Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The identification of genes associated with recurrent chromosomal abnormalities has revolutionized diagnostic surgical pathology. This is especially true for the hematopoietic diseases, loosely defined as hematolymphoid disorders that most commonly involve the bone marrow, spleen, and peripheral blood. These chromosomal aberrations result in altered cellular programs that, in concert with additional genetic or epigenetic changes, lead to characteristic tumor morphology and, perhaps more importantly, a particular clinical behavior and outcome. These molecular insights have resulted in several major transformations in the classification of hematopoietic malignancies over the past decades. For example, the current World Health Organization (WHO) classification schema incorporates numerous unique biomarkers and somatic mutations which, in addition to conventional cytogenetics, define distinct clinicopathologic myeloid and lymphoid neoplastic entities. This integrated approach has been validated in many instances. Examples include acute myeloid leukemia with NPM1 mutation or acute myeloid leukemia with biallelic mutation of CEBPA , which were once provisional entities but are now distinct well-defined disease subtypes in the current WHO revision.
In addition to providing information on more predictable clinical behavior and outcome, clinicians rely on the detection of these genetic aberrations to guide the use of unique, effective treatments targeting abnormal proteins and molecules. Examples include imatinib mesylate (Gleevec) in chronic myeloid leukemia (CML) and myeloid neoplasms associated with PDGFR abnormalities, all- trans retinoic acid (ATRA) in acute promyelocytic leukemia, or IDH inhibitors in acute myeloid leukemia (AML) associated with mutated IDH1 or IDH2 . These genetic defects also provide excellent biomarkers for identifying the presence of both new and persistent disease.
As molecular diagnostic techniques, including high-throughput genomic sequencing, polymerase chain reaction, fluorescence in situ hybridization (FISH), and karyotyping, become commonplace in the practice of pathology, an ever-increasing list of provisional diagnostic entities have been, and will be, proposed. Although the barrage of classification changes in hematopoietic malignancies has proved challenging for general surgical pathologists to adapt to, the intent has not been to confuse but to better define these entities with characteristics that provide useful therapeutic and prognostic information. This chapter provides a current overview of the most common categories of hematopoietic neoplasms—the myelodysplastic syndromes (MDSs), acute leukemias, myeloproliferative neoplasms (MPNs), and mature lymphoid neoplasms.
MDSs are a collection of heterogeneous clonal myeloid stem cell disorders that are unified by abnormalities in differentiation that result in ineffective hematopoiesis, bone marrow failure, and decreased normal peripheral blood cells. The term dysplasia refers to the altered myeloid cell morphology that is a consequence of the underlying stem cell defect. The affected myeloid cells can include cells of the erythroid, megakaryocytic, and granulocytic lineages, alone or in combination. Importantly, in addition to the decreased peripheral blood cell counts, there is often an associated increase in bone marrow blast forms in these disorders. The increase in blasts correlates with the risk of progression to acute leukemia. The terminology and diagnostic criteria of MDS have been updated in the 2016 revision of the WHO classification to address recent pivotal developments in the molecular biology of this group of disorders, and the current classification of MDS includes six distinct entities and one provisional entity ( Table 22.1 ).
| Disease | PERIPHERAL BLOOD FINDINGS | BONE MARROW FINDINGS | |||
|---|---|---|---|---|---|
| Cytopenia a | Blast Count (%) | Dysplasia b | Blast Count (%) | Ring Sideroblasts (%) | |
| MDS with single lineage dysplasia (MDS-SLD) | Anemia or neutropenia or thrombocytopenia; rarely bicytopenia | <1 | Unilineage | <5 | <15 |
| MDS with multilineage dysplasia (MDS-MLD) | Bicytopenia or pancytopenia | <1; no Auer rods | Two or more myeloid cell lines | <5; no Auer rods | <15 |
| MDS WITH RING SIDEROBLASTS (MDS-RS) | |||||
| MDS-RS with single lineage dysplasia (MDS-RS-SLD) | Anemia; rarely bicytopenia | <1 | Unilineage | <5 | ≥15 c |
| MDS-RS with multilineage dysplasia (MDS-RS-MLD) | Bicytopenia or pancytopenia | <1 | Two or more myeloid cell lines | <5 | ≥15 c |
| MDS WITH ISOLATED del(5q) | Macrocytic anemia; normal or increased platelet count | <1; no Auer rods | Increased abnormal, hypolobate megakaryocytes; other lineages maybe dysplastic | <5; no Auer rods | None or any |
| MDS WITH EXCESS BLASTS (MDS-EB) | |||||
| MDS-EB-1 | Monocytopenia, bicytopenia, or pancytopenia | <5; no Auer rods | Unilineage or multilineage | 5–9; no Auer rods | None or any |
| MDS-EB-2 | Monocytopenia, bicytopenia, or pancytopenia | 5-19; or Auer rods | Unilineage or multilineage | 10–19; or Auer rods | None or any |
| MDS, UNCLASSIFIABLE (MDS-U) | |||||
| With 1% blood blasts | Monocytopenia, bicytopenia, or pancytopenia | =1; recorded on at least two separate occasions | Unilineage or multilineage | <5; no Auer rods | None or any |
| With single lineage dysplasia and pancytopenia | Pancytopenia | <1 | Unilineage | <5 | None or any |
| Based on defining cytogenetic abnormality | None | <2 | Unilineage or multilineage | <5 | <15 |
| Refractory cytopenia of childhood (RCC); provisional | Macrocytic anemia, pancytopenia in most | <2; no Auer rods | Unilineage or multilineage | <5; no Auer rods | none |
a Monocyte count must be <1 × 10 9 /L, otherwise chronic myelomonocytic leukemia must be considered.
b Morphologic dysplasia is defined as >10% of lineage.
c Percent ring sideroblasts may be lower when SF3B1 mutation is detected.
MDS presents as either a primary (de novo) disease or as a disease that arises secondarily after chemotherapy or radiation treatment. It is predominantly a disease of the elderly, with a median age at onset of 72 years; however, the age range is wide (16–96 years). MDS can occur in childhood, particularly in the setting of inherited deoxyribonucleic acid (DNA) repair defect diseases, such as Fanconi anemia. The importance of MDS in childhood is reflected in the provisional WHO entity, refractory cytopenia of childhood (RCC), which is reserved for children with MDS who have fewer than 2% blasts in their peripheral blood, less than 5% in their bone marrow, and persistent cytopenias with dysplasia. The incidence of MDS rises steadily with increasing age. Its overall incidence is about 4 to 12 cases per 100,000 individuals per year, but it occurs at three to four times this rate in patients over 70. The incidence of MDS appears to be increasing in recent years, a finding that may reflect the aging of the population, greater physician awareness of the disease, and the extended use of diagnostic procedures in elderly patients. Patients with cancer who achieve a cure or long-term remission using intensive chemotherapy appear to be contributing to the rising incidence of therapy-related (secondary) MDS. This is a tragic consequence of genotoxic treatments, because survival in secondary MDS is considerably shorter than in primary MDS. With the exception of the isolated del(5q) syndrome, there is a slight male predominance in most forms of MDS.
The clinical features of MDS are related to bone marrow failure and include fatigue secondary to anemia, infection secondary to neutropenia, and hemorrhage secondary to thrombocytopenia. By definition, the anemia of MDS reflects an intrinsic defect in erythropoiesis that is unresponsive or refractory to hematinic therapy. The anemia is frequently macrocytic. Thrombocytosis can be present in patients with isolated del(5q) but is otherwise never seen. Death can occur from the related complications of marrow failure, transformation to acute leukemia, or occasionally complications of iron overload secondary to transfusion therapy. Extramedullary involvement is unusual and should raise suspicion for an alternative initial diagnosis or, in patients with well-documented myelodysplasia, extramedullary leukemic transformation.
Once a clinical suspicion of MDS arises, diagnosis requires careful assessment of the peripheral blood and bone marrow morphology for dysmorphic features. In the absence of clonal cytogenetic and molecular abnormalities, morphologic findings are the mainstay of diagnosis. Well-prepared Wright-Giemsa–stained peripheral blood or bone marrow aspirate smears on freshly isolated specimens with proper controls are essential to evaluate properly for MDS. The subjective nature of such findings can cause diagnostic difficulties and, even when present, dysplastic morphology can be unrelated to MDS and may stem from nutritional deficiencies, toxic exposures, and infectious agents. Thus diagnosis and classification of MDS require the careful correlation of morphologic findings with other clinical and laboratory data. In fact, the international working group has recently included persistent cytopenia as one of the prerequisite criteria for an MDS diagnosis. The other criterion is exclusion of other hematopoietic and nonhematopoietic conditions as primary causes of cytopenia and/or dysplasia.
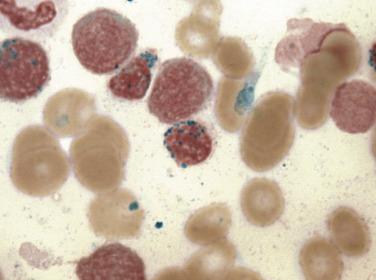
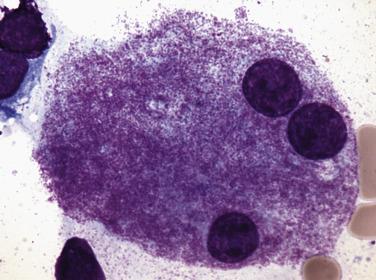
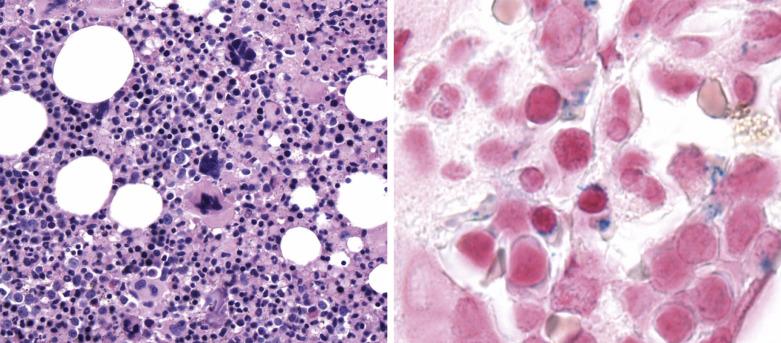
Common morphologic abnormalities in mature nonnucleated peripheral blood red cells include macrocytosis, anisocytosis and poikilocytosis, punctate basophilia, and cellular dimorphism. Within the marrow, abnormalities of nuclear morphology in red cell precursors are most commonly seen. This includes megaloblastoid maturation (dyssynchrony between nuclear and cytoplasmic maturation), multinucleation, and nuclear fragmentation, bridging, or budding ( Fig. 22.1 ). Cytoplasmic vacuolization, ring sideroblasts (erythroblasts with at least five Prussian blue–stained perinuclear iron granules encircling more than one-third of the nucleus) ( Fig. 22.2 ), and cytoplasmic periodic acid–Schiff (PAS)–positive granules are also commonly seen.
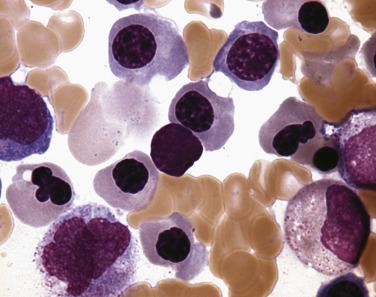
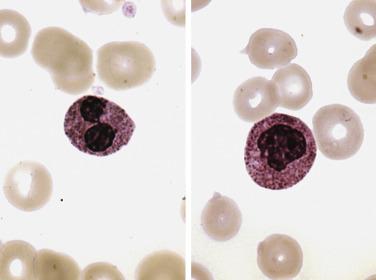
Among granulocytes, morphologic abnormalities are most commonly seen within the neutrophilic series. This includes peripheral blood neutrophils that demonstrate hypogranularity (loss of secondary granules and myeloperoxidase staining); nuclear hyposegmentation, including pseudo–Pelger-Huet forms (binucleated neutrophils with condensed chromatin) ( Fig. 22.3 ); or even nuclear hypersegmentation. Within the bone marrow, granulocytic precursors, which normally localize along the endosteal surface of the trabecular bone, may form clusters that are not contiguous with the bony trabeculae. These marrow precursors demonstrate a lack of synchrony between nuclear and cytoplasmic maturation, nuclear hyposegmentation ( Fig. 22.4 ), decreased primary or secondary granules, and giant primary granules.
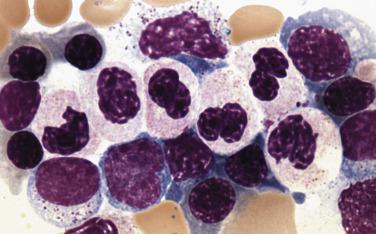
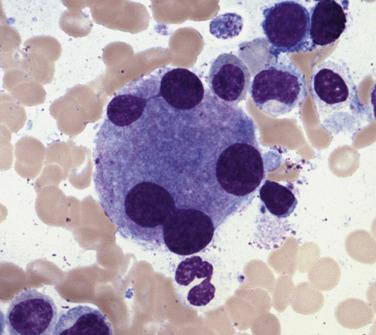
Megakaryocytes often demonstrate the most prominent dysplastic changes in MDS, including small hypolobated, micromegakaryocytic forms, decreased nuclear lobation, or (conversely) polyploidization, and widely spaced, noncontiguous nuclear lobes (so-called pawn ball nuclei to reflect their resemblance to the three-ball pawnbroker's symbol of ancient times) ( Figs. 22.5 and 22.6 ).
