Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There are three main categories of primary ovarian tumors. These are (1) epithelial tumors, which originate from the surface epithelium of the ovary, epithelial inclusions, or endometriosis, and some may actually be of tubal origin; (2) sex cord–stromal tumors, which arise from the ovarian stroma, from sex cord derivatives, or both; and (3) germ cell tumors, which mainly originate from germ cells. In addition, tumors that are not specific to the ovary, such as soft tissue tumors and lymphomas, can arise in the ovary, and tumors from extraovarian primary sites frequently metastasize to the ovaries.
Of the three main groups, epithelial tumors are the most common, comprising about 60% of all ovarian tumors ( Table 13A.1 ). Serous and mucinous cystadenomas are the most common epithelial tumors, and together account for about 30% of ovarian tumors. All types of carcinoma occur with some frequency, but only serous and mucinous borderline tumors are common. Sex cord–stromal tumors of the fibroma-thecoma group account for about 9% of ovarian tumors. The most common malignant sex cord–stromal tumor, the granulosa cell tumor, accounts for only about 1% of ovarian tumors. Other sex cord–stromal tumors are rare. The single most common ovarian tumor is a germ cell tumor, the benign cystic teratoma, which comprises 32% of ovarian tumors. All other types of germ cell tumors, including all of the malignant germ cell tumors, are rare.
| Tumor | % of Ovarian Tumors |
|---|---|
| Benign cystic teratoma | 32 |
| Serous cystadenoma | 16 |
| Mucinous cystadenoma | 14 |
| Serous carcinoma | 9 |
| Fibroma–thecoma | 9 |
| Borderline serous tumor | 4 |
| Endometrioid carcinoma | 3 |
| Borderline mucinous tumor | 1 |
| Clear cell carcinoma | 1 |
| Mucinous carcinoma | 1 |
Epithelial tumors comprise about 60% of all ovarian neoplasms and more than 90% of malignant tumors. Most can be classified according to their predominant pattern of differentiation as serous, mucinous, endometrioid, clear cell, Brenner/transitional cell, or undifferentiated ( Box 13A.1 ). Mixtures of cell types occur. Minor foci of cell types other than the predominant one can be ignored, but when significant amounts (>10%) of several cell types are present, the tumor is best classified as a mixed epithelial tumor. Rare epithelial tumors cannot be assigned to a specific category and are designated as unclassified.
Serous
Mucinous
Endometrioid
Clear cell
Brenner
Seromucinous (endocervical-like mucinous)
Undifferentiated
Rare types
Epithelial tumors are categorized as benign, malignant, or intermediate, based on their pathologic features ( Box 13A.2 ). A benign epithelial tumor is designated as a cystadenoma, a surface papilloma, a cystadenofibroma, or an adenofibroma, depending on its location in the ovary, the extent of cyst formation, and the amount of stroma present. Malignant epithelial tumors are mainly adenocarcinomas, although transitional cell carcinoma and, rarely, squamous cell carcinoma also occur in the ovary.
Controversy exists over the appropriate nomenclature for tumors in the intermediate group. Such tumors have been called borderline tumors or atypically proliferating epithelial tumors. In this chapter, intermediate epithelial tumors are called borderline tumors, since that name is the most widely used, although either terminology is acceptable under the current World Health Organization (WHO) classification. This category encompasses a spectrum of tumors ranging from tumors with a mild degree of proliferation and atypia equivalent to that seen in simple hyperplasia to tumors showing marked proliferation and/or severe cytologic atypia equivalent to in situ carcinoma but lacking invasion or confluent growth. Borderline epithelial tumors in which there is marked cytologic atypia are designated as borderline tumors with intraepithelial carcinoma. Pathologists should render their diagnoses using terminology that is clearly understood in their community. Other widely used diagnostic terms can be cited as appropriate to ensure that anyone who reads the pathology report will clearly understand the diagnosis.
With some exceptions, the clinical presentation, treatment, and results of treatment are similar for all types of epithelial tumors within a given category. An overview is given here, with more specific information, when available, discussed in the sections on the various tumor types.
Epithelial tumors occur mainly in adults. They are uncommon in children and teenagers. Benign and borderline tumors occur at all ages, but are often detected in premenopausal women. Carcinomas occur chiefly in perimenopausal and postmenopausal women. About 10% of patients with ovarian cancer have an inherited predisposition to develop the disease (BRCA mutation or Lynch syndrome). More than 70% of women with ovarian cancer have extensive extraovarian tumor spread at the time of diagnosis. This is partly because the symptoms caused by epithelial tumors are vague and nonspecific and do not prompt early diagnosis. The most common symptoms are pelvic discomfort or pain, a sensation of abdominal fullness or pressure, gastrointestinal disturbances, urinary frequency, and (occasionally) menstrual abnormalities. Torsion or rupture of the tumor can result in acute abdominal symptoms. Tumors greater than 15 cm in diameter are too large to fit in the pelvis; they rise into and distend the abdomen and may be palpated by the patient. Ascites is another cause of abdominal distention in women with ovarian tumors. Rarely caused by benign tumors, ascites is most suggestive of carcinoma. It interferes with gastrointestinal function, leading to nausea and vomiting. Ovarian enlargement of any degree, especially in a woman over 45 years of age, raises the question of ovarian cancer and calls for further evaluation. The identification of a solid or complex mass by sonography or some other imaging technique is particularly worrisome.
The CA125 monoclonal antibody blood test detects an antigenic site on MUC16, a high molecular weight glycoprotein of uncertain function. The test is most useful in women with serous carcinoma, although positive results are obtained in other histologic types as well. It is usually positive in women with advanced borderline and malignant epithelial tumors and in some women with localized disease. CA125 is sometimes used in a combined assay with HE4 (human epididymis protein 4) in an attempt to improve the sensitivity and specificity of testing for ovarian cancer. The response to therapy correlates with the serum CA125 level, with normalization of the CA125 titer in patients who experience a clinical remission and rising values if the tumor recurs. The CA125 test is not specific for ovarian cancer, as an increased serum concentration of the antigen is also detected in other cancers, and in association with benign conditions such as benign ovarian tumors and cysts, pregnancy, endometriosis, pelvic inflammatory disease, leiomyomas, liver disease, and some collagen-vascular disorders.
The treatment of ovarian tumors is primarily surgical. Benign tumors are cured by cystectomy or unilateral salpingo-oophorectomy. Borderline tumors, such as ovarian carcinomas, are surgically staged. The standard therapy is hysterectomy, bilateral salpingo-oophorectomy, and complete staging, but more conservative surgery such as unilateral salpingo-oophorectomy or even cystectomy is occasionally possible depending on the stage and histologic type. Current guidelines suggest that conservatively treated women with borderline tumors should be considered for completion surgery once childbearing is complete. Borderline tumors have a favorable prognosis, even in advanced stages, and only occasional patients, usually those with invasive implants, are treated with chemotherapy.
Invasive carcinoma of the ovary spreads by direct invasion into adjacent organs or via the peritoneal fluid to the omentum, the peritoneum, the serosal surfaces of the abdominal viscera, and the diaphragm. Tumor cells must be demonstrated outside of the ovary in patients with dense adhesions to warrant designating a cancer as stage II; patients with dense adhesions without demonstrable tumor cells have the same prognosis as those with stage I tumors. Lymph node metastases are common, and distant metastases are occasionally detected in the lungs and other sites. The clinical stage ( Table 13A.2 ) is the most important prognostic factor.
| STAGE | |
|---|---|
| I | Tumor confined to ovaries |
| IA | Tumor limited to one ovary, capsule intact, no tumor on surface, negative washings |
| IB | Tumor involves both ovaries, otherwise like IA |
| IC | Tumor limited to one or both ovaries |
| IC1 | Surgical spill |
| IC2 | Capsule rupture before surgery or tumor on ovarian surface |
| IC3 | Malignant cells in the ascites or peritoneal washings |
| II | Tumor involves one or both ovaries with pelvic extension (below the pelvic brim) or primary peritoneal cancer |
| IIA | Extension and/or implant on uterus and/or fallopian tube |
| IIB | Extension to other pelvic intraperitoneal tissues |
| III | Tumor involves one or both ovaries with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes |
| IIIA | Positive retroperitoneal lymph nodes and/or microscopic metastasis beyond the pelvis |
| IIIA1 | Positive retroperitoneal lymph nodes only |
| IIIA1(i) | Metastasis ≤10 mm |
| IIIA1(ii) | Metastasis >10 mm |
| IIIA2 | Microscopic, extrapelvic (above the brim) peritoneal involvement ± positive retroperitoneal lymph nodes |
| IIIB | Macroscopic, extrapelvic, peritoneal metastasis ≤2 cm ± positive retroperitoneal lymph nodes. Includes extension to capsule of liver/spleen |
| IIIC | Macroscopic, extrapelvic, peritoneal metastasis >2 cm ± positive retroperitoneal lymph nodes. Includes extension to capsule of liver/spleen |
| IV | Distant metastasis excluding peritoneal metastasis |
| IVA | Pleural effusion with positive cytology |
| IVB | Hepatic and/or splenic parenchymal metastasis, metastasis to extraabdominal organs (including inguinal lymph nodes and lymph nodes outside of the abdominal cavity) |
The treatment of ovarian cancer usually includes surgery and chemotherapy. The standard surgical treatment is hysterectomy, bilateral salpingo-oophorectomy, omentectomy, pelvic and paraaortic lymph node dissection, and staging biopsies and appendectomy if indicated. Gynecologic oncologists attempt to remove as much extraovarian tumor as possible (cytoreductive surgery) to enhance subsequent chemotherapy or radiotherapy. The prognosis is most favorable in early stage (I–II) disease. In this group, age, stage, tumor grade, and peritoneal cytology impact the prognosis. Tumor types of high-grade serous carcinoma and positive cytology are the most important unfavorable prognostic findings. A young woman with a stage IA or IC adenocarcinoma can be treated by unilateral salpingo-oophorectomy, omentectomy, and a thorough staging procedure if she wishes to retain her fertility. Some women with advanced ovarian cancer (stage IIIC–IV) are treated with chemotherapy prior to surgery to facilitate the subsequent surgical resection. Neoadjuvant chemotherapy can result in changes in the histologic appearance of a tumor such that it is difficult to accurately determine the histologic type, although immunohistochemical staining patterns are less altered and may be of some use in classifying treated tumors. Combination chemotherapy with carboplatin and paclitaxel is the usual type of first line chemotherapy administered to women with high-grade stage IA carcinomas and to those with extraovarian spread or positive peritoneal cytology. Optimally debulked patients with stage III carcinomas may be candidates for a treatment regimen in which intravenous administration of paclitaxel is followed by intraperitoneal chemotherapy with cisplatin and paclitaxel. This regimen is reported to result in increased survival compared to standard IV chemotherapy, but it is more toxic. A dose-dense protocol in which patients receive intensified therapy with paclitaxel along with intravenous or intraperitoneal carboplatin has been proposed and is being investigated. Chemotherapy results in a partial or complete clinical remission in about 85% of women with advanced cancer, but most patients relapse within 2 to 3 years, and the long-term survival rate is less than 20% to 30%. Radiation and radiocolloids were formerly used to treat ovarian cancer, but they have been largely supplanted by chemotherapy.
Serous tumors constitute about 30% of all ovarian tumors, making them the single most common group. They comprise 22% of benign and nearly 50% of malignant primary tumors of the ovary. Of all serous tumors, 50% are benign, 15% are borderline, and 35% are invasive carcinomas.
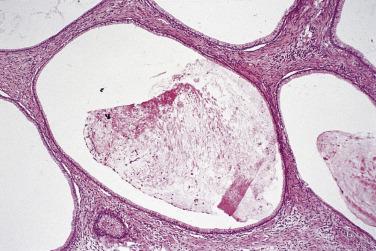
Serous cystadenoma may be unilocular or multilocular. It has a thin wall and contains clear fluid. The interior and exterior surfaces are usually smooth, but small papillary excrescences occasionally arise from the cyst lining. Serous adenofibroma is a solid tumor that has a firm white or tan fibrous cut surface. Scattered small cysts may be visible, or the tumor may have a spongy appearance due to the presence of many diminutive cysts. Serous cystadenofibroma is more common than adenofibroma; it is a unilocular or multilocular cystic tumor with solid adenofibromatous areas in its wall. Serous surface papilloma is an uncommon tumor that grows as papillary excrescences on the surface of the ovary. About 20% of benign serous tumors are bilateral.
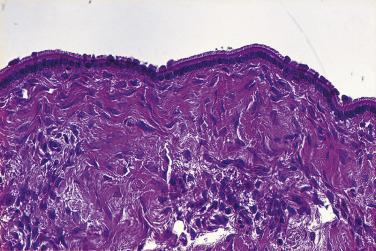
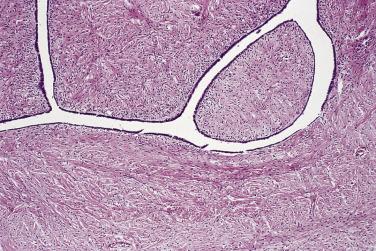
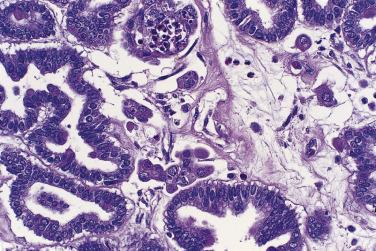
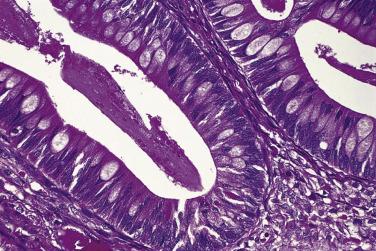
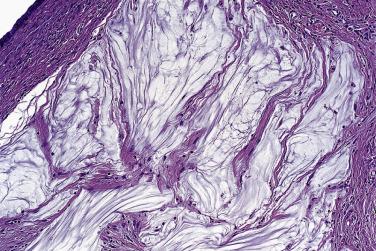
Microscopically, benign serous tumors are lined by ciliated and nonciliated low columnar cells with bland ovoid basal nuclei ( Fig. 13A.1 ). The epithelium becomes flattened if the cyst is under tension. Abundant fibrous stroma surrounds the glands and cysts in adenofibroma and cystadenofibroma ( Fig. 13A.2 ). In one study, only 14% of serous cystadenomas, usually the larger ones, were found to be monoclonal. This has led to the proposal that only tumors that exhibit proliferative features such as cellular stratification or papillations should be designated as cystadenomas or cystadenofibromas, although this view has not been widely adopted. There are small foci of mild to moderate nuclear atypia or branching papillary growth in occasional otherwise benign serous tumors. The behavior of serous tumors with small proliferative or atypical foci has not been adequately studied, but when these features are observed in only a few low-power fields ( lpf ) (<5%–10% of the tumor), the clinical evolution is generally benign, and such tumors are usually classified as a serous cystadenoma, cystadenofibroma, or adenofibroma with focal epithelial proliferation or atypia. In one study, all high-stage borderline serous tumors had areas of typical borderline growth greater than 1 cm or that constituted 20% or more of the tumor; the authors advocated a conservative cutoff point of 0.5 cm or 10% of the tumor as appropriate amounts of borderline growth to qualify for a diagnosis of borderline serous tumor in a serous tumor of the ovary. In some patients, a borderline serous tumor contains areas with a lesser degree of proliferative activity, or the contralateral ovary contains a serous tumor with focal proliferative activity, raising the possibility that serous tumors with focal proliferation may not be entirely inconsequential and that they may rarely progress.
Tumor is confined to the ovaries in 60% to 85% of cases. The remaining patients have pelvic or peritoneal implants. Lymph node involvement occurs in up to 40% of patients. Spread to parenchymal organs or beyond the abdominal cavity is uncommon. Long-term survival exceeds 90% for patients with all stages of disease, but the longer patients with extraovarian disease are followed the greater the number who will have recurrences, as most recurrences are detected more than 5 years after their initial diagnosis. Survival approaches 100% for patients with tumor confined to the ovaries. Tumor-related deaths fall into one of three main categories: (1) The patient develops low-grade serous carcinoma and dies of carcinoma; (2) the patient develops a fatal complication of a borderline tumor, such as fibrous adhesions leading to bowel obstruction; or (3) the patient dies of a complication of treatment.
Conservative therapy appears indicated for borderline tumors, except in a small cohort of women whose tumors exhibit features consistently associated with aggressive behavior or carcinoma. These features include invasive peritoneal implants or recurrence as low-grade serous carcinoma. While most oncologists administer chemotherapy to patients with invasive implants or low-grade serous carcinoma, these patients tend to have long survivals regardless of therapy, and tumor regression following chemotherapy is less than satisfactory.
The standard surgical treatment for a borderline tumor is total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and complete staging with resection of extraovarian tumor implants if any are present. Many women with borderline tumors are of reproductive age and wish to retain their childbearing capability. Unilateral salpingo-oophorectomy, or even cystectomy, is therefore considered as a treatment option in some circumstances, although patients treated in this way have about a 25% risk of recurrence in the contralateral ovary. Laparoscopic management has been utilized, even in women with small peritoneal implants, in an effort to conserve fertility. Conservative surgery to preserve fertility appears possible in some circumstances, even in patients with extraovarian tumor spread. Patients with micropapillary borderline serous tumors are more likely than those with typical borderline serous tumors to have tumor spread beyond the ovary. When corrected for stage and implant type, however, no difference in survival has been demonstrated. Restaging surgery for women who have been inadequately staged during their initial operation is controversial. The tumor stage is altered in about 15% of women who undergo restaging surgery, but the risk of recurrence appears to be similar in women who are restaged and those who are not. Restaging is of most value for women with micropapillary borderline serous tumors, since they have a greater risk of having invasive peritoneal implants. Recurrences are generally detected many years after primary therapy, and recurrent disease can be slowly progressive. Recurrent tumor can be borderline serous tumor, low-grade serous carcinoma, or, rarely, high-grade serous carcinoma. Some authors, but not others, have noted an increased rate of disease progression when recurrence is as low-grade serous carcinoma. Surgical resection of contralateral or extraovarian tumor deposits is the most effective treatment for women with progressive or recurrent tumors. Oncologists are not in complete agreement, but most administer chemotherapy or radiotherapy only in the face of progressive disease that is not amenable to surgical resection. The survival rate is high, even for women with advanced stage tumors.
Serous borderline tumors can harbor mutations in BRAF or KRAS . Tumors with KRAS mutations appear to be at higher risk for progression to low-grade serous carcinoma in contrast to tumors with BRAF mutation that do not progress. A recent study found invasive implants to frequently harbor KRAS but not BRAF mutations.
Borderline serous tumors are large, usually multilocular cystic neoplasms that are bilateral in 35% to 40% of cases. Coarse papillary excrescences arise from the cyst lining. Papillary growth is focal in some tumors, confluent in others, and present on the external surface of the ovary in 40% to 50% of cases. Areas of solid growth are unusual except in adenofibromatous borderline tumors, and zones of hemorrhage or necrosis are seldom seen.
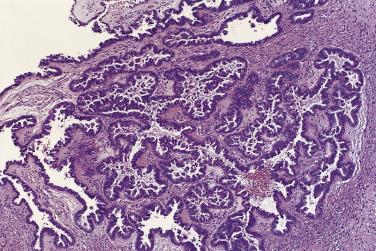
At low magnification, papillae with a hierarchic branching pattern grow from the cyst lining into the lumina ( Fig. 13A.3 ) or from the surface of the ovary. Complex papillary and glandular patterns and secondary cyst formation are typical. The papillae have fibrovascular cores that are conspicuous even in smaller branches and are surfaced by proliferating columnar cells that are stratified into several layers ( Fig. 13A.4 ). Ciliated cells may be present. Focally, the cells form tufts from which clusters of cells and single cells are detached into the cyst lumen. There is variable, but usually low grade, nuclear atypia, and scattered mitotic figures are present. Cells with abundant eosinophilic cytoplasm, the “indifferent” or “metaplastic” cells, are scattered singly or in small clusters among the columnar tumor cells; they tend to be most conspicuous at the tips of the papillae. Small foci of infarcted papillae are a common finding. Plaques or nodules of loose fibrous tissue containing glands and papillae are occasionally seen in borderline serous tumors. These can be found inside the tumor or on the surface of the ovary. They resemble desmoplastic peritoneal implants (see upcoming discussion) and have been termed autoimplants . Autoimplants tend to be detected in high stage tumors, but do not appear to have prognostic significance. The wall of a borderline serous tumor is generally thicker than that of a cystadenoma. Some tumors have sufficient fibrous stroma in their walls to be classified as a borderline serous adenofibroma or cystadenofibroma. The microscopic feature that differentiates a serous borderline tumor from a serous carcinoma is the absence of diffuse stromal invasion in the former. In a borderline tumor, papillae and glands that appear to be within the stroma are an artifact resulting from tangential cutting of complicated infoldings of the cyst lining. Such glands are not infiltrative and there is no stromal fibroblastic or inflammatory reaction around them.
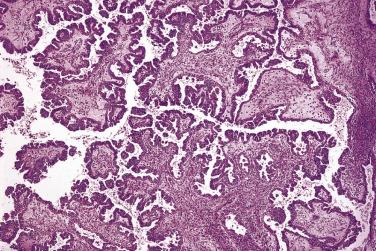
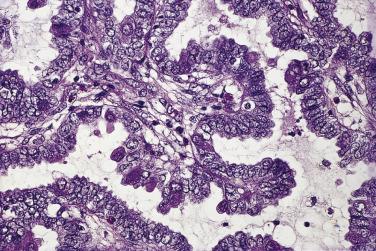
Foci of limited stromal invasion are occasionally identified in a borderline serous tumor. Various arbitrary size limits have been proposed for microinvasion, ranging from 3 to 5 mm, but in practice foci of microinvasion are almost always smaller than 3 mm. A largest dimension of invasion of 3 mm can therefore serve as a useful maximum size limit for microinvasion. Multiple foci of microinvasion are typically present, ranging from a couple to more than 10. Multiple patterns of microinvasive growth have been described. The most common is one in which small clusters and cords of cells with eosinophilic cytoplasm, round vesicular nuclei, and prominent nucleoli are haphazardly distributed in the fibrous stroma of the cyst wall or a papilla ( Fig. 13A.5 ). A stromal reaction is usually not seen around these cells. They are found occasionally within lymphatic spaces, but the clinical significance of this finding is not clear. Some authors believe that these eosinophilic cells are terminally differentiated senescent cells that are not capable of tumor progression. Other patterns of microinvasion include invasion by papillae, small glands or cribriform glandular structures, cords, or confluent nests of epithelial cells and one in which the tumor cells in the stroma line macropapillae with thick fibrous cores, also generally surrounded by clear spaces. Another pattern of stromal microinvasion is one in which micropapillae are present in the stroma surrounded by clear clefted spaces similar to low-grade serous carcinoma. Such foci have been referred to as microinvasive carcinoma to distinguish them from the usual microinvasion. Most patients whose tumors exhibit the usual pattern of stromal microinvasion have an uneventful course, but the presence of microinvasive carcinoma could be prognostically significant. For unknown reasons, microinvasion seems to be detected more frequently in tumors from pregnant women. Mucin secretion and stromal decidual reactions have also been noted in tumors removed from pregnant women. In contrast to borderline serous tumors with small areas of microinvasion, those with larger areas of invasion have an appreciable risk of progression if tumor has spread outside of the ovary.
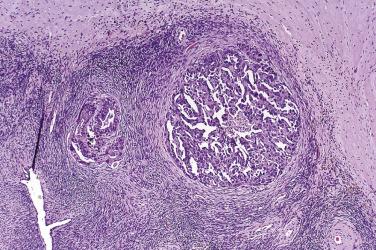
Peritoneal or omental tumor implants are found in 15% to 30% of women with serous borderline tumors. These are generally small superficial nodular excrescences of only a few millimeters in diameter, although larger solid or cystic implants are occasionally present. There is controversy as to whether these represent metastases from the ovarian tumor or sites of synchronous peritoneal neoplasia, although an increasing number of studies show similar genetic profiles in the ovarian and extraovarian tumors. Implants were originally classified as noninvasive or invasive based on the absence or presence of invasion into underlying tissue. Noninvasive implants are further divided into epithelial or desmoplastic types. Based on clinical outcome, the 2014 WHO Blue Book recommendation is that invasive implants should be designated as low-grade serous carcinoma and all noninvasive implants as “implants.”
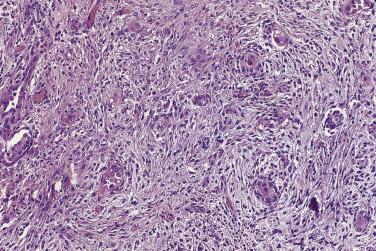
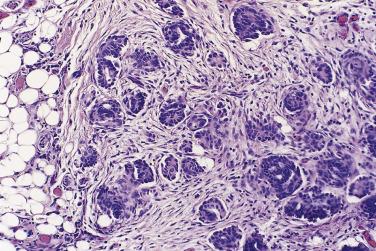
In the noninvasive epithelial implant, papillary serous borderline tumor grows on the surface of the peritoneum or in cystic spaces just beneath it ( Fig. 13A.6 ). The growths are circumscribed and do not invade the underlying stroma. Noninvasive desmoplastic implants are plaques of vascular fibrous stroma that contain a few epithelial cells, small clusters of cells, or scattered small glands lined by bland epithelial cells ( Fig. 13A.7 ). The implants appear plastered onto the peritoneal surface and show no invasion downward into the underlying stroma. Noninvasive papillary growths may overlie these desmoplastic implants. Although patients with noninvasive implants tend to have a favorable prognosis, long-term follow-up shows that a few of them develop progressive disease and die of tumor. Invasive implants are rare (5%–10%) but, together with advanced tumor stage, appear to be the most significant adverse prognostic findings in patients with borderline serous tumors. They are characterized by more abundant epithelium with an infiltrative pattern of growth into the subperitoneal tissues or surrounding omentum ( Fig. 13A.8 ). Expanded criteria for invasive implants have been proposed and include a micropapillary pattern of growth, clear spaces or clefts around clusters of tumor cells, and infiltrative glands that do not extend into underlying tissues, although these remain controversial. It can be difficult to classify implants; when it is unclear whether the implant is invasive or not, the term indeterminate implant is often used to categorize it.
Tumor is present in pelvic or paraaortic lymph nodes in up to a third of patients with advanced borderline tumors. While borderline serous tumors mainly involve intraabdominal lymph nodes, tumor is found in extraabdominal lymph nodes at, or subsequent to, primary surgery in occasional patients.
There are two main patterns of lymph node involvement. In one, the tumor grows in a branching papillary pattern similar to that observed in a primary ovarian borderline serous tumor. In the other, tumor cells are present singly or in small nests in the subcapsular sinuses. Lymph node involvement by hyperplastic mesothelial cells is a rare mimic of the latter pattern. Accompanying peritoneal mesothelial hyperplasia, the appearance of the cells, and positive immunostaining for mesothelial markers such as calretinin serve to differentiate hyperplastic mesothelial cells from tumor cells. A rare but prognostically unfavorable pattern of lymph node involvement by borderline serous tumor is one in which the lymph node parenchyma is replaced by a nodular proliferation of tumor cells growing in reactive fibroblastic stroma. This pattern has the appearance of low-grade serous carcinoma. Epithelial inclusions lined by low columnar cells, many of which are ciliated, are found in the pelvic or paraaortic lymph nodes in 5% to 25% of patients who are operated on for uterine cancers. These inclusions, designated as benign epithelial inclusions or endosalpingiosis, are more frequent in patients with borderline serous tumors, and it has been proposed that they may represent a precursor lesion or a type of lymph node involvement by a borderline serous tumor. For staging purposes this finding is designated as benign inclusions and is not by itself viewed as evidence of lymph node involvement. Some have proposed that lymph node involvement by borderline serous tumors may represent synchronous neoplasia arising in epithelial inclusions rather than metastasis from the ovarian tumor. Regardless of its origin, lymph node involvement by a borderline serous tumor is often found in women with peritoneal or omental implants; it does not appear to be an independent adverse prognostic finding in most studies. However, lymph node involvement in the form of nodular aggregates of tumor cells in reactive stroma, resembling low-grade serous carcinoma, appears to be associated with decreased survival.
The term micropapillary serous carcinoma or noninvasive low-grade serous carcinoma has been proposed for a group of proliferative serous tumors with excessive epithelial proliferation, the morphologic spectrum of which appears to encompass some noninvasive tumors in the borderline category as well as some low-grade invasive serous carcinomas. Gene expression analysis reveals that micropapillary borderline serous tumors are more closely related to low-grade serous carcinoma than to typical borderline serous tumors. Clinical follow-up of patients with micropapillary serous tumors indicates that most patients with stage I tumors are cured by surgery. Patients with micropapillary serous tumors appear more likely to have bilateral tumors, surface papillary growth, extraovarian disease, and invasive implants. Survival rates appear to be similar to those for conventional borderline serous tumors, once adjusted for stage and implant type. Although this remains a somewhat controversial entity, noninvasive micropapillary serous tumors are classified as proliferative variants of borderline serous tumors rather than as a type of serous carcinoma.
