal tumors of the GI tract. Around 50% of small intestinal GISTs are immunoreactive for CD34 or smooth muscle actin (SMA). S-100 protein is positive in around 20% of duodenal GISTs but only 5% of distal small intestinal GISTs. Desmin expression is rare in GISTs from these sites. Expression of succinate dehydrogenase subunit B (SDHB) is invariably retained in GISTs of the small intestine.
Nearly 90% of small intestinal GISTs harbor activating mutations in KIT , predominantly in exon 11 (around 75%) or exon 9 (10%–15%); 1% of KIT mutations are located in exons 13 or 17. In contrast to gastric GISTs, PDGFRA mutations are rarely found in small intestinal GISTs, mostly in the duodenum. GISTs that lack KIT and PDGFRA mutations are often referred to as wild-type GISTs. A small subset of small intestinal GISTs harbor BRAF V600E mutations (around 2% of GISTs at this site). GISTs of the small intestine that lack KIT , PDGFRA , and BRAF mutations often harbor NF1 mutations, which are usually constitutional; wild-type GISTs of the small intestine therefore may reflect unrecognized NF1 syndrome. The small molecule tyrosine kinase inhibitor imatinib mesylate is first line therapy for patients with metastatic or locally advanced GIST. Patients with KIT exon 11–mutant GISTs show the best response to imatinib. KIT exon 9–mutant tumors do not respond as well, but may respond better to high-dose imatinib (800 mg as opposed to standard 400-mg dose). Wild-type GISTs show limited responses. Imatinib is often also used as adjuvant therapy for patients with large primary tumors. Such an approach increases recurrence-free and overall survival. Most treated patients eventually develop resistance to imatinib, usually because of the selection of secondary mutations in the kinase domain of KIT . Many such patients benefit from treatment with another tyrosine kinase inhibitor such as sunitinib malate or regorafenib.
GISTs range from clinically indolent tumors to aggressive sarcomas. Around 40% of small intestinal GISTs are clinically malignant, much higher than the rate for gastric GISTs. Typical sites of metastasis are the liver and peritoneum. Even small tumors occasionally result in late metastases; the designation benign GIST should therefore be avoided. Risk of aggressive behavior can be predicted by a combination of anatomic site, tumor size, and mitotic rate ( Table 9.2 ). Of note, mitotic rate should be determined in 5 mm 2 , which corresponds to approximately 20 high-power fields (hpf) in most modern microscopes. Small intestinal GISTs have a higher risk of malignant behavior than gastric GISTs of similar size and mitotic rate. GISTs only rarely recur locally at anastomotic sites.
| Mitotic Rate (per 5 mm 2 ) | Tumor Size (cm) | Stomach | Small Intestine |
|---|---|---|---|
| ≤5 | ≤2 | No risk | No risk |
| >2 and ≤5 | Very low risk | Low risk | |
| >5 and ≤10 | Low risk | Moderate risk | |
| >10 | Moderate risk | High risk | |
| >5 | ≤2 | No risk | High risk |
| >2 and ≤5 | Moderate risk | High risk | |
| >5 and ≤10 | High risk | High risk | |
| >10 | High risk | High risk |
Leiomyomas of the small intestine are rare. Nearly all arise in the muscularis propria, similar to those in the esophagus and proximal stomach. They are benign and do not recur. Small polypoid leiomyomas of the muscularis mucosae (as seen in the colon and rectum) are extremely rare. Mural leiomyomas are well-circumscribed tumors composed of fascicles of uniform spindle cells with elongated, blunt-ended nuclei and abundant brightly eosinophilic cytoplasm. Mitotic activity is usually absent. Leiomyomas show strong, uniform expression of SMA, desmin, and caldesmon and are negative for KIT and DOG1.