Clonal chromosomal abnormalities are detected in approximately 40% of patients with MDS by standard karyotyping and in even greater numbers using high-resolution methods. FISH has not been shown to be of increased utility in the detection of clonal cytogenetic abnormalities in MDS. The most common cytogenetic abnormalities in de novo MDS are listed in Table 22.2 . Other less frequent recurrent chromosomal aberrations include balanced chromosomal translocations, unbalanced structural abnormalities (deletions and duplications), and numeric abnormalities. Clonal genetic defects are also typical in cases of therapy-related MDS, occurring in nearly 100% of cases and frequently involving chromosomes 5 and 7. The identification of clonal cytogenetic aberrations, in the proper clinical context, confirms a diagnosis of MDS and provides physicians with important prognostic information. Unlike conventional karyotype, up to 90% of MDS patients demonstrate at least one acquired somatic mutation. Mutations in more than 40 genes have been identified in MDS that fall into several general categories, including transcription factors, epigenetic regulators and chromatin-remodeling factors, pre-mRNA splicing factors, and signaling molecules ( Table 22.3 ). MDS-specific somatic mutations have the potential to improve diagnostic criteria, prognostication, and monitoring response to treatment. For example, the presence of SF3B1 mutation has already been included as a diagnostic criterion for MDS with ring sideroblasts in the revised fourth edition WHO classification. In addition, several mutations (e.g., EZH2 , ASXL1 , TP53 , RUNX1, and ETV6 ) have been shown to independently predict clinical behavior independent of other established prognostic variables. Moreover, certain mutations, such as DMNT3A and TET2, appear to be associated with superior response to hypomethylating agents, while the presence of TP53 mutation in addition to del(5q) predicts a worse response to lenalidomide. Despite high prevalence and diagnostic and prognostic significance, molecular status has not been incorporated into the recently revised International Prognostic Scoring System (IPSS-R) for newly diagnosed MDS patients. This widely accepted system is based on the degree of cytopenia(s), bone marrow blast counts, and cytogenetic findings that include five karyotype categories: very good (-Y, del[11q]), good (normal, del[5q], del[12p], del[20q], double including del[5q]), intermediate (del[7q], +8, +19, i[17q], any other single or double independent clones), poor (-7, inv[3]/t[3q]/del[3q], double including -7/del[7q], complex: 3 abnormalities), and very poor (>3 abnormalities). The calculated five risk groups show significantly different overall survival with 8.8 years for very low, 5.3 years for low, 3 years for intermediate, 1.6 years for high, and 0.8 year for very high risk groups. The IPSS-R has been widely adopted in clinical practice as a basis for treatment decisions and appears to predict outcome both at diagnosis and dynamically in patients being followed at later timepoints.
| Gain or Loss of Chromosomal Material (Relatively Common) | Other Translocations and Inversions (Relatively Uncommon) |
|---|---|
|
a Del(20q), +8, and -Y abnormalities are not considered MDS defining and cannot in isolation be used to make a diagnosis of MDS.
| Mutation Frequency | Effect on Prognosis | |
|---|---|---|
| RNA SPLICING FACTOR GENES | ||
| SF3B1 | 15%–32% | good |
| SRSF2 | 10%–17% | poor |
| U2AF1 | 7%–12% | poor |
| ZRSR2 | 3%–11% | neutral/poor |
| PRPF8 | 1%–4% | |
| EPIGENETIC REGULATORS | ||
| TET2 | 20%–32% | neutral |
| DNMT3A | 5%–13% | poor |
| IDH1/IDH2 | 4%–12% | poor |
| ASXL1 | 11%–23% | poor |
| EZH2 | 5%–12% | poor |
| UTX | 1% | |
| TRANSCRIPTION FACTORS | ||
| RUNX1 | 8%–15% | poor |
| TP53 | 4%–14% | poor |
| ETV6 | 2%–5% | poor |
| WT1 | <1% | |
| SIGNALING GENES | ||
| NRAS/KRAS | 5%–10% | poor |
| CCSNK1A1 | 7% | |
| CBL | 2%–5% | |
| JAK2 | 2%–5% | |
| FLT3 | 1%–4% | poor |
| GNAS | <1% | |
As mentioned previously, morphologic dysplasia is a common feature in megaloblastic anemias resulting from B 12 and folate deficiencies ( Fig. 22.7 ); toxic exposures, including chemotherapy; and certain infectious agents. These common causes of morphologic dysplasia must be excluded for a diagnosis to be made. It is also necessary to distinguish MDS from AML; cases with more than 20% blasts or with certain defining cytogenetic abnormalities (see “ Acute Myeloid Leukemia ” later in the chapter) should be considered AML. In cases in which aspirate smears are inadequate, CD34 immunostaining for blast forms in the core biopsy sample is useful in quantifying blasts; however, myeloblasts with absent CD34 expression can be seen (see Fig. 22.16 ). It is of clinical importance to distinguish MDS associated with marrow hypocellularity (which occurs in ~15% of cases ) from acquired aplastic anemia or hypoplastic AML. Careful inspection of cellular morphology for dysplastic nuclear features (typically absent in aplastic anemia) and accurate blast counts can aid in this differential.
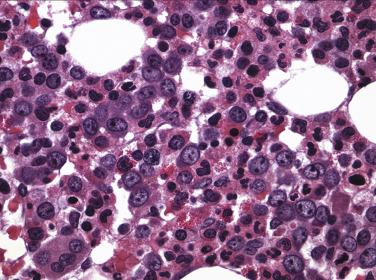
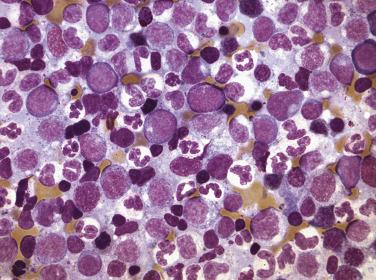
A group of myeloid neoplasms presents with features overlapping between myelodysplastic syndrome and myeloproliferative neoplasms preventing them from being classified as either MDS or MPN. For example, cytopenia(s) and dysplasia, the features of MDS, are seen in conjunction with leukocytosis, thrombocytosis, and organomegaly, the features commonly seen in MPN. This group includes five entities, chronic myelomonocytic leukemia (CMML); atypical chronic myeloid leukemia, BCR-ABL1 negative (see “ Differential Diagnosis ” under “Chronic Neutrophilic Leukemia” later in the chapter); juvenile myelomonocytic leukemia; MDS/MPN with ring sideroblasts with thrombocytosis (MDS/MPN-RS-T); and MDS/MPN, unclassifiable. Due to similarities in clinical, morphologic, and genetic features, these entities, especially CMML and MDS/MPN-RS-T, should be distinguished from pure MDS cases. CMML is defined as a clonal stem cell disorder characterized by prominent monocytosis in the peripheral blood (monocytes >1 × 10 9 /L) and marrow, accompanied by dysplasia involving one or more myeloid lineages, and the absence of a BCR-ABL fusion gene or rearrangements of PDGFRA , PDGFRB, FGFR1, or PCM1-JAK2 ( Fig. 22.8 and Box 22.1 ). Blasts do not constitute more than 20% of the marrow cellularity. If myelodysplasia is absent or minimal and the other requirements are met, a diagnosis of CMML may still be made especially in the presence of mutations in genes associated with CMML (e.g., TET2, SRSF2, ASXL1, SETBP1 ). A proportion of MDS with ring sideroblasts presents with thrombocytosis associated with proliferation of dysplastic megakaryocytes in the marrow and, in addition to SF3B1 mutation, a large subset of cases demonstrates the presence of JAK2 V617E. This entity, which displays both myelodysplastic and myeloproliferative features, has been identified as MDS/MPN with ring sideroblasts and thrombocytosis (MDS with RS-T) ( Fig. 22.9 and Box 22.2 ).
Persistent PB monocytosis ≥1 × 10 9 /L, with monocytes accounting for ≥10% of the white blood cell count
Not meeting WHO criteria for BCR-ABL1 –positive CML, primary myelofibrosis, polycythemia vera or essential thrombocythaemia a
a MPN can be associated with monocytosis simulating CMML. A previous documented history of MPN and the presence of MPN features in the bone marrow and/or of MPN associated-mutations ( JAK2, CALR, or MPL ) tend to support MPN with monocytosis rather than CMML.
No evidence of PDGFRA, PDGFRB, or FGFR1 rearrangement or PCM1-JAK2 (should be specifically excluded in cases with eosinophilia)
Fewer than 20% blasts in the blood and bone marrow b
b Blasts and blast equivalents include myeloblasts, monoblasts, and promonocytes. Abnormal monocytes, which can be present both in the PB and BM, are excluded from the blast count.
Dysplasia in one or more myeloid lineages. If myelodysplasia is absent or minimal, the diagnosis of CMML may still be made if the other requirements are met and
an acquired clonal cytogenetic or molecular genetic abnormality is present in hemopoietic cells (e.g., TET2, SRSF2, ASXL1, SETBP1 )
–OR–
the monocytosis (as previously defined) has persisted for at least 3 months and
all other causes of monocytosis have been excluded
Anemia associated with erythroid lineage dysplasia with or without multilineage dysplasia, ≥15% ring sideroblasts, <1% blasts in peripheral blood, and <5% blasts in the bone marrow
Persistent thrombocytosis with platelet count ≥450 × 109/L
Presence of a SF3B1 mutation or, in the absence of SF3B1 mutation, no history of recent cytotoxic or growth factor therapy that could explain the myelodysplastic/myeloproliferative features. A diagnosis of MDS/MPN-RS-T is strongly supported by the presence of SF3B1 mutation together with a mutation in JAK2 V617F, CALR, or MPL genes
No BCR - ABL1 fusion gene, no rearrangement of PDGFRA , PDGFRB, or FGFR1; or PCM1 - JAK2; no (3;3)(q21;q26), inv(3)(q21q26), or del(5q)
No preceding history of MPN, MDS (except MDS-RS), or other type of MDS/MPN
Recent genetic analysis has revealed that somatic mutations leading to clonal expansion are commonly acquired during human aging. However, these MDS-associated mutations by themselves do not currently define an MDS diagnosis. In the absence of diagnostic criteria for a myeloid neoplasm, a diagnosis of clonal cytopenia of undetermined significance (CCUS) should be considered in patients presenting with cytopenia(s) or clonal hematopoiesis of indeterminate potential (CHIP) in individuals with normal CBC counts. Mutation frequency increases with age, and individuals with clonal mutations are at a higher risk of development of a hematologic malignancy. Some patients with prolonged unexplained cytopenia(s) that do not meet diagnostic criteria for MDS (due to lack of sufficient dysplasia, increased blasts, or a defining cytogenetic abnormality) and do not demonstrate somatic mutations, can be designated as idiopathic cytopenia of undetermined significance (ICUS).
The diagnostic category of myelodysplastic syndrome with single lineage dysplasia (MDS-SLD; formerly refractory anemia) encompasses patients who typically have a unilineage cytopenia and corresponding dysplasia within that lineage (however, this correlation is not absolute). MDS-SLD therefore includes refractory anemia, refractory neutropenia, and refractory thrombocytopenia. The dysplasia should comprise more than 10% of the myeloid lineage, and blasts should be fewer than 5% in the bone marrow and fewer than 1% in the blood. Bicytopenia may occasionally occur, but in cases in which pancytopenia exists in conjunction with unlineage dysplasia, myelodysplastic syndrome–unclassifiable (MDS-U) is the proper designation. Recommended levels for defining cytopenias are hemoglobin less than 10 g/dL, absolute neutrophil count (ANC) fewer than 1.8 × 10 9 /L, and platelet count fewer than 100 × 10 9 /L; however, if definitive features of MDS exist, counts above these levels do not preclude this diagnosis. The majority of the patients present with anemia and dysplasia isolated to the erythroid lineage, accounting for approximately 9% of all cases of MDS. Patients have isolated anemia and no other peripheral blood abnormalities. Peripheral red blood cells are usually normochromic and either normocytic or macrocytic. The bone marrow is often normocellular or hypercellular, with a minority of cases demonstrating a hypoplastic appearance. Erythrodysplasia in aspirate smear preparations can vary from subtle to marked abnormalities, and megaloblastoid differentiation is common ( Figs. 22.10 and 22.11 ; also see Fig. 22.1 ). Nuclear irregularities in erythrocyte progenitors can readily be appreciated in most biopsy specimens. Cytoplasmic vacuolation and diffuse or granular PAS positivity are commonly seen. Morphologic abnormalities of granulocytic or megakaryocytic elements are not seen or are minimal in refractory anemia (always <10% of these cell lineages), and blasts constitute fewer than 5% of the marrow cellularity. Prussian blue staining frequently shows normal or increased iron stores but only occasional (<15%) ring sideroblasts (see Fig. 22.2 ). Approximately 50% of patients have an abnormal karyotype, the majority of which (80%–85%) fall into low-risk prognostic categories (see later discussion). Risk of leukemic transformation is low (~2%), and median survival is 66 months.
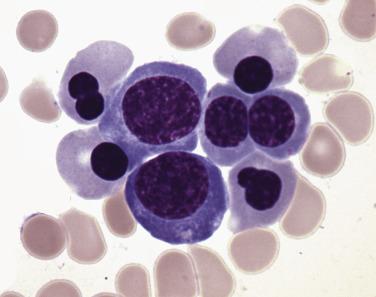
As with other forms of MDS, careful clinical investigation to rule out nonclonal causes of dysplasia (including drug, toxin, viral, immunologic, vitamin deficiencies, and congenital causes) must be undertaken. The presence of a clonal genetic abnormality is therefore critical for confirming this diagnosis.
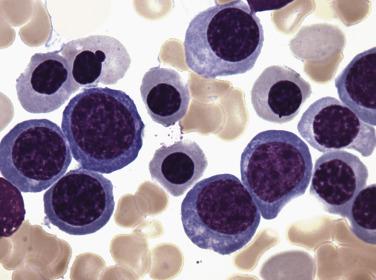
An acquired primary form of sideroblastic anemia, myelodysplastic syndrome with ring sideroblasts (MDS-RS; formerly refractory anemia with ring sideroblasts), is characterized by the presence of ring sideroblasts in at least 15% of the erythroid lineage cells or at least 5% ring sideroblasts in the presence of an SF3B1 mutation. MDS-RS accounts for approximately 11% of MDS. The majority of patients are elderly (median 60–73 years), without a sex predilection, and have moderate anemia and occasionally signs or symptoms of iron overload. Peripheral blood findings are variable. Sometimes the peripheral smear reveals dimorphic populations of normochromic and hypochromic cells; alternatively, normochromic, macrocytic, or normocytic anemia can be seen. Bone marrow cellularity ranges from normocellular to markedly hypercellular and most frequently shows erythroid hyperplasia with dyserythropoietic changes ( Fig. 22.12 ). If dysplasia is limited solely to the erythroid lineage, a diagnosis of MDS-RS with single lineage dysplasia (MDS-RS-SLD) should be made. Cases with granulocytic and/or megakaryocytic dysplasia should be diagnosed as MDS-RS with multilineage dysplasia (MDS-RS-MLD). By definition, numerous ring sideroblasts are seen (median 40% of nucleated erythroid elements in larger series); however, in the presence of SF3B1 mutation, a diagnosis of MDS-RS can be made if ring sideroblasts constitute as few as 5% of nucleated erythroid cells. At least 15% of ring sideroblasts are required for cases lacking this disease-defining mutation ( Fig. 22.13 ). PAS-positive erythroblasts are seen in slightly fewer than 10% of cases. Bone marrow myeloblasts number fewer than 5%, and granulocytic or megakaryocytic dysplasia is not seen.