On gross examination, micropapillary borderline serous tumors are cystic and solid tumors that average 8 to 9 cm in diameter. They tend to be bilateral, and they typically exhibit both intracystic and surface papillary tumor growth. Microscopically, micropapillary borderline serous tumors have a background of typical borderline serous tumor and have been defined arbitrarily as having foci of micropapillary growth measuring greater than 0.5 cm. Long tufts of epithelial cells with little or no supporting fibrous stroma sprout from bulbous fibrovascular stromal papillae ( Fig. 13A.9 ), from the cyst wall, or, when tangentially sectioned, are packed together with larger papillae that have more stroma. The papillae are five or more times longer than they are wide. Tumors with foci of cribriform growth along the surfaces of papillae are also classified as micropapillary borderline serous tumors. The tumor cells in the micropapillary variant are uniform and lack the heterogeneous cell types, including ciliated cells and eosinophilic cells that are often abundant in conventional serous borderline tumors. The tumor cells are cuboidal, hobnail shaped, or columnar, and they have uniform nuclei with only mild or moderate atypia and infrequent mitotic figures ( Fig. 13A.10 ).
Serous carcinoma is the most common type of ovarian cancer, accounting for 68% of ovarian cancers in one large population-based study. Mutations of the TP53 gene are present in most cases and are thought to be critical events in the pathogenesis of this type of carcinoma. The clinical presentation and treatment of serous carcinoma is discussed earlier in the section on the clinical features of epithelial tumors. Intraabdominal metastases are typically present at the time of diagnosis in patients with high-grade serous carcinoma, in the omentum, on peritoneal surfaces, or in abdominal lymph nodes. Occasionally, metastases are detected in unusual distant sites, such as the brain, lung, distant lymph nodes, or the breast.
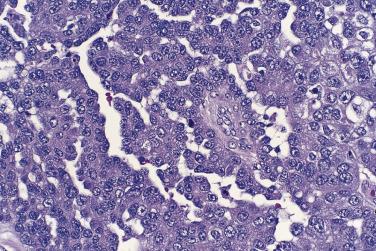
High-grade serous carcinoma is the type of ovarian cancer most associated with mutations in the BRCA genes. BRCA1 / BRCA2 mutations, both somatic and germline, can occur in up to 22% of high-grade serous carcinomas, and approximately 10% to 15% occur in women with germline BRCA mutations. This association has led to findings that have resulted in an ongoing reassessment of the nature of this tumor type. Large, clinically detected tumors in BRCA mutation carriers are mainly high-grade serous carcinomas, although other types occasionally occur. Many patients with BRCA mutations or with a family history of ovarian cancer are treated by risk-reducing bilateral salpingo-oophorectomy (RRSO), which has been shown to lower the patient's risk of developing carcinoma. Cancer risk is not eliminated as these patients are still at risk for peritoneal serous carcinoma and for breast cancer, although the risk for peritoneal cancer is very low. Microscopic foci of in situ or invasive carcinoma and even macroscopic carcinomas have been detected in 2% to 18% of asymptomatic women who undergo RRSO. Most of the carcinomas found in prophylactic salpingo-oophorectomy specimens are of serous type, measure only a few millimeters in diameter, and are of high grade ( Fig. 13A.11 ). While small tumors are occasionally identified in the ovaries, most of the intraepithelial and early invasive serous carcinomas detected in RRSO specimens have been found in the fallopian tubes. Tubal intraepithelial carcinoma is less common in salpingectomy specimens from patients without a BRCA mutation, but it is occasionally detected; in one study, tubal intraepithelial carcinoma was found in 8% of patients with a BRCA mutation and in 3% of patients with no mutation. Interestingly, complete sectioning of the fallopian tubes in patients with a clinical diagnosis of a primary ovarian or primary peritoneal serous carcinoma, regardless of whether it occurs in a woman with a germline BRCA mutation, frequently reveals foci of serous tubal intraepithelial carcinoma or small apparently primary foci of invasive serous carcinoma of the fallopian tube. This has led to the increasingly accepted hypothesis that many, if not all, serous carcinomas of the ovary and peritoneum are actually metastases from tubal tumors, not primary neoplasms of the ovary or peritoneum. Rare high-grade serous carcinomas appear to arise from a low-grade serous carcinoma or a borderline serous tumor. The histogenesis of serous carcinoma of the ovary and its relationship to tumors of the fallopian tube are areas of active research.
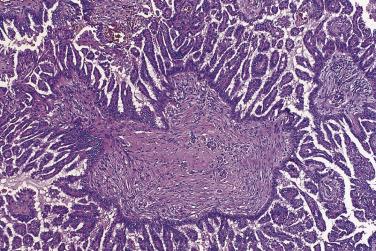
Serous carcinoma is a large, often bilateral neoplasm in which there is a mixture of cystic, papillary, and solid growth. The solid areas are tan or white and contain foci of hemorrhage and necrosis. Carcinoma frequently invades through the ovarian capsule and grows on the surface of the ovary. Serous surface papillary carcinoma grows predominantly on the surface of the ovary, with minimal parenchymal invasion and no intracystic growth.
The tumor cells exhibit marked nuclear atypia and frequent mitotic figures (30–50+ per 10 high-power fields [hpf]) ( Fig. 13A.12 ). The nuclei are hyperchromatic and variably pleomorphic, and often contain one or more prominent nucleoli. The nucleus to cytoplasm ratio is high, and the nucleus typically appears to occupy most of the cell. Papillary growth is usually present at least focally and is frequently the predominant growth pattern ( Fig. 13A.13 ). Some papillae have fibrovascular cores that are lined by stratified tumor cells. The tumor cells often pile up to form tufts that project above the surfaces of the papillae. A micropapillary pattern in which long, thin tufts of tumor cells grow into cystic spaces is also common. Tumor cells line glands and cystic spaces, diffusely infiltrate fibrotic stroma, or form solid nests and sheets. Elongated slitlike glands within foci of solid growth are a characteristic finding in high-grade serous carcinoma. Foci of microcystic growth, sometimes with admixed signet ring–type cells, are noted occasionally. Geographic zones of necrosis may be present. Rare examples of high-grade serous carcinoma apparently evolve from a borderline serous tumor or a low-grade serous carcinoma, and in these, areas of high-grade carcinoma are admixed with areas of borderline tumor or low-grade carcinoma.
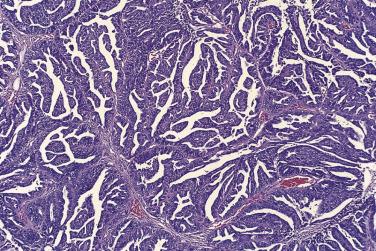
Traditionally, high-grade serous carcinomas were thought to be tumors with entirely or at least predominantly papillary architecture, and a more restrictive approach was used. However, recent immunohistochemical and molecular studies have demonstrated that high-grade serous carcinomas can display a variety of growth patterns, including solid, glandular, and transitional growth, and that some high-grade serous carcinomas may show none or only minimal papillary architecture.
Low-grade serous carcinoma is relatively uncommon, accounting for no more than 10% of serous carcinomas and accounting for 3% to 5% of all ovarian carcinomas. Although clearly of serous type, it is now recognized as a distinct type of ovarian carcinoma and is no longer viewed simply as the low-grade end of a continuum with high-grade serous carcinoma at the most malignant end of the spectrum. Low-grade serous carcinoma has been shown to have a different molecular basis than high-grade serous carcinoma. It is characterized by BRAF or KRAS mutations with the latter the most common, rather than TP53 mutations. It is thought to arise via stepwise progression of a serous tumor from benign to borderline to low-grade carcinoma. Low-grade serous carcinoma is typically admixed with areas of borderline serous tumor, particularly micropapillary borderline tumor, supporting its origin from the borderline tumor. In contrast to high-grade serous carcinoma, low-grade serous carcinoma does not appear to be linked to BRCA mutations. Low-grade serous carcinoma occurs in younger patients than high-grade serous carcinoma (average age in the mid-40s) and has a more indolent clinical course. Although the initial survival for low-grade serous carcinoma is better than high-grade serous carcinoma, over time this survival advantage decreases. In a recent study the estimated 5-year and 10-year survival rates for low-grade and high-grade serous carcinomas were 62.3% vs 43.9% and 21.2% vs 22.7%, respectively. Although women with low-grade serous carcinoma tend to have slowly progressive disease, primary and recurrent tumors are usually resistant to standard ovarian cancer chemotherapy regimens. Since low-grade serous carcinomas can harbor mutations in the MAPK pathway, MEK inhibitors are being investigated as targeted therapy. The clinical behavior of low-grade serous carcinoma that develops as a recurrence of a borderline serous tumor appears to be similar to that of de novo low-grade serous carcinoma.
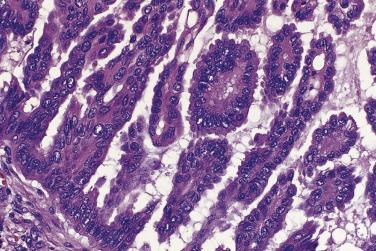
The tumor cells in low-grade serous carcinoma are cuboidal to low columnar with relatively uniform round to oval nuclei with evenly distributed chromatin and small nucleoli. There is mild to moderate nuclear atypia, and the mitotic rate is low. Crowded glands, simple or complex micropapillae, and solid or cribriform nests of tumor cells infiltrate a fibrous stroma ( Fig. 13A.14 ). Less common patterns of invasion include a so-called inverted macropapillary pattern in which large papillae with broad stromal cores lined by tumor cells invade the stroma, a solid pattern of growth, and a single cell pattern of invasion. Variable amounts of mucin are present, in tumor cell cytoplasm, in gland lumens, or in the stroma. Clusters of tumor cells are often surrounded by clefts or clear spaces. Zones of invasive carcinoma may be mixed with zones of serous borderline tumor, usually of the micropapillary type. Distant metastases from an ovarian low-grade serous carcinoma, such as those to lymph nodes, breast, or the mediastinum, can be difficult to diagnose because they can mimic borderline serous tumors or other types of carcinoma.

Psammoma bodies are small laminated calcifications that form around products of cellular degeneration. They are often present in serous tumors, particularly serous carcinomas, and are occasionally numerous. Rarely, psammoma bodies are so numerous in a low-grade serous carcinoma that they obscure the epithelial elements of the tumor. Such carcinomas, termed serous psammocarcinomas, have a highly favorable prognosis when they can be excised completely. Similar tumors can arise as primary neoplasms of the peritoneum. The prognosis for high-grade serous carcinoma with numerous psammoma bodies is unfavorable, and they are excluded from the “psammocarcinoma” category. While psammoma bodies are suggestive of a serous tumor, they can also be found in association with other types of tumors and such nonneoplastic conditions as epithelial inclusion cysts and endosalpingiosis.
Grading of ovarian carcinomas has not been standardized. Silverberg and colleagues proposed a universal grading system that they thought could be used for all types of ovarian carcinomas. With this system, the grade of a carcinoma is determined by the degree of nuclear atypia, the frequency of mitotic figures, and the extent to which the tumor cells form papillae or glands ( Box 13A.3 ). For ovarian serous carcinoma, a binary grading system, in which low-grade serous carcinoma almost always falls into grade 1 of the universal grading system, has gained greater acceptance than the universal grading system because it better reflects the current thinking that high-grade and low-grade serous carcinomas represent two different tumor types rather than two different grades of the same type of tumor. In the binary system, low-grade serous carcinoma is a tumor that exhibits mild to moderate nuclear atypia and 12 or fewer mitotic figures per 10 hpf. In contrast, high-grade serous carcinoma is characterized by marked nuclear atypia, usually with considerable variation in nuclear size and shape. Macronucleoli are common. Mitotic activity is high, with a median of 38 mitotic figures per 10 hpf in one study. Apart from differences in nuclear atypia and mitotic activity, low-grade and high-grade serous carcinomas grow in different architectural patterns, as described earlier, and most low-grade serous carcinomas are associated with a borderline serous tumor. Tumors with intermediate nuclear grade (grade 2 serous carcinoma) have similar growth patterns and molecular features as high-grade serous carcinoma and exhibit the same aggressive growth, so they are best grouped with the high-grade tumors.
|
|
|
1 point |
|
2 points |
|
3 points |
|
|
|
1 point |
|
2 points |
|
3 points |
|
|
|
1 point |
|
2 points |
|
3 points |
|
|
|
Grade 1 |
|
Grade 2 |
|
Grade 3 |
All serous tumors have immunohistochemical features in common. They usually show positive staining for cytokeratin 7 (CK7), and they do not stain for cytokeratin 20 (CK20) or CDX2. They show variable staining for estrogen and progesterone receptors, with less staining being present in high-grade serous carcinoma than in lower grade serous tumors. Borderline serous tumors and serous carcinomas generally show membrane staining for OC125. One of the most helpful features of serous tumors is that they show nuclear staining for WT1. This helps differentiate them from other types of ovarian tumor, such as endometrioid and clear cell carcinomas. Staining for WT1 is also helpful in differentiating ovarian serous carcinoma from endometrial serous carcinoma, which has a similar microscopic appearance but is less likely to stain for WT1. High-grade serous carcinoma is characterized by TP53 mutation, and immunohistochemical staining for p53 has been shown to correlate well with TP53 mutation status. The most common abnormal or aberrant p53 staining pattern is that of diffuse strong staining of nearly all tumor cell nuclei, which is seen in the presence of nonsynonymous TP53 mutations. Complete absence of staining for p53 is also an abnormal staining pattern that results from nonsense TP53 mutations. A third recently described aberrant staining pattern is that of strong cytoplasmic staining without nuclear overexpression that can occur with TP53 mutations that cause disruption in the nuclear localization domain. Tumors that do not have a TP53 mutation show wild-type staining characterized by staining in at least some tumor cell nuclei, a finding that contrasts with the patterns seen in tumors with a mutation. Some truncating or splice site TP53 mutations can result in nonfunctional p53 expression in a wild-type pattern. In addition, high-grade serous carcinoma is almost always p16 positive, with strong staining of the nuclei and cytoplasm of all or nearly all tumor cells. Low-grade serous carcinoma, in contrast, rarely shows the type of strong staining for p53 or p16 seen in high-grade serous carcinoma, and these markers are generally negative in borderline and benign tumors as well. PAX8 appears to be a reliable marker of female genital tract epithelial tumors, and it is almost always positive in serous tumors, although staining can vary in extent and intensity. HMGA2 is another marker that is positive in a majority of high-grade serous carcinomas but uncommon in other types of ovarian cancer. Patients with high-grade serous carcinoma are frequently treated with chemotherapy, either after or before surgery. Immunohistochemical staining for p53 and WT1 appears to be concordant before and after chemotherapy, although the amount and intensity of WT1 staining is often decreased in the setting of prior chemotherapy.
Pathologists occasionally need to differentiate between serous carcinoma and a mesothelioma. Useful stains for this differential diagnosis include calretinin, which is generally positive in mesothelioma, and estrogen receptors (ERs) and general adenocarcinoma markers such as MOC31 and Ber-EP4, which are positive in serous carcinoma. Staining for WT1 is not helpful, since it is likely to be positive in both tumors. D2-40 is a widely used marker for mesothelioma; it is positive in up to 20% of serous carcinomas, but staining tends to be weak and focal compared with the diffuse strong membranous staining seen in mesothelioma. Thus D2-40 can be useful in this differential diagnosis. PAX8 is almost always positive in serous carcinoma, but staining can be seen in benign and malignant peritoneal mesothelial cells; therefore this stain should be used with caution. Loss of BAP1 staining appears to be specific for malignant mesotheliomas and has not been observed in serous carcinoma.
Mucinous cystadenoma is the most common ovarian mucinous tumor; it is about equal in incidence to serous cystadenoma. Borderline and malignant mucinous tumors are less numerous than their serous counterparts, and borderline mucinous tumors greatly outnumber mucinous carcinomas.
Mucinous cystadenoma is generally unilateral. The average diameter is about 10 cm, but huge tumors have been reported. The cut surface reveals unilocular or multilocular mucin-filled cysts of varying size. Mucinous adenofibroma is a predominantly solid, white or tan, fibrous tumor that contains small mucin-filled cysts. Mucinous cystadenofibroma is a cystic tumor that has solid fibrous areas similar to a mucinous adenofibroma.
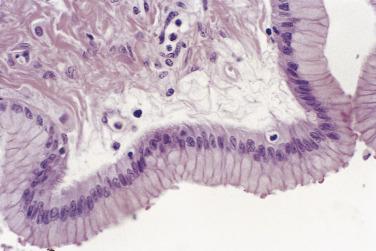
Microscopically, a layer of columnar cells lines the cysts, papillae, and cryptlike structures that are found in benign mucinous tumors ( Fig. 13A.15 ). A majority of the tumor cells are endocervical-like or gastric-like, with uniform round or oval basal nuclei and clear or amphophilic cytoplasm ( Fig. 13A.16 ). Stains for acid mucins and, to a lesser extent, neutral mucins color the cytoplasm. Intestinal-type epithelium with goblet cells or Paneth cells is present in many mucinous cystadenomas. Histochemical and immunohistochemical studies reveal argyrophilic cells that contain a variety of peptide hormones. Peripheral bands of luteinized stromal cells are noted occasionally around a mucinous cystadenoma. This finding can be associated with clinical evidence of steroid hormone secretion. Mucinous adenofibroma or cystadenofibroma has abundant fibrous stroma that surrounds the glands and dominates the histologic picture. An occasional mucinous cystadenoma or adenofibroma has small foci of mild to moderate nuclear atypia or nuclear stratification. When these involve only a few low power fields (5%–10% of the tumor) the clinical evolution is invariably benign. Such tumors are best classified as a mucinous cystadenoma, cystadenofibroma, or adenofibroma with focal low-grade atypia.
Two types of borderline mucinous tumors are recognized, an intestinal type and an endocervical-like type. The latter is also known as the Müllerian or seromucinous type of borderline mucinous tumor. Intestinal type borderline mucinous tumors (IBMT) are by far the most common, accounting for about 90% of borderline mucinous tumors. Intestinal type borderline mucinous tumors and carcinomas are discussed next, followed by the endocervical-like mucinous tumors.
Patients with intestinal-type borderline mucinous tumors have a highly favorable prognosis. The tumor is almost always confined to one ovary at diagnosis (stage IA). The risk of recurrence or metastasis is low in well-studied noninvasive mucinous tumors, with almost all patients with stage IA borderline mucinous tumors being cured. The rare tumors that do metastasize tend to be borderline mucinous tumors with intraepithelial carcinoma, and many of these are stage IC due to tumor rupture. Rare patients with microinvasion in a borderline mucinous tumor have recurrences, but it is unclear whether the recurrence is associated with the microinvasion or concurrent intraepithelial carcinoma. Development of metastases could be due to greater malignant potential of borderline tumors with microinvasion or intraepithelial carcinoma or it could reflect the presence of undetected invasive carcinoma in a large predominantly borderline neoplasm. In the past, it was thought that some patients with intestinal-type borderline mucinous tumors developed a form of extraovarian spread known as pseudomyxoma peritonei. Patients with pseudomyxoma peritonei accounted for nearly all deaths from tumors of this type. Pseudomyxoma peritonei is a progressive condition in which deposits of mucin containing strips of bland or borderline epithelium undergo fibrous organization, adhere to and dissect into the omentum and peritoneum, and eventually fill the abdomen and obstruct the gastrointestinal tract. Mucinous tumors having the appearance of IBMT are frequently associated with pseudomyxoma peritonei, but most of these are unusual forms of metastatic adenocarcinoma, usually secondary to primary neoplasms of the appendix or colon, that mimic borderline ovarian mucinous tumors of intestinal type. Pseudomyxoma peritonei is discussed in more detail in the section on metastatic tumors.
Borderline mucinous tumors of the intestinal type tend to be large, unilateral tumors with an average diameter of about 15 cm. Most are multilocular, although occasional tumors are unilocular or paucilocular. The cysts are filled with mucin and have a smooth or velvety lining. Intracystic papillae are uncommon in intestinal type borderline mucinous tumors. A variable amount of fibrous stroma surrounds the cysts.
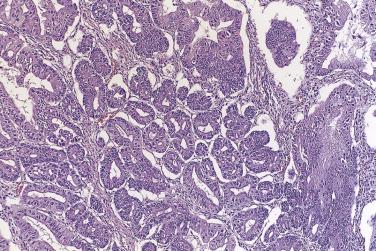
The growth pattern tends to be complicated, with crowding of glands and formation of secondary cysts, complex glands, and papillae supported by thin cores of fibrovascular connective tissue ( Fig. 13A.17 ). The glands, cysts, and papillae are lined by one to several layers of columnar tumor cells with basal nuclei. Some tumor cells resemble endocervical or pyloric cells, but goblet cells and cells resembling intestinal absorptive cells are conspicuous ( Fig. 13A.18 ). The tumor cells have round to oval vesicular nuclei, which usually display mild to moderate atypia. Nucleoli may be prominent. Occasional mitotic figures are present. They are generally most conspicuous in cryptlike glands in the walls of larger cysts. Noninvasive mucinous tumors with marked (grade 3) nuclear atypia are designated as borderline tumors with intraepithelial carcinoma. Intraepithelial carcinoma, which can be focal or extensive, is present in about 40% of IMBT. Pathologists should estimate the amount of intraepithelial carcinoma present, since there is some evidence that the amount of intraepithelial carcinoma may have prognostic significance; recurrence is most likely when more than 10% of the epithelium shows features of intraepithelial carcinoma. Reviews of the literature have shown a 2% to 6% risk of recurrence for a borderline mucinous tumor with intraepithelial carcinoma, depending on the time period studied.
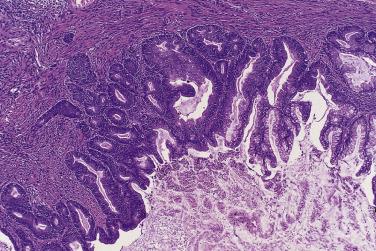
Small foci of stromal invasion occur in up to about 30% of IBMT. The definition of microinvasion used in studies of IBMT has varied, with some authors using 3 mm in maximum dimension, some 10 mm 2 , and others 5 mm in maximum dimension. The 10-mm 2 size limit is the most accepted, but the clinical outcome seems similar regardless of which definition is used, the presence of only very small foci of invasion being the significant finding. Microinvasive clusters of tumor cells or small complex glands are surrounded by inflamed or edematous stroma, a desmoplastic stromal reaction, or clear spaces. A few tumor cells with low-grade nuclei within a pool of extravasated mucus, often associated with histiocytes, chronic inflammatory cells, and multinucleated giant cells, should not be interpreted as microinvasion, as small numbers of tumor cells can be carried into the stroma along with the mucin when a cyst ruptures. In most cases, more than one focus of microinvasion is present. The average number of microinvasive foci is two to three, but five or more are occasionally identified. In determining the size of the microinvasion, the largest focus is measured; the sizes of the various foci are not added together. It may be worthwhile to divide microinvasion into two categories, although clear-cut clinical evidence for this distinction remains to be demonstrated. Hart proposed two categories: (1) borderline mucinous tumor with microinvasion, in which the tumor cell nuclei in the tumor and the associated microinvasive focus exhibit mild to moderate atypia, and (2) microinvasive carcinoma when the cells in the microinvasive focus exhibit high-grade nuclear atypia. Others have proposed a simpler and clearer division into low-grade and high-grade forms of microinvasion, with grade 3 nuclei being present in high-grade microinvasion. In most reports, patients with microinvasion have a favorable prognosis with only rare recurrences, but in one report 4 of 28 patients with microinvasion developed recurrences.
Acellular pseudomyxoma ovarii is a condition in which mucinous cysts or glands rupture, releasing acellular mucin into the surrounding ovarian stroma ( Fig. 13A.19 ). The pools of extravasated mucin are sometimes surrounded by histiocytes and multinucleated foreign-body giant cells. The mucin can partly or completely organize, with ingrowth of fibroblasts and blood vessels. Cellular pseudomyxoma ovarii, in which more extensive pools of mucin contain clusters and strips of epithelial cells, is a more ominous finding, particularly when the tumor cells in the mucin have high-grade nuclei. It can represent microinvasion or overt invasion developing in a primary intestinal-type borderline mucinous tumor, depending on its size, but when it is extensive it often represents a pattern of metastatic mucinous adenocarcinoma from some other site, such as the appendix or large intestine.
Mucinous carcinoma used to be a fairly common diagnosis, but it is now viewed as an unusual type of ovarian cancer, accounting for as few as 3% of primary ovarian carcinomas in some studies. Among patients with advanced stage ovarian tumors, mucinous carcinomas are particularly rare. This is because many tumors formerly classified as carcinomas are now classified as borderline tumors with intraepithelial carcinoma, while others can now be identified as metastatic carcinomas using immunohistochemistry or sophisticated clinical studies. The average patient with mucinous carcinoma is in her mid-50s. Mucinous carcinoma does not appear to be associated with BRCA mutations. It is generally treated the same way as other types of ovarian cancer, as detailed earlier. Stage I mucinous carcinomas have a highly favorable prognosis, and when they occur in young women they can be treated safely by unilateral salpingo-oophorectomy. Lymph node metastases are not detected in women whose mucinous carcinomas are confined to the ovary, indicating that lymph node dissection is unnecessary in these patients. Mucinous carcinomas showing an expansile type of invasion only rarely spread beyond the ovary or recur, and they are particularly amenable to conservative therapy. Advanced stage mucinous tumors, on the other hand, have a poor prognosis and are much less likely than serous carcinomas to respond to chemotherapy.
Mucinous carcinoma is a large multilocular cystic tumor averaging 15 to 20 cm in diameter. Firm, fleshy, white or tan solid areas may be present, and foci of hemorrhage or necrosis are often identified, particularly in larger tumors. Bilaterality and surface growth occur in fewer than 10% of cases, but rupture of the capsule is frequent because of the large size and mucin content of these tumors. Bilateral or small (<13 cm) mucinous carcinomas are likely to be metastatic, while large unilateral mucinous carcinomas are likely to be primary.
Mucinous glands and cysts are crowded and complex with irregular infoldings and protrusions into the surrounding stroma ( Fig. 13A.20 ). Intestinal-type cells predominate in most mucinous carcinomas; Müllerian mucinous carcinomas are discussed later. The cells are columnar, have eosinophilic cytoplasm, and tend to stratify into two or more layers ( Fig. 13A.21 ). The nuclei are enlarged and vesicular and have coarse chromatin and prominent nucleoli. Mitotic figures range from few to many, and atypical mitotic figures are often noted. Goblet cells are generally present but they can be infrequent and difficult to identify. Occasional endocrine cells with eosinophilic granules are sometimes seen. Areas of borderline and benign-appearing mucinous epithelia are frequently present in mucinous carcinomas, most often in those that are low grade.
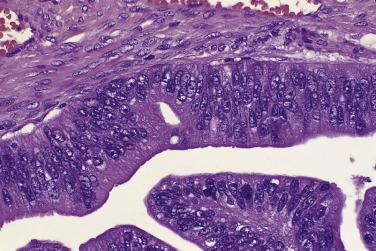
Only tumors that show stromal invasion by irregular cords and nests of tumor cells (destructive stromal invasion) or confluent expansile nodules of back-to-back glands or papillae (expansile stromal invasion) should be diagnosed as mucinous carcinoma. Expansile stromal invasion is far more common than infiltrative invasion. In one series of 31 cases, invasion was of the expansile type in 84% of the tumors. The pathologist should note which type of invasion is present in the pathology report. The risk of recurrence is thought to be greater if destructive stromal invasion is present, although when it is limited in amount and the tumor is of low stage the risk of recurrence appears to be low. An exclusively expansile pattern of invasion does not guarantee that there will be no recurrence, as some patients with expansile invasion have developed recurrences.
Signet ring cells are usually indicative that an ovarian tumor is metastatic but rare; apparently primary ovarian mucinous tumors contain signet ring cells. Some of these are unilateral tumors that predominantly resemble a primary ovarian mucinous tumor, usually a mucinous adenofibroma, but there are foci of signet ring cells. Others are mucinous carcinomas with signet ring cells that are associated with benign cystic teratomas. Finally, rare signet ring cell carcinomas with the appearance of Krukenberg tumors have been designated as primary to the ovary based on long clinical follow-up or absence of any extraovarian primary tumor at autopsy. Krukenberg tumors should always be suspected of being metastatic, as it can be difficult to identify a small extraovarian primary carcinoma in the stomach or elsewhere.