Most small intestinal tumors formerly designated leiomyosarcomas are now known to represent GISTs. True GI primary leiomyosarcomas are exceedingly rare (around 1% of GI mesenchymal tumors) but are most often encountered in the small intestine and colon. The possibility of a metastasis (e.g., from the uterus or retroperitoneum) should always be excluded. Leiomyosarcomas are usually large tumors that involve the full thickness of the bowel wall and present with GI bleeding and obstruction. They are aggressive sarcomas that often metastasize to the peritoneum, liver, lungs, and bone. Histologic features are similar to those of leiomyosarcomas at other sites (see Chapter 24 ). The degree of nuclear atypia, pleomorphism, and cytoplasmic eosinophilia serves to distinguish leiomyosarcoma from GIST. Leiomyosarcomas are often positive for SMA, desmin, and caldesmon to variable extents, but are negative for KIT and DOG1. Patchy expression of broad-spectrum keratins is common.
GI clear cell sarcoma–like tumor is a rare but increasingly recognized distinctive sarcoma type with a predilection for the small intestine (70% of cases). Although cytogenetic findings are similar, its histologic features, immunophenotype, and clinical behavior are distinct from those of conventional clear cell sarcoma of the distal extremities, arguing for classification in a separate diagnostic category. The alternate designation malignant gastrointestinal neuroectodermal tumor (GNET) has been proposed. Most tumors arise in young adults, who present with abdominal pain or bowel obstruction. Lymph node and liver metastases are common. The average size is 5 cm. The tumors show infiltrative borders through the bowel wall and often ulcerate the mucosa and invade the mesentery.
GI clear cell sarcoma–like tumor is composed of uniform, predominantly rounded to ovoid cells with indistinct or small nucleoli and palely eosinophilic to focally clear cytoplasm. In some areas, tumor cells may show a more epithelioid or spindle cell appearance. A sheetlike growth pattern usually predominates; a focally alveolar, pseudopapillary, or nested architecture is commonly observed ( Fig. 9.9 ). The uniform cytology and relatively low mitotic rate distinguish this tumor type from the much more common metastatic melanoma. Scattered osteoclast-like giant cells are seen in around half of cases. Strong, diffuse expression of S-100 protein ( Fig. 9.10 ) and SOX10 is typical, whereas HMB-45, melan A, KIT, and DOG1 are negative. GI clear cell sarcoma–like tumors usually have either the t(2;22)(q33;q12) translocation resulting in EWSR1–CREB1 or t(12;22)(q13;q12) involving EWSR1 and ATF1 , with an approximately equal distribution. Fluorescence in situ hybridization (FISH) for EWSR1 rearrangement can therefore be used to confirm the diagnosis.
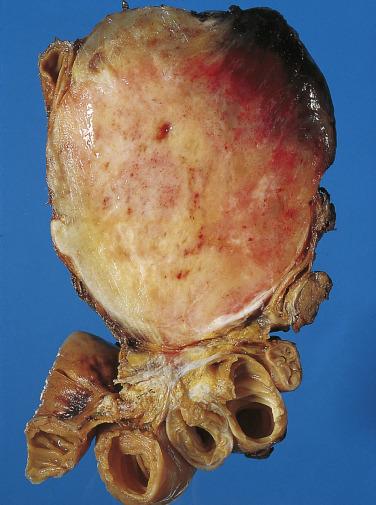
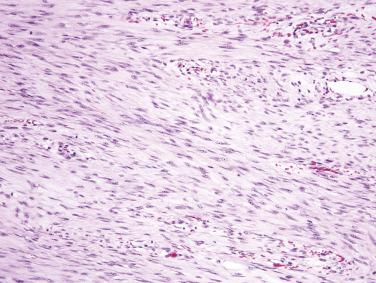
The mesentery of the small intestine is among the most common sites for intraabdominal desmoid fibromatosis. Such tumors occur over a wide age range and are often associated with a history of prior abdominal surgery. Mesenteric desmoid tumors are quite often associated with FAP; and in this subset of FAP patients, the Gardner variant is seen in more than 50%. Most patients present with vague abdominal symptoms. As desmoid fibromatosis often infiltrates deeply into the root of the mesentery, complete surgical excision is difficult, although the correlation between margin status and local recurrence is inconsistent. Mesenteric desmoid fibromatosis is usually large and grossly relatively circumscribed with a fibrous cut surface ( Fig. 9.11 ). The tumor is composed of long fascicles of spindle cells with ovoid or tapering nuclei, vesicular chromatin, small nucleoli, and indistinct palely eosinophilic cytoplasm, usually within a collagenous stroma ( Fig. 9.12 ). Desmoid tumors at this site often resemble nodular fasciitis with focally prominent myxoid stroma ( Fig. 9.13 ). Focal keloidal hyalinization is also common. Around 80% of desmoid fibromatosis cases show aberrant nuclear (and cytoplasmic) staining for β-catenin ( Fig. 9.14 ), reflecting the presence of CTNNB1 mutations (in sporadic tumors) or germline APC gene mutations (in patients with FAP). This finding is useful to confirm the diagnosis. Most tumors are positive for SMA, whereas KIT, DOG1, and caldesmon are negative. Focal reactivity for desmin may also be seen.