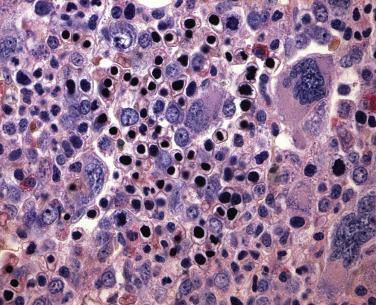
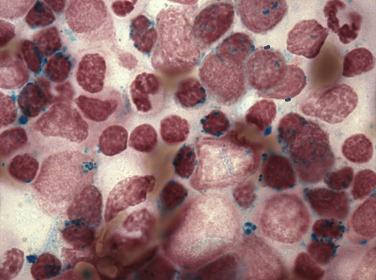
MDS-RS-MLD has an incidence of clonal cytogenetic abnormalities similar to that of MDS-MLD (50% for MDS-RS-MLD and MDS-MLD, and only 9% for MDS-RS-SLD). In addition, the rate of transformation to AML is nearly 10 times higher in MDS-RS-MLD than MDS-RS-SLD, which contributes to a significant difference in overall survival (32 months median survival for MDS-RS-MLD vs 69 months for MDS-RS-SLD).
MDS-MLD, formerly refractory cytopenia with multilineage dysplasia, accounts for approximately 25% to 30% of MDS cases. The peripheral blood must demonstrate either bicytopenia or pancytopenia, and the bone marrow must exhibit dysplastic features in more than 10% of cells from two or more myeloid cell lines ( Fig. 22.14 ; also see Figs. 22.4 and 22.5 ). Blasts are not present in the peripheral blood (<1%), and they are not increased in the bone marrow (<5%). Auer rods are not seen. Dysgranulopoiesis is considered present if cytoplasmic hypogranulation, or hyposegmented or hypersegmented nuclei, are observed in 10% or more of this lineage. Dysmegakaryopoiesis is diagnosed if more than 10% of a minimum of 30 megakaryocytes are micromegakaryocytes or have multiple, widely separated nuclei or a single, nonlobulated nucleus. Importantly, hematologic characteristics, genetic abnormalities, the rate of progression to AML, and overall survival differ significantly between MDS-SLD and MDS-MLD. Abnormal karyotypes are seen in up to 50% of MDS-MLD cases, and the frequency of progression to acute leukemia is approximately 11%. The overall median survival is 33 months.
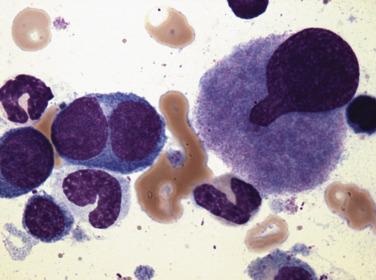
The key feature of myelodysplastic syndrome with excess blasts (MDS-EB; formerly refractory anemia with excess blasts) is the presence of both morphologic dysplasia and 5% to 19% blasts in the bone marrow. Increased blast forms strongly correlate with progressive bone marrow failure and an elevated rate of evolution to AML. In addition, a clear distinction in the rate of progression to acute leukemia and overall survival has been documented between patients possessing 5% to 9% marrow blasts and those with 10% to 19% blasts. As a result, the WHO classification divides MDS-EB into two subcategories, MDS-EB-1 (5%–9% marrow or 2%–4% peripheral blood blasts) ( Fig. 22.15 ) and MDS-EB-2 (10%–19% marrow or 5%–19% peripheral blood blasts) ( Fig. 22.16 ). The significance of Auer rods has been a source of controversy. Patients with Auer rods and fewer than 20% marrow blasts are placed in the MDS-EB-2 category in the WHO classification, based on studies suggesting that the clinical outcomes are similar in those two patient groups. It is important to note that this MDS category now includes the majority of cases formerly classified as acute erythroid leukemia, erythroid/myeloid type (previously defined as ≥50% erythroid precursors in bone marrow and ≥20% myeloblasts among nonerythroid cells) (see Figs. 22.49 and 22.50 ). This change in the revised 2016 WHO classification was based on the close biologic relationship between this AML subtype and MDS in terms of clinical presentation, morphologic features, genetic abnormalities, prognosis, and treatment. The revised 2016 WHO also recognizes MDS with significant degree of fibrosis (MDS-F). Such cases mostly belong to the MDS-EB category, and the presence of fibrosis (grade 2 or 3 according to the WHO grading system [see Box 22.11 ]) is considered an independent prognostic parameter in MDS. Approximately 35% of MDS-EB-1 and 42% of MDS-EB-2 cases demonstrate an abnormal karyotype and include +8, del(5q), -5, and -7. Progression to AML occurs at a high rate (21% of MDS-EB-1 and 35% of MDS-EB-2 cases). Although overall survival is poor, a significant difference exists in median survival between MDS-EB-1 and MDS-EB-2 (16 months vs 9 months, respectively).

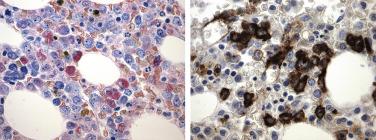
Myelodysplastic syndrome associated with an isolated del(5q) is a relatively uncommon disorder that accounts for only 2% of all MDSs. This entity is characterized by anemia (with or without other cytopenias), thrombocytosis, and a single genetic abnormality: a variably sized interstitial deletion of the distal region of the long arm of chromosome 5 involving bands q13-q33. The 5q- syndrome describes the clinical entity typically characterized by macrocytic anemia, normal to elevated platelet count, and bone marrow erythroid hypoplasia. This syndrome commonly, but not exclusively, occurs in middle-aged women (median 66 years; male to female ratio 1 : 1.7) and is not associated with prior exposure to chemotherapy or radiation. Macrocytic anemia is the most common feature, being seen in 80% of patients at presentation. Anemia is frequently accompanied by an elevated platelet count and modest leukopenia; significant neutropenia or thrombocytopenia is rare. The bone marrow is usually hypercellular and characteristically contains conspicuously increased numbers of megakarocytes, which are characteristically small and hypolobated ( Fig. 22.17 ). Variable degrees of erythroid and myeloid dysplasia are noted, and erythroid hypoplasia is common. Blasts number fewer than 5% in both the peripheral blood and bone marrow. Patients with isolated del(5q) have a very good prognosis (median survival 146 months with median follow-up of 67 months) and progression to AML is rare (8%). Cases showing one more chromosomal abnormality, with exception of -7/del(7q), can still be diagnosed as 5q- MDS. However, cases with numerous chromosomal abnormalities or with blasts greater than 5% should not be placed in this category, because these findings are associated with a much more aggressive clinical course.
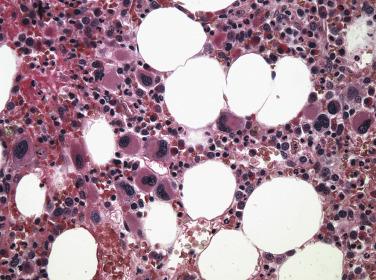
AML is characterized by a clonal increase in myeloid blasts or precursors within the bone marrow (>20% of the cellularity), blood, and other organs. These myeloid precursor forms include cells destined to contribute to mature neutrophilic, eosinophilic, and basophilic granulocytic lineages, as well as the monocytic, erythroid, and megakaryocytic lineages. Therefore the increase in immature myeloid elements occurs at the expense of residual normal hematopoiesis and frequently results in hematopoietic insufficiency (i.e., granulocytopenia, anemia, and thrombocytopenia). The term acute denotes the rapidly progressive clinical course of the disease in the absence of any treatment. The WHO classification includes AML with specific recurrent genetic abnormalities to emphasize their importance in diagnosing, predicting clinical behavior, and (increasingly) directing therapy. The current revised WHO classification ( Box 22.3 ) has expanded this concept even further to include both balanced translocations—for example, the previously defined t(8;21)(q22;q22), inv(16)(p13.1q22), t(16;16)(p13.1;q22), t(15;17)(q22;q12), t(9;11)(p22;q23), t(6;9)(p23;q34), inv(3)(q21q26.2), t(3;3)(q21;q26.2), and t(1;22)(p13;q13)—as well as AML with specific genetic mutations. AML with mutated NPM1 and AML with biallelic mutations of CEBPA have become true entities, while two new provisional categories of AML with BCR-ABL1 and AML with mutated RUNX1 have been introduced. The hope is that as we progress toward an entirely molecularly defined classification system for AML, pathologists will help direct therapy and provide clinicians with important prognostic information. In addition to these genetic subclasses of AML, the WHO recognizes the clinical importance of preexisting MDS or prior chemotherapy by incorporating AML subclasses based on these clinical associations. The remaining WHO AML, not otherwise specified (NOS) category is based on the blast and precursor cell morphology and blast count of more than 20%, which in the revised AML classification is always counted as percentage of total marrow cells. This approach has resulted in elimination of acute erythroid leukemia, erythroid/myeloid type from the AML category . Most of these cases are now classified as MDS with excess blasts due to the overall blast count of less than 20% and similar clinical, morphologic, and genetic features between these two entities (see “ Myelodysplastic Syndrome With Excess Blasts ” earlier in the chapter). With the continuing discovery of new clinically important genetic markers, less entities will be included in this NOS category, which already constitute less than 25% of all AMLs.
AML with t(8;21)(q22;q22.1); RUNX1 - RUNX1T1
AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB/MYH11
Acute promyelocytic leukemia with PML/RARA
AML with t(9;11)(p21.3;q23.3); MLLT3-KMT2A
AML with t(6;9)(p23;q34.1); DEK-NUP214
AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2 , MECOM
AML (megakaryoblastic) with t(1;22)(p13.3;q13.3); RBM15–MKL1
AML with mutated NPM1
AML with biallelic mutations of CEBPA
Provisional entity: AML with BCR-ABL1
Provisional entity: AML with mutated RUNX1
Following MDS or MDS/MPD
Without antecedent MDS
Alkylating agents
Topoisomerase type II inhibitor
Other agents
AML with minimal differentiation
AML without maturation
AML with maturation
Acute myelomonocytic leukemia
Acute monoblastic and monocytic leukemia
Pure erythroid leukemia
Acute megakaryoblastic leukemia
Acute basophilic leukemia
Acute panmyelosis with myelofibrosis
Blastic Plasmacytoid Dendritic Cell Neoplasm
Transient abnormal myelopoiesis
Myeloid leukemia associated with Down syndrome
Although a clear link exists between AML and prior exposure to radiation, cytotoxic chemotherapy, and certain environmental toxins, such as benzene, the vast majority of cases of AML are idiopathic. AML can occur throughout life but is primarily a disease of older adults (median 60 years). Based on data prior to the era of genetic subclassification, for both sexes, the incidence rises between the ages of 25 and 65 years over 10-fold (1–10.6 per 100,000 population) and climbs to 15.7 per 100,000 per year in individuals between 70 and 74 years old. Over the age of 60, incidence in males increases; no sex predilection is seen at younger ages.
The clinicopathologic features of AML are related to the proliferation of blasts in the bone marrow and their infiltration into normal tissues, such as the liver and spleen. The most common consequence in the marrow is the replacement of normal hematopoietic elements, which results in cytopenias. Fatigue, pallor and dyspnea on exertion, infection and fever, and bleeding can occur as a result of anemia, neutropenia, and thrombocytopenia, respectively. Anemia and thrombocytopenia are almost invariably present. Laboratory evidence of disseminated intravascular coagulation is often associated with acute promyelocytic or monoblastic leukemias. The majority of patients have elevated white blood cell counts (>10 × 10 9 /L); leukocyte counts above 100 × 10 9 /L occur in 15% to 20% of patients (most commonly in monocytic leukemias). Leukocyte counts greater than 150 × 10 9 /L can lead to leukostasis, aggregates of leukemia cells in the microvasculature of various organs (especially brain and lung), that can result in life-threatening tissue infarction and hemorrhage. Leukopenia can be observed in elderly patients, hypergranular acute promyelocytic leukemia (APL), AML associated with multilineage dysplasia, or Fanconi anemia. Peripheral blasts are commonly observed, but are absent in up to 10% of patients (aleukemic presentation).
Infiltration of other tissues (lymph nodes, skin, gums, meninges) occurs less frequently, most often in leukemias with monocytic differentiation. Uncommonly, AML can occur as an isolated tumor mass, in which case it is usually designated a myeloid sarcoma or, because of the green coloration resulting from the presence of myeloperoxidase, a chloroma (see Chapter 21 ). Such tumors may be detected months or even years in advance of any other evidence of AML and can also be the first evidence of relapse. Bones (particularly the skull and facial bones), breast, testis, ovary, and brain are favored sites.
In the majority of cases, marrow cellularity is increased and mainly comprises blasts or immature granulocytic or monocytic forms. A corresponding decrease occurs in maturing granulocytic forms and megakaryocytes (with a few notable exceptions). The numbers of erythroid precursors are more variable; increased erythroid forms are frequently seen in pure erythroid leukemia or AML arising from MDS. Under the WHO guidelines, blasts or blast equivalents constitute more than 20% of the total nucleated marrow cells with the exception of AML with t(8;21)(q22;q22.1), inv(16)(p13.1q22), t(16;16)(p13.1;q22), or APL with PML-RARα . In these subtypes, a diagnosis of AML should be made based on the defining genetic abnormality even when blasts are less than 20%. For diagnostic purposes, the atypical promyelocytes of acute promyelocytic leukemia, the monoblasts and promonocytes of acute monoblastic and monocytic leukemias, and the megakaryoblasts of acute megakaryoblastic leukemias are all counted as blast equivalents. Erythroblasts are not included in the blast count except for the rare pure erythroid leukemia. In all other subtypes, once a blast count greater than 20% has been established, cytochemistry, immunophenotyping, and clinical history are all used in final subclassification ( Fig. 22.18 ).