In general, it is advisable to prepare one section per centimeter of tumor diameter from a complex mucinous tumor, paying particular attention to areas of solid growth. Destructive stromal invasion can be difficult to identify in complex mucinous tumors, and other criteria have been proposed for the diagnosis of carcinoma in the absence of invasion. Features such as cellular stratification into four or more layers, limited cribriform growth, papillae without stromal cores, and marked nuclear atypia suggest that a neoplasm may be a carcinoma but are not, by themselves, diagnostic. If such features are present in a tumor in which no invasion is identified, an even more thorough microscopic evaluation may be warranted with some suggesting preparation of up to two slides per centimeter of tumor diameter. At present, stromal invasion is the only criterion for classifying an ovarian mucinous tumor as a carcinoma. Tumors in which invasion is not identified are classified as borderline tumors, noting when appropriate that they show features of intraepithelial carcinoma. Clinicians should be informed that there is a low risk of recurrence or metastasis in such cases, particularly if the tumor has been sampled inadequately.
Mural nodules of sarcoma-like connective tissue, sarcoma, or anaplastic carcinoma occasionally occur in mucinous tumors, usually borderline tumors or carcinomas of intestinal type. Reactive mural nodules grow as single or, more often, multiple circumscribed masses up to 5 cm in diameter. They consist of spindle-shaped fibroblasts, myofibroblasts, and possibly submesothelial cells, histiocytes, inflammatory cells, and occasional multinucleate cells. Three subtypes have been described: an epulis-like type composed of mononuclear stromal cells and multinucleate giant cells; a pleomorphic type, composed of spindle cells and giant cells; and a histiocytic type. The stroma is typically edematous with foci of hemorrhage and necrosis. Immunostains for vimentin and CD68 are positive. Cytokeratin is expressed in a weak and focal pattern if at all, in contrast to the diffuse strong staining typical of carcinoma.
The average diameter of mucinous tumors containing mural nodules of anaplastic carcinoma is about 20 cm. Most are borderline tumors or carcinomas of intestinal type. The nodules can be single or multiple, and range from less than 1 cm to more than 10 cm in diameter; sometimes they are not grossly visible and are detected on microscopic examination only. The nodules have an infiltrative border and consist of nests and sheets of undifferentiated polygonal epithelial cells or sheets and fascicles of malignant spindle cells ( Fig. 13A.22 ). Mitotic figures are always present, but they vary in number with some tumors having fewer than 4 per 10 hpf. These nodules have been subclassified into a rhabdoid type, in which the tumor cells are polygonal with moderate to abundant eosinophilic cytoplasm and eccentric nuclei with prominent nucleoli, a sarcomatoid type in which the tumor cells are spindled, and a pleomorphic type in which both rhabdoid and spindle cells are present. Immunoreactivity for cytokeratin and epithelial membrane antigen (EMA) is an important diagnostic finding; staining tends to be strong and diffuse in the rhabdoid type and patchier in the sarcomatoid type. The tumor cells coexpress vimentin in some cases. Rare carcinosarcoma-like nodules have also been reported. Patients with stage IA tumors appear to have a favorable prognosis with only occasional tumor-related deaths, while the outlook is unfavorable if tumor has ruptured or spread beyond the ovary.
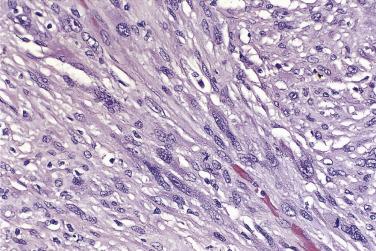
Mural nodules of sarcoma are composed of malignant spindle cells and usually resemble fibrosarcoma or undifferentiated sarcoma. The tumor cells are cytokeratin negative, but positive for vimentin, and depending on the type of sarcoma they may stain for other intermediate filaments such as actin or desmin.
Stains for cytokeratins 7 and 20 and for CDX2 are of great value in the diagnosis of mucinous tumors of the ovary, particularly borderline tumors and carcinomas, and can be essential for differentiating them from metastatic carcinomas. Primary mucinous tumors tend to show strong positive staining for CK7. Staining for CK20 is generally present, but it tends to be moderate and patchy, as is staining for CDX2. More mucinous tumors stain for CK20 than for CDX2. Metastatic adenocarcinomas from the lower gastrointestinal tract, which can mimic primary mucinous tumors, are generally CK7 negative, but they show diffuse strong staining for CK20 and CDX2, and they are more likely to show nuclear staining for β-catenin. SATB2 is a newer marker of lower gastrointestinal tract origin that is negative in mucinous tumors of ovarian and upper gastrointestinal tract origin. Among lower gastrointestinal tract adenocarcinomas that metastasize to the ovary, those from the rectum are the most likely to be CK7 positive. Primary mucinous carcinomas generally stain for MUC5AC and for the pancreas cancer–associated antigen DPC4. The latter is lost in 40% to 50% of pancreatic adenocarcinomas, so loss of staining for DPC4 raises the possibility that a mucinous tumor is metastatic from the pancreas, not primary in the ovary. Diffuse strong positive staining for p16 is generally interpreted as suggesting that a mucinous tumor in the ovary is metastatic from the cervix, since this type of staining is uncommon in primary ovarian neoplasms. The authors of one study, however, reported that 29% of primary mucinous carcinomas showed diffuse strong staining for p16, while none contained human papillomavirus (HPV) deoxyribonucleic acid (DNA). This result has not been duplicated by others, but if metastatic cervical adenocarcinoma is suspected based on positive staining for p16, the diagnosis should be confirmed by other microscopic features, the identification of HPV DNA in the tumor cells using in situ hybridization or polymerase chain reaction (PCR), or by identifying a primary tumor with similar morphology in the cervix. Argyrophilic cells are found in more than 50% of intestinal-type borderline mucinous tumors, and immunohistochemical stains reveal peptides such as serotonin, adrenocorticotropic hormone (ACTH), synaptophysin, glucagon, gastrin, and chromogranin in them. Other stains that are commonly positive include carcinoembryonic antigen (CEA) and villin, which shows staining along the luminal borders. Staining for CA19-9 is ubiquitous in mucinous tumors. Estrogen and progesterone receptors are absent in intestinal mucinous tumors. Some mucinous carcinomas show positive staining for Her-2/Neu, as well as Her-2/Neu gene amplification as demonstrated by fluorescence in situ hybridization (FISH), potentially providing a therapeutic option for patients with Her-2/Neu–positive tumors. Intestinal-type mucinous tumors generally do not stain for common female genital tract markers such as estrogen and progesterone receptors, CA125, and PAX8. If staining for CA125 is present, the possibility of a metastasis from the pancreas or biliary tract should be suspected.
Endocervical-like borderline mucinous tumors (EBMT), also known as Müllerian mucinous or seromucinous borderline tumors, comprise 5% to 15% of borderline mucinous tumors. Most patients are premenopausal, with an average age in the early 30s. The tumors are bilateral in up to 50% of cases. The prognosis is favorable and similar to that of a borderline serous tumor, with a benign evolution even when noninvasive extraovarian tumor implants are present. Patients who are treated conservatively by cystectomy sometimes develop recurrences in the ipsilateral ovary.
Grossly, there are one or a few cysts that contain mucinous fluid. Branching papillae grow from the cyst linings into the lumens, reminiscent of a borderline serous tumor ( Fig. 13A.23 ). Tumor growth on the surface of the ovary is noted occasionally. Microscopically, the cysts and papillae are lined predominantly by columnar mucinous endocervical-like cells, with variable numbers of indifferent cells with eosinophilic cytoplasm ( Fig. 13A.24 ). Foci of endometrioid and serous differentiation are commonly present, and tufts of polygonal indifferent cells are often located at the tips of the papillae, from which clusters of cells detach into the cyst lumen. Neither goblet cells nor intestinal absorptive-type cells are present. Mitotic figures are infrequent in most tumors, and there is usually minimal nuclear atypia. There is greater nuclear atypia and mitotic activity in a few EMBT, and these are designated as showing intraepithelial carcinoma. Polymorphonuclear leukocytes are typically focally numerous in the luminal mucin, the epithelium, and the stroma. Endometriosis is present in the ovary adjacent to the tumor in 30% to 70% of cases, and in some instances the tumor appears to originate in the endometriosis. Stromal microinvasion of less than 10 mm 2 in maximum dimension is present in 15% to 30% of EBMT. This most often takes the form of clusters of eosinophilic or pale cells, usually with little surrounding reaction, within the stroma. EBMT are frequently bilateral, and peritoneal implants are present in 20% to 30% of cases. The implants are generally of the noninvasive epithelial type and frequently are surrounded by desmoplastic stroma. Neither intraepithelial carcinoma nor stromal microinvasion has been associated with an unfavorable outcome.

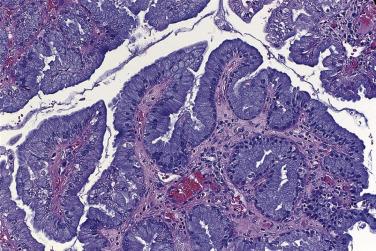
Some mucinous carcinomas lack intestinal differentiation and contain a prominent component of endocervical-like cells. The tumors often contain, in addition to the carcinoma, areas with features of an endocervical-like borderline mucinous tumor. Endocervical-like mucinous carcinomas appear to be somewhat smaller than intestinal mucinous carcinomas, with an average diameter of about 12 cm. About half the cases are bilateral, and most are cystic with solid areas. The tumor cells line glands, cysts, and papillae. There is a prominent component of columnar cells with mucinous cytoplasm that resemble endocervical cells, although other patterns of differentiation, especially endometrioid, are also commonly present. Nuclear atypia and mitotic activity tend to be less than what is seen in intestinal-type mucinous carcinomas. The pattern of invasion can be expansile or infiltrative, or both patterns can be present. The immunophenotype is similar to that of endocervical-like borderline mucinous tumors, with strong staining for CK7 and for estrogen and progesterone receptors, and absent or at most weak staining for CK20. The category of seromucinous carcinoma of the ovary was added to the 2014 WHO Classification of Tumors of Female Reproductive Organs, but recent studies have suggested that seromucinous carcinoma is not a distinct entity, and these tumors usually represent endometrioid carcinomas or occasionally low-grade serous carcinoma.
Endocervical-like borderline mucinous tumors have a different immunophenotype than intestinal-type mucinous tumors. Their immunophenotype is more like that of serous and endometrioid (Müllerian) borderline tumors than intestinal-type mucinous borderline tumors. They show strong positive staining for CK7, but they are CK20 and CDX2 negative. Endocervical-like tumors are mainly CEA negative, although the eosinophilic indifferent cells that are often present in borderline seromucinous tumors can exhibit strong staining for CEA. These tumors are typically positive for estrogen and progesterone receptors, and there is positive cytoplasmic/membranous staining for vimentin in about half of such tumors. They differ from borderline serous tumors in that they are WT1 negative, although they can show positive staining for CA125. Molecular studies have shown mutations in KRAS, PIK3CA, PTEN, and ARID1A and there is often loss of expression of ARID1A on immunohistochemical stains.
There is a close association between mucinous tumors and some other types of ovarian tumor, including Brenner tumors, Sertoli-Leydig cell tumors, and teratomas. Brenner tumors and mucinous tumors frequently coexist. Mucinous epithelium can be prominent in some Brenner tumors, known as metaplastic Brenner tumors, and expansion of this mucinous component may give rise to some mucinous tumors. Sertoli-Leydig cell tumors can contain heterologous elements, the most common of which is mucinous epithelium. The mucinous epithelium is generally a focal finding, but it can be prominent enough to suggest a mucinous cystadenoma, and in a few cases it is proliferative and atypical enough to be compatible with a borderline mucinous tumor or even a carcinoma. In such cases the Sertoli-Leydig cell tumor growing in the stromal compartment between the mucinous cysts may be overlooked. Ovarian mucinous tumors are occasionally associated with teratomas, and some mucinous tumors appear to be derived from teratomas. Any type of mucinous tumor can be associated with a teratoma. In two large series there were 41 mucinous cystadenomas, 44 borderline mucinous tumors (including 4 with intraepithelial carcinoma), and 11 mucinous carcinomas. Any combination of staining for cytokeratin 7 and cytokeratin 20 can be seen. About half of cystadenomas are CK7-/CK20+, but they can also be CK7+/CK20- or CK7+/CK20+. Borderline tumors are usually either CK7+/CK20+ or CK7-/CK20+ while carcinomas are usually CK7-/CK20+. Some carcinomas have a signet ring cell component. Tumors with a lower gastrointestinal immunophenotype (CK7-/CK20+) may originate from intestinal epithelium in the teratoma. The others may arise from nonintestinal mucinous epithelium in the teratoma, but they may also be collision tumors in which the mucinous tumor is of epithelial, rather than germ cell, origin. Pseudomyxoma ovarii is often prominent, especially in women with borderline tumors or carcinomas, and some women have pseudomyxoma peritonei, presumably of ovarian origin in this unusual setting. The pseudomyxoma peritonei may have a more favorable prognosis than the more common form of pseudomyxoma peritonei associated with an appendiceal or colonic tumor. It is always necessary to rule out the possibility that the ovarian mucinous tumor could be a metastasis from an appendiceal or colonic tumor, particularly in patients whose tumors have a CK7-/CK20+ immunophenotype and in those with pseudomyxoma peritonei. If there is no evidence of a potential extraovarian primary and sections of the ovary reveal evidence of a teratoma as well as a mucinous neoplasm, it is reasonable to conclude that the mucinous tumor is of primary ovarian origin, presumably arising in the teratoma, despite an unusual immunophenotype or clinical setting. Tissue genotyping can be useful in the distinction of ovarian mucinous tumors arising in teratomas from metastatic somatic mucinous tumors.
Endometrioid tumors of the ovary have an epithelial component that resembles proliferative, hyperplastic, or malignant endometrium. Benign and borderline endometrioid tumors are rare. Borderline endometrioid tumors occur at an average age of 50 to 51 years, are generally unilateral and confined to the ovary, and have a favorable outcome with no recorded deaths from tumor even when microinvasion is present. Endometrioid carcinoma, which histologically resembles endometrial adenocarcinoma, accounts for about 11% of all malignant epithelial tumors of the ovary. The percentage of ovarian cancers that are of endometrioid type is lower in contemporary than in older reports, probably because many tumors formerly classified as high-grade endometrioid carcinomas are now classified as high-grade serous carcinomas. Endometrioid carcinoma has a favorable prognosis, relative to other types of adenocarcinoma of the ovary, at least partly because it tends to be low grade and to be detected at an early stage. Low-grade stage I endometrioid carcinomas with confluent invasive growth only rarely progress, although those with a destructive pattern of invasion can be more aggressive, as are higher grade tumors. Endometrioid carcinoma commonly occurs in women with endometriosis, and as many as a third of endometrioid carcinomas arise in, or adjacent to, endometriosis, with possibly a greater risk in patients with atypical endometriosis. About a third of endometriosis-associated endometrioid carcinomas have mutations in the ARID1A tumor suppressor gene. Endometrioid adenocarcinoma can occur in women with Lynch syndrome (hereditary nonpolyposis colon cancer syndrome), in whom it is related to mutations in the mismatch repair proteins. In a study of 63 members of Lynch syndrome families, cancers developed at a mean age of 48 years. Endometrioid carcinoma was overrepresented, accounting for 35% of the cases.
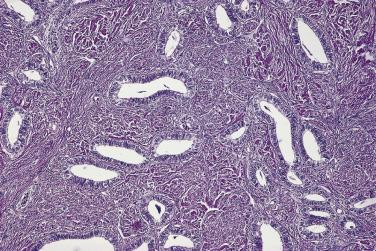
Benign endometrioid tumors are all adenofibromas and they comprise about 10% of ovarian adenofibromas. They are solid fibrous tumors that average 8 to 10 cm in diameter. The cut surfaces are tan or white and contain small cysts ranging from a few millimeters to several centimeters in diameter. Microscopically, tubular or cystic glands are surrounded and separated by fibrous stroma ( Fig. 13A.25 ). The glands are lined by a single layer of endometrial-type cells. These are columnar, with basophilic or amphophilic cytoplasm and uniform round or oval nuclei. The nuclei may be pseudostratified, as in proliferative endometrium, but mitotic figures are usually rare or absent. Squamous metaplasia may be present, and in rare tumors the glands are lined by ciliated cells.
Various names have been used for borderline endometrioid tumors, including proliferative, atypical, atypical proliferative, borderline, and tumor of low malignant potential. At the low end of the spectrum of borderline endometrioid tumors are adenofibromas in which the glandular component resembles simple hyperplasia of the endometrium. In the middle are adenofibromas in which glands are more crowded and irregular, as seen in complex hyperplasia of the endometrium. The epithelium in the highest-grade borderline endometrioid tumors exhibits about the same degree of proliferative activity and nuclear atypia as is seen in atypical hyperplasia of the endometrium.
Borderline endometrioid tumors are usually unilateral (only 8% were bilateral in one large series), and many are adenofibromas or cystadenofibromas. They are predominantly solid, but some, especially higher grade tumors, contain cysts. The average diameter is 6 to 10 cm, and the cut surfaces are tan or white. Papillary excrescences are grossly visible within the cysts in some tumors. Microscopically, low-grade borderline endometrioid tumors are adenofibromas with glands of increased density relative to benign endometrioid tumors, showing varied architecture and focal crowding. There may be small foci of cribriform growth or limited areas in which the glands are closely packed. The epithelial cell nuclei are pseudostratified or stratified, there is mild to moderate nuclear atypia, and occasional mitotic figures are present (<3 per 10 hpf). More proliferative borderline endometrioid tumors exhibit two main patterns of growth. Some are adenofibromas with increased glandular density; conspicuous areas of glandular crowding ( Fig. 13A.26 ), usually exceeding 5 mm in diameter; foci of cribriform growth; and prominent nuclear atypia, stratification, and mitotic activity. The second pattern is one of papillary growth into cystic spaces. The papillae often have a villoglandular appearance with broad fibrovascular cores. The cells covering the papillae show variable degrees of stratification and atypia. Mitotic activity is usually low (<3 per 10 hpf), but in some tumors mitotic figures are more frequent. Proliferative activity can be conspicuous in borderline endometrioid tumors, but the degree of glandular crowding is usually less than in endometrioid carcinoma, and the proliferative nests have a smooth contour and are surrounded by stroma. If there is extensive confluent glandular or papillary growth the tumor should be classified as a low-grade endometrioid adenocarcinoma, not as a borderline tumor. Squamous and mucinous metaplasia is common, particularly in adenofibromatous tumors, and rare neoplasms contain numerous ciliated cells. Endometriosis is often present in the involved ovary and elsewhere in the pelvis, and an origin from endometriosis can be demonstrated in occasional cases. Stromal microinvasion is seen in 5% to 15% of borderline endometrioid tumors, in which small irregular glands infiltrate the stroma or there are foci of confluent glandular growth in an area less than 10 mm 2 . No patients with microinvasion have had recurrences.

Endometrioid carcinoma is a cystic and solid or completely solid tumor, typically measuring 10 to 20 cm in diameter. The solid areas can be firm or soft, and they are gray or tan. Areas of hemorrhage and necrosis are common. Only 10% to 20% are bilateral.
Endometrioid carcinoma is a glandular or papillary tumor that resembles adenocarcinoma of the endometrium. The growth pattern is glandular, papillary, or a mixture of the two, and invasion can take the form of haphazard infiltration of the stroma or confluent growth. The glands tend to be small and relatively uniform in size and shape ( Fig. 13A.27 ). The papillae often have a villoglandular appearance, with prominent fibrovascular supporting cores. The glands and papillae are lined by columnar cells with amphophilic to basophilic cytoplasm. The nuclei are basally located, round or oval, and may contain nucleoli ( Fig. 13A.28 ). The degree of atypia, the amount of nuclear stratification, and the extent to which the glands coalesce into foci of solid growth increase as the grade increases. Endometrioid carcinoma can be graded using the same criteria as endometrial adenocarcinoma. Grade 1 tumors grow in a glandular or papillary pattern with less than 5% of solid tumor growth. Grade 2 tumors show 5% to 50% solid growth, and grade 3 tumors show more than 50% solid tumor growth. Areas of squamous or spindle cell differentiation are not counted in the determination of percentage of solid growth. If the nuclei are grade 3 and the growth pattern is grade 1 or 2, the final grade is increased by one level. Endometrioid carcinoma can also be graded using the previously discussed universal grading system for ovarian cancer (see Box 13A.3 ).
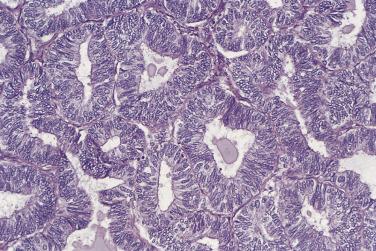
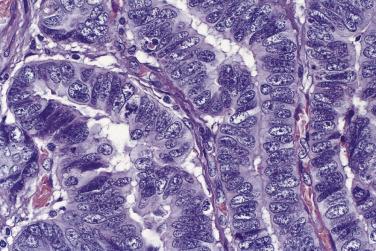
Foci of squamous differentiation, which can be either cytologically benign or malignant, are present in 25% to 50% of endometrioid carcinomas. Keratin granulomas, which the surgeon may mistake for tumor implants, are found occasionally on the peritoneum in such patients. Mucinous differentiation is commonly present in endometrioid carcinomas. Endocervical-like columnar cells with basophilic mucinous cytoplasm or cells with intermediate features between endocervical and endometrioid type cells, but with mucinous cytoplasm, may be present. The cytoplasmic mucin shows diffuse staining with the periodic acid–Schiff diastase (PASD) stain. Goblet cells or other features of intestinal-type differentiation are not present.
High-grade endometrioid adenocarcinoma needs to be differentiated from high-grade serous carcinoma. Both can grow in glandular and solid patterns. Findings that favor interpreting a high-grade adenocarcinoma as an endometrioid adenocarcinoma include the presence of differentiated glands with a clearly endometrioid appearance, the presence of squamous metaplasia, an adenofibromatous component in the tumor, and an association with endometriosis. Immunohistochemical staining can also help correctly classify the tumor. Positive staining for WT1 and diffuse strong staining for p53 and p16 are more in keeping with serous carcinoma than endometrioid carcinoma, although rare endometrioid carcinomas show nuclear staining for WT1. A type of high-grade carcinoma known as undifferentiated carcinoma has received increasing attention. Undifferentiated carcinoma can be either a pure type or coexist with endometrioid adenocarcinoma. This type of carcinoma is discussed in a subsequent section, but it is important to recognize it and, particularly when it is combined with glandular endometrioid adenocarcinoma, not confuse it with a form of intermediate-grade or high-grade endometrioid carcinoma. It appears to be a more aggressive variant of ovarian carcinoma that warrants specific diagnosis.
There are several unusual and potentially confusing variants of endometrioid carcinoma, some of which mimic sex cord–stromal tumors. A microglandular pattern is uncommon in endometrioid carcinoma, but when one is present, the carcinoma can be mistaken for a granulosa cell tumor. Sertoliform endometrioid carcinomas have prominent areas in which the tumor cells grow in long, branching, tubular glands or trabeculae ( Fig. 13A.29 ). This variant mimics a Sertoli or Sertoli-Leydig cell tumor, particularly when the stroma is abundant and fibrous and luteinized stromal cells are present. There are also rare variants of Sertoli-Leydig cell tumor that mimic endometrioid carcinoma because they contain open tubules that resemble endometrioid glands. Clinical, microscopic, and immunohistologic features all serve to differentiate these variants of endometrioid carcinoma from a sex cord–stromal tumor. They occur in perimenopausal or postmenopausal women and are seldom associated with clinical evidence of steroid hormone production. Grossly, they usually lack the golden or yellow appearance of the cut surface that typifies a sex cord–stromal tumor, and they may be bilateral. Microscopically, careful search usually reveals foci of typical endometrioid carcinoma as well as squamous metaplasia or adenofibromatous regions.
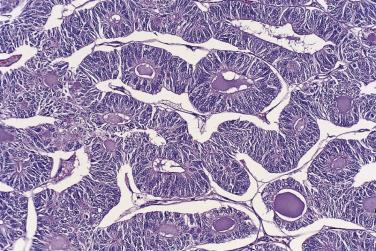
Other variants of endometrioid carcinoma that can cause diagnostic problems include the oxyphilic variant, tumors with spindle cells, and tumors with clear cells. The oxyphilic variant of endometrioid carcinoma has a prominent component of large polygonal tumor cells with abundant eosinophilic cytoplasm and round central nuclei that contain large nucleoli. The tumor cells grow in nests and sheets or line tubular glands. The spindle cell variant has a prominent component of bland spindle cells. The combination of ribbons and cords of tumor cells admixed with lobulated nests of spindle cells can resemble a sex cord–stromal tumor. Some endometrioid carcinomas contain foci of clear cells, usually growing in nests or lining glands. The characteristic patterns of clear cell carcinoma, such as tubulocystic glands, papillae, and large sheets of clear cells are not present, and focal clear cell changes in an endometrioid carcinoma are not viewed as a manifestation of a clear cell carcinoma component.
Rare tumors have been described in which there is a mixture of endometrioid carcinoma and yolk sac tumor. These occur in the same age group as endometrioid carcinoma, are large, and have an unfavorable prognosis. Immunostains for α-fetoprotein (AFP) and other yolk sac tumor markers, such as glypican-3 and SALL4, are strongly positive in the yolk sac tumor component, while epithelial markers such as CK7 and EMA are positive in the carcinoma.
Endometrioid adenocarcinoma is found in the endometrium in 10% to 20% of women with ovarian endometrioid carcinoma ( Fig. 13A.30 ). In cases where the endometrial cancer is superficial and well differentiated and the carcinomas are confined to the uterus and ovary, the patients have excellent clinical outcomes. In this setting, the ovarian and endometrial carcinomas were thought to be more compatible with separate, synchronous primary tumors than with advanced endometrial adenocarcinoma with ovarian metastasis or advanced ovarian cancer with endometrial metastasis. Recent sequencing data, however, have shown that such tumors are in fact clonally related and that endometrium is the likely primary site for the ovarian tumor. In some patients with simultaneous tumors, there are many histologic features suggesting that the ovarian neoplasm is a metastasis from the endometrial carcinoma. These include a high-grade or deeply invasive endometrial carcinoma, presence of ovarian hilar lymphatic vascular invasion, small ovarian tumor, multinodular and solid ovarian tumor, bilateral ovarian involvement, presence of surface implants on the ovary, and presence of extraovarian metastases in a distribution characteristic of endometrial adenocarcinoma (i.e., lymph node metastases more likely than peritoneal metastases).
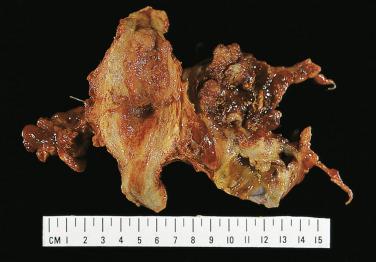
All endometrioid tumors have similar immunohistochemical features. Most endometrioid ovarian tumors are endometrioid carcinomas, and they have accordingly been studied most extensively so this discussion focuses on them. Endometrioid carcinoma is strongly immunoreactive for cytokeratin and epithelial membrane antigen. It is cytokeratin 7 positive and generally negative for cytokeratin 20. There is basal or perinuclear staining for vimentin in about a third of endometrioid carcinomas. Nuclear staining for estrogen and progesterone receptors is present in most endometrioid tumors, including carcinomas that exhibit glandular differentiation.
Most epithelial tumors, including endometrioid tumors, show membranous staining for β-catenin. Nuclear staining, which is more common in endometrioid tumors than in other types of epithelial neoplasm, is the only type of staining that is associated with β-catenin mutation. Borderline endometrioid tumors, particularly those with morules, appear to be particularly likely to show nuclear staining for β-catenin, most prominently in the cells in squamous morules. The cells in squamous morules can also exhibit nuclear staining for CDX2 and cytoplasmic staining for CD10.
The growth patterns in some endometrioid carcinomas mimic sex cord–stromal tumors such as Sertoli cell tumors, Sertoli-Leydig cell tumors, or granulosa cell tumors; the Sertoliform variant of endometrioid carcinoma is the most common of these. Immunohistochemistry is very helpful in the differential diagnosis between an endometrioid carcinoma and a sex cord–stromal tumor. Endometrioid carcinomas generally show positive staining for EMA and estrogen and progesterone receptors, and they do not stain for inhibin or calretinin or for FOXL2. They are also more likely to stain for CK7. While some sex cord–stromal tumors stain for cytokeratin, they are negative for EMA and show cytoplasmic staining for inhibin, cytoplasmic and nuclear staining for calretinin, and, typically, nuclear staining for FOXL2. The tubules in pseudoendometrioid Sertoli-Leydig cell tumor show a similar staining pattern as is seen in typical Sertoli-Leydig cell tumors: They are negative for EMA and CK7, but positive for inhibin and calretinin, as well as other sex cord–stromal markers.