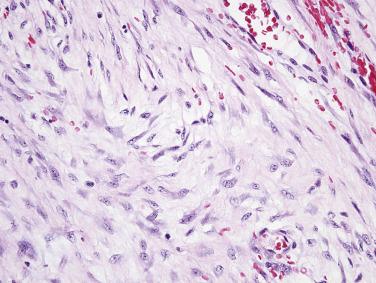
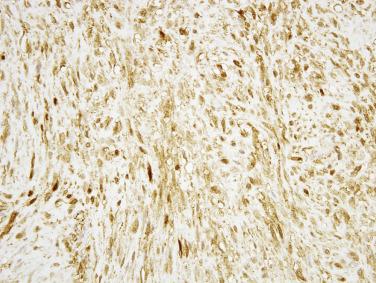
Inflammatory fibroid polyp is a distinctive benign mesenchymal neoplasm with a predilection for the gastric antrum and ileum, but which may arise anywhere in the tubal GI tract and affects mainly older adults. Patients with small intestinal tumors may present with abdominal pain, GI bleeding, or more typically intussusception ( Fig. 9.15 ). Inflammatory fibroid polyps are usually mural masses centered in the submucosa, often between 3 and 6 cm in size, with infiltrative borders into the mucosa and bowel wall. The tumors are composed of bland, stellate to short spindle cells arranged haphazardly in an edematous to myxoid stroma, with prominent inflammatory cells, including conspicuous eosinophils ( Fig. 9.16 ). Scattered small stromal blood vessels typically show perivascular fibrosis. Most tumors are positive for CD34 and PDGFRA, whereas KIT, DOG1, SMA, desmin, and S-100 protein are negative. The majority of inflammatory fibroid polyps harbor activating mutations in PDGFRA . Exon 12 mutations predominate in ileal tumors, in contrast to exon 18 mutations in gastric tumors. Recurrence is very rare.
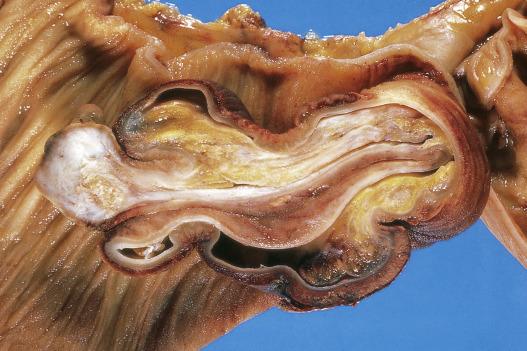
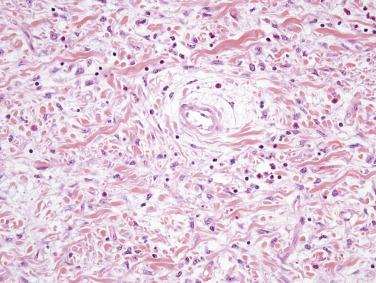
Hemangiomas of the small intestine are rare and infrequently come to clinical attention. Larger hemangiomas and vascular malformations may present with GI tract bleeding. Most hemangiomas of the small intestine are of cavernous type and involve the submucosa.