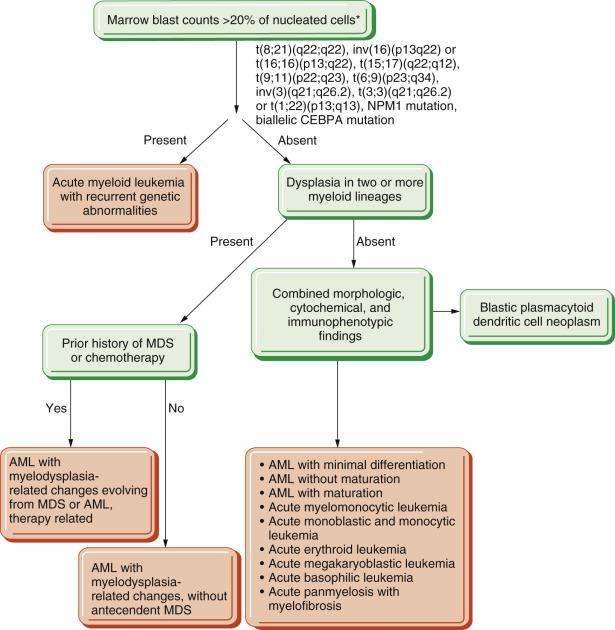
Myeloblasts are relatively large cells (14–18 µm) with single round to oval nuclei, fine evenly distributed chromatin, and one to several distinct nucleoli ( Fig. 22.19 ). The nucleus is surrounded by moderate amounts of basophilic cytoplasm, which often contains several azurophilic granules. In some cases, numerous cytoplasmic granules or fused granules forming rodlike structures (Auer rods) are observed (see Fig. 22.19 ). Auer rods occur only in blasts of myeloid derivation. For minimally differentiated AMLs, the blasts are often smaller and have inconspicuous nucleoli and scant agranular cytoplasm, making the cells difficult to distinguish morphologically from lymphoblasts.
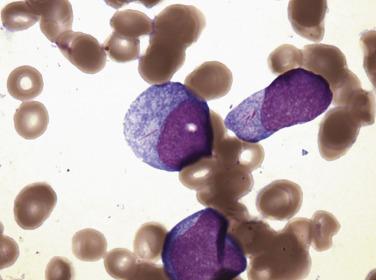
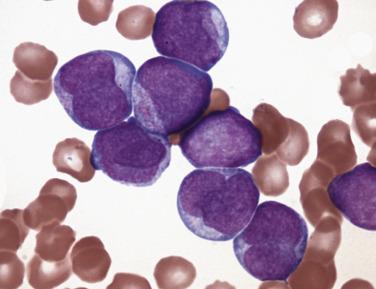
Monoblasts are large cells (15–20 µm) with a round nucleus; prominent, often single nucleoli; and abundant basophilic cytoplasm with fine, evenly distributed azurophilic granules and occasional fine vacuoles ( Fig. 22.20 ). The cytoplasm may show “blebbing” or pseudopod formation. Promonocytes have irregular or lobulated nuclei, with delicate folds or creases but fine chromatin, inconspicuous nucleoli, and abundant blue-gray, finely granulated, often vacuolated cytoplasm. These immature monocytic forms are often difficult for inexperienced practioners to differentiate from mature abnormal monocytes (such as in CMML); however, some useful distinguishing features are the more condensed nuclear chromatin and more heavily granulated cytoplasm of the mature monocyte.
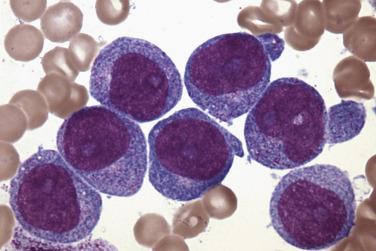
Erythroblasts are medium to large cells with round nuclei; fine, dispersed chromatin; one to several distinct nucleoli; and deeply basophilic cytoplasm with occasional vacuoles ( Fig. 22.21 ) that contain PAS-positive material.
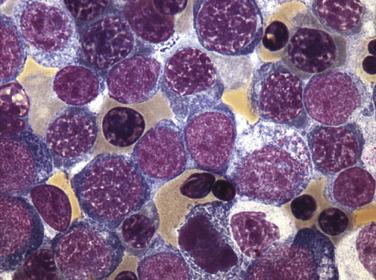
Megakaryoblasts are medium to large cells with round to irregular, occasionally bilobed nuclei with fine, dispersed chromatin and 1 to 3 distinct nucleoli ( Fig. 22.22 ). The cells possess ample pale, basophilic cytoplasm that usually lacks granules and often demonstrates projections or pseudopods.
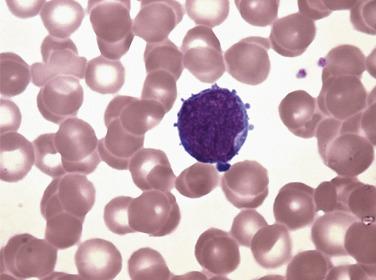
Cytochemical stains take advantage of the specific enzymatic and cytochemical properties of cells within a hematopoietic lineage. Unfixed specimens, such as air-dried aspirate smears or touch preparations, are generally used for these tests; however, the specific esterase (naphthol AS-D chloroacetate esterase [CAE] or Leder) stain or immunohistochemistry for myeloperoxidase can be performed on formalin-fixed, paraffin-embedded tissue. In general, myeloperoxidase, Sudan black B, and specific esterase stains are useful for identifying granulocytic myeloid forms, whereas nonspecific esterase stains cells of the monocytic lineage ( Table 22.4 ). PAS reactivity can confirm the presence of erythroblasts in pure erythroid leukemia and can differentiate between the blasts of AML with minimal differentiation (PAS negative) and precursor B-cell lymphoblasts (PAS blocklike positive).
| Stain | Positive Normal Cell | Positive Neoplastic Cell |
|---|---|---|
| Myeloperoxidase (MPO) (primary granules) | Early myeloblasts (weak +) Myeloblast → neutrophil Eosinophils Monocytes (few granules) |
Myeloblasts Promyelocytes (intense) |
| Sudan black B (SBB) (primary granules and intracellular lipid) | Early myeloblasts (weak +) Myeloblast → neutrophil Eosinophils Monocytes (few granules) |
Myeloblasts Promyelocytes (intense) |
| SPECIFIC ESTERASE (SE) (PRIMARY GRANULES) | ||
| Naphthol AS-D chloracetate esterase (CAE/Leder) | Early myeloblasts (weak +) Myeloblast → promyelocytes Mast cells Monocytes (few granules) |
Myeloblasts (weak +) Promyelocytes (intense) Monoblasts (± fine scattered granules) Eosinophils in AML with the inv(16) or t(16;16) |
| NONSPECIFIC ESTERASE | ||
| α-Naphthyl acetate esterase (ANA) | Monocytes and macrophages (inhibited by sodium fluoride) Granulocytes (negative to weak) Megakaryocytes |
Monoblasts (inhibited by sodium fluoride) Promyelocytes in APL (25%) Lymphoblasts (multifocal punctate or Golgi pattern) Megakaryoblasts and erythroblasts (multifocal punctate) Prolymphocytes in T-PLL |
| α-Naphthyl butryate esterase (ANB) | Monocytes and macrophages Granulocytes (negative to weak) Megakaryocytes (weak) |
Monoblasts Lymphoblasts (multifocal punctate) |
| Periodic acid–Schiff (PAS) | Neutrophils (diffuse granular pattern) Monocytes (few granules) |
Lymphoblasts (perinuclear, coarse, blocklike granular pattern) Erythroleukemia (chunky positivity in blasts and erythroid precursors) |
Immunophenotypic analysis is an essential adjunct to morphology and genetic testing and in many institutions has replaced cytochemical staining for lineage assignment ( Tables 22.5 and 22.6 ). Flow cytometric analysis is the preferred technique for immunophenotyping because of its specificity, its multiparametric quantitative nature, and the availability of a wide array of antibody reagents. Although a great degree of immunophenotypic heterogeneity exists among AML subtypes, certain characteristic patterns exist (e.g., HLA-DR negativity in t(15;17) APL and CD19/CD56 positivity in t(8;21) AML).
| Antigen or CD | Reactivity in Hematopoietic Cells | Detectable by Flow Cytometry | Detectable by Immunohistochemistry | |
|---|---|---|---|---|
| Unfixed | Fixed | |||
| HEMATOPOIETIC PRECURSORS | ||||
| CD45(dim) | Leukocyte blasts | + | + | + |
| CD117 | Leukocyte blasts (± monoblasts), mast cells (very bright) | + | + | + |
| CD34 | Leukocyte blasts | + | + | + |
| TdT | B and T lymphoblasts, some myeloid blasts | + | + | + |
| HLA-DR | B lymphoblasts, myeloid blasts (not promyelocytes) | + | + | − |
| MYELOMONOCYTIC | ||||
| CD33 | CFU-GEMM to promyelocytes, monocytes | + | − | − |
| CD13 | CFU-GM to granulocytes, monocytes | + | − | − |
| CD14 | Monocytes | + | − | − |
| CD68 | Monocytes, macrophages | − | + | + |
| CD15 | Granulocytes | + | + | − |
| Myeloperoxidase (MPO) | Neutrophils, eosinophils | − | + | − |
| Lysozyme | Myelomonocytic cells | − | + | + |
| MEGAKARYOCYTIC | ||||
| CD41 (GPIIb/IIIa) | Platelets and megakaryocytes | + | + | − |
| CD42 (GPIb) | Platelets and megakaryocytes | + | + | − |
| CD61 (GPIIIa) | Platelets and megakaryocytes | + | + | + |
| von Willebrand factor | Platelets and megakaryocytes | − | + | + |
| ERYTHROID | ||||
| Glycophorin A | Red blood cells and precursors | + | + | + |
| Hemoglobin | Red blood cells and precursors | − | + | + |
| CD71 (transferin receptor) | Early red cell precursors | + | − | + |
| B LYMPHOID | ||||
| CD20 | B lymphocytes | + | + | + |
| CD19 | B lymphocytes | + | + | − |
| CD10 | B-lymphocyte subsets, granulocytes | + | + | + |
| CD23 | B lymphocytes | + | + | + |
| Surface Ig (sIg) | Mature B lymphocytes | + | + | − |
| Cytoplasmic Ig (cIg) | Plasma cells | + | + | + |
| CD138 | Plasma cells | + | + | + |
| T LYMPHOID | ||||
| CD2 | T lymphocytes, neoplastic mast cells | + | + | + |
| CD3 | T lymphocytes | + | + | + |
| CD5 | T lymphocytes | + | + | + |
| CD4 | T-lymphocyte helper subset | + | + | + |
| CD8 | T-lymphocyte cytotoxic subset | + | + | + |
| CD1a | Thymocytes, Langerhans cells | − | + | + |
| WHO Subtype | Morphology | Cytochemistry | Immunophenotype |
|---|---|---|---|
| AML with minimal differentiation | >20% agranular, midsize, primitive-appearing blasts without Auer rods; often difficult to distinguish from lymphoblasts | <3% MPO, SBB, SE, or NSE-positive blasts | CD117±, CD13±, CD33±, TdT±, HLA-DR+, CD34+, CD7−/+, CD2−/+, CD19−/+ |
| AML without maturation | >20% myeloid blasts; <10% of marrow nucleated cells are promyelocytes or more mature granulocytic forms; Auer rods may be seen | >3% MPO, SBB-positive blasts | CD117+, CD13+, CD33+, HLA-DR+, CD34+ |
| AML with maturation | >20% myeloid blasts ± azurophilic granules; >10% of marrow nucleated cells are promyelocytes or more mature granulocytic forms; Auer rods typically seen | >3% MPO, SBB-positive blasts | CD13+, CD33+, CD15+, CD34±, CD117±, HLA-DR± |
| Acute myelomonocytic leukemia | >20% myeloblasts, monoblasts, promonocytes; >20% monocytes and monocytic precursors; >20% neutrophils and granulocytic precursors | NSE-positive monocytic cells; MPO, SBB-positive myeloblasts | Varying proportions of myeloblasts or monoblasts and monocytic and granulocytic cells: CD13+, CD33+, CD11b+, CD14+, CD4+, CD117±, CD34± |
| AML with monocytic differentiation | >80% monocytic cells; either monoblasts predominate (>80%) or promonocytes predominate with <80% monoblasts | NSE positive, and MPO, SBB-negative monocytic cells | CD13±, CD33+, CD117±, CD34-, CD36+, CD11b+, CD14±, CD4+ |
| Pure erythroid leukemia | >80% immature erythroid precursors ≥30% proerythroblasts No significant myeloblastic component |
ANA and PAS-positive erythroid blasts | Erythroid precursors: CD71+ glycophorin± (stronger in more differentiated forms), hemoglobin+, CD34-, HLA-DR- |
| Acute megakaryoblastic leukemia | >20% blasts with >50% demonstrating megakaryocytic derivation by morphology, immunophenotypic, or electron microscopic studies | MPO, SBB-negative; PAS ± in megakaryoblasts | HLA-DR+, CD33+, CD34+, CD61+, CD41+, vWf+, CD42+ |
| Acute basophilic leukemia | >20% midsize cells with oval to bilobed nucleus, one to three nucleoli and basophilic cytoplasm with multiple coarse basophilic granules | Metachromatic toluidine blue staining, acid phosphatase-positive | CD13+, CD33+, CD34+, HLA-DR+, CD9+, TdT± |
| Acute panmyelosis with myelofibrosis | Panmyelosis with clusters of blasts, including dysplastic megakaryocytes in a background of marked reticulin fibrosis | MPO in myeloid precursors; PAS ± in megakaryoblasts | Blasts CD117+, CD13+, CD33+, MPO+, CD41+, CD61, and vWf+ in megakaryocytic precursors |
| Blastic plasmacytoid dendritic cell neoplasm a | >20% midsize blasts with round to folded, bilobed nuclei and scant agranular cytoplasm; may resemble promonocytes | Negative for MPO and NSE | CD4+, CD56+, CD123+, TCL1+, CD33±, TdT± |
a Blastic plasmacytoid dendritic cell neoplasm is no longer included with acute myeloid leukemias and represents a separate entity.
In addition, immunophenotyping is required for defining mixed phenotype acute leukemia that contains more than one blast cell lineage (i.e., T cell/myeloid or B cell/myeloid). Multiparameter flow cytometry is the method of choice for recognizing this entity, allowing detection of specific myeloid and lymphoid lineage markers on the same blast population ( Table 22.7 ).
| Myeloid Lineage | T-Cell Lineage | B-Cell Lineage |
|---|---|---|
| Myeloperoxidase Or |
Strong a cytoplasmic CD3 (with antibodies to CD3 epsilon chain) Or Surface CD3 |
Strong CD19 with at least one of the following strongly a expressed: CD79a, cytoplasmic CD22, or CD10 |
| Monocytic differentiation (at least two) NSE, CD11c, CD14, CD64, lysozyme |
Weak CD19 with at least two of the following strongly a expressed: CD79a, cytoplasmic CD22, or CD10 |
a Strong expression is defined as equal or brighter than the normal T or B cells in the sample.