Various types of metastatic adenocarcinoma can mimic endometrioid adenocarcinoma. Metastatic colorectal adenocarcinoma is the most common of these. Diffuse strong staining for CK7 and lack of staining for CK20 are characteristics of endometrioid carcinoma. Metastatic colorectal adenocarcinoma is generally negative for CK7 and shows diffuse strong cytoplasmic staining for CK20 and nuclear staining for CDX2 and SATB2. Colorectal adenocarcinoma typically stains for carcinoembryonic antigen, while endometrioid carcinoma does not, although it must be noted there can be focal staining for CEA in areas of squamous differentiation. Other stains that can be helpful in this differential diagnosis include PAX8 and CA125, which are generally positive in endometrioid carcinoma but not in metastatic colorectal carcinoma. Metastatic cervical adenocarcinoma can also mimic endometrioid adenocarcinoma. Positive immunostaining for carcinoembryonic antigen, p16, and a positive molecular test for HPV (in situ hybridization or PCR) support a diagnosis of metastatic cervical adenocarcinoma. Staining for p16 must be strong and diffuse (nearly all tumor cells positive) to be indicative of metastatic cervical adenocarcinoma, as primary ovarian carcinomas, including endometrioid adenocarcinomas, can show lesser degrees of staining, usually with a mosaic pattern.
Carcinosarcoma, or mixed mesodermal tumor (MMT), and endometrioid stromal sarcoma arise from endometriosis or by metaplasia. Adenosarcoma can arise directly from ovarian epithelium and stroma, or in endometriosis. The clinical behavior of these tumors is similar to that of their more frequent uterine counterparts.
Carcinosarcoma, also known as mixed mesodermal (or mixed Müllerian) tumor, occurs predominantly in postmenopausal women of low parity. A few patients have had a history of tamoxifen therapy. The most common symptoms are pelvic or abdominal pain, abdominal distention, bowel symptoms, and weight loss. The serum concentration of CA125 is usually elevated. Most patients have a palpable adnexal mass and many have ascites. More than 70% of carcinosarcomas have spread beyond the ovaries at diagnosis. The pattern of spread, as in ovarian carcinoma, is primarily to the peritoneum, omentum, and regional lymph nodes. Treatment is by hysterectomy, bilateral salpingo-oophorectomy, and excision of as much extraovarian tumor as possible, followed by chemotherapy. The most common chemotherapy includes a platinum-based drug, a taxane, and sometimes ifosfamide. Patients with disease of limited extent should be thoroughly staged. The prognosis is poor; in studies of more than 400 patients the median survival was only 6 to 12 months and more than 70% of patients were dead at 1 year. In most studies there has been a poor response to chemotherapy and a worse prognosis than for comparably staged carcinomas, although a few authors have reported a more favorable response to chemotherapy. Nearly all patients eventually relapse, and the 5-year survival rate in some studies is less than 10%. The stage is the most important prognostic factor. Histologic parameters, such as the type and grade of the carcinomatous and sarcomatous elements, do not appear to be of prognostic significance. Rare patients present with early stage tumors (stage I–II); they have longer survival and are the most likely to be cured.
Carcinosarcomas tend to be large, with an average diameter of 15 cm. They are either cystic and solid or entirely solid tumors. The solid portions are gray or tan, and areas of hemorrhage and necrosis are usually prominent.
Microscopically, carcinosarcoma is a biphasic neoplasm with intermixed epithelial and mesenchymal elements ( Fig. 13A.31 ). The epithelial component can be any type of carcinoma, but serous, endometrioid, and undifferentiated carcinoma are most common. The mesenchymal component is a pure sarcoma or a mixture of various types of sarcoma. Homologous sarcomatous elements include fibrosarcoma, leiomyosarcoma, and undifferentiated sarcoma. Heterologous elements, such as rhabdomyosarcoma, chondrosarcoma or osteosarcoma are present in a majority of ovarian carcinosarcomas ( Fig. 13A.32 ). Eosinophilic hyaline globules are often present in carcinosarcomas, scattered among either the epithelial or mesenchymal cells. These globules are PAS positive and some are immunoreactive for α1-antitrypsin. Singular cases of MMT have been reported to show trophoblastic or neuroectodermal differentiation or to express AFP. Microscopic study of peritoneal and omental metastases present at diagnosis generally reveals a combination of carcinoma and sarcoma, while lymph node metastases are usually pure carcinoma. Late metastases reportedly contain an increased proportion of the sarcomatous component or are entirely sarcomatous.
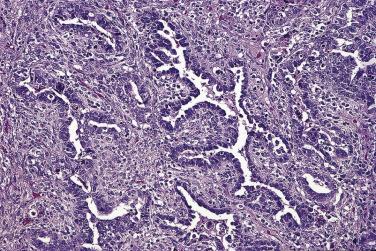
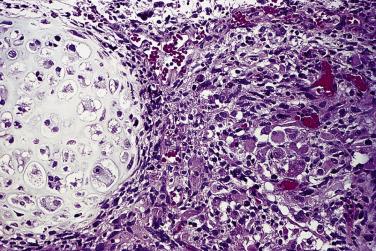
Adenosarcoma occurs in younger women than carcinosarcoma, the average age being in the mid-50s. The symptoms are nonspecific, and most tumors are unilateral. In contrast with carcinosarcoma, a majority of patients present with early stage tumors. Adenosarcoma tends to recur within the pelvis and abdomen, and a majority of patients eventually die of tumor. The role of postoperative radiation and chemotherapy has yet to be clearly defined.
Adenosarcoma is a large, partly cystic tumor 10 to 15 cm in diameter. Microscopically it is a mixed tumor in which the mesenchymal component is malignant, but the epithelium is benign. The epithelium lines cysts, covers intracystic papillary or polypoid stromal excrescences, and lines elongated clefts and simple tubular glands in solid portions of the tumor. The epithelium ranges from indifferent cuboidal cells to columnar endometrioid or ciliated tubal-type cells. Foci of squamous or mucinous metaplasia are present in some tumors. The mesenchymal component is usually fibrosarcoma or endometrioid stromal stroma, although heterologous sarcomatous elements, such as rhabdomyosarcoma or chondrosarcoma, are present occasionally. In one case, the stroma was decidualized. The stroma tends to be hypercellular beneath the cyst lining and around the glands, and less cellular and more collagenous away from them. The degree of nuclear atypia is usually mild or moderate. Mitotic figures are invariably present, and usually number 4 or more per 10 hpf. Sex cord–like differentiation is occasionally present within the stroma, and a minority of adenosarcomas exhibit sarcomatous overgrowth, in which epithelium is absent and there is diffuse growth of malignant cellular stroma. Recurrent tumor can be either pure sarcoma or adenosarcoma. Unfavorable prognostic findings include young age at diagnosis, tumor rupture, high-grade sarcomatous elements, and sarcomatous overgrowth.
The uterus is the usual primary site for stromal sarcoma, but it can arise in extrauterine sites, including the ovaries. The average patient is 50 to 55 years old and presents with abdominal distention or pain. Extraovarian spread is often present at diagnosis. Women with low-grade stromal sarcoma have a relatively favorable short-term prognosis, but a significant percentage of tumors recur, including a substantial number more than 5 years after diagnosis, and some patients die of tumor. Analysis of the clinical evolution is difficult because of limited follow-up. The value of treatment with progesterone, radiotherapy, or chemotherapy is unclear, although there are anecdotal reports of favorable responses to progesterone therapy. High-grade stromal sarcoma, also known as undifferentiated endometrioid sarcoma, appears to pursue a more aggressive clinical course.
Stromal sarcoma averages 11 cm in diameter. Most tumors are solid or solid and cystic and have tan, yellow, or white cut surfaces with foci of hemorrhage or necrosis. Microscopically, low-grade stromal sarcoma is composed of small cells that resemble proliferative phase endometrial stromal cells. They have uniform round, oval, or spindled dark nuclei with fine chromatin and inconspicuous nucleoli ( Fig. 13A.33 ). The cytoplasm is scanty, and the cell borders are ill defined. Cytologic atypia is minimal, and mitotic figures are infrequent. Fibromatous or sex cord–like differentiation may be present. Nests and cords of tumor cells infiltrate the ovarian parenchyma, and vascular invasion can be prominent, particularly once the tumor invades beyond the ovary. An origin in (or association with) ovarian endometriosis can occasionally be demonstrated. High-grade stromal sarcoma is composed of cells with a resemblance to endometrial stromal cells but that are more atypical or that exhibit greater mitotic activity (>10 mitotic figures per 10 hpf).

The main differential diagnostic considerations are endometrial stromal sarcoma of the uterus metastatic to the ovary, metastatic gastrointestinal stromal tumor (GIST), and thecoma. Endometrial stromal sarcoma frequently invades the adnexa and extends to the ovary, and stromal sarcoma is considerably more likely to develop in the uterus than the ovary. Any woman with stromal sarcoma of the ovary should have careful evaluation of the uterus, and hysterectomy should be part of the surgical treatment. Low-grade stromal sarcoma may contain fibrous areas that mimic thecoma. Thorough histologic evaluation usually reveals areas typical of stromal sarcoma, and immunohistochemical stains for inhibin and other sex cord–stromal markers, which are usually positive in a thecoma, are negative in stromal sarcoma.
Accurate diagnosis of these tumors can require identification of an epithelial component or correct identification of various mesenchymal cell types. The epithelial components of carcinosarcoma and adenosarcoma include various types of ovarian epithelia, and they accordingly stain for cytokeratin, cytokeratin 7, EMA, and often for other female genital tract markers such as CA125 and PAX8. Immunohistochemical testing for cytokeratin or EMA can help the pathologist identify an epithelial component in a carcinosarcoma or adenosarcoma that consists predominantly of mesenchymal cells. The type of epithelial elements present can sometimes be delineated with immunohistochemistry. For example, serous carcinoma is likely to be p53 and p16 positive, while clear cell carcinoma may stain for hepatocyte nuclear factor-1β. Often, when the epithelial component is p53 positive so is the mesenchymal component. Immunostains for desmin and myogenin are useful to confirm the presence of rhabdomyoblasts, and chondroid elements are often S100 positive; smooth muscle elements stain for smooth muscle actin and desmin, and endometrioid stromal cells are CD10 positive. The mesenchymal component of an adenosarcoma is often CD10 and ER positive, and sometimes positive for progesterone receptors.
Endometrioid stromal sarcoma exhibits a distinctive positive staining reaction for CD10, while sex cord–stromal tumors, with which it may be confused, are typically positive for inhibin and calretinin. Endometrioid stromal sarcomas that contain foci of epithelioid or sex cord–like differentiation pose particular diagnostic problems since these can express inhibin or calretinin. In addition to diffuse staining for CD10, endometrioid stromal sarcomas tend to show strong positive staining for estrogen and progesterone receptors.
Initially thought to be of mesonephric origin, clear cell tumors were shown by Scully and Barlow to arise from endometriosis or from the surface epithelium. Most clear cell tumors are carcinomas, but benign and borderline clear cell tumors also occur. Women with borderline clear cell tumors generally have a favorable prognosis, but exceptional patients develop metastases or die of their tumor.
More than 90% of clear cell tumors are carcinomas. This type of carcinoma comprises from 5% to 12% of ovarian cancers in North America and other Western countries, with the highest percentages reported most recently. Since clear cell carcinoma is often detected at an early stage, studies that concentrate only on advanced stage tumors contain a lower percentage of clear cell carcinomas. Interestingly, in Japan, clear cell carcinoma constitutes a higher percentage of epithelial ovarian cancers (20%–25%) than in western countries. Survival rates that are favorable, or at least equivalent to those observed with other malignant epithelial tumors, have been found in many studies of clear cell carcinoma, particularly for patients with early stage tumors. The trend recently, however, has been to view clear cell carcinoma as an unfavorable histologic type because of a worse prognosis in advanced stages compared to other common epithelial tumors and a poor response to platinum-based chemotherapy. Recurrent clear cell carcinoma appears to be particularly resistant to chemotherapy and it is accordingly difficult to treat; some studies show radiation to be beneficial. Several paraneoplastic syndromes occur in women with clear cell carcinoma. Clear cell carcinoma is more likely than other types of epithelial carcinoma to be associated with hypercalcemia, and women with clear cell carcinoma also are more likely to have thromboembolic events such as deep venous thrombosis and pulmonary emboli. There is a strong association between clear cell carcinoma and endometriosis, with clear cell carcinoma patients often having endometriosis in the ovaries or elsewhere in the pelvis. Atypical endometriosis has been proposed as a possible precursor of clear cell carcinoma, and recently mutations in the ARID1A gene and loss of staining for BAF250a by immunohistochemistry have been detected in ovarian cancers associated with endometriosis, including 46% to 57% of patients with clear cell carcinoma. Clear cell carcinoma appears overrepresented among women with Lynch syndrome.
Benign clear cell tumors are unilateral solid tumors 3 to 15 cm in diameter. Small cysts can usually be identified on close inspection of the white or tan cut surfaces. Microscopically all benign clear cell tumors are adenofibromas. Small tubules or cysts are lined by cuboidal or hobnail cells with clear or granular eosinophilic cytoplasm ( Fig. 13A.34 ). The stroma is composed of spindle-shaped fibroblasts and collagen bundles. There is no cytologic atypia or mitotic activity.
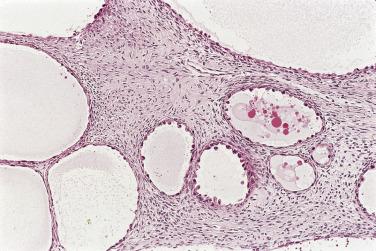
Borderline clear cell tumors are unilateral and predominantly solid. They typically measure 10 to 15 cm in diameter. The cut surface is white, gray, or tan and contains small to midsize cysts. Microscopically, borderline clear cell tumors are all adenofibromas. Tubules and cysts lined by cuboidal or hobnail cells with clear or eosinophilic cytoplasm are irregularly distributed in a fibrous stroma. Occasionally, the epithelium is stratified or tufted, or grows as small solid circumscribed nests. The presence of mild to moderate nuclear atypia and scattered mitotic figures (usually <1 per 10 hpf) differentiate a borderline clear cell tumor from a benign one. Rare clear cell tumors grow in a parvilocular pattern in which cysts lined by clear cells are surrounded by stroma. Most of these cases exhibit sufficient atypia or mitotic activity to be classified as borderline tumors. The absence of stromal invasion or confluent growth differentiates clear cell adenofibroma from clear cell carcinoma. Noninvasive clear cell adenofibromas with marked nuclear atypia or frequent mitotic figures can be classified as borderline tumors with intraepithelial carcinoma, although it should be noted that very few of these have been reported, and as a result their clinical behavior is not well defined. The diagnosis of a benign or borderline clear cell tumor should be made only after thorough histologic study since bland-appearing areas are often seen in clear cell carcinoma.
Clear cell carcinomas are usually large tumors, ranging from 10 to 30 cm in diameter. They are solid or, more often, partly cystic with solid gray-tan nodular areas in their walls. The cut surfaces are soft and tan or gray-white. Some clear cell carcinomas may arise from clear cell adenofibromas, while others appear to arise from endometriosis. In one study, cystic tumors were more likely to be diagnosed at an early stage, to be associated with endometriosis, to exhibit a papillary growth pattern, and to have a more favorable outcome than solid adenofibromatous tumors; in another study, however, adenofibromatous tumors were more likely to be of low stage at diagnosis and to have a more favorable prognosis. The significance, if any, of the various growth patterns needs further study. Tumors that are confined to the ovaries (stage I) are usually unilateral, but when tumors of all stages are considered, about 30% are bilateral.
Microscopically a variety of cell types are present, including clear cells, cells with granular eosinophilic cytoplasm, and hobnail cells ( Fig. 13A.35 ). Usually a mixture of cell types is present. Clear cells are cuboidal, low columnar, or polygonal and have abundant clear cytoplasm, central vesicular nuclei, and usually conspicuous nucleoli. PAS stains and electron microscopic studies reveal that they contain abundant cytoplasmic glycogen. Cells with eosinophilic cytoplasm are similar in size, shape, and nuclear features to the clear cells, but they have granular eosinophilic cytoplasm. Hobnail cells are columnar and have either granular eosinophilic or clear cytoplasm. They have hyperchromatic apical nuclei that bulge into the lumina. Mitotic activity tends to be lower in clear cell carcinoma than in other types of ovarian carcinoma; the low rate of cell proliferation has been proposed as one possible reason for the poor response to chemotherapy.
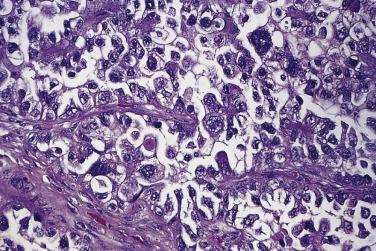
The tumor cells grow in a variety of patterns, and most clear cell carcinomas show a mixture of patterns. In some areas, the tumor cells line glands, tubules ( Fig. 13A.36 ), or cysts, or form papillae. Ringlike tubules lined by cuboidal cells with clear cytoplasm and filled with eosinophilic secretions are particularly characteristic. Often the tubules and papillae are lined only by one or two layers of tumor cells. Elsewhere there are areas of solid growth composed of polygonal cells with clear or eosinophilic cytoplasm ( Fig. 13A.37 ). Eosinophilic hyaline globules are often scattered among the tumor cells, and amorphous eosinophilic hyaline material is a frequent finding in the stroma or the connective tissue cores of the papillae. The hyaline appears to be of basement membrane–like material, based on its ultrastructural appearance and immunohistochemical staining for type IV collagen and laminin. The rare oxyphilic clear cell carcinoma is composed predominantly of large polygonal cells with abundant eosinophilic cytoplasm. Thorough sampling is necessary to reveal areas that are more typical of clear cell carcinoma, thereby establishing the diagnosis. Some clear cell carcinomas contain minor admixtures of endometrioid carcinoma or other types of surface epithelial carcinoma. Other types of epithelial ovarian tumor occasionally contain clear cells, and the diagnosis of clear cell carcinoma should be restricted to those showing a combination of characteristic cytologic features, architectural growth patterns, and immunohistochemical findings, rather than simply the presence of tumor cells with cytoplasmic clearing. Carcinomas with overlapping features of clear cell carcinoma and serous carcinoma are occasionally encountered; it has been shown that these are best regarded as variants of serous carcinoma. Papillary clear cell carcinomas are occasionally mistaken for other types of tumors, especially for borderline serous tumors. Features that suggest the correct diagnosis include a unilateral tumor, nonbranching papillae, a monotonous tumor cell population, the presence of more characteristic patterns of clear cell carcinoma, and an association with endometriosis.
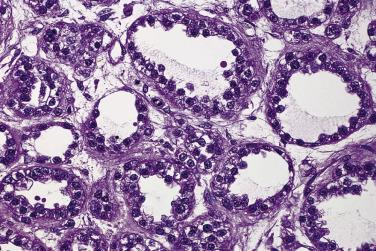
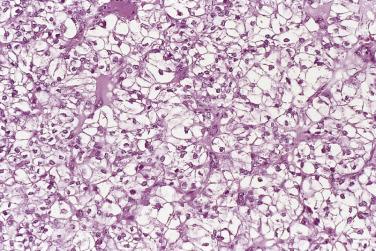
Clear cell carcinoma can be graded using the universal grading system (see Box 13A.3 ), but histologic grading has not proven to be of prognostic value in most studies.
Clear cell carcinoma is strongly associated with endometriosis, either within the involved ovary or elsewhere in the pelvis, and occasional examples of clear cell carcinoma arise directly from endometriosis. The percentage of cases associated with endometriosis, including atypical endometriosis, approaches or exceeds 50% in some series.
Clear cell tumors share many immunohistochemical features with other primary epithelial tumors of the ovary. The tumor cells show positive staining for cytokeratin, cytokeratin 7, and EMA. They stain for CD15 but are generally CK20 negative. Staining for estrogen and progesterone receptors is minimal or totally absent. Recently, hepatocyte nuclear factor-1β (HNF1β) has emerged as a sensitive marker for clear cell tumors, particularly clear cell carcinoma. Diffuse strong nuclear staining for HNF1β is observed in more than 80% of clear cell carcinomas. More recently, Napsin-A (Nap-A) has been introduced as a specific, but not sensitive, marker for clear cell carcinoma. Alpha-methylacyl-coenzyme A racemase (AMACR, P504S) is also positive in clear cell carcinoma of the ovary. A panel that includes HNF1β, Nap-A, WT1, and ER may help resolve a differential diagnosis between clear cell carcinoma and serous carcinoma. HNF1β and Nap-A are likely to be positive in clear cell carcinoma, while WT1 and ER are likely to be positive in serous carcinoma. While HNF1β has been reported as being relatively specific for clear cell tumors, we have noted that serous and endometrioid tumors sometimes show strong nuclear staining for HNF1β. At present, we view HNF1β positivity as supporting a diagnosis of a clear cell tumor, but do not think positive staining can be used to completely exclude a serous or endometrioid tumor. Diffuse positive nuclear staining for p53 has been reported by some but not by others. We find that staining for p53, when present, is usually irregular in distribution and less intense than is observed in serous carcinoma, where nuclear staining tends to be strong and diffuse when present. Clear cell carcinoma frequently contains waxy eosinophilic hyaline material in its stroma. This material appears to be basement membrane material based on its electron microscopic appearance and positive staining for laminin and type IV collagen. In summary, the typical clear cell tumor immunophenotype is cytokeratin 7, EMA, Nap-A, and HNF1β positive and estrogen receptor, progesterone receptor, WT1, and p53 negative.
In some cases, yolk sac tumor needs to be differentiated from a borderline clear cell tumor or a clear cell carcinoma. Knowledge of the clinical details of the case, especially the patient's age and the serum α-fetoprotein level, is helpful in arriving at the correct diagnosis. Clear cell carcinoma is generally AFP negative, while positive staining for this marker is one of the hallmarks of yolk sac tumor; staining for AFP can be weak or focal. Yolk sac tumors generally stain for glypican-3, which less often stains clear cell carcinoma, and then focally and weakly, and for the nuclear antigen SALL4. Clear cell carcinoma stains strongly for epithelial markers such as CD15, EMA, and cytokeratin 7, which are generally negative in yolk sac tumor, although exceptions occasionally occur.
Metastatic clear cell renal cell carcinoma can potentially mimic clear cell carcinoma of the ovary, although this is an infrequent diagnostic issue as renal cell carcinoma rarely spreads to the ovaries. Ovarian clear cell carcinoma shows positive staining for CK7, Nap-A, and p504s, while metastatic clear cell renal cell carcinoma is rarely CK7 positive but shows positive staining for CA-IX and CD10. Renal cell carcinoma antigen (RCC) and PAX2 are generally positive in metastatic renal cell carcinoma, but ovarian clear cell tumors also occasionally stain for these markers. Both renal and ovarian clear cell carcinomas show positive nuclear staining for PAX8 and HNF1β.
The Brenner tumor is a type of adenofibroma in which nests of transitional epithelium grow in a fibrous stroma. Brenner tumors comprise around 2% of all ovarian tumors. Most are small and are incidental findings. About 20% occur together with a mucinous or serous cystadenoma or a benign cystic teratoma or some other form of benign teratoma, such as a struma ovarii.
Grossly, Brenner tumors are circumscribed, firm, pale yellow or gray-white, solid fibrous tumors. Foci of calcification are present in many of them. Occasional Brenner tumors are partly cystic and about 5% are bilateral. The size ranges from microscopic to more than 10 cm, but they tend to be small; the average tumor measures only 1 to 2 cm. Microscopically, nests and cords of oval or polygonal epithelial cells resembling urothelial cells of the bladder grow in a fibrous stroma ( Fig. 13A.38 ). The nuclei are round or oval and have small nucleoli. A longitudinal nuclear groove is often present and is a characteristic feature of the Brenner tumor. The cytoplasm ranges from clear to dense and eosinophilic. Microcysts lined by transitional cells or metaplastic columnar endocervical-like mucinous cells occasionally develop within the cell nests, and there are occasional foci of squamous metaplasia. The stroma is composed of spindle-shaped fibroblastic cells and collagen in varying proportions. Luteinized cells are present in the stroma in 10% to 15% of Brenner tumors and, in some cases, are associated with evidence of steroid hormone secretion, such as endometrial hyperplasia.
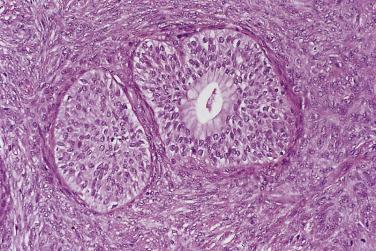
Borderline Brenner tumors were traditionally referred to as proliferating Brenner tumors because of their benign clinical evolution.
Borderline Brenner tumors are usually unilateral. They are circumscribed white or tan tumors that are considerably larger than benign Brenner tumors. The diameter ranges from 8 to 30 cm, with an average of 14 cm. Most are partially or largely cystic with polyps or friable papillae projecting into the lumens of cysts or in some cases growing as nests of cells in a fibromatous background. Microscopically the epithelium is proliferative and resembles low-grade papillary transitional cell carcinoma of the urinary tract. Much of the cyst lining consists of broad papillae with fibrovascular cores covered by stratified transitional cells ( Fig. 13A.39 ). Similar epithelium lines the nonpapillary parts of the cysts. Nests of low-grade transitional cells surrounded by fibrous stroma may be present in the wall of the tumor. The tumor cells have uniform, mildly atypical nuclei with slightly increased nucleus to cytoplasm ratios. Mitotic figures are usually not numerous, although they can occasionally be conspicuous. Squamous differentiation is seen in some proliferating Brenner tumors. A greater degree of nuclear atypia is the main feature distinguishing high-grade borderline Brenner tumors from low-grade ones. A diagnosis of intraepithelial carcinoma is warranted if there is significant nuclear atypia and mitotic activity in a noninvasive Brenner tumor. Epithelial proliferation may be remarkable in a borderline Brenner tumor, but it is completely circumscribed and usually intracystic, with no stromal invasion. Foci of benign Brenner tumor are mixed with, or adjacent to, most borderline Brenner tumors.
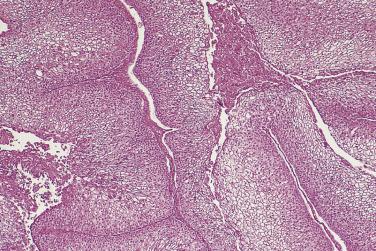
A malignant Brenner tumor has been defined as a carcinoma in which features of transitional cell, squamous cell, or undifferentiated carcinoma are associated with foci of benign or borderline Brenner tumor. A carcinoma with a transitional cell appearance, but with no associated benign Brenner tumor, was called transitional cell carcinoma in the past and is now thought to represent high-grade serous carcinoma or less frequently endometrioid carcinoma with a transitional growth pattern. It is possible that some transitional cell carcinomas of the ovary could represent a malignant Brenner tumor in which the benign or borderline Brenner tumor component has been overrun, but these would be rare. Malignant Brenner tumors are negative for WT1, lack TP53 mutation, and show activation of the PI3K pathway. TERT promoter mutations, which are frequently seen in urothelial carcinomas, are absent in benign and malignant Brenner tumors of the ovary.
Malignant Brenner tumor is unilateral and ranges from 5 to 25 cm in diameter. Most are large, partly cystic tumors with an average diameter of 15 cm. Solid regions are gray, yellow, or tan and frequently contain calcifications. Microscopically, malignant Brenner tumor usually resembles a high-grade transitional cell carcinoma of the urinary tract. The tumor cells are polygonal with moderate amounts of amphophilic cytoplasm and pleomorphic, atypical nuclei ( Fig. 13A.40 ). Nuclear grooves are not seen, and mitotic figures are numerous. The transitional epithelium lines cysts and broad papillae and invades the stroma in confluent masses or irregular cords and nests of cells. Squamous and glandular differentiation is common. Benign or borderline Brenner tumor must be intermixed with or adjacent to the carcinoma for a diagnosis of malignant Brenner tumor.
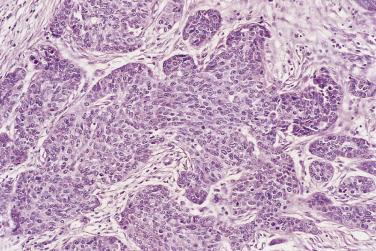
Immunohistochemical study of Brenner tumors of the ovary reveals that they are immunoreactive for carcinoembryonic antigen, the CA19-9 antigen, cytokeratin, epithelial membrane antigen, and cytokeratin 7 ( Table 13A.3 ). Brenner tumors have a staining pattern that suggests a urothelial line of differentiation, including staining for uroplakin III, thrombomodulin, CK20, S100P, and GATA3. Immunoreactivity for p63 is generally present in Brenner tumors. Immunostains for p53 and p16 are negative in benign, borderline, and malignant Brenner tumors.
| CK7 | CK20 | WT1 | CA125 | CEA | CDX2 | VIM | |
|---|---|---|---|---|---|---|---|
| Serous | + | - | + | + | - | - | - |
| TCC | + | - | + | + | - | - | - |
| Endometrioid | + | - | - | + | - | - | V |
| Mucinous, intestinal type | + | V | - | - | + | V | - |
| Clear cell | + | - | - | - | - | - | - |
| Metastatic colorectal | - | + | - | - | + | + | - |
Occasional ovarian cancers are too poorly differentiated to classify and are designated as undifferentiated carcinomas. It has been estimated that about 5% of ovarian tumors fall into this category, but the percentage of undifferentiated carcinomas has been less in more recent studies using different diagnostic strategies, and the use of immunohistochemistry has decreased the number of cases that cannot be classified.