Lymphangiomas of the GI tract are rare; the duodenum is the most common site. They are usually detected incidentally at endoscopy as a white spot or plaque. Lymphangiomas are circumscribed lesions composed of thin-walled dilated channels, often containing clear fluid, involving the mucosa and submucosa ( Fig. 9.17 ). Such lesions should be distinguished from lymphangiectasia, which is generally diffuse.
Primary angiosarcomas of the small intestine are very rare. Some may arise in a prior radiation field. Metastasis from more common sites (e.g., the scalp or face) should always be excluded. Small intestinal angiosarcomas often present with GI tract bleeding and are usually large mural masses with hemorrhage and necrosis. The tumors range from vasoformative lesions composed of irregular dissecting channels lined by hyperchromatic, atypical cells to more solid tumors containing spindled or epithelioid cells with amphophilic cytoplasm. ERG and CD31 are the most sensitive endothelial markers. Keratin expression is often seen in angiosarcomas.
Neurofibromas of the small intestine are rare and essentially diagnostic of NF1. Intraabdominal neurofibromas in NF1 are often plexiform, may present with GI bleeding, and may extend through the muscularis propria into the mesentery. In the older literature, many NF1-associated GI tumors thought to represent neurofibromas were likely in fact GISTs.
Diffuse ganglioneuromatosis is associated with NF1 and MEN-2B. Patients may present with either constipation or diarrhea. Ganglioneuromatosis is often a grossly ill-defined thickening of the bowel wall and may involve the small intestine, appendix, and colon. MEN-2B–associated ganglioneuromatosis usually extends from the myenteric plexus, which is expanded in a bandlike fashion, into the muscularis propria, whereas NF1-associated ganglioneuromatosis is more often centered in the submucosa. The lesion is composed of a diffuse proliferation of S-100 protein–positive Schwann cells, ganglion cells, and their cellular processes.
Intestinal benign lymphoid polyps (representing reactive lymphoid follicular hyperplasia) are often encountered in children, especially in the duodenum. Diffuse nodular lymphoid hyperplasia of the small intestine, which appears endoscopically as multiple mucosal polyps, is a rare finding in adults that usually is associated with a variety of immunodeficiency disorders.
Although primary lymphomas of the small and large intestines are uncommon, they represent a relatively high proportion of malignant neoplasms at these sites (nearly 50%) because carcinomas and mesenchymal neoplasms are also uncommon. Many systemic nodal lymphoma types may secondarily involve the intestines and other extranodal sites; a relatively limited group of lymphomas arises primarily in the intestines ( Table 9.3 ). Other than mantle cell lymphoma and Burkitt lymphoma, which relatively commonly present in the colon, most of these lymphomas are much more frequent in the small intestine and will therefore be discussed in this section of the chapter.
| B-cell lymphomas | Diffuse large B-cell lymphoma |
| Marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) | |
| Immunoproliferative small intestinal disease | |
| Duodenal-type follicular lymphoma | |
| Mantle cell lymphoma | |
| Burkitt lymphoma | |
| T-cell lymphomas | Enteropathy-associated T-cell lymphoma (EATL) |
| Monomorphic epitheliotropic intestinal T-cell lymphoma |
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma of the intestines, accounting for around 50% of all lymphomas at this site. DLBCL may present with abdominal pain, obstruction, a palpable mass, or perforation. A large polypoid mass or ulcerating circumferential lesion is typical ( Fig. 9.18 ). The tumor is composed of sheets of large lymphoid cells (usually at least three times the size of normal lymphocytes) with vesicular chromatin, variably prominent nucleoli, irregular nuclear contours, and small amounts of cytoplasm ( Fig. 9.19 ). Unlike in gastric DLBCL, a concomitant low-grade extranodal marginal-zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is uncommon. The tumor cells almost invariably express the pan–B-cell markers CD20, CD79a, and PAX5. Either a germinal center phenotype (CD10 and BCL6) or an activated B-cell phenotype (MUM1) may be observed, although the former is more common. Similar to DLBCL at other sites, translocations involving BCL6 and the immunoglobulin heavy chain gene locus are relatively common ; MYC rearrangements may also be detected in a small subset of cases.