As already emphasized, cytogenetic and other molecular genetic studies are required for the complete characterization of suspected AML. Routine karyotyping should be performed in all cases, and FISH or reverse-transcriptase polymerase chain reaction (RT-PCR) can be performed to confirm the presence of the t(8;21), inv(16), or t(16;16), t(15;17), or other recurrent genetic abnormalities associated with rearrangement of KMT2A (MLL). With the use of several next generation sequencing (NGS) platforms, numerous somatic mutations in myeloid-associated genes have been identified ( Box 22.4 ). These findings indicate that AML is a multistep genetic process requiring cooperation of mutations of several classes that may be present much before clinical presentation of AML. Recent studies have shown the presence of at least one driver mutation in 96% of AML cases and two or more driver mutations in 86% of AML cases. Addition of genomic sequencing to conventional cytogenetic analysis has allowed subdivision of AML into at least 11 molecular and clinically distinct classes defined by t(15;17), t(8;21), inv(16)/t(16 : 16), t(6;9), inv(3)/t(3;3), KMT2A (MLL) rearrangements, CEBPA biallelic mutations, NPM1 mutations, TP53 /complex karyotype, chromatin/splicing factor mutations, and provisionally with less than three aneuploidies. In addition, the mutational profile between de novo AML and therapy-related/secondary AML is different, with the latter showing mutations in genes that are commonly detected in MDS, such as RNA splicing and chromatin modifying genes (see Table 22.3 ). The most common mutated genes currently known to have clinical (prognostic and therapeutic) significance in AML are FLT3, NPM1, CEBPA, RUNX1, KMT2A (MLL), NRAS, WT1, IDH1 and IDH2 , DNMT3A, and ASXL1 . It is important to mention that similar gene mutations are present in the newly recognized group of myeloid neoplasms with germline predisposition, which has been included in the revised WHO classification ( Box 22.5 ). Since these molecular aberrations are not unique and shared between sporadic and familial cases, screening family members may be required to recognize this group of myeloid disorders.
Class I (provide proliferative advantage)
FLT3, NRAS, BCR-ABL1 fusion
Class II (impair hematopoietic differentiation and subsequent apoptosis)
CEBPA, NPM1, RUNX1
Class III (epigenetic regulators)
DNMT3A, IDH1, IDH2, ASXL1, TET2
Class IV (tumor suppressor genes)
TP53, WT1
Class V (RNA splicing genes required for RNA maturation)
SF3B1, SRSF2, U2AF1, ZRSR2
Myeloid neoplasms with germline predisposition without a preexisting disorder or organ dysfunction
AML with germline CEBPA mutation
Myeloid neoplasms with germline DDX41 mutation a
a Lymphoid neoplasms also reported.
Myeloid neoplasms with germline predisposition and preexisting platelet disorders
Myeloid neoplasms with germline predisposition and other organ dysfunction
Myeloid neoplasms with germline GATA2 mutation
Myeloid neoplasms associated with BM failure syndromes
Myeloid neoplasms associated with telomere biology disorders
JMML associated with neurofibromatosis, Noonan syndrome, or
Noonan syndrome–like disorders
Myeloid neoplasms associated with Down syndrome a
With rapidly increasing knowledge of AML biology and the development of targeted therapies, molecular profiling is expected to become standard of care for diagnosis and disease monitoring.
The balanced chromosomal translocation t(8;21)(q22;q22.1); RUNX1-RUNX1T1 accounts for approximately 10% of all cases of AML and nearly 40% of cases of AML with maturation. The translocation joins the 5′ portion of the RUNX1 gene on chromosome 21, with the 3′-terminal portion of the eight-twenty-one (ETO) or RUNX1T1 gene on chromosome 8. The resulting RUNX1-RUNX1T1 fusion protein represses the transcriptional activation of AML1 target genes that are normally responsible for granulocyte differentiation. The t(8;21) can be detected by standard karyotypic analysis ( Fig. 22.23 ) and confirmed by FISH ( Fig. 22.24 ). AML with this abnormality is commonly seen in younger individuals and is typically associated with a good response to therapy, a high complete remission rate, and good long-term survival after consolidation with multiple cycles of high-dose cytarabine chemotherapy. Additional chromosomal and genetic abnormalities are also frequently present and can include mutations in KRAS, NRAS, and KIT . The presence of KIT mutations is associated with worse prognosis.
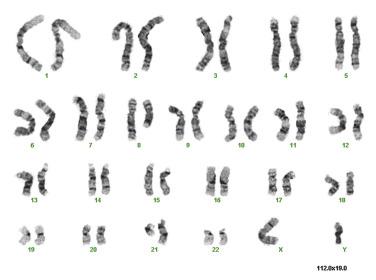
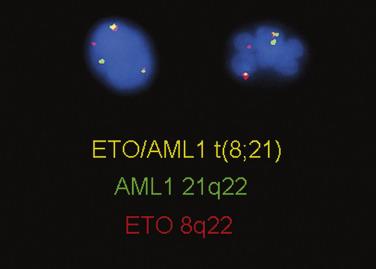
The most common morphologic appearance of the bone marrow in cases with t(8;21) is acute myeloid leukemia with maturation beyond the blast stage ( Fig. 22.25 ). Occasionally, absence of maturation is noted or, rarely, monocytic differentiation. Blasts typically possess prominent azurophilic granules, and Auer rods are readily detectable. Frequently, maturing granulocytes show marked cytoplasmic or nuclear abnormalities, including hypergranulation, abnormal clumping of granules (resembling the Chédiak-Higashi anomaly), or pseudo–Pelger-Huët nuclear abnormalities. It is important to appreciate that these changes can be seen in AML with t(8;21) and are not indicative of preexisting MDS. Rarely, the extent of differentiation will be such that blasts constitute less than 20% of the cellularity. In this event, the diagnosis of AML t(8;21) should still be rendered.
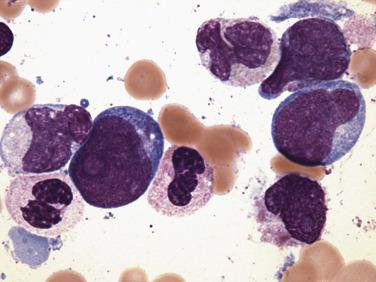
In cases without monocytic differentiation, blasts typically show staining for myeloperoxidase and Sudan black B of variable intensity. In some cases with abnormal granulation, however, this staining is absent. Most cases express CD34, CD13, CD33, CD15, and/or CD65 and HLA-DR. Interestingly, the B-cell markers CD19 and PAX5 are frequently coexpressed in the blast forms in this entity, and CD19/CD56 coexpression is fairly specific for this chromosomal anomaly. Terminal deoxynucleotidyl transferase (TdT) can be positive in a minority of blasts in over 50% of cases.
The inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB/MYH11 abnormality results in an acute myelomonocytic leukemia that is associated with marked marrow eosinophilia. The genetic defect involves the CBFB gene located at chromosome 16q22 and the smooth muscle myosin heavy-chain gene, also on chromosome 16 at p13.1. Either a chromosomal inversion or translocation results in the breakage and rejoining of these genes to create an in-frame fusion gene product ( CBFB/MYH11 ). CBFB is the normal heterodimeric partner to RUNX1, and the fusion to MYH11 results in repression of RUNX1- mediated myeloid cell differentiation. Similar to the t(8;21), inv(16)(p13.1q22) is more common in younger patients and constitutes approximately 5% to 10% of all cases of AML. The detection of inv(16)(p13.1q22) is often difficult in routine karyotypes; RT-PCR or FISH methods for detection of the CBFB/MYH11 fusion are favored ( Fig. 22.26 ). The presence of this genetic abnormality is associated with a good clinical response and long-term survival with multicycle high-dose cytarabine therapy ; however, patients with inv(16)(p13.1q22) may have a higher risk of relapse than similarly treated patients with t(8;21). The presence of KIT mutation adversely affects the prognosis.
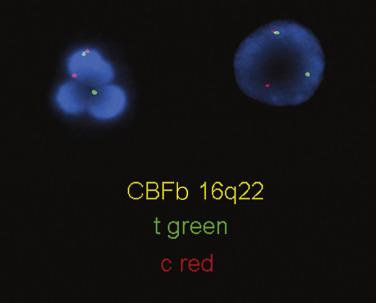
The peripheral blood will frequently show increases in myeloblasts and monocytic precursors; however, interestingly, often no increase in peripheral blood eosinophils is found. The bone marrow shows a combination of myeloblasts, monoblasts, and promonocytes that constitute more than 20% of the marrow cellularity ( Figs. 22.27 and 22.28 ). In the majority of cases, eosinophils are increased without significant maturation arrest, and in all cases abnormal eosinophils are present. The characteristic abnormalities include large eosinophilic granules within early eosinophilic prescursors and, more strikingly, large basophilic granules within eosinophilic precursors (so-called eo-basos) (see Fig. 22.28 ). Auer rods are frequently present within the myeloblasts. Occasional cases, however, may completely lack eosinophilia or a monocytic component or may have blasts that account for less than 20% of the marrow cellularity. Regardless of the phenotypic appearance, if inv(16)(p13.1q22) or t(16;16)(p13.1;q22) is identified cytogenetically or molecularly, a diagnosis of AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22) should be rendered.
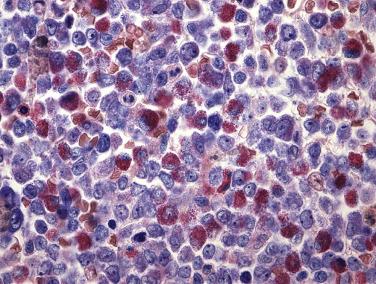
Eosinophils show atypical cytochemical reactions with PAS and naphthol AS-D CAE positivity in most cases (see Table 22.4 ). Reactivity with naphthol AS-D CAE is of particular note because normal eosinophils are typically negative for this stain. In addition, myeloperoxidase activity is seen in more than 3% of myeloid blasts, and weak nonspecific esterase (NSE) activity can be identified within the monocytic elements. Evidence of monocytic differentiation is also observed by immunophenotypic analysis, including CD14, CD11b, CD4, CD36, and lysozyme expression in monocytoid forms. Typical myeloid antigens, CD13 and CD33, are noted within the blasts as well. Although not specific, the coexpression of the T-cell marker CD2 in myeloblasts is common and should prompt a search for inv(16)(p13.1q22).
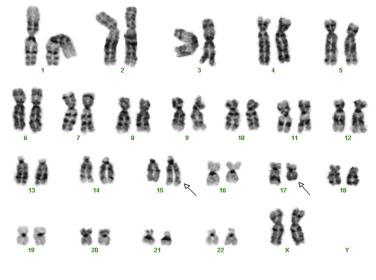
APL with t(15;17)(q24.1;q21.2) has been renamed to recognize the significance of PML-RARα fusion that may be cryptic or result from complex genetic rearrangements other than the classic t(15;17)(q24.1;q21.1). This type of leukemia is characterized by the accumulation of atypical promyelocytes within the bone marrow. APL is most commonly seen in middle-aged adult patients and accounts for 5% to 10% of all forms of AML. In 98% of all cases of APL, the t(15;17)(q24.1;q21.2) abnormality is present, which fuses the RARα gene on chromosome 17 and PML gene on chromosome 15, resulting in a PML-RARα fusion protein. The PML-RARα fusion protein inhibits the function of RARα, which is critical for normal myeloid cell development and results in a block in differentiation at the promyelocytic stage. This abnormality can be overcome by pharmacologic doses of ATRA or arsenic trioxide, which induce the neoplastic promyelocytes to differentiate and undergo apoptosis. ATRA therapy, together with an anthracyline, confers a high likelihood of long-term survival. In a minority of cases, alternative balanced chromosomal translocations are observed that result in novel RARα fusion genes. The variant partner proteins include promyelocytic leukemia zinc finger (PLZF 11q23), nucleophosmin (NPM; 5q35), nuclear mitotic apparatus (NUMA1; 11q13), signal transducer and activator of transcription 5B (STAT5B; 17q21), and others. Importantly, tumors harboring some of these variant fusion proteins (PLZF - RARα and STAT5B-RARα) are not responsive to ATRA differentiation therapy. Interestingly, in addition to t(15;17), over 30% of cases of APL also harbor internal tandem duplication (ITD) or Asp835 mutations of the receptor tyrosine kinase FLT3, which may not only play a role in the pathogenesis of the disease but also may be a second viable therapeutic target. Standard karyotypic analysis is routinely used in the diagnosis of APL ( Fig. 22.29 ), as are FISH and RT-PCR ( Fig. 22.30 ). RT-PCR for the PML-RARα fusion transcript is particularly useful in monitoring minimal residual disease. Of note, APL is frequently associated with a hemorrhagic diathesis similar to that of disseminated intravascular coagulation, which may be related to a hyperfibrinolytic state that occurs in conjunction with limited activation of the blood coagulation system.
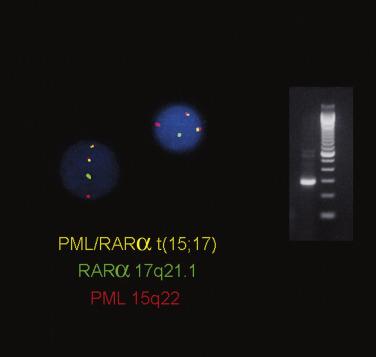
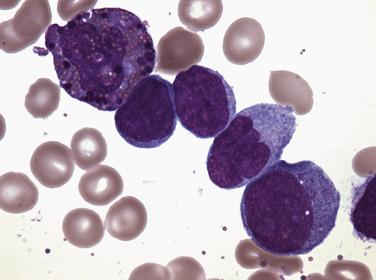
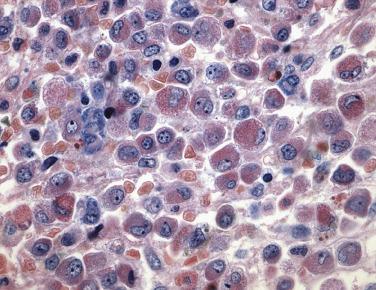
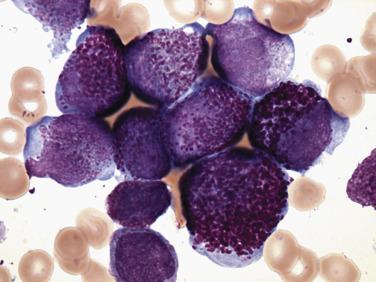
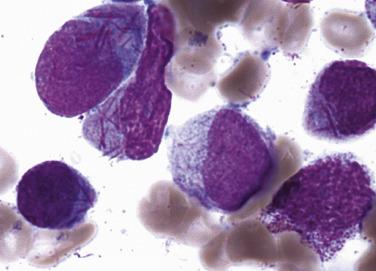
Two morphologic subtypes of APL exist, a common hypergranular or typical variant and a less common microgranular form. There is no correlation between the various RARα rearrangements and the hypergranular or microgranular subtypes. The hypergranular form is commonly associated with leukopenia, whereas the microgranular form is more frequently associated with leukocytosis. Bone marrow core biopsy samples are typically markedly hypercellular and show limited terminal myeloid differentiation associated with numerous atypical promyelocytes ( Fig. 22.31 ). In aspirate smears, the atypical promyelocytes of hypergranular APL show great variation in nuclear size and shape, with frequently bilobed or indented nuclei. The cytoplasm is packed with numerous large, bright pink or red to purple granules that may obscure the nuclei ( Fig. 22.32 ). Auer rods are numerous and occasionally occur in large collections that resemble bundles of sticks (so-called faggot cells) ( Fig. 22.33 ). In the microgranular variant of APL, the azurophilic granules are submicroscopic (<200 nm in size) and not readily identified by light microscopy. The granules in these cases are dustlike, or seemingly absent, and the more easily seen nuclei show prominent bilobed or dumbbell shapes ( Fig. 22.34 ). On careful inspection, small numbers of more typical hypergranulated forms are seen in cases of microgranular APL, as are Auer rods or faggot cells.