In most cases, undifferentiated carcinoma has spread beyond the ovary by the time of diagnosis, and most patients cannot be optimally debulked. Undifferentiated carcinoma has the worst prognosis of any type of surface epithelial carcinoma. In one study, 29 of 35 patients died of tumor in less than 3 years. Of the 6 who survived more than 3 years, 5 eventually died of tumor.
Grossly, undifferentiated carcinoma is usually a large, predominantly solid tumor with foci of hemorrhage and necrosis. Most are bilateral. Microscopically, the carcinoma grows as sheets of poorly cohesive epithelial cells, typically with relatively uniform medium to large vesicular nuclei, variably prominent nucleoli, and scant cytoplasm ( Fig. 13A.41 ). The nuclei are markedly atypical, mitotic figures are numerous and may be abnormal, and pleomorphic cells or bizarre tumor giant cells or rhabdoid cells may be present. Poorly formed glands, areas with vague squamoid or transitional features, or nests and cords of malignant cells have been described in some tumors, although nowadays such findings would likely result in classification of the tumor as a high-grade serous carcinoma. The combination of undifferentiated carcinoma with well-differentiated endometrioid carcinoma is one that pathologists should be on the alert for, as such tumors frequently occur in young women and are associated with abnormalities of the mismatch repair proteins.
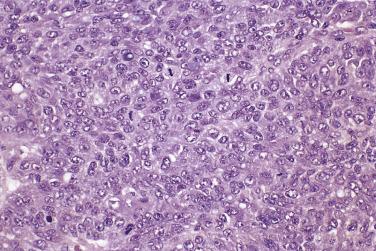
Immunohistochemical stains showing reactivity for cytokeratin or epithelial membrane antigen may be needed to prove that the tumor is a carcinoma. Staining for these markers is often weak and focal, and it is important to stain for both keratin and EMA as only one or the other may be expressed. Tumors classified as undifferentiated carcinomas on routine hematoxylin and eosin (H&E)–stained slides frequently show positive staining for p16 and WT1, and frequently for p53 as well, and such tumors are more appropriately classified as high-grade serous carcinomas. Immunostains for B72.3 are positive in most cases, and a stain for OC125 is positive in about 50%.
Ovarian carcinoma can usually be classified as one of the common types. Unusual kinds of carcinoma that occur only rarely include neuroendocrine carcinoma, squamous cell carcinoma, hepatoid carcinoma, and carcinoma with focal differentiation into a malignant germ cell tumor.
Neuroendocrine carcinoma of the ovary has a clinical presentation and metastatic pattern similar to other types of ovarian cancer and has a poor prognosis. Patients with neuroendocrine carcinomas of the ovary occasionally develop paraneoplastic syndromes, such as hypercalcemia caused by secretion of parathyroid hormone by the tumor cells.
The most common type of ovarian neuroendocrine carcinoma is a small cell carcinoma morphologically identical to small cell carcinoma of the lung and other body sites. This type of carcinoma is often referred to as small cell carcinoma of “pulmonary” or “neuroendocrine” type to differentiate it from the hypercalcemic type of small cell carcinoma that occurs only in the ovary. Neuroendocrine small cell carcinoma is composed of nests and sheets of small to midsize cells with darkly stained round to oval nuclei, finely granular nuclear chromatin, and inconspicuous or absent nucleoli ( Fig. 13A.42 ). The nuclei of adjacent cells are often molded to each other. The cytoplasm is pale and scanty, and the cell borders are ill defined. The immunophenotype is variable. About half of the cases stain for cytokeratin or epithelial membrane antigen. Stains for neuron-specific enolase or CD56 are generally positive, but staining for chromogranin or synaptophysin is detected in only a minority of tumors.
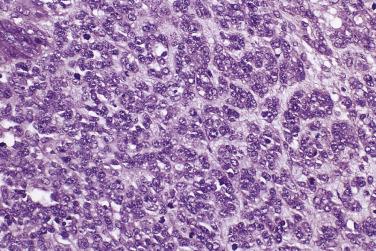
Neuroendocrine carcinomas composed of larger or more pleomorphic cells also occur in the ovary. These are designated as large cell neuroendocrine carcinoma and are composed of cells with vesicular nuclei, large nucleoli and abundant cytoplasm, or of cells with hyperchromatic nuclei with coarse chromatin, multiple nucleoli, and moderate amounts of amphophilic cytoplasm. The cells grow in nests, sheets, trabeculae, and poorly formed glands. Stains for epithelial and neuroendocrine markers are generally positive in large cell neuroendocrine carcinoma.
Most neuroendocrine carcinomas are intimately associated with tumors of surface epithelial type, most typically of either endometrioid or mucinous type, and may arise from neuroendocrine cells within the epithelial tumors. Metastatic neuroendocrine carcinoma from the lung and other sites must be considered in the differential diagnosis. Expression of thyroid transcription factor 1 (TTF1) does not necessarily indicate that a small cell carcinoma is metastatic from the lung, since a significant subset of nonpulmonary small cell carcinomas also express TTF1.
Squamous cell carcinoma occurs infrequently in the ovary. Most examples are secondary neoplasms arising in a benign cystic teratoma; this kind of ovarian squamous cell carcinoma is discussed in the section on teratomas. Squamous cell carcinoma in the ovary can be metastatic from the cervix or another primary site; it can occur in a mixed surface epithelial tumor, in a malignant Brenner tumor, or in an adenosquamous carcinoma of primary or metastatic endometrioid type, so a careful search for other elements is mandatory before a tumor is accepted as a pure primary ovarian squamous cell carcinoma. Nonteratomatous squamous cell carcinoma of the ovary originates in endometriosis, in an epidermoid cyst, or from the surface epithelium. It tends to be a predominantly solid tumor with small cysts. The malignant squamous cells grow in solid sheets, infiltrate the stroma, line cysts or papillae, and (in some tumors) grow in a spindle cell pattern. The prognosis is most closely correlated with the stage and grade, and since a majority of squamous cell carcinomas of the ovary are discovered at an advanced stage the overall prognosis is poor.
Some rare, apparently primary ovarian carcinomas that occur predominantly in older women histologically resemble hepatocellular carcinoma. These tumors, which have been designated as hepatoid carcinomas, are composed of polygonal cells with vesicular nuclei and abundant eosinophilic or clear cytoplasm. Additional features of hepatoid differentiation, identified in some or all such tumors, include hyaline globules in the tumor cells and stroma, immunoreactivity with antihepatocyte antibody, α-fetoprotein and albumin, a canalicular pattern of staining with polyclonal carcinoembryonic antigen, and a positive bile stain. The main differential diagnostic considerations are the hepatoid variant of yolk sac tumor and metastatic hepatocellular carcinoma. All of these tumors can be immunoreactive for antihepatocyte antibody and α-fetoprotein, so the differential diagnosis must be based on a combination of histologic and clinical findings. The finding of an admixture of other types of surface epithelial carcinoma in some tumors is indicative of ovarian origin.
Surface epithelial carcinomas of the ovary, like adenocarcinomas in other organs, occasionally differentiate focally into malignant germ cell elements. Rare carcinomas secrete human chorionic gonadotropin (hCG) from activated stromal cells or contain scattered trophoblastic cells, but only a few carcinomas with differentiation into choriocarcinoma have been reported. In addition to the underlying carcinoma, these patients’ tumors contain hemorrhagic nodules within which the typical biphasic pattern of choriocarcinoma, with cytotrophoblast and syncytiotrophoblast, is present. There is positive immunohistochemical staining for hCG in the syncytiotrophoblastic cells. Carcinomas with differentiation into choriocarcinoma appear biologically aggressive and capable of the type of hematogenous spread characteristic of choriocarcinoma. A small number of cases of yolk sac tumor arising in association with a malignant epithelial tumor, usually an endometrioid or high-grade serous carcinoma, have been reported. These exhibit typical histologic patterns of yolk sac tumor, are immunoreactive for α-fetoprotein, and secrete α-fetoprotein into the blood. Such tumors appear to have a poor prognosis.
Tumors derived from the sex cords or ovarian mesenchyme ( Box 13A.4 ) comprise 5% to 12% of all ovarian neoplasms. Tumors in the fibroma-thecoma group are relatively common, but other sex cord–stromal tumors and mesenchymal tumors are rare.
Granulosa cell tumor
Adult type
Juvenile type
Thecoma
Typical
Luteinized
Fibroma
Typical
Cellular
Fibrosarcoma
Fibroma or thecoma with minor sex cord elements
Sclerosing stromal tumor
Sertoli-Leydig cell tumor
Well differentiated
Intermediate differentiation (moderately differentiated)
Poorly differentiated
With retiform tubules
With heterologous elements
Sertoli cell tumor
Sex cord tumor with annular tubules (SCTAT)
Leydig cell tumor
Steroid cell tumor
Stromal luteoma
Gynandroblastoma
Soft tissue tumor not specific to the ovary
Lymphoma and leukemia
Unclassified
Around 1% to 2% of all ovarian tumors are granulosa cell tumors, the most common malignant sex cord–stromal tumor. There are two types of granulosa cell tumor: an adult type that occurs mainly in perimenopausal and postmenopausal women and a juvenile type that occurs mainly in children.
While most common in postmenopausal women, these tumors occur over a wide age range, from teenagers to the elderly. The average age is 45 to 55 years. Granulosa cell tumors typically secrete estrogens, which stimulate the endometrium to proliferate. The usual presenting symptom is postmenopausal bleeding in older women and menorrhagia, metrorrhagia, or amenorrhea in those who are premenopausal. Endometrial biopsy reveals hyperplasia in about 30% of patients and endometrial adenocarcinoma in 5% to 10% of them; most patients with endometrial pathology are older than 40. Rare adult-type granulosa cell tumors, most occurring in young women 15 to 35 years of age, secrete androgens and induce virilization. Typical symptoms include hirsutism, enlargement of the clitoris, deepening of the voice, and amenorrhea. About 25% of patients with granulosa cell tumors present with nonspecific symptoms such as abdominal distention, pain, or a palpable mass. Rupture or torsion of the tumor and intratumoral hemorrhage can cause acute abdominal symptoms. Rare granulosa cell tumors occur in pregnant women. Most women with a granulosa cell tumor have a palpable unilateral adnexal mass; bilateral tumors are uncommon.
A recent study of data in the Surveillance, Epidemiology, and End Results (SEER) database revealed that of 739 patients with granulosa cell tumors, 77% had stage I neoplasms, 12% stage II, and 11% stage III. The standard treatment is total abdominal hysterectomy and bilateral salpingo-oophorectomy. Unilateral salpingo-oophorectomy can be considered for treatment of a stage IA tumor, particularly in a young woman who wishes to conserve her fertility; laparoscopic and open surgical techniques appear to yield comparable results. All granulosa cell tumors have malignant potential, although most do not recur or metastasize. The recurrence rate is 10% to 15% for stage IA tumors and at least 20% to 30% overall. Extraovarian spread is to the peritoneum and omentum and occasionally to the liver or lungs. Lymph node metastases are uncommon, and routine dissection of the pelvic and abdominal lymph nodes is not necessary. When intraabdominal spread is present at diagnosis (stage III) or the tumor recurs, as many as two-thirds of patients die of tumor. Granulosa cell tumors grow slowly, and metastases are typically detected more than 5 years after initial treatment, so long-term follow-up is necessary. Recent studies in which molecular testing has been performed on all tumors have revealed that about 20% of tumors were misclassified. The misclassified cases, about half of which were carcinomas, accounted for most early recurrences and tumor-related deaths. Surgical resection of metastases can be beneficial as it affords symptom relief and can prolong survival. Some patients with advanced or recurrent granulosa cell tumor respond to combination chemotherapy that includes cisplatin, but responses are seldom durable, and convincing evidence of extended overall survival in patients treated with chemotherapy is lacking. Antiangiogenesis therapy with bevacizumab has resulted in partial or complete remissions in some patients. The value of radiotherapy is unclear, but there is some evidence that, in selected cases, adjuvant radiotherapy can result in a longer disease-free survival.
Several potential tumor markers have been identified in the sera of women with granulosa cell tumors, including estradiol, Müllerian-inhibiting substance, follicle regulatory protein, and inhibin. Inhibin has emerged as the most widely used tumor marker, as serum inhibin levels are elevated in nearly all patients with primary or recurrent granulosa cell tumor. Inhibin is not specific for granulosa cell tumors, as elevated serum concentrations can be observed in women with other types of ovarian tumor, but once the diagnosis has been established it can be used for monitoring treatment and detecting recurrence.
Granulosa cell tumors range from small, incidentally discovered nodules only a few millimeters in diameter to large tumors more than 30 cm in diameter. The average size is about 10 cm. Some are entirely solid, but most are partly cystic. The solid portions are pink, tan, brown, or light yellow and vary from soft to firm in consistency. Rare granulosa cell tumors grow as large cysts with a wall only a few millimeters thick. These are more likely than other granulosa cell tumors to be androgenic.
The tumor cells resemble normal granulosa cells. They are small and round, cuboidal, or spindle shaped with pale cytoplasm and ill-defined cell borders. The nuclei are round or oval with fine chromatin and a single small nucleolus ( Fig. 13A.43 ). Longitudinal folds or grooves are present in many nuclei and are a characteristic feature of adult granulosa cell tumor. Mitotic figures and nuclear pleomorphism and atypia are unusual findings in these tumors, but may be present. Cells with bizarre nuclei are detected in some tumors ( Fig. 13A.44 ), but when present only focally, as is usually the case, do not appear to affect the prognosis adversely. In occasional tumors the cells have more abundant pale cytoplasm, and there is often a nodular growth pattern resulting in some resemblance to a thecoma. The tumor cells are extensively (>50% of cells) luteinized in about 1% of adult granulosa cell tumors. Luteinized granulosa cells have abundant eosinophilic cytoplasm, well-defined cell borders, central nuclei with conspicuous nucleoli, and the background tumor stroma is usually myxoid. Luteinized granulosa cell tumors occur in pregnancy, in patients with androgenic tumors, and as an idiopathic finding.
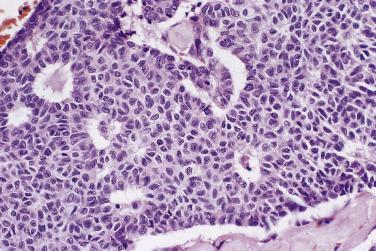
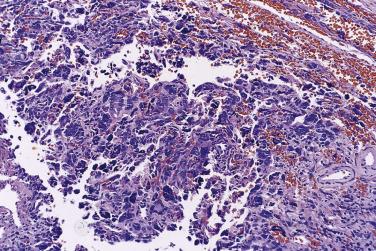
A number of histologic patterns have been described. These are often mixed and do not have prognostic significance. The microfollicular pattern is the most characteristic one and consists of nests and sheets of granulosa cells punctuated by small spaces lined by granulosa cells that contain eosinophilic secretions and cellular debris ( Fig. 13A.45 ). The spaces resemble the Call-Exner bodies of developing follicles. The macrofollicular pattern is one in which large, often irregularly shaped follicles are lined by stratified granulosa cells. Granulosa cells grow in anastomosing bands, ribbons, and cords in the trabecular pattern ( Fig. 13A.46 ); in irregular undulating ribbons in the gyriform or watered-silk pattern; and in circumscribed nests and islands in the insular pattern. The cells grow in large, irregular sheets with no organized substructure in the solid or diffuse pattern. Many granulosa cell tumors contain large cysts lined by one or more layers of granulosa cells. The cysts frequently contain blood, and hemosiderin-laden macrophages are often present in the cysts and the lining. Rare cystic granulosa cell tumors grow as large unilocular cysts lined by stratified granulosa cells, among which are microfollicles or areas of trabecular growth. Occasionally there are areas in cystic granulosa cell tumors where the tumor cells line blunt or branching papillae that project into cystic spaces; this finding is more common in juvenile granulosa cell tumors than in adult-type tumors.
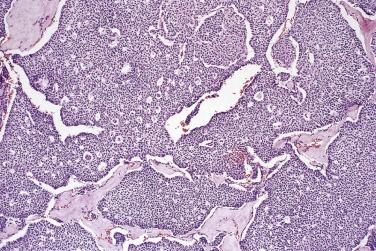

Granulosa cell tumors contain a variable amount of fibrous or thecomatous stroma. Tumors with a prominent fibrothecomatous stroma are designated as granulosa-theca cell tumors; they appear to have a different frequency of FOXL2 mutation and a more favorable prognosis. In current practice, a tumor in which granulosa cells comprise 10% to 50% of the cellular population is classified as a granulosa-theca cell tumor. Spindle cell gonadal stromal tumors with only a minor (<10%) granulosa cell component are best classified as a thecoma or fibroma with minor sex cord elements.
Rare granulosa cell tumors contain heterologous mucinous epithelium or are composite tumors with mucinous elements. Conversely, some mucinous tumors or other types of epithelial tumor contain prominent proliferations of granulosa cells in their stroma. FOXL2 mutation testing of the granulosa cells has revealed that in some cases the granulosa cells are a presumably nonneoplastic proliferation of mutation-negative granulosa cells in the stroma of an epithelial tumor, while in others there is a FOXL2 mutation in the granulosa cells supporting the interpretation of a granulosa cell tumor admixed with a mucinous or other epithelial neoplasm. In such cases FOXL2 mutation testing is necessary to determine the nature of the granulosa cell proliferation. A few granulosa cell tumors with focal heterologous hepatocellular differentiation have been reported.
It is difficult to predict the prognosis of a granulosa cell tumor by pathologic means, although some pathologic findings correlate to a degree with the clinical outcome. The prognosis is less favorable for large tumors more than 15 cm in diameter, for bilateral tumors, and for those that have ruptured or spread beyond the ovary (i.e., International Federation of Gynecology and Obstetrics [FIGO] stage >IA). The stage is the single most powerful prognostic indicator. Tumors in which there is diffuse moderate or marked nuclear atypia or a high mitotic rate (variably defined as >2 or 4 mitotic figures per 10 hpf) have been considered more likely to recur, but in more recent studies neither the mitotic index nor the Ki67 proliferation rate has correlated with the clinical outcome. There is no correlation between the microscopic pattern and the clinical outcome.
Small nonneoplastic granulosa cell proliferations, which can mimic small adult granulosa cell tumors, occur occasionally in the ovaries of women who are pregnant or postpartum. In contrast to a granulosa cell tumor, these granulosa cell proliferations are small, multifocal, and confined to the antra of atretic follicles. They do not have the appearance of a granulosa cell tumor in pregnancy. Strips and clusters of nonneoplastic granulosa cells are observed occasionally within vascular channels, perhaps being misplaced during surgery or at ovulation. These small aggregates of benign granulosa cells, which are often seen within vascular spaces adjacent to follicles, represent an artifact and should not be mistaken for tumor cells.
A missense somatic point mutation that is characteristic of adult granulosa cell tumors has been identified in the FOXL2 gene (402C–G). This mutation has been identified in 70% to 97% of adult granulosa cell tumors, as well as in occasional sex cord–stromal tumors of other types, mainly thecomas, but so far not in other tumor types. In one study, the tumor cells in granulosa cell tumors without the mutation were spindled and interspersed with theca cells and might have been viewed as granulosa-theca cell tumors, and the tumor cells in the thecomas with the mutation were luteinized thecomas. In a study conducted by our group, granulosa-theca cell tumors had a FOXL2 mutation in only 50% of cases. FOXL2 mutation testing can be helpful in cases where the diagnosis remains unresolved after histologic and immunohistochemical studies are complete. In one study, testing revealed TERT C228T promoter mutations in a significant percentage of granulosa cell tumors; patients whose tumors had a TERT promoter mutation had a worse prognosis.
Immunohistochemical and special stains are useful in the diagnosis of granulosa cell tumor. Granulosa cell tumors tend to have an epithelial-like growth pattern in which reticulin-poor nests of tumor cells are surrounded by rims of stromal cells in which reticulin fibers invest individual stromal cells. In thecomas and other cellular stromal tumors that sometimes mimic granulosa cell tumor, the tumor cells are likewise invested by reticulin fibers.
Nearly all granulosa cell tumors are vimentin positive. The results of keratin stains depend on the antibody used and how the tissue is pretreated. Most granulosa cell tumors are keratin negative, but occasional tumors show focal or, less often, diffuse positive staining for keratin, particularly with antibodies directed against low molecular weight cytokeratins 8 and 18 (such as Cam 5.2 and AE1/3). Dotlike or globoid perinuclear staining is particularly suggestive of a granulosa cell tumor, but extensive perinuclear or diffuse cytoplasmic staining can be seen. Absence of staining for epithelial membrane antigen is an important characteristic of granulosa cell tumors and other sex cord–stromal tumors that helps to differentiate them from various types of epithelial tumor. There is positive staining for smooth muscle actin in most tumors, but granulosa cells generally do not stain for desmin. About 50% of granulosa cell tumors show positive nuclear or cytoplasmic staining for S100 protein. There is membrane staining for CD99 (MIC2 gene product) in about 70% of granulosa cell tumors.
There are a number of positive markers for granulosa cell tumor; most of these are also positive in other types of sex cord–stromal tumors. Most granulosa cell tumors show cytoplasmic staining for inhibin ( Fig. 13A.47 ). Staining is often patchy and of variable intensity, and a positive reaction is not specific for granulosa cell tumor, as other types of sex cord–stromal tumor are also immunoreactive. Most epithelial tumors are inhibin negative, but focal or diffuse positive staining for inhibin is detected occasionally. Calretinin is an even more sensitive marker for granulosa cell tumors but, like inhibin, it is not specific as it is positive in other types of sex cord–stromal tumor and in mesothelioma. Staining for calretinin is both nuclear and cytoplasmic. As with inhibin, there is occasional staining in epithelial tumors, and mesotheliomas are diffusely and strongly positive. Two newer immunostains, steroidogenic factor-1 (SF1) and FOXL2, are almost invariably diffusely and strongly positive in granulosa cell tumors. These antibodies react with a nuclear antigen, so positive staining is in the tumor cell nuclei. FOXL2 staining is seen in granulosa cell tumors regardless of whether they have a FOXL2 mutation. None of these stains is specific for granulosa cell tumor, as all other types of sex cord–stromal tumors show positive staining as well. Staining for FOXL2 is absent in steroid cell tumors, but steroid cell tumors show positive staining for SF1. Other immunostains that are typically positive in granulosa cell tumors are WT1, which shows nuclear staining, and CD56, which tends to show strong membrane staining, but these antibodies react with various other tumor types as well. Granulosa cell tumors can show cytoplasmic staining for CD10, but it tends to be weak and focal. An immunohistochemical staining panel used for the diagnosis of granulosa cell tumor could include inhibin, calretinin, SF1, and FOXL2 as positive markers and EMA, which should be negative. It can also be helpful to stain for cytokeratin (including CK8 and CK18) and, in certain circumstances, for leukocyte-common antigen to exclude a lymphoma and for synaptophysin and chromogranin to exclude a carcinoid tumor ( Table 13A.4 ).
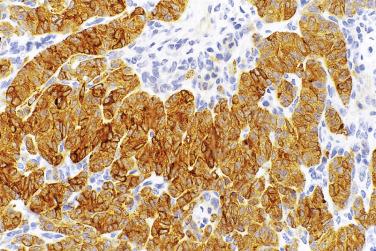
| Inhibin | FOXL2 | CK | EMA | LCA | Synapt | BRG1 | |
|---|---|---|---|---|---|---|---|
| Granulosa cell tumor | + | + | -/+ | - | - | - | + |
| Small cell carcinoma | - | - | +/- | +/- | - | +/- | Lost |
| Sertoli-Leydig cell tumor | + | + | + | - | - | - | + |
| Carcinoma | - | - | + | + | - | - | + |
| Carcinoid | - | - | + | + | - | + | + |
| Lymphoma | - | - | - | - | + | - | + |
Fewer than 5% of granulosa cell tumors occur in children and teenagers. Most of those have distinctive clinicopathologic features, and they have been termed juvenile granulosa cell tumors, the most common sex cord–stromal tumors in children.
Juvenile granulosa cell tumor can occur at any age, from infancy to old age, but most arise in children. The average patient age is 15 years, but in a study limited to children in a pediatric tumor registry, the average age was only 7.6 years.
Symptoms are caused by estrogens secreted by the tumor. Premenarcheal girls often (50%–75%) have isosexual precocious pseudopuberty, with development of the breasts, growth of pubic and axillary hair, vaginal bleeding, and increased bone age. An estrogen effect is seen in vaginal cytology specimens. Older children and premenopausal women have menstrual abnormalities, including amenorrhea. A third to half of all patients present with nonspecific symptoms such as abdominal distention, pain, or a palpable abdominal mass. Occasional patients develop acute abdominal symptoms due to torsion or rupture of their tumors. An adnexal mass is palpable in more than 70% of patients. With rare exceptions, juvenile granulosa cell tumors are unilateral, and more than 95% of them are confined to the ovary (stage I). The prognosis appears to be worse for patients with stage IC tumors, so it is important to collect peritoneal washings for cytologic evaluation. There is an association between juvenile granulosa cell tumor and Ollier syndrome (enchondromatosis) and Mafucci syndrome (enchondromatosis and multiple subcutaneous hemangiomas).
Juvenile granulosa cell tumor is typically encapsulated and confined to one ovary at diagnosis (stage IA), and it is adequately treated by unilateral salpingo-oophorectomy. Since most patients are young, hysterectomy and bilateral salpingo-oophorectomy should be reserved for the few who have advanced disease. Pelvic and abdominal lymph node metastases are generally not present at the time of initial surgery, and there does not appear to be any need to dissect them for staging or treatment purposes. The long-term survival is good, but patients whose tumors rupture or who have positive peritoneal cytology or extraovarian tumor spread have a significant risk of recurrence. Recurrence, if it does occur, is usually detected within 3 years of diagnosis, although rare patients with later recurrences have been reported. Some patients with advanced, persistent, or recurrent disease respond to platinum-based combination chemotherapy. In a study of 23 children with juvenile granulosa cell tumors, in which patients with tumor rupture, malignant ascites, or peritoneal implants were usually treated with VIP chemotherapy (etoposide, ifosfamide, and cisplatin), only 1 patient, who initially had disease confined to the ovary and did not receive adjuvant chemotherapy, died of tumor. Inhibin and Müllerian inhibitory substance are useful as tumor markers for the detection and follow-up of patients with juvenile granulosa cell tumors.
Juvenile granulosa cell tumors vary from 2.5 to 30 cm in diameter, with an average of about 12 cm. Most tumors have a mixed solid-cystic appearance, but some are completely solid and others largely cystic. Solid areas are yellow or tan. Hemorrhage is frequent, but necrosis is uncommon.
There is typically a mixture of cysts and solid lobular or nodular areas ( Fig. 13A.48 ). Macrofollicular, solid, and cystic growth patterns are characteristic of juvenile granulosa cell tumor. In some tumors, the macrofollicles are rounded and relatively uniform in size, while in others, the follicles vary markedly in size and have irregular shapes ( Fig. 13A.49 ). The macrofollicles contain mucinous material, are lined by one or more layers of granulosa cells, and may be surrounded by a rim of theca cells. Solid areas consist of sheets of granulosa cells with a variable admixture of spindle-shaped thecal or fibroblastic stromal cells. Vague or well-defined papillae lined by granulosa cells occasionally grow into cystic spaces. Growth patterns that are common in adult granulosa cell tumors, such as the microfollicular and insular patterns, are usually not seen, although some tumors have foci in which the granulosa cells grow in a trabecular or tubelike pattern. The granulosa cells in juvenile granulosa cell tumors differ from those seen in adult granulosa cell tumors. They are large, polygonal to spindled in shape, and have a variable but usually ample amount of amphophilic or pink cytoplasm. Focal or extensive luteinization is a typical finding. The tumor cell nuclei are large, round, and usually darkly stained ( Fig. 13A.50 ). They lack grooves and may contain conspicuous nucleoli. Some cells have greatly enlarged pleomorphic nuclei and others are multinucleate. Mitotic figures tend to be numerous and average around 6 per 10 hpf.