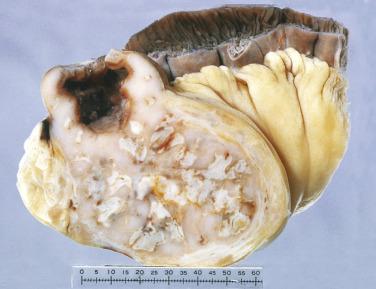
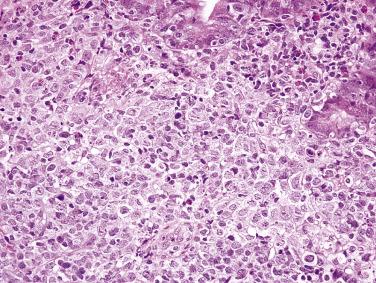
Although much less common than gastric MALT lymphoma, MALT lymphoma is one of the most common primary intestinal lymphomas after DLBCL. Patients usually present with vague abdominal symptoms and weight loss. Some lesions are discovered incidentally. MALT lymphoma may be seen as a polyp or mass. The distinction from normal or hyperplastic MALT (e.g., Peyer patches) may sometimes be difficult, particularly in small biopsies. Similar to gastric MALT lymphoma, the tumor often infiltrates around residual lymphoid follicles and is composed of uniform small lymphoid cells, with either rounded or irregular nuclei, inconspicuous nucleoli, and variable amounts of pale cytoplasm, sometimes with a monocytoid appearance ( Fig. 9.20 ). Plasma cell differentiation may be seen. Unlike with gastric MALT lymphomas, lymphoepithelial lesions are usually not prominent. An infiltrative growth pattern (beyond the confines of the normal marginal zone) distinguishes MALT lymphoma from reactive hyperplasia. The neoplastic cells express pan–B-cell markers and are usually negative for CD5, CD10, and CD23. Aberrant expression of CD43 may be observed. Demonstration of immunoglobulin light chain restriction (by immunohistochemistry) or monoclonality may be required to confirm the diagnosis of lymphoma in histologically equivocal cases. The t(11;18)(q21;q21) translocation resulting in API2–MALT1 is detected in a subset of cases.
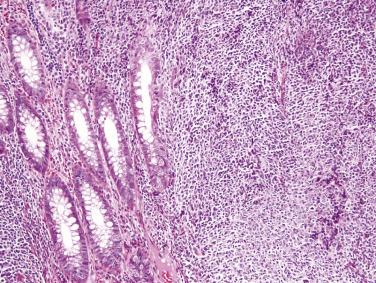
Immunoproliferative small intestinal disease (IPSID) (also known as alpha heavy chain disease) is a distinctive variant of MALT lymphoma that is nearly exclusive to the Mediterranean region and the Middle East. Campylobacter jejuni infection is strongly associated with IPSID and is believed to invoke a chronic antigenic stimulus for lymphoma development, analogous to Helicobacter pylori in gastric MALT lymphoma. Early lesions may be cured by antibiotic therapy. IPSID has a predilection for young adults and often presents with chronic diarrhea and weight loss. A serum paraprotein (alpha heavy chain) is frequently detected. The lymphoma shows similar cytologic features to other MALT lymphomas, with the exception that plasma cell differentiation is often extensive ( Fig. 9.21 ). In early phases of disease, the small intestinal mucosa is grossly normal, and the infiltrate is confined to the mucosa. Later lesions show mucosal thickening and nodularity and histologic evidence of infiltrative growth. Some cases progress to DLBCL. The neoplastic cells show a similar immunophenotype to other forms of MALT lymphoma, with the exception that immunoglobulin A heavy chain is consistently detected without light chains.