Myeloperoxidase is intensely positive in both the hypergranular and microgranular forms of the disease ( Fig. 22.35 ). This finding should prompt investigation for the PML-RARα fusion, particularly in microgranular cases, in which such intense myeloperoxidase staining is disproportionate to the degree of granularity observed. By flow cytometry, CD33 is homogeneously and brightly positive, with CD13 being more variably expressed. HLA-DR is characteristically absent, and CD34 is usually negative. CD15 and CD65 are rarely or weakly expressed, and CD34/CD15 double-positive cells are never seen. The preferential T-cell marker CD2 is commonly expressed. CD34, HLA-DR, and CD2 expression, if present, is more often noted in the microgranular APL variant.

Because of the inconspicuous nature of the granules in the microgranular variant, this entity is easily missed. Important clues include intense myeloperoxidase staining, the absence of HLA-DR expression, or the identification of occasional hypergranulated or Auer rod–laden forms. In general, the morphologic features in such cases suggest monocytic leukemias, which are ruled out by histochemical or immunophenotypic studies. Cytogenetics, FISH, and RT-PCR will detect the PML-RARα fusion in most cases, confirming a diagnosis of APL. Occasionally, drug-induced agranulocytosis or large granular lymphocyte leukemia will mimic APL as a result of arrest in maturation at the promyelocytic stage. These cases can be distinguished by the absence of atypical promyelocytes and the absence of Auer rods. A small subset of cases with variant translocations involving RARα may show occasional phenotypic features that resemble acute promyelocytic leukemia. These fusion partner genes include the ZBTB-16 gene at 11q23 (formerly known as PLZF ), the NUMA1 gene at 11q13, the NPM1 gene at 5q35, and the STAT5B gene at 17q11.2. Importantly, these variant RARα fusion genes show differential responsiveness to standard APL therapy, with only the NPM1-RARα fusion showing some responsiveness to ATRA.
Abnormalities of 11q23 are seen in 6% to 8% of primary AML cases. The gene involved is the human homologue of the Drosophila trithorax gene, lysine [K]–specific methyltransferase 2A ( KMT2A; previously called mixed-lineage leukemia [ MLL ]). The most common abnormalities take the form of balanced chromosomal translocations. Over 80 different partner genes have been observed in KMT2A translocations. In de novo AML, 11q23 abnormalities are found most often in infantile AML; however, the vast majority of KMT2A rearrangements are associated with prior chemotherapy (see later discussion). The t(9;11)(p22.3;q23.3) rearrangement appears to represent a distinct entity among AML cases with 11q23 abnormalities and therefore has been assigned its own diagnostic category in the WHO classification system. Cases with t(9;11) may occur at any age, but are most frequently observed in pediatric AML, constituting approximately 10% of AMLs. Adult cases of t(9;11) abnormality are uncommon and account for less than 5% of all AMLs. AMLs with this genetic abnormality are frequently detected by conventional cytogenetics ( Fig. 22.36 ). The karyotypic abnormality represents a balanced translocation involving the MLLT3 (AF9) gene on chromosome 9 and the KMT2A gene at 11q23. FISH does not increase the detection yield when good-quality metaphases are obtained but is useful when chromosome preparations are suboptimal or complex rearrangements are present ( Fig. 22.37 ).
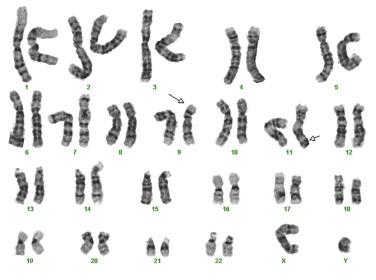
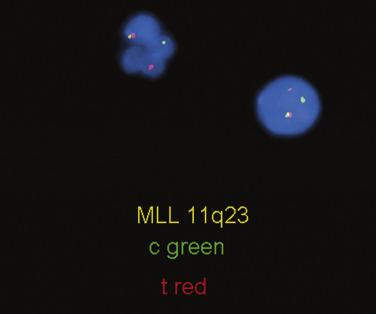
Similar to acute leukemias with KMT2A rearrangements, most AMLs with a t(9;11) abnormality show evidence of monocytic or myelomonocytic differentiation. Monoblasts show a round nucleus; prominent, often single nucleoli; and abundant basophilic cytoplasm with fine, evenly distributed azurophilic granules and occasional fine vacuoles (see Fig. 22.20 ). The cytoplasm may show blebbing or pseudopod formation. Promonocytes have irregular or lobulated nuclei, with delicate folds or creases but fine chromatin, inconspicuous nucleoli, and abundant blue-gray, finely granulated, often vacuolated cytoplasm. Both cell types typically show strong reactivity to nonspecific esterase. Immunophenotypic findings also correlate with monocytic differentiation and include high levels of expression of HLA-DR, CD33, and CD4, with low expression of CD34, CD13, and CD14. Importantly, survival in adult patients with the t(9;11) abnormality is superior to that in AMLs with other KMT2A translocations and intermediate to all other forms of AML. The t(9;11) abnormality is often accompanied by additional cytogenetic abnormalities (commonly including trisomy 8), but these additional abnormalities do not appear to affect survival.
Other genetic abnormalities that have been recognized by the WHO as warranting inclusion into the AML with recurrent genetic abnormalities category include t(6;9)(p23;q34.1), inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2), and t(1;22)(p13.3;q13.3). These three AML subtypes are very rare, accounting for less than 3% as a group. AML with t(6;9)(p23;q34.1): DEK-NUP214 abnormality typically results in AML with monocytic differentiation associated with basophilia, multilineage dysplasia, and a high incidence of FLT3 internal tandem duplications. Patients with t(6;9) have poor outcomes. AML with t(1;22)(p13.3;q13.3): RBM15-MKL1 results in a megakaryoblastic leukemia with features similar to those seen in the acute megakaryoblastic leukemia of AML, NOS. The inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2) abnormality can show features of any morphologic subtype of AML (excluding APL).
In addition to the balanced translocations and inversions that are commonly associated with AML, it has been recognized that a series of gene mutations that are specific to AML can affect the clinical outcome of the cases in which they are encountered. These specific genetic alterations include abnormalities in NPM1 and CEBPA that recently have been assigned the status of true entities. It is important to note that improved prognosis is associated only with biallelic CEBPA mutation, which is reflected in the definition of this entity as AML with biallelic CEBPA mutation. It is important to mention that AML with mutated NPM1 represents the largest category in AML with recurrent genetic abnormalities group, with NPM1 mutations being identified in approximately 30% of cases of adult AML. NPM1 mutations typically occur in de novo AML and are associated with a normal karyotype and a relatively favorable prognosis. Recent studies have shown that NPM1 mutations typically do not occur in isolation, with the majority of cases harboring at least one co-mutation, such as FLT3 (including both FLT3 -ITD and FLT3 -TKD mutations) and in genes regulating DNA methylation ( DNMT3A, TET2, IDH1, and IDH2 ). In addition, the revised WHO classification has included two provisional AML categories: AML with BCR-ABL1 and AML with mutated RUNX1 . Distinction between AML with BCR-ABL1 and CML blast crisis relies on adequate clinical information; preliminary data suggest that deletion of TCR , IGH , IKZF1, and/or CDKN2A may support the de novo AML with BCR-ABL1 . Other mutations described in AML include FLT3, KIT, WT1, MLL, NRAS, and KRAS . These abnormalities are typically, but not exclusively, noted in AMLs with normal karyotypes and therefore are detected by PCR and sequencing strategies. Identification and separation of these genetic subtypes of AML, as suggested by the WHO classification, will help researchers better understand the clinical consequences of these mutations.
AML with myelodysplasia-related changes (AML-MRC), most commonly seen in the elderly, is defined by the presence of more than 20% blasts in the blood or bone marrow together with dysplasia in more than 50% of the cells in at least two myeloid lineages. This diagnosis can be made in patients with de novo AML showing myelodysplastic morphologic features, in patients with a known history of MDS, or MDS/MPN in the presence of an MDS-related cytogenetic abnormality (with the exception of del(9q), which has been shown to be associated with NPM1 and biallelic CEPBA mutations). Detection of these two mutations excludes this AML subtype even in the presence of dysplasia. Importantly, this diagnosis must be excluded if there is a prior history of cytotoxic therapy for an unrelated disease (see later discussion, “ Therapy-Related Myeloid Neoplasms ”) or in the presence of a recurrent cytogenetic abnormality, as described earlier (AML with recurrent genetic abnormalities). The presence of multilineage dysplasia in either the de novo or prior MDS setting does correlate with adverse outcome.
Patients are almost exclusively adults and typically have severe pancytopenia. Bone marrow cellularity is often decreased. Blast counts tend to be between 20% and 50%. Cases with lower blast counts very often show clinical findings of somewhat less aggressive disease. Dysplasia of the type seen in typical MDS is noted in maturing forms from all lineages. CD34 staining is useful in identifying the blasts, which coexpress the myeloid antigens CD13 or CD33; however, in cases with antecedent MDS, CD34 staining frequently identifies only a subpopulation of blasts in these cases, and reliance on CD34 for blast quantification may underestimate the number of blasts in many cases. Aberrant expression of CD7 or CD56 is often noted. Immunopositivity for p53 is quite frequent and may also predict a poor prognosis.
The presence of morphologic dysplasia alone is not clearly associated with poor clinical prognosis in the setting of a marrow with greater than 20% blasts and therefore, when present, should be considered a potential indicator of high-risk cytogenetic abnormalities. High-risk chromosomal abnormalities identical to those seen in MDS are common and, if identified, are sufficient to diagnose AML-MRC (with the exception of del(9q), as discussed earlier). These defining abnormalities include the unbalanced abnormalities monosomy 5 and 7, del(5q), del(7q), i(17q)/t(17p), monosomy 13 or del (13q), del(11q), del(12p)/t(12p), or idic(X)(q13), or the balanced abnormalities t(11;16)(q23.3;p13.3), t(3;21)(q26.2;q22.1), t(1;3)(p36.3;q21.2), t(2;11)(p21;q23.3), t(5;12)(q33;p13.2), t(5;7)(q32;q11.2), t(5;17)(q32;p13.2), t(5;10)(q32;q21.2), or t(3;5)(q25.3;q35.1). The monosomal and/or complex karyotype is very often associated with TP53 mutation.
Cases of AML with recurrent cytogenetic abnormalities may show dysplastic morphology. Erythroid leukemia or MDS with significant erythroid hyperplasia is also frequently in the differential diagnosis. When blasts are increased, but account for less than 20% of the nucleated elements, and dysplasia is present, MDS with excess blasts is the appropriate diagnosis.
Therapy-related forms of AML, MDS, or MDS/MPNs result directly from prior exposure to cytotoxic agents and radiation therapy. They are important to recognize because of their poor prognosis and to document the adverse effects of these therapies. The offending agents include alkylating drugs (e.g., melphalan, cyclophosphamide, nitrogen mustard, chlormabucil, busulfan, cisplatin, carboplatin), γ-radiation (especially large field involving bone marrow), DNA topoisomerase II inhibitors (e.g., epipodophyllotoxins, etoposide, teniposide), and antimetabolites (e.g., thiopurines, fludarabine) or antitubulin agents (e.g., vincristine, vinblastine, paclitaxel, docetaxel). Classification can be based on the appropriate corresponding WHO category, including associated genetic abnormality, with the addition of the qualifying term therapy-related to denote this etiologic link.
Alkylating agent–related and radiation-related AML usually occur more than 5 years after therapy, with the risk being related to the patient's age and cumulative dose of therapy. In addition, most commonly, an antecedent dysplastic phase occurs with associated cytopenias and marrow failure (often taking the form of MDS-MLD or MDS-EB-1 or MDS-EB-2). Myeloblastic or myelomonocytic AMLs are most common. Some cases take the appearance of acute panmyelosis with myelofibrosis (see later discussion). Although no genetic abnormalities specific for alkylating or radiation therapy have been described, karyotypic analysis commonly reveals partial or complete deletion of chromosome 5 or 7. Many patients treated with high amounts of alkylating agents also present with 11q23 and 21q22 abnormalities.
AML after treatment with DNA topoisomerase II–inhibitor therapy shows a short interval to disease onset (1–3 years) and absence of an antecedent MDS. Acute lymphoblastic leukemias (ALLs) associated with t(4;11) are also observed. AML related to DNA topoisomerase II inhibitor occurs less frequently than other types of treatment-related AML, usually shows monocytic or myelomonocytic differentiation, and is very often associated with 11q23 abnormalities. Numerous translocation partners for 11q23 have been identified; t(6;11)(q27;q23), t(9;11)(p22;q32), t(10;11)(p12;q23), and t(11;19)(q23;p13.1-p13.3) abnormalities are most frequently seen. Other abnormalities also have been described in this setting, including t(8;21), inv(16), and t(15;17). TP53 mutation is seen in up to 50% of therapy-related AML/MDS, a much higher frequency than in de novo cases, and is associated with poor outcome. Although the overall survival in therapy-related MDS/AML is worse than in de novo AML/MDS, it largely depends on the associated genetic alterations and the presence of TP53 mutation.