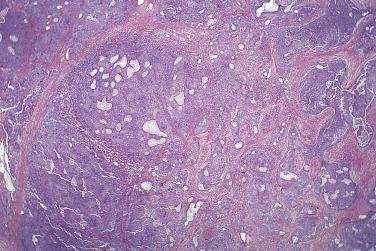
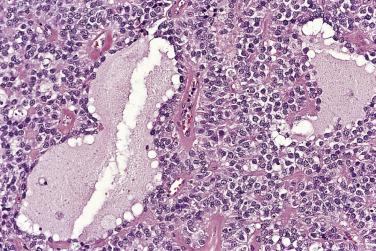
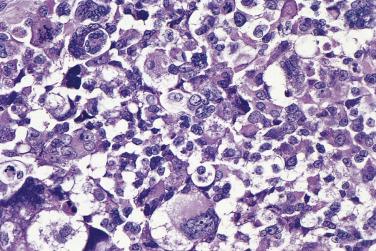
The FOXL2 (C402G) mutation that occurs in adult granulosa cell tumors is, with occasional exceptions, absent in juvenile granulosa cell tumors, indicating that these two tumors, both involving granulosa cells, likely have a different histogenesis. Occasional juvenile granulosa cell tumors with DICER1 mutations, which are more typical of Sertoli-Leydig cell tumors, have also been identified.
The immunohistochemical features of juvenile granulosa cell tumor are similar to those of adult granulosa cell tumors, with a few differences. The tumor cells are vimentin positive and they stain, usually focally and weakly, for low molecular weight cytokeratin in a quarter to half of the cases. Sex cord–stromal markers are generally positive. There is cytoplasmic staining for inhibin, nuclear and cytoplasmic staining for calretinin, nuclear staining for FOXL2 and SF1, and membrane staining for CD99 and CD56. Staining for CD99 tends to be more intense in juvenile granulosa cell tumors than in the adult type, and a greater percentage of tumors stain. EMA tends to be negative, but in another difference from adult-type granulosa tumors, some juvenile granulosa cell tumors show staining for EMA, usually in a focal and weak pattern. Some authors have reported that greater intensity of staining for FOXL2 is associated with more aggressive clinical behavior, while others have reported the opposite, namely that tumors that show loss of staining for FOXL2 are more aggressive. The prognostic significance of the presence of and intensity of staining for FOXL2, if any, thus remains to be determined.
Thecoma is a gonadal stromal tumor in which the cells resemble those of the theca interna. It is uncommon and comprises 7% or less of sex cord–stromal neoplasms.
Thecomas arise in patients of all ages, but most occur in perimenopausal or postmenopausal women. The average patient age is between 50 and 55 years. Thecomas occur only rarely in children. The luteinized variant of thecoma occurs at a younger age, with a significant proportion affecting women in their 20s or 30s. Older patients typically present with postmenopausal bleeding, but as many as 25% of patients have nonspecific symptoms such as pelvic or abdominal pain or abdominal distention. The latter can be caused by a large tumor or be due to ascites. Some patients have a Meigs-like syndrome, with ascites and hydrothorax. Premenopausal women have either endocrine-associated symptoms, such as irregular bleeding or amenorrhea, or less specific complaints such as pelvic or abdominal pain or abdominal distention. Some patients, usually those with luteinized thecomas, are virilized. Estrogens secreted by the tumor cause endometrial hyperplasia or adenocarcinoma of the endometrium, so it is important to assess the endometrium in a woman with a thecoma. Thecoma is almost invariably benign, and excision is the appropriate treatment. For all practical purposes extraovarian spread does not occur, but about 5% of patients have bilateral tumors. Because many thecomas are associated with endometrial hyperplasia or carcinoma, hysterectomy with bilateral salpingo-oophorectomy is the standard treatment. Unilateral salpingo-oophorectomy is adequate treatment for a young woman as long as the endometrium is normal. Rare malignant tumors with unequivocal thecomatous differentiation have been reported. However, most neoplasms reported as malignant thecoma have proven to be sarcomatoid or luteinized granulosa cell tumors, fibrosarcomas, or other types of malignant mesenchymal tumor.
Thecomas are firm or hard tumors that vary from small, incidentally discovered nodules less than 1 cm in diameter to masses more than 20 cm in diameter. The average diameter is 5 to 7 cm. The cut surface is gray or tan, typically with focal to extensive yellow or golden areas. Degenerative changes such as cysts and calcifications may be present.
The tumor is composed of fascicles or sheets of plump, spindled, or ovoid stromal cells that resemble the cells of the theca interna ( Fig. 13A.51 ). The neoplastic cells have pale round or fusiform nuclei with fine, dispersed chromatin. The nuclei are generally bland, but rare tumors contain bizarre nuclei, a finding that does not appear to have any impact on the prognosis. Mitotic figures are generally absent or rare. The cytoplasm is amphophilic or gray, or lightly eosinophilic. Some cells have clear cytoplasm or contain lipid vacuoles that are best demonstrated with fat stains applied to unfixed frozen sections ( Fig. 13A.52 ). A variable number of fibroblastic cells are intermixed among the theca-like cells. Reticulin stains reveal a network of fibrils surrounding individual tumor cells. Hyalinized connective tissue plaques and microcalcifications are common, and extensive calcifications are occasionally present in thecomas affecting young women, and rare tumors contain fat. In some tumors, zones of edema separate the tumor cells resulting in a pale appearance at low magnification. The ovarian cortex adjacent to a thecoma is often hyperplastic.
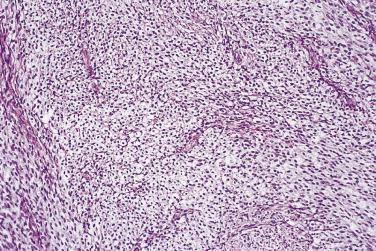
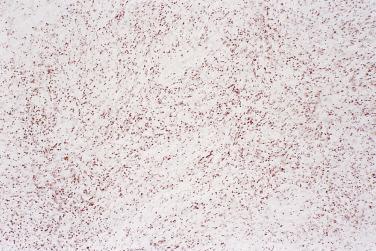
Electron microscopic studies suggested that fibromas and thecomas are derived from the same cell type and that they differ only in the proportion of collagen-forming and steroid-forming cells. It is therefore not surprising to find intermediate forms that are difficult to classify either as thecoma or fibroma. Nowadays these are most often designated as fibromas, although some designate them as fibrothecomas ( Fig. 13A.53 ). From a practical point of view, the diagnosis of thecoma is restricted to tumors that show evidence of steroid hormone secretion, or that have a conspicuous component of cells with clear or vacuolated cytoplasm, or that contain luteinized cells, as discussed later. A tumor that contains many cells that are immunoreactive for inhibin can also be designated as a thecoma. In daily practice, thecoma is an uncommon diagnosis, while fibromas are relatively common.
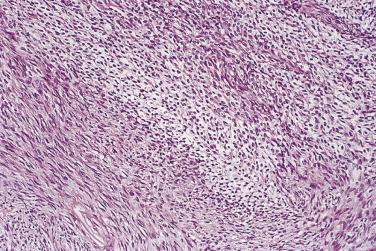
Clusters, nests, or sheets of luteinized cells are found in some tumors in the thecoma-fibroma group ( Fig. 13A.54 ). In the past, regardless of whether the tumor was a thecoma or a fibroma, if luteinized tumor cells were present, it was classified as a luteinized thecoma. However, it seems perfectly reasonable to base the diagnosis of such neoplasms on the overall appearance of the tumor, allowing for both luteinized thecomas and fibromas. Luteinized thecomas can be estrogenic (50%), androgenic (11%), or nonfunctional (39%). Nearly all androgenic thecomas are luteinized thecomas. A luteinized thecoma is differentiated from a histologically similar neoplasm, the stromal Leydig cell tumor, only by the presence of cytoplasmic crystalloids of Reinke in the latter.

Rare gonadal stromal tumors that contain theca cells or luteinized cells are clinically malignant. Features that raise the possibility of malignancy include large size, hypercellularity, diffuse moderate to severe nuclear atypia, and frequent mitotic figures (≥4 per 10 hpf), but definitive criteria for the histologic diagnosis of malignant thecoma have not been established. Tumors that contain many luteinized cells should be scrutinized carefully for features suggestive of malignancy. The presence of slightly increased numbers of mitotic figures (>4 per 10 hpf) does not, in the absence of other features of malignancy, indicate that the tumor is a malignant thecoma, and such tumors can be designated as mitotically active thecomas. Tumors in this category are viewed as tumors of uncertain malignant potential, like cellular fibromas.
A cytogenetic abnormality, trisomy 12, can be detected in some thecomas. A mutation in the FOXL2 gene has been recently discovered in granulosa cell tumors. While the mutation appears to be relatively specific for adult-type granulosa cell tumors, rare tumors of other types, including a few thecomas, have been reported to harbor the mutation. However, the finding of this mutation in a thecoma raises the question of whether the tumor might be a granulosa cell tumor variant that has been misclassified.
Immunostains for vimentin are positive in thecoma, while those for cytokeratin and EMA are negative. Inhibin and calretinin have been the most widely used markers for thecoma and other sex cord–stromal tumors; the percentage of thecomas that show positive staining varies depending on the threshold that is set for a positive result. In general, there is at least patchy staining for inhibin and calretinin in a majority of thecomas. Other markers that are useful in the diagnosis of thecoma are SF1 and FOXL2, which are positive in most tumors. Positive staining for FOXL2 appears to be present in most sex cord–stromal tumors, and it does not depend on the presence of the (402 C–G) mutation that is found only in adult granulosa cell tumors. CD10 is frequently positive in thecomas, but staining is generally focal and weak, and thecomas can also show staining for WT1.
An unusual syndrome in which sclerosing peritonitis is coupled with tumoral ovarian gonadal stromal proliferation can occur at any age, but it is most often found in young women. The typical presentation is with abdominal distention, symptoms of chronic intestinal obstruction, or an acute abdomen. Most patients have ascites and some have hydrothorax. The omentum, mesentery, and the serosa of the small intestine typically show fibrous thickening and nodularity, often with adhesions and intestinal obstruction. This condition appears to be self-limited and is treated adequately by excision of the ovarian lesions and limited abdominal surgery, including lysis of adhesions, omentectomy, and bowel resections as necessary to relieve obstruction. In several patients, the sclerosing peritonitis appears to have responded to treatment with various combinations of toremifene, steroids, and a gonadotropin-releasing hormone (GnRH) agonist, including regression of the ovarian tumors in one case. No recurrences of the ovarian lesions or metastases have been reported to date, although several patients have died of intestinal obstruction and sepsis related to the peritoneal pathology. The initiating cause of the syndrome is unknown. Several cases have been reported in patients who were taking anticonvulsant medications, suggesting a possible association, but in the largest series it appeared that anticonvulsant usage could not be implicated as the cause of the syndrome in most cases.
The average diameter of affected ovaries is about 10 cm. The surfaces tend to be smooth but often nodular or lobulated. The cut surfaces are tan or gray, often with grossly edematous or hemorrhagic areas.
The microscopic appearance of the lesions depends at least partly on their size. When the ovaries are small (<9.5 cm) the histologic picture is that of a bilateral nodular cortical spindle cell proliferation that results in a thick cortical rind with a polypoid surface contour but no discrete tumor mass. Larger lesions replace the entire ovary, although entrapped follicles, corpora albuginea, or epithelial inclusion glands may be present among the proliferated spindle cells. The low magnification appearance is one of alternating cellular and hypocellular edematous zones, with separation of the cells by edema sometimes resulting in a microcystic appearance. Extravasation of erythrocytes is a typical finding. The proliferating cells are spindle cells with bland pale nuclei and variable amounts of amphophilic or eosinophilic cytoplasm. Mitotic activity is variable but usually brisk in the spindle cells ( Fig. 13A.55 ), with an average of 18 mitotic figures per 10 hpf. The spindle cells generally show no staining for classic sex cord–stromal markers such as inhibin and calretinin, but positive staining for the newer nuclear markers SF1 and FOXL2 is present, supporting the sex cord–stromal nature of the cells. The spindle cells also often stain for smooth muscle actin or desmin. Clusters of luteinized cells with eosinophilic or clear foamy cytoplasm are almost invariably present among the spindle cells. The luteinized cells show cytoplasmic staining for inhibin, calretinin and CD56, and Oil Red O. The ovarian tumors were originally designated as luteinized thecomas despite the fact that they differ significantly from the usual luteinized thecoma in that they are bilateral, do not stain for cytoplasmic markers of sex cord–stromal neoplasia, and the luteinized cells occur in small, ill-defined clusters. Despite the worrisome mitotic activity, metastatic spread has not occurred in the limited number of cases with follow-up. The histologic appearance and clinical behavior, including complete regression with medical therapy in at least one case, suggest that these ovarian masses may be tumorlike proliferative lesions rather than true neoplasms, and that luteinized thecomatosis may be a more appropriate name for them than luteinized thecoma .
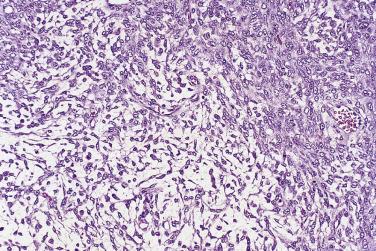
The sclerosing peritonitis involves the omentum, peritoneum, and intestinal serosa. There is serosal thickening, bands of fibrous tissue containing fibroblasts and myofibroblasts surround lobules of fat, chronic inflammatory cells are present in the fibrous tissue, and there is mesothelial hyperplasia and (in some cases) fibrin deposition on the surface.
The ovarian fibroma is a benign tumor composed of fibroblasts and collagen fibers. It is by far the most common sex cord–stromal tumor, accounting for 1% to 5% of all ovarian tumors. Cellular fibromas are much less common, and fibrosarcoma is rare.
The clinical presentation is nonspecific. Fibromas occur in patients 20 to 80 years of age, with an average age of more than 50, while patients with cellular fibromas appear to be somewhat younger with an average age of 40 to 50 years. Fibromas are often small and asymptomatic and are discovered only when the patient is operated on for some other condition. Large fibromas, cellular fibromas, and fibrosarcomas tend to be symptomatic and cause abdominal pain or distention; a pelvic mass is detected, and some patients have ascites. Demons-Meigs syndrome is a rare condition in which a large ovarian fibroma is accompanied by ascites and hydrothorax. About 17% of patients with the nevoid basal cell carcinoma syndrome (multiple basal cell carcinomas of the skin, odontogenic keratocysts, and other abnormalities), which is caused by a germline mutation of the PTCH1 gene on chromosome 9, have ovarian fibromas, which can be multifocal and bilateral. The discovery of an ovarian fibroma or fibromas in a child or young patient should prompt consideration as to whether the patient could have the syndrome.
Fibromas are adequately treated by surgical excision. Occasional patients with cellular fibromas (up to 13%) have adhesions or peritoneal tumor implants at diagnosis, but in a large series all patients with follow-up were well at last contact, including some with adhesions or implants. Nevertheless, cellular fibromas have been reported to recur, and one tumor-related death has been recorded, so these tumors are viewed as tumors of uncertain malignant potential, particularly those that are adherent to other structures or associated with peritoneal implants. Appropriate clinical follow-up is accordingly necessary. Fibrosarcoma is a malignant mesenchymal tumor that has a poor prognosis. Treatment is complete resection followed by chemotherapy.
Fibroma is a firm tumor with a smooth, lobulated surface. It ranges from less than 1 cm to more than 10 cm (average ~6 cm) and has a solid white or tan cut surface. It is bilateral in 5% to 10% of cases. Cellular fibroma is almost invariably unilateral, averages 8 to 10 cm in diameter, and is mainly solid with a white or tan cut surface. Some are polypoid growths from the surface of the ovary. Cysts are present in about a third of cellular fibromas. Fibrosarcoma is large and soft, and may contain areas of hemorrhage and necrosis.
Fibromas are composed of thin spindle cells growing in whorled and anastomosing bundles ( Fig. 13A.56 ). The nuclei are fusiform and uniform from cell to cell. The cytoplasm is scanty and lightly eosinophilic. There is a variable amount of collagenous stroma around the tumor cells.
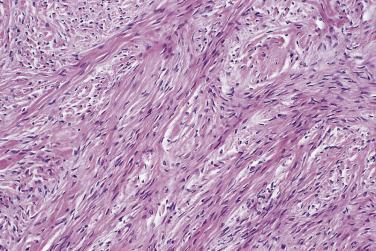
About 10% of fibroblastic tumors are hypercellular with little intercellular collagen. The tumor cells have oval to spindled bland nuclei and variable amounts of amphophilic or eosinophilic cytoplasm. The tumor cells grow in herringbone, cross-stitch, and storiform patterns. Zones of edema may be present, and there may be areas of lower cellularity compatible with a typical fibroma. Some cellular fibromas contain conspicuous hyaline globules. Necrosis is occasionally present, especially in pedunculated tumors where there may be compromise of the blood supply. Such tumors are designated as cellular fibromas when nuclear atypia is mild to moderate and there are 3 or fewer mitotic figures per 10 hpf ( Fig. 13A.57 ) and as mitotically active cellular fibromas when there are 4 or more mitotic figures per 10 hpf. Mitotic counts are between 4 and 9 per 10 hpf in 85% of mitotically active cellular fibromas and between 10 and 19 per 10 hpf in the rest. The clinical behavior of cellular fibromas appears to be similar regardless of the level of mitotic activity, and all tumors in this category are viewed as tumors of uncertain malignant potential, as noted earlier. Solitary fibrous tumors (SFTs) are rare neoplasms that are most common in the pleura, peritoneum, and soft tissues, but they can occasionally arise in the ovary and might be mistaken for a cellular fibroma. Solitary fibrous tumors are composed of bland spindle cells growing in a patternless arrangement with a hemangiopericytoma-like vascular pattern. The diagnosis can generally be confirmed using immunohistochemistry, as SFTs show nuclear staining for STAT6, and they are often CD34 positive. A mitotic count of 4 or more mitotic figures per 10 hpf is considered to be indicative of a malignant SFT.
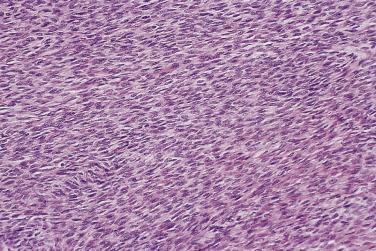
Small, irregular nests or tubules of sex cord cells are occasionally found in a fibroma or cellular fibroma. The sex cord cells are polygonal and have uniform nuclei and small amounts of cytoplasm; they resemble indifferent sex cord cells or granulosa cells. When they comprise less than 10% of the tumor, the sex cord cells do not appear to have any prognostic significance. Tumors that contain them are called fibromas or cellular fibromas with sex cord elements.
Many ovarian tumors that have been reported as fibrosarcomas in the past would now be considered to be mitotically active cellular fibromas. Fibrosarcoma is exceptionally rare in the ovary. The diagnosis should be considered for a cellular spindle cell fibromatous neoplasm with moderate to marked nuclear atypia and 4 or more mitotic figures per 10 hpf; tumor cell necrosis and atypical mitotic figures are frequently present. Fibrosarcomas are typically large tumors that have often spread beyond the ovary at diagnosis. The differential diagnosis includes leiomyosarcoma, a gastrointestinal stromal sarcoma, and various types of primary or metastatic soft tissue sarcoma.
Trisomy 12 is a consistent cytogenetic finding in ovarian fibromas. Molecular testing has not revealed FOXL2 mutations in any cellular or mitotically active cellular fibromas. Absence of the mutation can help differentiate a cellular fibroma from a spindle cell granulosa cell tumor.
Immunohistochemistry is frequently not considered as an aid in the diagnosis of fibroma and cellular fibroma, since these tumors show little or no staining for such standard immunohistochemical markers of sex cord–stromal tumors as inhibin and calretinin. More recently introduced markers such as FOXL2, SF1, CD56, and WT1 are more likely to show positive staining in fibromas and can be helpful in differentiating them from smooth muscle tumors and other nongonadal stromal mesenchymal neoplasms. These markers stain most types of sex cord–stromal tumor and are not specific for fibromas. Interestingly, it is not uncommon to see staining for smooth muscle actin in fibromas and cellular fibromas, a finding that should be kept in mind when the differential diagnosis includes a smooth muscle tumor. Fibromas are CD10 negative, which helps differentiate them from endometrial stromal tumors.
The sclerosing stromal tumor is an uncommon benign tumor that occurs mainly in teenagers and young women in their 20s, although it is occasionally encountered in young girls or in older perimenopausal or postmenopausal women. The usual presentation is with disturbances of menstruation or pelvic pain, although rare examples cause virilization. Sclerosing stromal tumor is one of the types of ovarian tumor rarely associated with Meigs syndrome. A sclerosing stromal tumor is occasionally detected during pregnancy, when it may cause virilization. Sclerosing stromal tumor is benign and, since most examples are found in young women, the usual treatment is by excision or unilateral salpingo-oophorectomy.
Sclerosing stromal tumors range from 1.5 to 17 cm in diameter. Most are solid or predominantly solid with small cysts, but an occasional tumor is predominantly cystic. The solid areas are firm, lobulated, and tan or yellow.
Sclerosing stromal tumor has a variable appearance due to the juxtaposition of cellular nodules and zones of edema or hypocellular fibrous stroma. Blood vessels are prominent and often have a staghorn or hemangiopericytoma-like appearance. The tumor cells include spindle-shaped fibroblasts, myoid cells, and polygonal theca-like cells with vacuolated eosinophilic cytoplasm ( Fig. 13A.58 ). Rarely, vacuolated signet ring–like cells are present, but the vacuoles contain lipid, not mucin, and the tumor cells are keratin negative, differentiating them from the cells of a metastatic signet ring cell carcinoma. Mitotic figures are generally infrequent in sclerosing stromal tumors, but rare tumors exhibit increased mitotic activity up to the range of 7 to 12 mitotic figures per 10 hpf. One such tumor recurred in the pelvis, as occasionally happens with cellular fibromas, and it has been suggested that tumors with increased mitotic activity should be designated as mitotically active sclerosing stromal tumors and that they should be viewed as tumors of uncertain malignant potential. Tumors that are removed from pregnant women can exhibit prominent luteinization of the polygonal cells, sometimes to such an extent that the tumor is difficult to differentiate from a steroid cell tumor. The collagenous stroma, ectatic vessels, and spindle cells that characterize sclerosing stromal tumors are generally still recognizable, however, and provide clues to the correct diagnosis. In some cases, transitions have been identified between other types of sex cord–stromal tumor and sclerosing stromal tumors, and in other cases sclerosing stromal tumors have appeared to evolve into difficult to classify sclerotic calcified tumors.
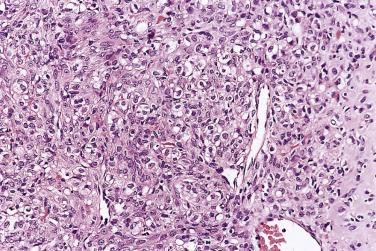
Immunostains for vimentin, inhibin, calretinin, and melan-A are positive, and most tumors contain myoid cells that are immunoreactive for smooth muscle actin or desmin. Positivity for estrogen or progesterone receptors can often be identified in tumor cell nuclei; staining for progesterone receptors is usually most prominent. Vascular endothelial growth factor (VEGF) has been identified in the theca-like polygonal cells and its receptor localized to the vascular endothelial cells, suggesting that VEGF may play a role in the vascularity and edema that characterize this tumor. Nuclear staining for FOXL2 and SF1 is present in most sclerosing stromal tumors. Diffuse moderate to strong staining for TFE3 has been reported to be present in most sclerosing stromal tumors. A few cases have been shown to have trisomy 12.
Sertoli-Leydig cell tumors constitute less than 1% of ovarian tumors. There are two main clinicopathologic categories:
Well-differentiated Sertoli-Leydig cell tumors, which comprise 10% of all such tumors
Moderately and poorly differentiated Sertoli-Leydig cell tumors, which make up the remaining 90% of such tumors
Sertoli-Leydig cell tumors occur mainly in young women, but they occur over a wide age range and are occasionally detected in children or postmenopausal women. In the largest studies the average age at diagnosis was 24 years, and 75% of patients were under 30 years of age. Similar patient ages have been recorded in other reports, although series in which the patients have been older have also been reported. Women with well-differentiated Sertoli-Leydig cell tumors average 40 years of age, 10 to 15 years older than those with the more common moderately or poorly differentiated tumors. Retiform Sertoli-Leydig cell tumors have been reported to occur mainly in younger patients, with an average age of 16 to 17 years, although a series has been reported in which the patients had a mean age of 31 years, illustrating that retiform tumors can occur in mature and even elderly women as well as in the young.
About 50% of Sertoli-Leydig cell tumors secrete steroid hormones in amounts sufficient to cause symptoms, and 40% of patients are virilized. Virilized patients have various combinations of symptoms, including amenorrhea, deepening of the voice, hirsutism, temporal alopecia, hypertrophy of the clitoris, and acne. Serum testosterone and urine 17-ketosteroids are increased in virilized patients. Rare Sertoli-Leydig cell tumors secrete α-fetoprotein, but the precise frequency with which this occurs is unknown, since testing for AFP is usually not performed.
Nonvirilized patients present with nonspecific symptoms, such as abnormal vaginal bleeding, abdominal distention, an abdominal mass, or abdominal or pelvic pain; 5% to 10% of patients have acute abdominal symptoms caused by torsion or rupture of the tumor. Occasional Sertoli-Leydig cell tumors are incidentally discovered in asymptomatic women being operated on or evaluated for some other reason. DICER1 mutations are frequently present in Sertoli-Leydig cell tumors, and these tumors can be one of the manifestations of the DICER1 syndrome, which is discussed further on p. 745 . In some patients, Sertoli-Leydig cell tumors are diagnosed when the patient is investigated for another DICER1-associated disease. A smaller subset of these tumors have FOXL2 mutations, and recent data suggest clinicopathologic differences from the DICER1 -mutant group.
Sertoli-Leydig cell tumors tend to be unilateral and confined to the ovary (stage IA) at diagnosis. There is extraovarian spread at diagnosis in less than 20% of cases but, in 5% to 15% of cases, the tumor is ruptured or there are tumor cells in the peritoneal fluid (FIGO stage IC). Unilateral salpingo-oophorectomy is adequate treatment for most patients. Chemotherapy has been advocated for patients with advanced disease, including for those with tumor rupture and those with malignant cells in ascites fluid or pelvic/abdominal washings. A total abdominal hysterectomy and bilateral salpingo-oophorectomy is appropriate treatment for older patients and can be considered in younger women when there are unfavorable prognostic findings, such as rupture, extraovarian spread, a poorly differentiated neoplasm with frequent stromal cell mitoses, or heterologous mesenchymal differentiation (cartilage or skeletal muscle, or foci of neuroblastoma). Lymph node metastases are exceptional, and routine dissection of the pelvic and abdominal lymph nodes is not necessary. Regression of virilization and resumption of normal menses follow tumor removal.
Well-differentiated Sertoli-Leydig cell tumor is a clinically benign tumor that does not recur after complete excision. The prognosis is generally favorable for patients with moderately and poorly differentiated Sertoli-Leydig cell tumors. Only 18% of patients had a recurrence in one study, while a 5-year survival rate of 92% was found in another. Retiform Sertoli-Leydig cell tumors may have a slightly worse prognosis than the overall group. Recurrences are generally detected within the first few years after treatment. Chemotherapy is helpful in about half of the patients who receive it.
Well-differentiated Sertoli-Leydig cell tumors are solid, unilateral, encapsulated tumors that range from 1.5 to 10 cm in diameter. The average size is 5 cm. The cut surface is yellow or yellow-tan. Moderately and poorly differentiated tumors are larger, with an average diameter of 15 cm. Poorly differentiated tumors tend to be larger than those that are moderately differentiated, but there is considerable overlap. Sertoli-Leydig cell tumors are usually partly solid and partly cystic, but tumors that are predominantly solid or cystic are not unusual. Solid areas are firm or soft and gray-pink, yellow, or orange. Papillae are sometimes visible in tumors with retiform differentiation.
Hollow or closed tubules lined by columnar Sertoli cells are surrounded by fibrous stroma in well-differentiated Sertoli-Leydig cell tumors ( Fig. 13A.59 ). Tumors with prominent aggregates of open tubules can mimic an endometrioid adenocarcinoma, but such tumors generally have more typical areas of Sertoli-Leydig cell tumor growth and they are immunohistochemically different from endometrioid adenocarcinoma, as the tubules stain for inhibin and calretinin, and staining for cytokeratin 7 and EMA is absent. Aggregates of Leydig cells with abundant eosinophilic cytoplasm and centrally placed round or oval nuclei are present in the stroma. Crystalloids of Reinke are rarely identified within the Leydig cells. One unusual tumor had central ossification. Cytologic atypia and mitotic activity are insignificant, and immature Sertoli cells, indifferent gonadal stroma, retiform tubules, and heterologous elements are absent.