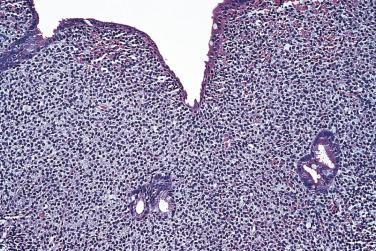
Primary small intestinal follicular lymphoma (duodenal-type follicular lymphoma) has a predilection for the duodenum. It usually is discovered incidentally as small polyps. Localized disease is clinically indolent and rarely spreads beyond the intestine, even without therapy. The histologic features are similar to those of low-grade follicular lymphoma at other sites, characterized by a nodular infiltrate of small to intermediate-sized cells with irregular to folded nuclei and scant cytoplasm ( Fig. 9.22 ). The neoplastic cells are positive for pan–B-cell markers, CD10, and BCL2 but negative for CD5, cyclin D1, and CD43. Coexpression of CD10 and BCL2 and a low Ki67 index distinguish follicular lymphoma from reactive follicular hyperplasia. The typical t(14;18)(q34;q21) translocation with BCL2 rearrangement is usually found. Duodenal-type follicular lymphoma must be distinguished from secondary intestinal involvement by systemic follicular lymphoma.
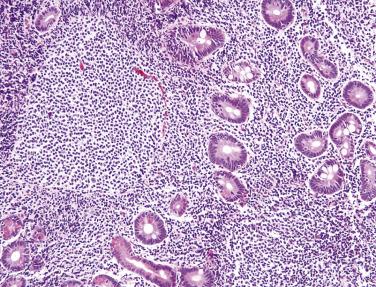
Mantle cell lymphoma may present primarily in the small intestine but usually is subsequently discovered systemically in lymph nodes, spleen, and other sites. A classic presentation of mantle cell lymphoma is that of multiple lymphomatous polyposis, wherein numerous polyps are detected throughout the GI tract by endoscopy, preferentially in the small intestine ( Fig. 9.23 ). A similar appearance may also be observed with follicular lymphoma or MALT lymphoma. Some cases present as a single polyp or mass. Mantle cell lymphoma is an aggressive disease with poor response to chemotherapy. In most cases, a diffuse architecture is observed. The tumor is composed of uniform, small to intermediate-sized lymphoid cells with slightly irregular nuclei, indistinct nucleoli, and scant cytoplasm ( Fig. 9.24A ). Scattered epithelioid histiocytes are a typical feature. In addition to pan–B-cell markers, CD5, cyclin D1 (see Fig. 9.24B ), and SOX11 are positive, whereas CD10 is consistently negative. CD5 expression is often weak. Nearly all cases harbor the t(11;14)(q13;q32) translocation with CCND1 (cyclin D1) rearrangement.
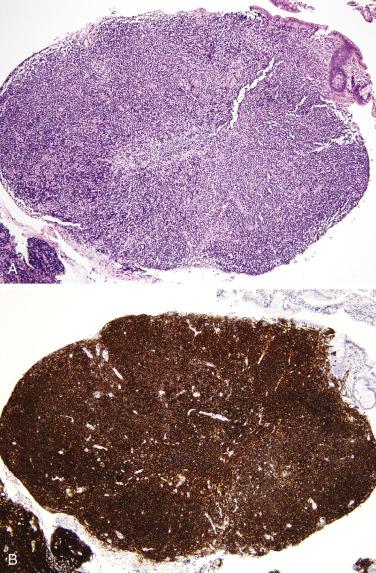
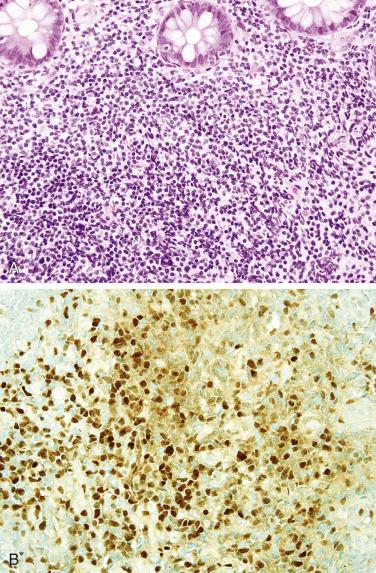
Burkitt lymphoma, including endemic, sporadic, and human immunodeficiency virus (HIV)–associated forms, has a predilection for the small intestine, particularly the ileum, and often also involves the cecum and appendix. Endemic Burkitt lymphoma is strongly associated with Epstein-Barr virus (EBV) infection and usually affects children. Burkitt lymphoma typically presents acutely with obstruction or perforation but may also manifest as a large mass. Advanced stage is common at diagnosis. Most patients can be cured by high-dose combination chemotherapy. The tumor is composed of sheets of midsize cells with rounded nuclei, several distinct nucleoli, and small amounts of basophilic cytoplasm, often with a high mitotic rate ( Fig. 9.25 ). The classic starry-sky