Cases that do not fit into the preceding categories are placed into the various WHO subgroups that largely correspond to former French-American-British (FAB) categories ( Figs. 22.38 through 22.52 ; see also Table 22.6 ). In contrast to the FAB classification, several distinct entities are also included in the not otherwise specified group, including acute basophilic leukemia—excluding all cases with t(6;9)—abnormality and acute panmyelosis with myelofibrosis (see Table 22.6 ). The latter entity is characterized by the expansion of all myeloid lineages, marked marrow fibrosis, and increased blasts. It is unclear whether this is a distinct entity or a heterogeneous group that includes primary or secondary hyperfibrotic MDS, rare primary AMLs with fibrosis, or even unusual toxic myelopathies. As already mentioned, acute erythroid leukemia type has been revised to include only cases of pure erythroid leukemia ( Fig. 22.53 ; also see Table 22.6 ). Cases of acute megakaryoblastic leukemia that develop in infants with Down syndrome are included as a separate entity in the WHO classification (i.e., myeloid proliferations of Down syndrome) and should also be excluded from the AML, NOS category. This megakaryocytic proliferation, which could be transient, is characterized by GATA1 mutation and mutations in the JAK-STAT pathway (see Box 22.3 ).
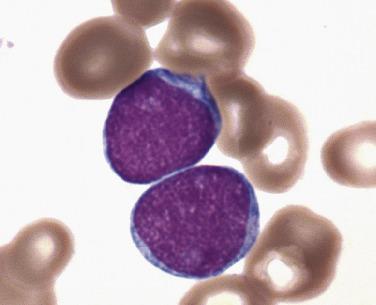
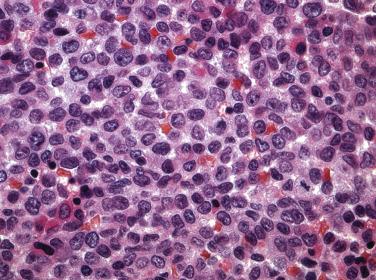
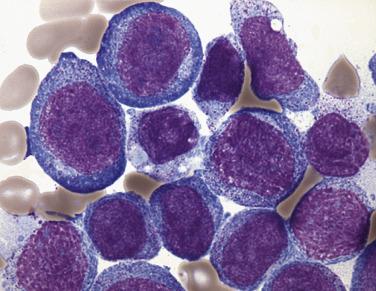
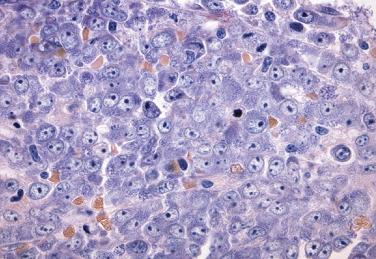
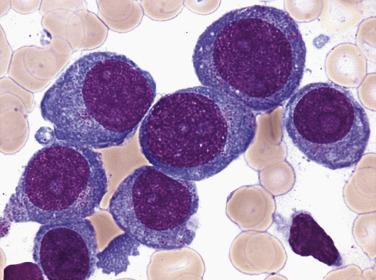
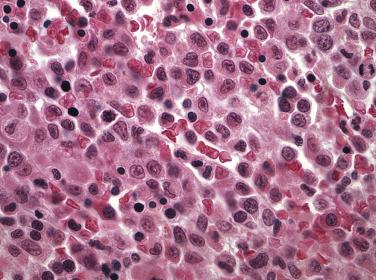
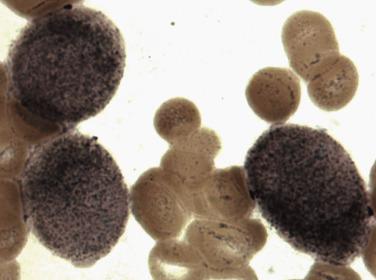
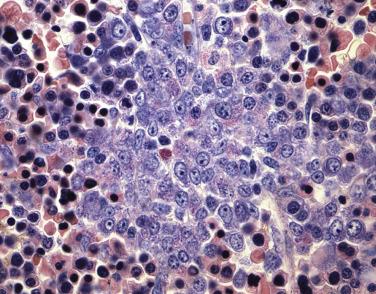
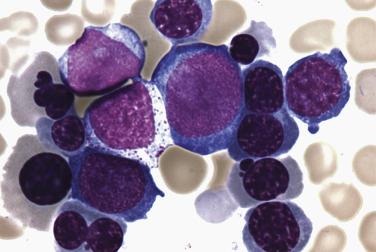
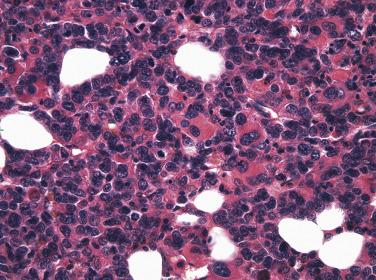
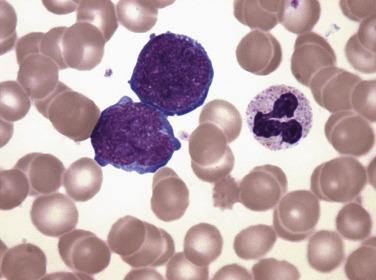
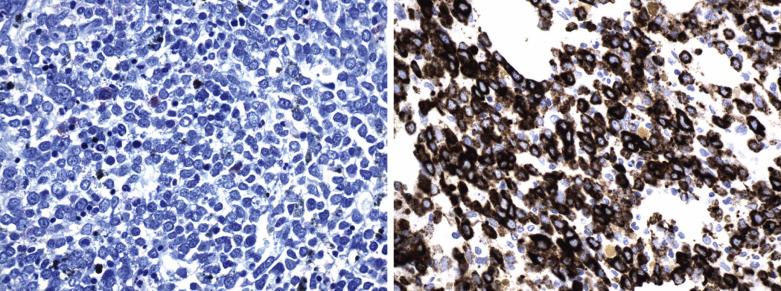
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare clonal myeloid disorder with high frequency of cutaneous and marrow involvement that represents a separate entity and is no longer included among other AML subtypes. BPDCN originates from plasmacytoid dendritic cell precursors. Because of the significant clinical and genetic overlap with AML, immunophenotyping is essential in establishing the diagnosis of BPDCN. BPDCN is characterized by the expression of plasmacytoid dendritic markers BDCA-2/CD303, IL-3Ra/CD123, CD2AP, TCL1, BCL11A, and SPIB with aberrant CD4 and CD56 in the absence of lineage-specific markers for myeloid, T-cell, B-cell, or NK-cell lineages (see Table 22.6 ). It has been proposed that BPDCN may be confidently diagnosed when the neoplastic cells express four of the five principal markers (CD4, CD56, CD123, TCL1, and CD303). The prognosis of this disease remains poor. However, recently a new targeted therapy with a CD123-directed recombinant fusion protein composed of IL-3 conjugated to modified diphtheria toxin via amide linkage (tagraxofusp) has been successfully used in patients with BPDCN ( Fig. 22.54 ; also see Box 22.3 and Table 22.6 ). While genetic abnormalities are present in 60% of BPDCN, there are no cytogenetic changes that are typical or diagnostic and represent abnormalities seen in myeloid and lymphoid malignancies. In BPDCN, cytogenetic abnormalities predominantly involve genomic losses rather than gene-specific rearrangements; the key recurrent chromosomal abnormalities include 5q, 12p, 17p, 13q, 6q, 15q, and 9 (monosomy). The genome wide array-based comparative genomic hybridization analysis identified commonly deleted chromosome regions that represent tumor suppressor genes involved in cell cycle regulation, such as CDKN2A/CDKN2B, RB1, CDKN1B, LATS2, and IKZF1 . Recently, monoallelic deletion of the NR3C1 locus at 5q31 has been described as a recurrent abnormality in 28% of BPDCN patients, and this finding is associated with a poor clinical outcome.
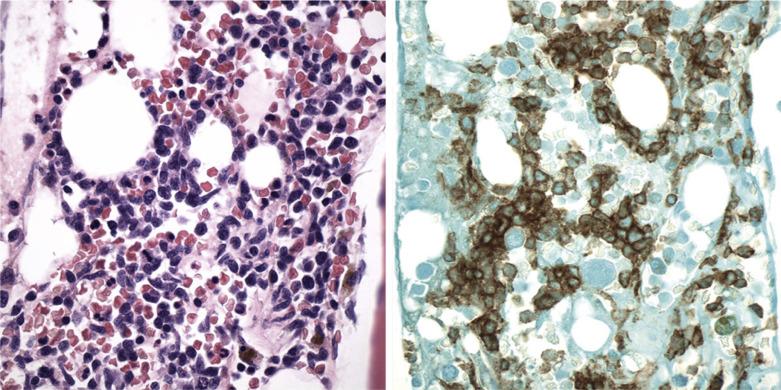
B-lymphoblastic leukemia/lymphoma is a clonal neoplasm of immature lymphoid cells (B lymphoblasts) that are committed to the B-cell lineage. In most cases the blood and bone marrow are primarily involved (90% of cases), and the term B lymphoblastic leukemia (B-ALL) is preferred. Although no consensus has been reached on the lower limit of marrow blasts to make a diagnosis of B-ALL, in general more than 20% lymphoblasts should be present to consider this entity. The remaining cases present as an extramedullary tumor mass, with or without bone marrow involvement (<25% of marrow cellularity, if present) and are designated B-lymphoblastic lymphoma (B-LBL) . It is unknown whether there is a distinct biologic basis for either presentation, and because B-ALL and B-LBL are morphologically and immunophenotypically indistinguishable, use of either term is somewhat arbitrary. Within the WHO classification, this category does not separate precursor lymphoid tumors that correspond to the various stages of early B-cell maturation (i.e., early precursor B-ALL, common B-ALL, and precursor B-ALL). However, more mature B-cell neoplasms with surface immunoglobulin light-chain expression and Burkitt morphology (formerly the ALL FAB L3 subtype) are no longer placed within this category.
The majority of cases of ALL are of precursor B-cell phenotype (80%–85%), with the remainder being of T-cell derivation (see later discussion). Approximately 3000 cases of ALL are diagnosed in the United States each year. The age-adjusted incidence rate for all forms of ALL is 1.5 cases per 100,000 individuals. This incidence varies greatly with age, being highest in the pediatric age group (2.7 cases/100,000 individuals from birth to 19 years old), followed by a second peak in the elderly (1.4 cases/100,000 individuals 65 years and older). Overall, ALL is primarily a pediatric disease, with three-fourths of all cases occurring in patients under the age of 6. There is a slight predilection in males and an increased incidence among whites and Hispanics.
As is the case with AML, most symptoms relate to the consequences of progressive replacement of normal marrow elements by blasts. This includes nonspecific infections from neutropenia, fatigue, and lethargy resulting from anemia and mucocutaneous bleeding secondary to thrombocytopenia. Leukocyte counts are often in the normal range but may be decreased or elevated. Circulating blast forms and mature lymphocytes comprise the majority of the peripheral blood cellularity; however, it is not uncommon for patients to present without peripheral blasts (i.e., an aleukemic presentation). Bone and joint pain are frequently noted, and generalized adenopathy or hepatosplenomegaly can be seen. Overt testicular involvement is found in 2% of cases at the time of diagnosis. Central nervous system (CNS) involvement is common in B-ALL, necessitating aggressive CNS prophylactic treatment and frequent monitoring of cerebrospinal fluid cytology. Relapse often occurs in extramedullary tissues (CNS, testis, ovary, or kidney) before the bone marrow. As mentioned earlier, prior treatment with etoposide therapy predisposes individuals to B-ALL with KMT2A (MLL) abnormalities.
B-LBL, by definition, involves extramedullary sites, most often the skin, bone, and soft tissues and less commonly the lymph nodes (see Chapter 21 ). It is uncommon, constituting only 10% to 15% of all cases of LBL. Unlike T-lymphoblastic lymphoma (T-LBL) (see later discussion), B-LBL rarely presents as a mediastinal mass. If marrow involvement is present in B-LBL, it is less than 25% of the cellularity (by definition) and more typically less than 5%.
Peripheral blood and aspirate smear morphology is essential for the proper identification of lymphoid blast forms. Marrow may be difficult to aspirate when packed with leukemic cells, occasionally resulting in an inadequate smear. In these cases, core biopsy examination is required. B lymphoblasts have a somewhat varied appearance, ranging from a homogeneous population of small cells with a round to slightly irregular nucleus, condensed chromatin, and inconspicuous nucleoli, to a more heterogeneous population of larger cells with irregular, clefted, or indented nuclei; variably distributed chromatin; and one or more distinct nucleoli ( Fig. 22.55 ). Cytoplasm is scant to moderate and can be slightly basophilic to more deeply basophilic as cell size increases. Small vacuoles are variably seen, and a few coarse granules may be observed. Occasionally, blasts characterized by the presence of an asymmetric cytoplasmic projection called a uropod are observed, producing so-called hand mirror cells.
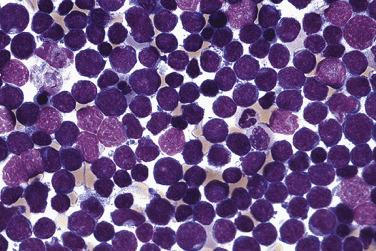
Core biopsy samples or tissue sections are typically hypercellular and demonstrate numerous tightly packed lymphoblasts with round to irregular, occasionally convoluted nuclei, with cleared-out or dispersed chromatin, small nucleoli, and nearly undetectable cytoplasm ( Fig. 22.56 ). In bone marrow cores, if residual hematopoietic cells are apparent, their morphology is normal. Areas of necrosis are common in B-ALL, and expansile tumor masses can cause lytic bone lesions. Mitotic figures are usually frequent, and tingible-body macrophages (imparting a starry-sky appearance) can sometimes be seen in tissues involved by B-LBL.
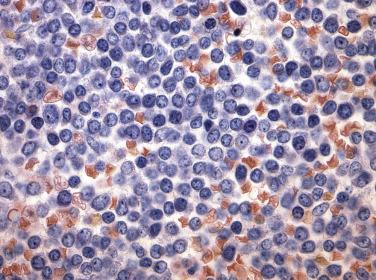
The morphologic appearance of pre-B lymphoblasts may resemble that of myeloblasts seen in minimally differentiated AML. In addition, in histologic sections, small B lymphoblasts are identical to pre-T lymphoblasts and can be difficult to distinguish from small mature lymphocytes. For these reasons, cytochemical and immunophenotypic characterization are essential for diagnosis and classification. PAS stains cytoplasmic glycogen within lymphoblasts in a perinuclear, coarse, blocklike pattern ( Fig. 22.57 ; also see Table 22.4 ). Lymphoblasts are negative for myeloperoxidase and only stain faintly for Sudan black B. A multifocal, punctuate, or Golgi pattern of reactivity for NSE can be seen, but it is usually much weaker than that observed in monoblasts and is variably inhibited by treatment with sodium fluoride.