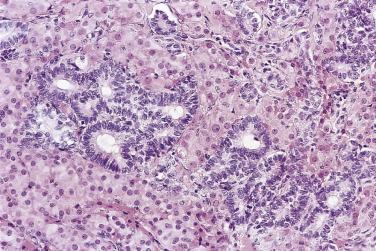
In moderately and poorly differentiated Sertoli-Leydig cell tumors, mature and immature Sertoli cells line well-formed tubules, ill-defined tubules, trabeculae, and cordlike arrangements reminiscent of the embryonal sex cords ( Fig. 13A.60 ). The tubules have a retiform appearance in 10% to 25% of moderately and poorly differentiated tumors, and the retiform tubules occasionally dominate the histologic picture to the extent that it is difficult to recognize that the neoplasm is a Sertoli-Leydig cell tumor. Retiform tubules are long and branched and may be dilated with prominent areas of papillary growth ( Fig. 13A.61 ). They are lined by low columnar to cuboidal cells with scanty cytoplasm and oval hyperchromatic nuclei. Mitotic figures are uncommon in Sertoli cells, but numerous mitotic figures are seen occasionally in the cells lining retiform tubules.
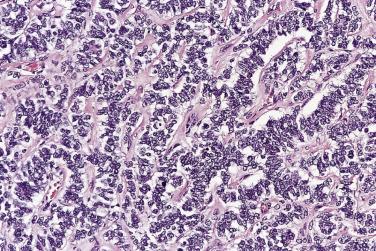
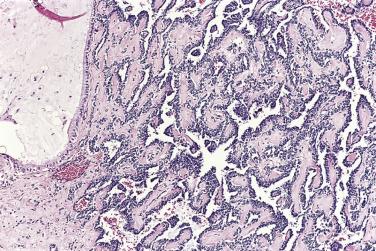
Sertoli cells with bizarre atypical nuclei are present in some tumors, but this does not appear to be an adverse prognostic finding. The tubules are surrounded by stroma that ranges from fibrous connective tissue to immature mesenchyme with the appearance of indifferent gonadal stroma, as seen in the developing gonad ( Fig. 13A.62 ). The presence of immature Sertoli cells and gonadal stroma is the main feature that distinguishes moderately and poorly differentiated Sertoli-Leydig cell tumors from well-differentiated lesions. Stroma is most abundant in poorly differentiated tumors, where it constitutes the bulk of the tumor, resulting in a sarcomatoid appearance ( Fig. 13A.63 ). The stromal cells are usually not strikingly atypical, but mitotic figures, which average 4 to 5 per 10 hpf, are easy to find. Leydig cells, found singly or in small or midsize clusters, are present in most tumors. They are often most conspicuous at the periphery of cellular tumor nodules. They are polygonal, with central round nuclei and abundant eosinophilic cytoplasm. Contradictory evidence has been presented regarding the nature of the Leydig cells, with some authors concluding that they are reactive and not part of the neoplastic process, and others concluding that they are neoplastic at least in some cases.
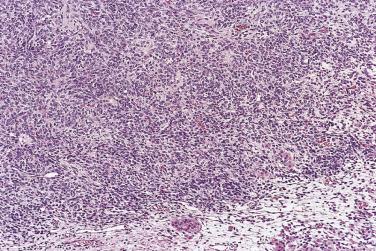
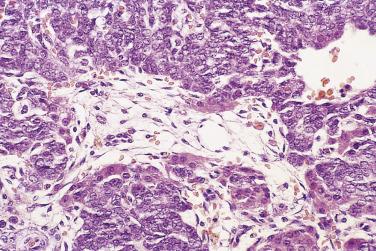
There are heterologous elements in 20% to 25% of moderately and poorly differentiated Sertoli-Leydig cell tumors. Mucinous epithelium of intestinal type is the most common heterologous element ( Fig. 13A.64 ). It can be present as scattered glands or it can be a prominent or even the predominant component of the tumor, obscuring the more typical elements of a Sertoli-Leydig cell tumor that are present between the mucinous glands. The mucinous component can resemble a mucinous cystadenoma, a borderline mucinous tumor, or (rarely) a mucinous adenocarcinoma. Carcinoid, neuroblasts, cartilage, and rhabdomyoblasts have also been described as heterologous elements in Sertoli-Leydig cell tumors. Clusters of polygonal cells with eosinophilic cytoplasm are often present in Sertoli-Leydig cell tumors that secrete α-fetoprotein. Although these were interpreted as Leydig cells by some authors, most now view them as foci of heterologous hepatic differentiation. Heterologous hepatic differentiation can occur in any moderately to poorly differentiated Sertoli-Leydig cell tumor, but it appears to be most frequent in those with retiform tubules. Occasionally hepatic cells are absent, and heterologous mucinous epithelium appears to be the source of the AFP.
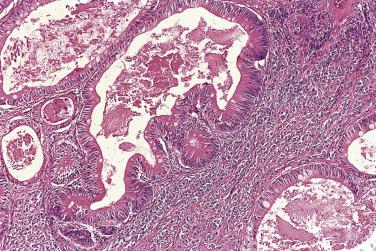
An uncommon sertoliform variant of endometrioid adenocarcinoma can mimic a Sertoli-Leydig cell tumor. Such tumors are characterized by the presence of small tubular glands or trabecular patterns of growth, occasionally with intermixed luteinized stromal cells that raise the question of Leydig cells. Diagnostic clues to the correct diagnosis include older patient age, associated endometriosis or an adenofibromatous growth pattern, the presence of areas with the typical confluent glandular pattern of endometrioid adenocarcinoma, intracytoplasmic mucin in tumor cells, greater nuclear atypia and mitotic activity than expected in the tubules of a Sertoli-Leydig cell tumor, squamous metaplasia, and ciliated cells.
Immunohistochemical stains for cytokeratin are positive in most mature and immature Sertoli cells, but these cells generally do not stain for epithelial membrane antigen. Keratin stains are particularly helpful in confirming the identity of nests and cords of immature Sertoli cells in these tumors. Staining for keratin is typically strongest in retiform tubules. EMA is negative and CK7 is generally negative in Sertoli-Leydig cell tumors, although staining for CK7 is sometimes seen in retiform tubules.
Immunostains for inhibin and calretinin are typically positive in both the Sertoli cells and Leydig cells in Sertoli-Leydig cell tumors, with stronger staining in the Leydig cells. Positive nuclear staining for SF1 is generally present in both Sertoli and Leydig cells. Positive nuclear staining for FOXL2 was initially reported to be present in the Sertoli cells in only about 50% of Sertoli-Leydig cell tumors, but our experience is that the Sertoli cells in most of these tumors show positive staining for FOXL2 while the Leydig cells are negative. CD56 shows strong membranous and cytoplasmic staining, mainly in the Sertoli cells. Sertoli cells show membrane staining for CD99, and most of them show nuclear staining for WT1. Stromal cells and Leydig cells are vimentin positive. Immunostains for estrogen and progesterone receptors are positive in a majority of Sertoli-Leydig cell tumors, with the most common pattern being one of diffuse staining for estrogen receptors and focal staining for progesterone receptors.
Immunostains for α-fetoprotein reveal positive staining of Leydig cells, Sertoli cells, and hepatoid cells in many tumors that secrete AFP. Hepatoid cells also typically show positive granular cytoplasmic staining with antihepatocyte antibody and positive cytoplasmic staining for glypican-3. The hepatoid cells can be keratin positive, and bile canaliculi between them can be stained with polyclonal CEA. When the serum AFP is elevated but hepatoid cells cannot be identified, heterologous gastrointestinal epithelium can often be shown to be the source of the AFP. The intestinal type glands can show positive staining for AFP, but also for SALL4, glypican-3, CDX2, and villin. While these can be positive in the intestinal type epithelium in Sertoli-Leydig cell tumors, they are also markers of yolk sac tumor, and it is important not to mistake the heterologous mucinous epithelium for yolk sac tumor because of its immunophenotype. The gastrointestinal epithelium in heterologous tumors contains argyrophilic cells that stain for chromogranin, serotonin, and other peptides, and heterologous carcinoid elements stain for chromogranin or synaptophysin or both. Staining for desmin and myogenin can help confirm the presence of heterologous rhabdomyosarcoma.
Sertoliform endometrioid adenocarcinomas are an important mimic of Sertoli-Leydig cell tumors. They are almost invariably EMA and CK7 positive, while these stains tend to be negative or very limited in Sertoli-Leydig cell tumors. Sertoli-Leydig cell tumors with hollow tubules can also mimic endometrioid adenocarcinomas. They are distinguished from adenocarcinomas by the presence of more typical areas of Sertoli-Leydig cell tumor, including Leydig cells, and by the absence of squamous metaplasia and associated endometriosis. Sertoli-Leydig cell tumors with pseudoendometrioid tubules exhibit the characteristic immunophenotype of a Sertoli-Leydig cell tumor, including positive staining for keratin, but absence of staining for CK7 and EMA, and positive staining for sex cord–stromal markers such as inhibin and calretinin.
Sertoli-Leydig cell tumors only rarely contain the type of FOXL2 mutation that occurs in adult granulosa cell tumor, but in recent years it has become evident that there are frequently somatic or germline mutations in DICER1 in Sertoli-Leydig cell tumors. The frequency of DICER1 mutations has ranged from 33% to 100% of moderately and poorly differentiated tumors in reported series. In one small study there was a suggestion that DICER1 -mutated tumors had an increased risk of recurrence, and in another it was noted that some tumors contained juvenile granulosa cell tumorlike areas or unclassifiable areas. Well-differentiated Sertoli-Leydig cell tumors do not appear to have DICER1 mutations. Other diseases linked to the DICER1 syndrome include thyroid multinodular goiters, thyroid carcinoma, cystic nephroma of the kidney, nasal chondromesenchymal hamartoma, ciliary body medulloepithelioma, pituitary blastoma, pineoblastoma, Wilms tumor, pleuropulmonary blastoma, and embryonal rhabdomyosarcoma of the cervix. Given the strong association between Sertoli-Leydig cell tumors and DICER1 mutations, patients with Sertoli-Leydig cell tumors should undergo genetic counseling and testing for germline DICER1 mutations to determine whether they may have the DICER1 syndrome, and whether they or their relatives or offspring may be at risk for development of DICER1-associated diseases. Interestingly, most DICER1 mutation carriers appear unaffected by tumors associated with the syndrome.
Sertoli cell tumors are among the rarest sex cord–stromal tumors. They differ from Sertoli-Leydig cell tumors in that they do not contain Leydig cells or immature gonadal stroma. Tumors with complex annular tubules have been classified by some authors as Sertoli cell tumors with complex tubular patterns, while other authors classify these tumors separately, as sex cord tumors with annular tubules. In this chapter, sex cord tumors with annular tubules (SCTAT) are discussed in a subsequent section.
Sertoli cell tumors occur most often in women of reproductive age, but they occasionally arise in children and postmenopausal women. The average patient age is about 30 years. Occasional Sertoli cell tumors occur in patients with the Peutz–Jeghers syndrome (~10%), although, as will be discussed, the sex cord tumor with annular tubules is a more characteristic finding in patients with the Peutz–Jeghers syndrome. Two-thirds of Sertoli cell tumors secrete steroid hormones. Most secrete estrogens but a few secrete androgens. Girls with hormonally active tumors present with precocious pseudopuberty and vaginal bleeding. Older women have irregular bleeding, postmenopausal bleeding, or (rarely) virilization, depending on the type and amount of hormone secreted. Patients with hormonally inactive neoplasms have nonspecific symptoms such as pain or abdominal swelling, or their tumors are incidental findings. Sertoli cell tumors are unilateral and most are clinically benign tumors that can be treated by unilateral salpingo-oophorectomy. Occasional poorly differentiated or invasive Sertoli cell tumors recur or metastasize, and cause the patient's death. Most of these have spread beyond the ovary at diagnosis, but a few stage IA Sertoli cell neoplasms have metastasized.
Sertoli cell tumors are unilateral, encapsulated tumors that average 8 cm in diameter; most are between 4 and 12 cm. The cut surfaces are gray, tan, brown, or yellow and are predominantly solid, although there are small cysts in some tumors.
These are tumors composed of Sertoli cells that grow in mature fibrous or hyalinized stroma. A tubular pattern is characteristic. The tubular pattern tends to be a simple one, consisting of round or oval open glands with a central lumen or long closed cordlike tubules two or three cells thick. Mixed tubular patterns are often present. In some tumors, there are cords, trabeculae, or areas of diffuse tumor cell growth.
Sertoli cells are cuboidal or columnar and have round to oval nuclei, which sometimes contain small nucleoli. Bizarre but degenerate nuclei do not appear to adversely affect the prognosis. The cytoplasm varies from clear to eosinophilic ( Fig. 13A.65 ). Uncommon variants of Sertoli cell tumor include a lipid-rich type, in which the cells have abundant clear foamy cytoplasm, and an oxyphilic type composed of cells with abundant granular eosinophilic cytoplasm. Most Sertoli cell tumors are well differentiated and the neoplastic cells have uniform bland nuclei and few mitotic figures. Sertoli cell tumors that are confined to the ovary at diagnosis and that exhibit no or minimal nuclear atypia and mitotic activity are likely to have a benign clinical evolution. Less than 10% of Sertoli cell tumors are clinically malignant. These have a poorly developed pattern of tubules, the Sertoli cells have enlarged atypical nuclei, and there are 5 or more mitotic figures per 10 hpf. Malignant Sertoli cell tumors can bear a resemblance to endometrioid adenocarcinoma but can be differentiated from it by the presence of more typical patterns of Sertoli cell growth and by differences in immunophenotype.
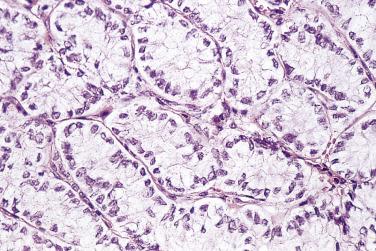
Small ovarian hilar proliferations consisting of aggregates of Sertoli tubules, sometimes admixed with rete ovarii tubules or hilar Leydig cells, have been described. The cells lining the tubules have been bland with no mitotic activity. The immunophenotype has been compatible with cells of sex cord–stromal type. In several cases, the proliferations have been bilateral, leading to speculation that they may be benign nonneoplastic proliferations rather than small neoplasms. In any event, the bland cytology and small size suggests that this finding is likely to be benign.
Sertoli cell tumors tend to be immunoreactive for vimentin and cytokeratin, but not for epithelial membrane antigen, and they rarely stain for cytokeratin 7, estrogen receptors, or progesterone receptors. There is frequently staining for inhibin, calretinin, and CD99 with inhibin being the most likely to show staining, and SF1, FOXL2, and WT1 stain the tumor cell nuclei in virtually all Sertoli cell tumors. Occasional tumors show focal weak staining for smooth muscle actin, S100 protein, or neuron-specific enolase, but staining for chromogranin is absent. Sox9 is a nuclear transcription factor that is involved in Sertoli cell differentiation in the testis. Although Sox9 mRNA has been detected in ovarian Sertoli cell tumors, fewer than 50% show positive nuclear staining for Sox9. Moreover, staining is present in some tumors in the differential diagnosis, such as endometrioid adenocarcinoma. Sox9 therefore does not appear to be particularly helpful in the diagnosis of Sertoli cell tumors of the ovary.
DICER1 mutations have been identified in a few Sertoli cell tumors. In one report, they were found in five of eight Sertoli cell tumors, so Sertoli cell tumors likely represent another tumor type that may be found in patients with the DICER1 syndrome.
The SCTAT is designated as an unclassified sex cord–stromal tumor because its histogenesis is controversial. Nevertheless, its microscopic appearance and clinical features are distinctive. As noted earlier, some authors view these as a complex type of Sertoli cell tumor.
The SCTAT occurs in two clinical settings. About a third are detected in women with the Peutz–Jeghers syndrome, a hereditary condition with an autosomal dominant pattern of inheritance in which there is mutation of the STK11 tumor suppressor gene on chromosome 19. Those affected have mucocutaneous melanin pigmentation and hamartomatous intestinal polyps. Palpable ovarian tumors of various types are detected in 10% to 20% of women with the Peutz–Jeghers syndrome, but if the ovaries are examined microscopically, virtually all contain SCTAT. In this clinical setting, the tumors are generally microscopic in size, multicentric, and bilateral, and show mutations of STK11 . SCTAT are almost always an incidental finding in women with the Peutz–Jeghers syndrome since they are too small to palpate and rarely secrete sufficient steroid hormones to cause symptoms. Microscopic SCTAT in women with the Peutz–Jeghers syndrome are clinically benign, and asymptomatic women do not need to have an oophorectomy. A clinically detectable SCTAT in a woman with Peutz–Jeghers syndrome can usually be treated by unilateral salpingo-oophorectomy, although rare patients with bilateral or malignant SCTAT have been reported. A small percentage of women with the Peutz–Jeghers syndrome develop a well-differentiated adenocarcinoma of the endocervix, so lifetime surveillance by a gynecologist is appropriate. An extremely rare type of sex cord–stromal tumor of the ovary, distinct from the SCTAT, occurs in children with the Peutz–Jeghers syndrome and causes sexual precocity. Sertoli cell tumors, including unusual variants with oxyphilic or lipidized cytoplasm, also occur rarely in patients with the Peutz–Jeghers syndrome.
About two-thirds of SCTAT occur in patients who do not have the Peutz–Jeghers syndrome (nonsyndromic SCTAT). Mutations of the STK11 gene are usually not detected in nonsyndromic SCTAT. Nonsyndromic SCTAT occur over a wide age range, from 6 to 76 years, with an average age of 36 years. The presenting symptoms depend on the patient's age. Premenarcheal girls often have precocious pseudopuberty. Older women may have menstrual dysfunction or postmenopausal bleeding. There is evidence that these tumors are able to produce progesterone as well as estrogen, since the endometrium exhibits progesterone effect in some patients, a peritoneal decidual reaction has been observed in others, and serum levels of progesterone may be elevated. A unilateral adnexal mass is palpable in about 50% of patients. Increased serum concentrations of anti-Müllerian hormone (Müllerian-inhibiting substance) and inhibin have been found in a few women with SCTAT. These substances may prove to be useful tumor markers for the follow-up of patients with SCTAT. Young women with localized tumors can be treated by unilateral salpingo-oophorectomy, while older women and those with advanced disease are treated by hysterectomy, bilateral salpingo-oophorectomy, and resection of extraovarian tumor. About 15% of nonsyndromic SCTAT are clinically malignant, with higher percentages of malignancy reported in some series. Recurrences occur early in some patients, but in many they are detected late, 5 or more years after initial treatment. It is difficult to predict which tumors will metastasize. Combination chemotherapy has been effective, at least in the short term, in some patients, but not in others.
In patients with the Peutz–Jeghers syndrome, SCTAT are small and are usually not grossly visible. Those that can be seen are solid tan or yellow tumors that may contain calcifications. They tend to be multifocal and bilateral. Nonsyndromic SCTAT are generally unilateral and vary in size from microscopic tumors or small nodules less than 1 cm in diameter to large tumors more than 20 cm in diameter. They are mainly solid, although cysts are present in some, and they have fleshy, tan, or yellow cut surfaces.
Rounded, sometimes coalescent nests of tumor cells growing in simple or complex closed annular tubules enclose cores of eosinophilic hyaline material ( Fig. 13A.66 ). The tumor cells grow in a fibrous stroma and are surrounded by basement membrane–like material that is continuous with the hyaline cores. The cores and the basement membrane–like material are PAS positive. There is typically a paired cell arrangement in the tubules, with the nuclei of the apposed cells located at opposite ends of the cells. The tumor cells are columnar and have clear or foamy cytoplasm and round or oval hyperchromatic nuclei with small nucleoli. Nuclear grooves are occasionally present. Atypia and mitotic figures are uncommon. Large tumors from patients who do not have the Peutz–Jeghers syndrome may contain long closed tubules, coalescent nests of tumor cells, foci of solid growth, cysts, or acellular areas of amorphous hyaline material ( Fig. 13A.67 ). Small areas of granulosa cell or Sertoli cell differentiation are noted occasionally. Metastases from some SCTAT resemble a granulosa cell tumor, and metastases from some granulosa cell tumors resemble a SCTAT, emphasizing the relationship between various types of sex cord–stromal tumors. One unique tumor contained areas with endometrioid glandular differentiation. Features associated with aggressive behavior include stromal invasion, diffuse moderate to marked nuclear atypia, and increased numbers of mitotic figures.
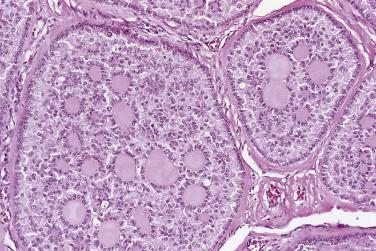
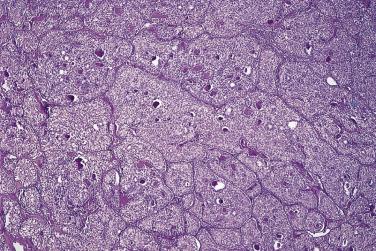
The immunophenotype of the SCTAT is similar to that of a Sertoli cell tumor. It shows positive staining for vimentin, inhibin, and calretinin, and it stains for cytokeratin but not for epithelial membrane antigen. Other immunostains that are positive in Sertoli cell tumors, such as FOXL2, SF1, WT1, and CD56, are also positive in SCTAT. The electron microscopic features of the SCTAT were studied by numerous investigators with the hope that the ultrastructure might provide a clue to its histogenesis. Basement membrane material surrounds tumor cell nests and fills the central spaces within the nests, forming the hyaline cores seen by light microscopy. Many tumor cells contain cytoplasmic filaments, which, in some cells, form perinuclear aggregates. These have been interpreted by some as Charcot–Böttcher filaments, leading them to conclude that SCTAT are Sertoli cell tumors. Others have not been able to identify Charcot–Böttcher filaments and have concluded that the appearance of the tumor cells is more similar to that of a granulosa cell tumor. The immunophenotype, specifically positive staining for cytokeratin, which is typically not present in granulosa cell tumors, favors a closer relationship to Sertoli cell tumor.
This category of ovarian tumors encompasses a somewhat heterogeneous group of gonadal stromal tumors. Some tumors in the group can be classified as Leydig cell tumors based on the identification of crystalloids of Reinke in the tumor cell cytoplasm or by their characteristic location in the hilum of the ovary. Others lack crystalloids of Reinke and occur in an extrahilar location; these are designated as steroid cell tumors, not otherwise specified (NOS). Finally, the stromal luteoma, a usually small tumor composed of luteinized stromal cells, also falls into this category.
Most ovarian Leydig cell tumors originate in the hilum, presumably from hilus cells, leading to the occasional designation of hilar Leydig cell tumors as hilus cell tumors . Less often, Leydig cell tumors arise outside the hilum, from the ovarian stroma. This category includes nonhilar and stromal Leydig cell tumors.
Leydig cell tumors typically occur in postmenopausal women. The average age is 58, and almost all patients are over 30 years of age. The usual clinical presentation is with hirsutism or signs of virilization such as acne, hair loss, deepening of the voice, a male body contour, or hypertrophy of the clitoris. The serum testosterone concentration is elevated in virilized patients, but urinary 17-ketosteroids are generally within normal limits. Patients who are not virilized may have amenorrhea or postmenopausal bleeding, depending on their age. Some Leydig cell tumors are found incidentally during surgery for some other condition. The endometrium can show hyperplasia or even adenocarcinoma, most likely secondary to peripheral conversion of testosterone to estrogen. Symptoms are often present for several years before the diagnosis is made. This is because Leydig cell tumors are usually small and difficult to localize. Nonpalpable tumors can be detected by imaging studies in some cases but, in others, it is necessary to measure hormone concentrations in blood obtained by selective catheterization of the ovarian veins. Although most Leydig cell tumors are unilateral, rare patients with bilateral tumors have been reported. Virtually all Leydig cell tumors are benign and are cured by surgery. Signs of virilization usually regress following removal of the tumor. Malignant Leydig cell tumors are very rare; only a few cases have been reported.
Leydig cell tumors are unilateral, small solid brown or yellow-brown tumors located in the hilum of the ovary or, rarely, in the medulla or cortex. The average diameter is between 3 and 5 cm, but tumors as small as 0.7 cm and as large as 15 cm have been reported.
Hilar Leydig cell tumors are circumscribed but not encapsulated. The tumor cells resemble Leydig cells of the testis, and the hilus cells normally present around nerves in the hilum of the ovary. They are round or polygonal and have abundant granular eosinophilic cytoplasm ( Fig. 13A.68 ). Many cells contain yellow or brown lipochrome pigment. The nuclei are uniform and of medium size, range from vesicular to hyperchromatic, and may contain conspicuous nucleoli. Crystalloids of Reinke are characteristic of Leydig cells, but they are identified in only about 50% of hilar Leydig cell tumors. They are intracytoplasmic eosinophilic rods with blunt or tapered ends ( Fig. 13A.69 ). Intracytoplasmic eosinophilic hyaline globules, which are thought to be precursors of Reinke crystals, are often easier to find. Other findings of note include perivascular clustering of nuclei and a peculiar fibrinoid change in the walls of blood vessels within the tumor.
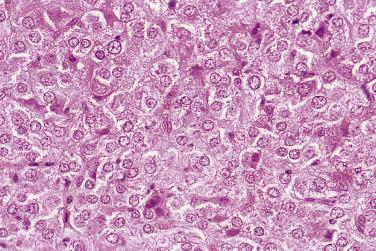
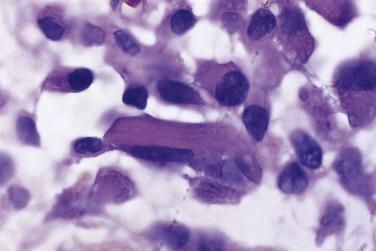
There are two types of nonhilar Leydig cell tumors. Pure nonhilar Leydig cell tumors are circumscribed tumors that are similar to hilar Leydig cell tumors except for their location; they are usually centered in the medulla. Stromal Leydig cell tumors are fibromas or thecomas that contain clusters, nests, or sheets of Leydig cells. By definition, crystalloids of Reinke must be identified in the tumor cells to diagnose a nonhilar Leydig cell tumor. Since only 50% of hilar Leydig cell tumors contain crystalloids, some nonhilar Leydig cell tumors are unrecognized and are misdiagnosed as a stromal luteoma or a luteinized thecoma.
This category of ovarian tumors encompasses stromal neoplasms that cannot be classified more specifically, hence the designation not otherwise specified . Tumors of this type have also been called lipid cell tumors because many of them have clear foamy cytoplasm. Cells resembling Leydig cells, but lacking crystalloids of Reinke, and cells resembling adrenal cortical cells are present in variable proportions in most of them.
Steroid cell tumors occur over a wide age range, from 3 to 80 years. The average patient is 45 years of age, but steroid cell tumors occasionally occur in children and in the elderly. Steroid cell tumors often secrete androgenic steroids in amounts sufficient to cause hirsutism or virilization. Serum testosterone concentrations and urinary 17-ketosteroids are elevated in virilized patients. Several unusual tumors secreted renin and were associated with secondary polycythemia or hypertension. Nonvirilized patients present with abdominal distention or pain, menstrual irregularities, or postmenopausal bleeding. Rare steroid cell tumors secrete cortisol and cause Cushing syndrome.
Most tumors are confined to the ovaries at diagnosis, and bilateral tumors are rare (6%). Pelvic, peritoneal, or distant metastases are present at diagnosis in 10% to 20% of cases. Young patients with stage IA neoplasms can be treated by salpingo-oophorectomy. Older patients and those with advanced tumors are generally treated by hysterectomy and bilateral salpingo-oophorectomy. Hirsutism and signs of virilization usually regress after removal of the tumor. A significant proportion of steroid cell tumors (25%–43%), including a majority of those that cause Cushing syndrome, are clinically malignant. Recurrences are most often detected within the first few years after treatment, but about 20% are detected more than 5 years later.
Steroid cell tumors are solid and range from less than 1 cm to more than 20 cm in diameter, with an average of about 7 cm. The cut surface is tan, yellow, or orange, and about 25% have areas of hemorrhage and necrosis. A few parovarian steroid cell tumors, possibly originating in ectopic ovarian tissue, have been reported.
A mixture of Leydig-like and adrenal cortical–like cells is generally present, although one of these may predominate. The Leydig-like cells are round or polygonal and have abundant, sometimes vacuolated eosinophilic cytoplasm ( Fig. 13A.70 ). The nucleus is round, centrally located, and typically contains a small nucleolus. Crystalloids of Reinke are never identified. The adrenal cortical–like cells are also round or polygonal and have abundant pale or clear vacuolated cytoplasm ( Fig. 13A.71 ). The nucleus is vesicular and may contain a small to midsize but conspicuous nucleolus. Fat stains are positive in adrenal-type cells. In most cases, mitotic figures are infrequent, and nuclear atypia is absent or modest. Pathologic features found in malignant steroid cell tumors include large size (>7 cm), hemorrhage or necrosis, 2 or more mitotic figures per 10 hpf, and moderate or marked nuclear atypia.