appearance is due to numerous interspersed histiocytes phagocytizing nuclear debris. The neoplastic cells are positive for pan–B-cell markers and CD10, but consistently negative for BCL2, CD5, and cyclin D1. The Ki67 index is nearly always greater than 95%. In situ hybridization for EBV-encoded RNA (EBER) is positive in endemic cases but is rarely positive in sporadic Burkitt lymphoma. Nearly all cases harbor MYC gene rearrangements, most commonly with t(8;14)(q24;q32) ; FISH for MYC can therefore be used to confirm the diagnosis. Immunohistochemistry for MYC correlates moderately well with translocation.
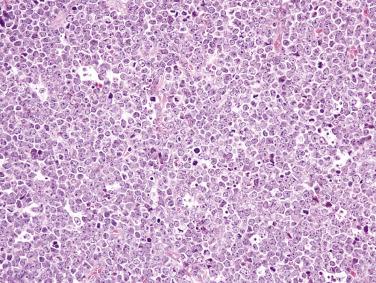
Enteropathy-associated T-cell lymphoma (EATL) is very uncommon (although constituting nearly 90% of intestinal T-cell lymphomas), other than in populations with a high prevalence of celiac disease, which is a major risk factor. Most cases of EATL follow adult-onset celiac disease (or are diagnosed concurrently), with a male predominance and a predilection for the jejunum and proximal ileum. Patients usually present emergently with bowel perforation or obstruction. In a small subset of patients, overt lymphoma develops after progression to refractory celiac disease (ulcerative jejunitis). Refractory celiac disease may be classified as either type I, in which intraepithelial lymphocytes have a normal (CD8+) phenotype and are polyclonal, or type II, in which intraepithelial lymphocytes lose expression of CD8 (which can be demonstrated by immunohistochemistry or flow cytometry) and show monoclonal T-cell receptor gene rearrangement. Type II refractory celiac disease has also been referred to as EATL in situ and intraepithelial T-cell lymphoma . Type I refractory celiac disease only rarely progresses to EATL. EATL is an aggressive lymphoma; most patients succumb to the disease.
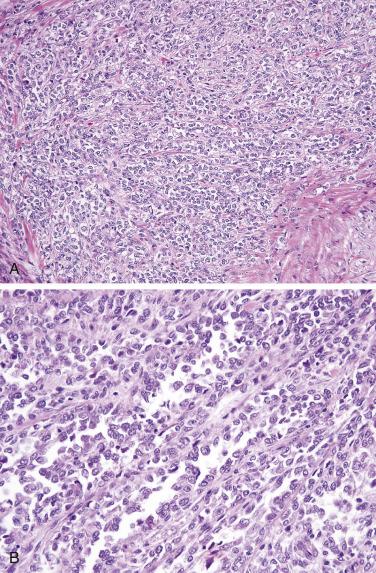
The tumor often appears as multiple ulcerative masses ( Fig. 9.26 ), sometimes with perforation. The tumor is composed of sheets of midsize to large lymphoid cells with rounded nuclei, vesicular chromatin, prominent nucleoli, and moderate amounts of pale cytoplasm, often admixed with numerous inflammatory cells, which may obscure the neoplastic nature of the infiltrate. Some cases show marked pleomorphism ( Fig. 9.27 ). The adjacent mucosa shows enteropathy, usually with villous blunting, an increased lymphoplasmacytic infiltrate in the lamina propria, and often a striking intraepithelial lymphocytosis. The neoplastic cells are positive for CD3, CD7, and CD103 but are usually negative for CD4, CD5, CD8, and CD56. CD30 is variably positive. The intraepithelial lymphocytes in the adjacent mucosa show a similar aberrant phenotype (i.e., CD8 negative). Lack of CD5 expression in intraepithelial T cells per se should not be overinterpreted as evidence of lymphoma, because normal cells in this compartment are often negative or weakly positive for CD5. Monoclonal T-cell receptor gene rearrangement can be detected.