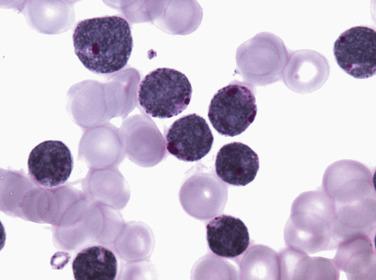
Flow cytometric analysis is the most accurate way to immunophenotype the leukemic blasts and distinguish them from T lymphoblasts and myeloid blasts. Immunoreactivity for B-lineage–specific antigens CD19, CD79a, and cytoplasmic CD22 and coexpression of TdT and HLA-DR are almost invariably present in B-ALL/LBL. Surface immunoglobulin light-chain expression is characteristically negative. Several distinct patterns of antigen expression are noted within B-ALL/LBL, correlating with the degree of differentiation of the neoplastic cells (early precursor, common, or precursor B-ALL) ( Table 22.8 ). In addition, certain common cytogenetic observations, including t(4;11) involving the KMT2A ( MLL) gene, t(1;19), t(9;22), and t(12;21) are associated with characteristic immunophenotypic patterns. KMT2A gene rearrangement and t(9;22) cases are often associated with the presence of myeloid antigen expression, such as CD15, CD13, or CD33. Cases with the t(1;19) abnormality show a classic precursor B-cell immunophenotype, which is present in 25% of cases with this immunophenotype. The t(12;21) abnormality characteristically shows high levels of expression of CD10 and HLA-DR. Flow cytometric analysis can also be used to monitor minimal residual disease and to distinguish normal marrow B-cell precursors (hematogones) from leukemic cells (see later discussion). TdT immunostaining on paraffin sections can be helpful in confirming the presence of leukemic infiltrates within organs and soft tissues.
| Antigen | Early Precursor | Common | Precursor | t(4;11) | t(1;19) | t(12;21) |
|---|---|---|---|---|---|---|
| TdT | + | + | + | + | + | + |
| HLA-DR | + | + | + | + | + | + |
| CD10 | − | + | + | − | + | + |
| CD19 | + | + | + | + | + | + |
| CD20 | − | − | ± | − | −/+ | − |
| cCD22 | + | + | + | ± | + | + |
| cCD79a | + | + | + | + | + | + |
| cµ-Ig | − | − | + | − | + | + |
| sIg | − | − | − | − | − | − |
| CD34 | + | + | − | + | − | |
| CD15 | − | – | − | + | − | − |
| CD24 | + | + | + | − | + | + |
Specific genetic abnormalities are found in the blast cells of 60% to 75% of cases. Karyotyping and other molecular genetic techniques are sometimes diagnostically helpful and are prognostically informative ( Fig. 22.58 ). The most common genetic abnormalities in B-ALL are listed in Table 22.9 . These molecular genetic findings impart distinctive clinical and immunophenotypic properties to the leukemias and also may have important prognostic and biologic implications for the disease. Therefore these entities are recognized as B-lymphoblastic leukemia or lymphoma with recurrent genetic abnormalities in the current WHO classification, and some prognostic features associated with these genetic subtypes are listed in Table 22.9 . The 2016 WHO revision has recognized two provisional entities: B-ALL with intrachromosomal amplification of chromosome 21 (iAMP21) and B-ALL with translocations involving tyrosine kinase or cytokine receptors ( BCR-ABL1– like). These two provisional entities, as well as B-ALL with t(12;21), cannot be detected by conventional karyotype and require FISH, PCR, and/or NGS analyses. Five or more copies of RUNX1 gene, as detected by FISH, characterizes B-ALL with iAMP21. BCR-ABL1– like B-ALL shows translocation with over 30 different partner tyrosine kinase genes, requiring NGS platforms for the diagnosis. In addition, the classification of hypodiploid B-ALL highlights the unique association with TP53 mutation that is often constitutional.

| Chromosomal Abnormality | Involved Genes | Frequency (%) | Prognostic Implications |
|---|---|---|---|
| Hyperdiploidy | >50 but <65 chromosomes | 25 children; rare adults | Favorable |
| t(12;21)(p13.2;q22.1), | ETV6 , RUNX1 | 22 children; rare adults | Favorable |
| KMT2A ( MLL ) rearrangements | AF4 , ENL , AF9 , KMT2A (MLL) | Uncommon children over age 1; 10 adults | Unfavorable |
| t(1;19)(q23;p13.3) | TCF3, PBX1 | 5 slightly more common in children | No significance |
| t(9;22)(q34.1;q11.2) | ABL1 , BCR | 3 children; 25 adults | Unfavorable |
| Hypodiploidy | <45 chromosomes | 1 child; rare adults | Unfavorable |
| t(5;14)(q31.1;q32.3) | IL3 , IGH | <1 overall | No significance |
| BCR - ABL1– like | CRLF2, EPOR, JAK, IKZF1, CDKN2A/B | 13 children, 21 adults | Unfavorable |
| iAMP21 | Multiple gains of the long arm of 21 | 5 children; rare adults | Unfavorable |
In adult B-ALL, t(9;22)(q34;q11) is present in 25% of cases, and rearrangements of KMT2A (MLL) in an additional 10%, whereas abnormalities typical of pediatric B-ALL (hyperdiploidy, t[1;19], or t[12;21]) are seen in only a small fraction of cases. In addition to cytogenetic findings, age, leukocyte count, sex, and initial response to therapy, including the detection of minimal residual disease after induction chemotherapy, play a role in risk stratification in children.
In general, B-ALL/LBL is a poor prognostic disease in adults compared with children, with cure rates using modern treatments approaching 80% for children and only 40% for adults. This likely reflects, in part, the relatively frequent occurrence of poor-risk and the infrequent presence of good-risk cytogenetic abnormalities in the latter age group. As our understanding of B-ALL biology increases, the combined analysis of flow cytometry, cytogenetic, FISH, molecular mutation, and deletion studies is required not only for prognostic but also diagnostic stratification and for the selection of targeted treatment strategies. For example, the addition of tyrosine kinase inhibitors (TKI) to treatment in ALL with t(9;22) and the subset of BCR-ABL –like B-ALL has resulted in a significantly higher rate of remission and overall survival.
In addition to the obvious differential diagnosis of AML and T-ALL, B-ALL can occasionally be confused with the many small round blue cell tumors of childhood that present with marrow involvement, particularly when there is no clinically obvious mass. Examination of the aspirate smear material for tumor cell clumping, characteristic of nonhematopoietic tumors, and immunophenotyping of aspirate and core biopsy material is usually sufficient to arrive at the correct diagnosis. Since certain B-ALL with recurrent genetic abnormalities are associated with aberrant myeloid marker expression, complete genetic and immunophenotypic analyses are required to exclude mixed phenotype acute leukemia, such as B/myeloid leukemia (see Table 22.7 ). A subset of Ph-like B-ALL shows rearrangements involving PDGFRB or JAK2 that demonstrate genetic overlap with the group of myeloid and lymphoid neoplasms with eosinophilia rearrangements of PDGFRB or PCM1-JAK2 . However, in the presence of an antecedent or concurrent myeloproiferative neoplasm, a diagnosis of B lymphoblastic crisis of a myeloproliferative neoplasm with a specific rearrangement should be rendered. The atypical lymphocytes that occur in the peripheral blood during viral infections can be distinguished from leukemic blasts by their mature chromatin pattern, low nucleus to cytoplasm ratio, and prominent nucleoli. Finally, in infants and young children, lymphocytes can constitute up to 50% of the marrow cellularity. A subpopulation of these lymphocytes in young children shows a spectrum of immature lymphoid cell morphology, with small to frequently larger round nuclei showing fine, immature-appearing chromatin and scant to small amounts of basophilic cytoplasm ( Fig. 22.59 ). These functional B-lymphoid precursors (hematogones) can be difficult to distinguish from malignant lymphoblasts. This is particularly true after chemotherapy, even in adult patients, when hematogone numbers can be greatly increased. Various findings, particularly the presence of a heterogeneous population of pre-B cells showing a full range of B-cell differentiation (including mature B cells), heterogeneous adhesion molecule expression, and nonclustered bone marrow architectural distribution can be helpful in recognizing normal hematogones. Additionally, cytogenetic abnormalities and aberrant immunophenotypic markers are not present in reactive immature lymphoid proliferations.
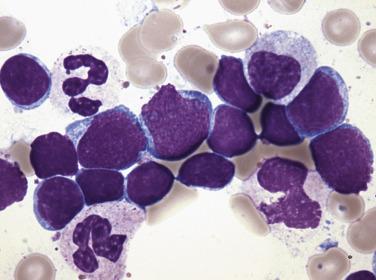
In lymphoid tissues, B-LBL must be distinguished from T-LBL, myeloid sarcoma, Burkitt lymphoma/leukemia ( Fig. 22.60 ), and the blastoid variant of mantle cell lymphoma.
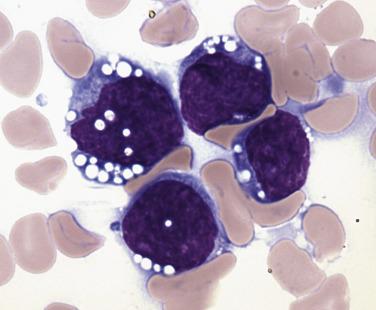
T-lymphoblastic leukemia/lymphoma is the T-lineage counterpart of B-ALL and LBL, being a neoplasm of clonal immature T-cell progenitors (T lymphoblasts). Terminology and definitions identical to those for B-ALL or B-LBL apply to T-ALL and T-LBL.
T-ALL constitutes 15% of childhood cases of acute lymphoblastic leukemia and 25% of adult cases. By definition, blood and bone marrow are the principal sites of involvement. T-ALL has an older average median age than B-ALL and affects predominantly males. It is associated with high white blood cell counts, the presence of an anterior mediastinal mass in the majority of cases, and frequent involvement of the CNS.
The most common presentation of a T-cell lymphoblastic neoplasm is as T-LBL, comprising approximately 85% of all forms of LBL. T-LBL also most commonly affects adolescent males and typically presents as an anterior mediastinal (thymic) mass (~50% of cases) with less than 25% bone marrow involvement (by definition). Peripheral lymph nodes, liver, spleen, skin, gonads, and the CNS are other sites that may be involved.
Rarely, T-LBL could be a presentation of a distinct group of hematologic disorders characterized by T-LBL, peripheral, and/or bone marrow atypical eosinophilia and bone marrow involvement by a myeloproliferative neoplasm with or without leukemic involvement. These groups of disorders are often associated with PDGFRA or FGFR1 rearrangements, which are present in both lymphoid and myeloid neoplastic cells (see Figs. 22.70 to 22.72 ).
As previously mentioned, both T-ALL and T-LBL are associated with leukocytosis (often over 50 × 10 9 /L) and circulating blasts. The marrow aspirate and biopsy sample are hypercellular, but more often show residual normal hematopoiesis than does B-ALL. Morphologic distinctions among T-ALL, T-LBL, and B-ALL/LBL are subtle at best and unreliable (see earlier discussion). Compared with B lymphoblasts, T lymphoblasts may show greater nuclear convolution and significant nuclear hyperchromasia ( Fig. 22.61 ). The mitotic rate is frequently very high in the bone marrow biopsies or tissue sections.
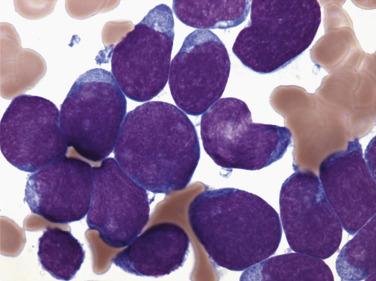
The leukemic cells in more than 90% of cases of T-ALL exhibit strong, focal paranuclear acid phosphatase activity in the cytoplasm. No staining for PAS, myeloperoxidase, specific esterase, or NSE is seen. T lymphoblasts commonly express markers that correspond to the various stages of T-cell differentiation. Nuclear TdT is always present and is most useful in confirming the immature nature of the infiltrate. Other markers of the precursor nature of T lymphoblasts include CD34, CD99, and CD1a. T-cell and thymocyte markers CD2, CD3 (cytoplasmic), CD4, CD5, CD7, and CD8 are variably present. CD4/CD8 double-positive lymphoblasts are frequently noted, and CD10 expression can occasionally be seen. Interestingly, T-LBL has a greater likelihood of expressing a more mature thymocyte immunophenotype than does T-ALL. Markers of myeloid differentiation (CD13 and CD33) are present in up to one-third of adult and one-fourth of childhood cases of ALL, and c-KIT (CD117) expression can also be seen in association with activating mutations of FLT3 . This feature has no clinical implications but can be useful in immunologic monitoring of minimal residual disease. The 2016 WHO revision has recognized a new provisional entity, early T-cell precursor (ETP) ALL. This ALL subtype has a unique immunophenotype with the blasts always positive for CD7, coexpression of one or more myeloid stem cell marker, such as CD34, CD117, HLA-DR, CD13, CD33, CD11b, or CD65, and negative for CD1a and CD8. The blasts are frequently positive for CD2 and cytoplasmic CD3 with variable CD4 expression, but these are not the markers that define this entity. ETP ALL often demonstrates mutations in many myeloid-associated genes and should be distinguished from mixed phenotype acute leukemia, such as T/myeloid (see Table 22.7 ).
Three major classes of genetic abnormalities have been shown to be associated with T-ALL/LBL, occurring with nearly equal frequency. These involve (1) translocations of multiple genes with either the β T-cell receptor locus at 7q35, the α and δ T-cell receptor locus at 14q11.2, or the γ locus at 7p14-15 (with HOX11 or HOX11L2 translocations occurring in up to 30%–40% of cases); (2) a deletion in 9p, resulting in the loss of the CDKN2A tumor suppressor (30% of cases); or (3) a variety of activating mutations in the NOTCH1 gene (50% of cases). The mutations in NOTCH1 provide a gain of function for the gene, a protein that is critical for early T-cell development, pointing to a central role for NOTCH1 dysregulation in T-ALL. NOTCH1 mutations are rare in ETP ALL, which typically demonstrates high frequency of the myeloid-associated gene mutations, such as FLT3, NRAS/KRAS, DNMT3A, IDH1, and IDH2 . In contrast to the risk stratification observed in B-ALL/LBL that correlates with various cytogenetic abnormalities, to date there is less well-documented clinical impact regarding genetic changes in T-ALL/LBL.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here