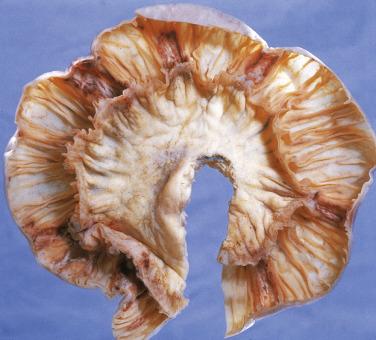
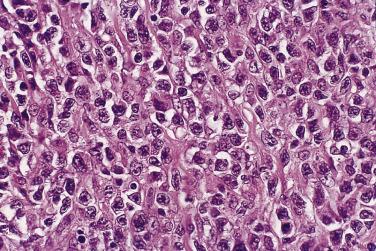
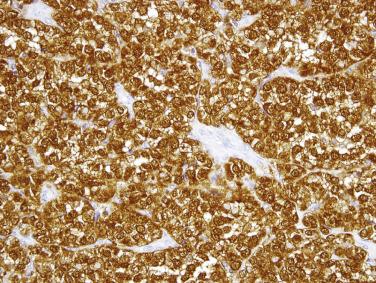
This rare type of intestinal T-cell lymphoma, previously referred to as type II EATL, is rarely associated with celiac disease and shows distinct histologic and immunophenotypic features. The prognosis is, however, similarly poor. In contrast to classic EATL, the tumor cells are usually small, with hyperchromatic nuclei and scant cytoplasm, although the adjacent mucosa often shows enteropathy-like features, including a striking intraepithelial lymphocytosis ( Fig. 9.28A ). The neoplastic cells are positive for CD3, CD8 (see Fig. 9.28B ), and CD56 (the latter two markers distinguishing this subtype from EATL). EBER is negative (distinguishing this lymphoma from nasal-type NK/T-cell lymphoma, which may also occasionally present in the small intestine).
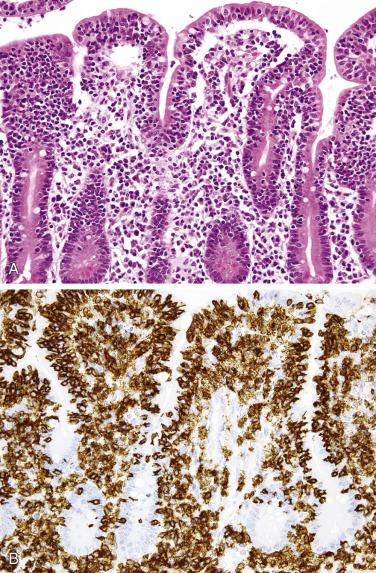
The small intestine is the site of predilection for metastases to the GI tract. Of note, secondary carcinomas are much more common than primary small intestinal adenocarcinomas. The most common tumor to metastasize to the small intestine is melanoma ( Fig. 9.29 ), followed by breast, lung, and ovarian carcinomas. Direct extension from the pancreas, stomach, and colon is also relatively common. Colonization of the native small intestinal epithelium by metastatic adenocarcinoma may mimic a primary tumor. The possibility of a metastasis from the colon should always be excluded when an intestinal-type adenocarcinoma is encountered, particularly when the small intestinal mucosa is uninvolved and when a precursor lesion or significant risk factor is absent (e.g., celiac disease or Crohn disease).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here