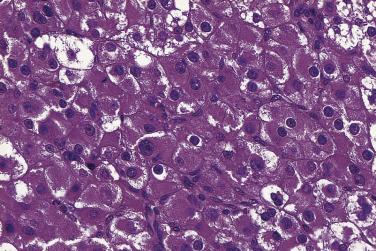

Stromal luteoma is a rare estrogen-secreting tumor that occurs mainly in postmenopausal women. The most common presentation is with abnormal uterine bleeding, and an endometrial biopsy often reveals hyperplasia. Rare stromal luteomas are virilizing. A third of cases are incidental findings at operation or autopsy. The stromal luteoma is clinically benign.
Stromal luteoma is a small unilateral neoplasm; all reported examples have been less than 3 cm in diameter. The cut surface is gray, white, yellow, or brown.
The tumor is located in the ovarian stroma and is composed of luteinized stromal cells that grow diffusely or form nests or cords. The cells are polygonal, with granular eosinophilic cytoplasm and small round centrally placed nuclei. There is no nuclear atypia, and mitotic figures are rare or absent. Differentiation from a Leydig cell tumor is based upon the nonhilar location of the stromal luteoma and the absence of cytoplasmic crystalloids of Reinke. Other ovarian abnormalities are typically present, including stromal hyperthecosis, which is often bilateral, and hilus cell hyperplasia.
Steroid cell tumors differ from other types of sex cord–stromal tumors in that they rarely show immunostaining for FOXL2 or WT1, which are generally positive in sex cord tumors. On the other hand, they typically show strong granular cytoplasmic staining for melan-A, and, in some studies, for the related marker MART1. Regardless of their site of origin within the ovary, steroid cell tumors show strong cytoplasmic staining for inhibin, cytoplasmic and nuclear staining for calretinin, and nuclear staining for SF1. In one study there was strong membranous staining for CD99 in cells with eosinophilic cytoplasm, but cells with clear vacuolated cytoplasm were generally negative, while in other studies CD99 stained only a minority of steroid cell tumors. The tumor cells are vimentin positive; staining for cytokeratin is present in 30% to 50% of tumors, most often in cells with clear vacuolated cytoplasm. Staining for keratin is usually focal, and sometimes there is a perinuclear globoid staining pattern. Steroid cell tumors are EMA negative. Immunostains for androgen receptors are positive in about two-thirds of cases. An immunostain for smooth muscle actin is positive in about a third of cases. Steroid cell tumors frequently show positive cytoplasmic staining for CD10. The best combination of stains to confirm the diagnosis is inhibin, calretinin, and SF1; lack of staining for EMA helps to exclude a carcinoma.
Gynandroblastoma is a rare tumor with substantial (>10%) areas of both Sertoli or Sertoli-Leydig cell and granulosa cell differentiation. Tumors of this type are placed in the unclassified sex cord–stromal tumor category by some. Patients with a gynandroblastoma are typically virilized, although peripheral conversion of testosterone to estrogen can cause abnormal vaginal bleeding due to endometrial proliferation or hyperplasia. The treatment is surgical excision. Most reported examples have had a benign clinical evolution, but recurrences have been reported. Given the composition of the tumor, it should probably be viewed as having the same malignant potential as a granulosa cell tumor or intermediate grade Sertoli-Leydig cell tumor. One reported example recurred, illustrating the malignant potential of these tumors. Gynandroblastoma is unilateral and measures 1 to 18 cm in diameter. It can be solid or partly cystic. Solid areas are white, tan, or yellow. Microscopically, open and closed tubules and cords lined by Sertoli cells are intermixed with nests and sheets of granulosa cells ( Fig. 13A.72 ). Microfollicles and other typical patterns of granulosa cell tumor may be present. The granulosa cell component is usually of the adult type, although in some cases it has been of the juvenile type. The stroma may contain spindle-shaped cells resembling theca cells, luteinized cells, or Leydig cells. Neither significant cytologic atypia nor frequent mitotic figures have been present in reported examples of gynandroblastoma. These tumors express inhibin and calretinin. Vimentin and androgen receptor are preferentially expressed in the granulosa cell component, and low molecular weight keratin and CD10 are more likely to be expressed by the Sertoli cell component. DICER1 gene mutations have been documented in some gynandroblastomas. Several studies have demonstrated DICER1 mutations in a proportion of the gynandroblastomas that were studied. On the other hand, while several groups failed to demonstrate FOXL2 mutations in any of their cases, another group found FOXL2 mutations in both components of two of three DICER1 wild-type tumors.
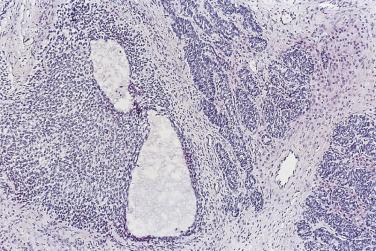
Microcystic stromal tumor is a rare neoplasm of gonadal stromal derivation that occurs mainly in middle-age women (mean age 45 years). Most patients present with nonspecific findings, usually a pelvic mass. Hormonally related symptoms are uncommon. Several microcystic stromal tumors have occurred in patients with familial adenomatous polyposis whose tumors exhibited mutations in the APC gene, but not in CTNNB1 ; mutations in both of these genes occur in microcystic stromal tumors, but they appear to be mutually exclusive, with mutations in CTNNB1 being far more frequent. The tumors are unilateral, and only one microcystic stromal tumor is known to have recurred after treatment, although the number with follow-up is limited. Unilateral salpingo-oophorectomy is appropriate treatment for a young patient.
Microcystic stromal tumor averages 8.7 cm in diameter and is usually partly solid and partly cystic. The cut surfaces tend to be firm and tan or white. Microscopic examination reveals a lobulated tumor with variably prominent fibrous bands. The tumor cells are round to spindled and have monotonous round, oval, or fusiform dark nuclei with inconspicuous nucleoli. Mitotic activity is low, usually in the range of 0 to 2 mitotic figures per 10 hpf. The tumor cells have moderate amounts of eosinophilic finely granular cytoplasm. In some places, the tumor cells grow in solid sheets reminiscent of a fibroma or thecoma, but the most characteristic finding, which is usually present extensively, is the formation of microcystic, and sometimes larger, spaces filled with lightly basophilic or clear material among the tumor cells. Clusters of cells with large bizarre often smudged, or degenerate nuclei have been present in a majority of the reported cases.
The tumor has the morphology of a sex cord–stromal neoplasm, but immunohistochemical analysis reveals absent or only weak staining for inhibin and calretinin. On the other hand, there is strong positive nuclear staining for FOXL2 and SF1, supporting the sex cord–stromal nature of the tumor, and there is positive nuclear staining for WT1 and cyclin D1, and nuclear and cytoplasmic staining for β-catenin. The tumor cells show positive staining for vimentin and CD10, there is dotlike cytoplasmic staining for CD99 in a few cases, and weak focal staining for cytokeratin is seen in about a quarter of cases. Positive staining for EMA has not been observed. Mutations in the CTNNB1 gene have been identified in about two-thirds of microcystic stromal tumors, suggesting that mutation of this gene may be involved in the genesis of microcystic stromal tumors.
The signet ring–stromal cell tumor is a rare nonfunctioning gonadal stromal neoplasm that occurs in patients of all ages. It has a conspicuous component of vacuolated cells that resemble signet ring cells. Microscopically there is usually a mixture of spindle cells and signet ring cells, both having bland nuclei and exhibiting little or no mitotic activity. The cytoplasmic vacuoles do not contain mucin. Immunostains for vimentin are positive in the signet ring cells while those for inhibin, calretinin, and SF1 are variable but often negative. Immunostains for keratin and EMA are negative. Ultrastructural and histochemical studies have demonstrated that the vacuoles can be cytoplasmic or cytoplasmic pseudoinclusions of extracellular matrix, and they have confirmed the absence of lipid and glycogen as well as mucin. Occasionally, hyaline globules are present in the cytoplasm of tumor cells. Molecular studies conducted on one tumor revealed a deletion in the CTNNB1 gene, and immunohistochemical studies documented nuclear staining for β-catenin and cyclin-D1, which could potentially be used for diagnostic purposes if positive staining for these markers can be documented in additional cases. The tumor cells are differentiated from signet ring carcinoma cells by their positive staining for vimentin and lack of staining for mucin and epithelial markers such as keratin and EMA. Signet ring–like stromal cells are occasionally seen in other types of sex cord–stromal tumors, such as sclerosing stromal tumors and thecomas, and even in the stroma of some epithelial tumors.
Tumors that do not fit into any of the named categories are called unclassified sex cord–stromal tumors . The clinical presentation is nonspecific, with abnormal bleeding, an abdominal mass, or abdominal pain. Sex cord–stromal tumors removed from pregnant women are often difficult to classify, and some must be designated as unclassified . In most cases, the tumor is confined to the ovary at diagnosis, and the prognosis is favorable. A few tumor-related deaths have been reported, so these tumors are viewed as having low malignant potential, along the lines of a granulosa cell tumor or an intermediate Sertoli-Leydig cell tumor. Unclassified sex cord–stromal tumors consist of variable admixtures of spindle cells, cords and trabeculae of sex cord cells, and vague tubelike structures. They do not show sufficiently distinctive patterns of growth to be classified as granulosa cell or Sertoli-Leydig cell tumors, although, as mentioned earlier, gynandroblastoma is included in this category by some.
Mesenchymal tumors of all types occur in the ovary. The gross and microscopic appearance of these tumors are the same in the ovary as in the soft tissues or uterus, as is the clinical behavior. Leiomyomas are the most common mesenchymal tumors of the ovary. They occur mainly in middle-aged women who also have uterine leiomyomas. Lipoleiomyoma is a variant of leiomyoma in which the smooth muscle is mixed with fat. Other benign soft tissue tumors that occur in the ovary from time to time are hemangioma and myxoma. Hemangiomas tend to be small and mainly occur in older women. Most are of the cavernous type, although some have an admixture of cavernous and capillary features. Rare cases of anastomosing hemangioma, a neoplasm characterized by anastomosing capillary vessels that contain fibrin thrombi and are lined by endothelial cells with hyaline cytoplasmic globules, mild nuclear size variability, and occasional hobnailed features, have also been reported in the ovary. Ovarian myxoma has a matrix that is rich in hyaluronic acid, and it is more cellular and more vascular than soft tissue myxoma.
Primary sarcomas of the ovary are rare. Fibrosarcoma and endometrioid stromal sarcoma, discussed earlier, are the most common ovarian sarcomas. Leiomyosarcoma occurs in the ovary and can be diagnosed by evaluating similar features to those used for the diagnosis of uterine leiomyosarcoma. Leiomyosarcoma of the ovary exhibits diffuse moderate to severe nuclear atypia, frequent mitotic figures, and tumor cell necrosis. In the largest reported study, it was found that tumors with diffuse significant nuclear atypia and 5 or more mitotic figures per 10 hpf were likely to behave as leiomyosarcomas; many, but not all, had foci of tumor cell necrosis. Other types of primary ovarian sarcoma are rhabdomyosarcoma, chondrosarcoma, osteosarcoma, malignant schwannoma, liposarcoma, and angiosarcoma. Rhabdomyosarcoma occurs in patients of all ages, including in children, and at least one childhood tumor was interpreted as being a manifestation of the DICER1 syndrome. Thus the diagnosis of a primary ovarian rhabdomyosarcoma, at least in a child, may be an indication for referral for genetic counseling to exclude a germline DICER1 mutation.
Lymphoma presents as an ovarian tumor in less than 1% of cases. The clinical presentation in such cases is with pelvic or abdominal pain, abdominal distention, disturbances of menstruation, or lymphoma B symptoms such as fever, night sweats, or weight loss. On examination, unilateral or bilateral adnexal masses are palpable. Although the clinical presentation is as an ovarian tumor, the lymphoma generally also involves the pelvic or abdominal lymph nodes, the liver or spleen, or other organs, indicating that the ovaries are involved as part of disseminated disease. A few patients treated only by oophorectomy never develop extraovarian lymphoma, indicating that rare lymphomas are primary in the ovary. The Ann Arbor lymphoma stage provides more prognostic information than the FIGO stage. The survival of patients who receive modern combination chemotherapy is greater than 70% and is similar to that attained overall in lymphomas of comparable grade and stage.
Both ovaries are involved in more than 50% of cases, although primary lymphomas tend to be unilateral. The size ranges from microscopic to more than 20 cm, with an average diameter of 10 to 15 cm. Rare ovarian lymphomas are incidental microscopic discoveries in ovaries removed for some other reason. The cut surface is fleshy and pink, tan, or gray.
Ovarian lymphomas are almost always of non-Hodgkin type. Burkitt and Burkitt-like lymphomas are most common in children and young women, but can also occur in older women. The tumor cells have uniform, round, small to midsize nuclei with coarse chromatin and one to three nucleoli. Mitotic figures are frequent. The cytoplasm is scanty and basophilic. The cells grow in sheets punctuated by spaces that contain phagocytic histiocytes, producing the starry sky appearance typical of Burkitt lymphoma, or in cords. The tumor cells tend to surround, rather than replace, follicles and other ovarian structures. Large cell lymphoma of B-cell type is the most common ovarian lymphoma in adults, followed by follicular lymphoma. In large cell lymphomas the tumor cells have round, oval, or cleaved nuclei and scanty to moderate amphophilic cytoplasm ( Fig. 13A.73 ). The nuclei are hyperchromatic with coarse chromatin, or vesicular with a prominent nucleolus. Mitotic figures are numerous. The cells grow in sheets and may focally infiltrate the stroma in cords. Other types of non-Hodgkin lymphoma, including lymphoblastic lymphoma and T-cell lymphoma, occasionally involve the ovary. Vascular invasion and focal stromal sclerosis is common in all types of lymphoma. The most useful immunohistochemical markers are CD45 (leukocyte common antigen), CD20 for B cells, and CD3 for T cells. Other markers of B-cell and T-cell differentiation help to immunophenotype ovarian lymphomas. As noted earlier, immunohistochemical studies generally reveal a B-cell phenotype.
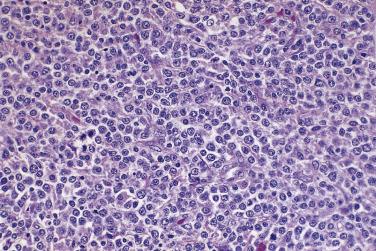
Leukemia not infrequently involves the ovaries, but it is usually an incidental microscopic finding in a patient with known leukemia and seldom presents as an ovarian tumor. Ovarian masses composed of leukemic cells are rare, but they do occur, both in children and adults. Ovarian masses have been reported in patients with lymphoblastic and granulocytic leukemia.
Granulocytic sarcoma is an extramedullary mass of immature myeloid cells. It is a rare cause of unilateral or bilateral ovarian enlargement. The cut surface is lobulated, firm or fleshy, and ranges in color from white or tan to a green color that, although characteristic of granulocytic sarcoma, is rarely seen in the ovary.
Microscopically, granulocytic sarcoma consists of sheets or cords of immature hematopoietic cells with midsize round nuclei, fine chromatin, inconspicuous nucleoli, and scanty amphophilic cytoplasm. Evidence of myeloid differentiation, such as cytoplasmic eosinophilia or an admixture of eosinophilic myelocytes, is typically inconspicuous and easy to overlook. Histochemical stains for naphthyl chloracetate esterase are usually positive, although often only focally. A variety of immunohistochemical stains are positive in granulocytic sarcoma and are easier to interpret than histochemical stains. Myeloperoxidase and lysozyme are the most sensitive and specific. Immunostains for markers of B and T lymphocyte differentiation, such as CD20 and CD3, are negative. Leukemia may or may not involve the peripheral blood and bone marrow at the time of diagnosis of granulocytic sarcoma; if leukemia is not present at diagnosis, it generally develops subsequently.
The neoplasms in this group are derived from germ cells ( Box 13A.5 ). Some are composed of undifferentiated cells (dysgerminoma, embryonal carcinoma), while in others there is differentiation toward embryonic (teratoma) or extraembryonic (choriocarcinoma, yolk sac tumor) structures. Benign cystic teratomas are common, but other types of germ cell tumor, including all the malignant ones, are rare.
Dysgerminoma
Yolk sac tumor
Embryonal carcinoma
Polyembryoma
Choriocarcinoma
Teratoma
Mature cystic
Mature solid
Malignant tumor arising in a mature teratoma
Immature teratoma
Neuroectodermal tumors
Monodermal teratoma
Struma ovarii
Carcinoid tumor
Mixed germ cell tumor
Gonadoblastoma
Mixed germ cell–sex cord–stromal tumor, unclassified
Unclassified germ cell tumor
Dysgerminoma is one of the two most common malignant germ cell tumors of the ovary, but it accounts for only 1% to 2% of all malignant ovarian tumors. Dysgerminoma is a tumor of children and young women. The average age is 22 years, and 90% of patients are less than 30 years of age. About 20% of malignant ovarian tumors detected during pregnancy are dysgerminomas. The typical presentation is with abdominal distention, an abdominal mass, or abdominal pain. Some patients have menstrual abnormalities or gastrointestinal or urinary symptoms. Rare patients have hypercalcemia. Serum lactic dehydrogenase (LDH) is frequently elevated, and can serve as a useful tumor marker. Increased levels of serum α-fetoprotein or human chorionic gonadotropin suggest the presence of other germ cell elements, although rare patients with pure dysgerminoma have increased amounts of βhCG in the blood. Dysgerminoma is the most common malignant gonadal tumor in patients with gonadal dysgenesis.
Dysgerminoma is confined to the ovaries (stage I) at diagnosis in 60% to 80% of patients. It is usually unilateral, but both ovaries contain tumor (stage IB) in 5% to 15% of cases. The tumor in the contralateral ovary is microscopic in half of the bilateral cases, leading some oncologists to recommend biopsy of an apparently normal contralateral ovary if treatment is to be by unilateral salpingo-oophorectomy. Dysgerminoma metastasizes via the lymphatics to the paraaortic lymph nodes, with subsequent spread to the mediastinal lymph nodes, and by transperitoneal spread to the pelvic and abdominal peritoneum.
Unilateral encapsulated dysgerminoma (FIGO stage Ia) can be treated by salpingo-oophorectomy with a 5-year survival rate of greater than 90%. Postoperative therapy has been advocated for patients with localized disease, but there is an increasing trend to manage such patients with close surveillance and administer chemotherapy only to those who develop a recurrence. Fortunately, recurrences usually are managed successfully. When dysgerminoma arises in a dysgenetic gonad, the appropriate treatment is bilateral gonadectomy. The standard treatment for advanced disease (stage >IA) is total abdominal hysterectomy, bilateral salpingo-oophorectomy, limited debulking, and postoperative chemotherapy or radiotherapy. If they are not involved by tumor, the uterus and the contralateral ovary may be conserved in advanced cases where preservation of fertility is important. Chemotherapy with platinum-based regimens is highly effective against dysgerminoma and is less likely than radiation to cause ovarian failure and infertility. Overall survival of optimally treated patients now exceeds 90%. Recurrences usually become evident within 2 years of primary treatment.
Dysgerminoma is a large solid tumor, usually more than 10 cm in diameter, with a smooth outer surface. On cross section, it is fleshy, homogeneous or nodular, and gray, tan, or white. Hemorrhage and necrosis are often present in large tumors.
The microscopic appearance of dysgerminoma is the same as that of seminoma of the testis. The tumor cells are polygonal, with distinct cell membranes and abundant granular to clear cytoplasm ( Fig. 13A.74 ). Glycogen can often be demonstrated in the cytoplasm with the PAS stain. The nuclei are central, round, and vesicular, and they contain prominent nucleoli. Mitotic figures are usually numerous. The cells grow in nests, lobules, and trabeculae that are surrounded by fibrous septa. There is occasionally loss of intercellular cohesion with formation of glandlike spaces. Lymphocytes are frequently present in the fibrous septa and are occasionally seen among the tumor cells ( Fig. 13A.75 ). Lymphocytes are numerous in some tumors, in which they form sheets or nodules that may contain reactive germinal centers. Epithelioid cells and multinucleate Langhans giant cells are often seen, and some tumors contain sarcoid-like granulomas. A fibrous or granulomatous reaction can be so intense that it obscures the tumor cells. Zones of necrosis are present in some dysgerminomas, especially large ones. Neoplasms with a degree of atypia greater than usual and a high mitotic rate (>30 mitotic figures per 10 hpf) have been termed anaplastic dysgerminomas . The prognosis in such cases is comparable to that of a typical dysgerminoma. About 3% of dysgerminomas contain syncytiotrophoblastic giant cells but exhibit no other types of nongerminomatous differentiation. Such tumors are classified as dysgerminomas with syncytiotrophoblastic giant cells and have the same prognosis as tumors that lack syncytiotrophoblastic giant cells.

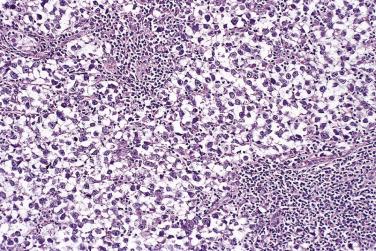
Chromosome 12p abnormalities are a characteristic of malignant germ cell tumors. An isochromosome, i12p, is the most common finding, but overrepresentation of chromosome 12p material is sometimes found either in addition to or instead of an i12p. In one study, an i12p was identified in 16 of 21 dysgerminomas, and overrepresentation of chromosome 12 material was detected in 5 dysgerminomas. KIT mutations are detected in about a quarter of dysgerminomas, but they are located in exon 17, not the exon 11 location that confers sensitivity to imatinib.
Dysgerminoma has an immunophenotype similar to that of seminoma of the testis. Several categories of antibodies are useful in the diagnosis of dysgerminoma. One group of antibodies is characterized by strong and diffuse membranous and, to a lesser extent, cytoplasmic staining of tumor cells. The principal antibodies in this group are placental alkaline phosphatase (PLAP), CD117, and D2-40. Staining for CD117 is present in almost all dysgerminomas and is not dependent on the presence of a KIT mutation. A second group of antibodies reacts with nuclear antigens. This group includes OCT4, NANOG, and SALL4. Staining of tumor cell nuclei is generally strong and diffuse. Monoclonal antibodies against cytokeratin, particularly those against low molecular weight cytokeratins, may be positive in dysgerminoma. Staining is usually weak and focal with a dotlike or rimlike appearance, and it is not likely to be confused with the stronger membrane or cytoplasmic staining seen in other tumors in the differential diagnosis. Dysgerminoma is EMA negative. Positive staining for human chorionic gonadotropin is seen in the syncytiotrophoblastic giant cells that are noted occasionally in dysgerminoma. Immunostains for AFP, glypican-3, SOX2, and CD30, which mark other types of germ cell tumor, are negative. The tumor-infiltrating lymphocytes in dysgerminoma are predominantly T cells, as has been shown by staining for various markers of T-cell differentiation.
Yolk sac tumor, formerly also known as endodermal sinus tumor, is a malignant germ cell tumor in which there is differentiation into yolk sac structures. Yolk sac tumor is the third most common malignant germ cell tumor of the ovary and comprises approximately 1% of ovarian malignancies.
Yolk sac tumor occurs principally in children and young women, although it rarely occurs in women older than 40 years of age, sometimes in association with an epithelial neoplasm. The median age is 19 years. The most common presenting complaints are abdominal pain, abdominal enlargement, or an abdominal mass. About 10% of patients have acute abdominal symptoms due to rupture or torsion of the tumor. High levels of AFP are present in the serum of most patients with yolk sac tumors, and some have increased levels of CA125 as well.
Yolk sac tumor appears limited to the ovary at diagnosis in about 50% of patients. When tumor disseminates beyond the ovary, it spreads to the peritoneum and omentum, the paraaortic lymph nodes, and the liver. Tumor appears confined to the pelvis (stage II) in about 10% of cases, while the remaining 40% have more widespread metastases (stages III and IV).
The recommended initial surgical treatment for yolk sac tumor is unilateral salpingo-oophorectomy with limited debulking of extraovarian tumor. Bilateral involvement is rare in patients with localized (stage I) tumors, so it is not necessary to biopsy the contralateral ovary. If they are uninvolved, the contralateral ovary and uterus need not be removed in patients with advanced disease. Despite apparently adequate surgery, the prognosis for individuals with yolk sac tumor was dismal prior to the availability of combination chemotherapy. The clinical course was characterized by rapid development of metastases and high mortality, even when the tumor appeared limited to the ovary at operation. Radiotherapy proved ineffective for treating these tumors. The 3-year survival was only 13% in one large study of patients treated prior to 1975. The development of VAC (vincristine, dactinomycin, and cyclophosphamide) combination chemotherapy completely changed the outlook for patients with yolk sac tumor. When treated with VAC chemotherapy, about 80% of patients with stage I tumors survived, as did about 50% of those with advanced disease. Chemotherapy regimens containing platinum provide even better results, and standard therapy is now with a combination of bleomycin, etoposide, and platinum (BEP) or some other similar regimen. The serum AFP level can be used to monitor the response to treatment and to detect tumor recurrence.
Yolk sac tumors tend to be large, with an average diameter of 16 cm. The cut surface is tan, white, or gray with small cysts and areas of hemorrhage and necrosis. A honeycomb appearance due to the presence of numerous cysts suggests an area of tumor with a polyvesicular vitelline pattern.
There are numerous histologic patterns of yolk sac tumor growth, and a mixture of patterns is present in most tumors, often resulting in a confusing histologic appearance. The two most common and distinctive patterns are the reticular, or microcystic, pattern and the endodermal sinus pattern. The reticular pattern consists of a loose meshwork of microcystic spaces lined by a single layer of flattened or cuboidal cells ( Fig. 13A.76 ). These have clear or amphophilic cytoplasm and atypical, hyperchromatic nuclei. The endodermal sinus pattern is also known as the festoon or pseudopapillary pattern. It consists of labyrinthine anastomosing glands and papillae lined by columnar cells with clear or amphophilic cytoplasm and fusiform, hyperchromatic nuclei. Schiller-Duval bodies are papillary or glomeruloid structures in which fibrovascular cores covered by columnar tumor cells project into glands or cystic spaces lined by cuboidal cells. They are a characteristic finding in this pattern of yolk sac tumor growth ( Fig. 13A.77 ) and are observed in about two-thirds of cases. They are diagnostic of yolk sac tumor. The endodermal sinus pattern often merges into the closely related alveolar-glandular pattern, in which anastomosing tubules or glands are surrounded by a myxoid or spindle cell stroma. The glands are lined by cuboidal or columnar cells that are often stratified into multiple layers or form small papillae. The solid pattern is characterized by nests or sheets of small to midsize undifferentiated cells with a moderate amount of amphophilic or clear cytoplasm. In the polyvesicular vitelline pattern, cysts bearing a resemblance to yolk sac vesicles are lined by cuboidal, columnar, or mucinous epithelial cells ( Fig. 13A.78 ). The cysts are surrounded by immature cellular mesenchymal stroma. Rare patterns include a hepatoid pattern composed of sheets or trabeculae of large cells with central vesicular nuclei with prominent nucleoli and abundant granular eosinophilic cytoplasm. These cells are reminiscent of those seen in hepatocellular carcinoma. Although large areas of hepatoid differentiation are present in some yolk sac tumors, this pattern is most often a focal microscopic finding. A glandular pattern, in which there are zones of glands of endometrioid or intestinal type, is a rare but important pattern in yolk sac tumor. In the endometrioid-like variant, which can be mistaken for endometrioid carcinoma, the glands are lined by a single layer of columnar cells, which have clear supranuclear or subnuclear cytoplasmic vacuoles ( Fig. 13A.79 ). Some authors think that this pattern would be more accurately designated as an enteroblastic pattern, and others note a resemblance to fetal lung. The intestinal pattern can mimic a primary or metastatic mucinous tumor. It is characterized by aggregates of primitive endodermal glands lined by low columnar cells growing in loose or cellular stroma. Glandular patterns of differentiation in yolk sac tumors of germ cell origin must be differentiated from rare tumors occurring in older women in which a yolk sac tumor arises from, or in association with, an epithelial tumor, such as an endometrioid carcinoma. Two rare tumors in which yolk sac tumor was admixed with mucinous carcinoid have been described.
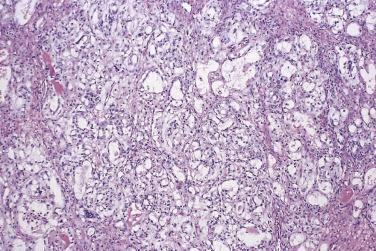
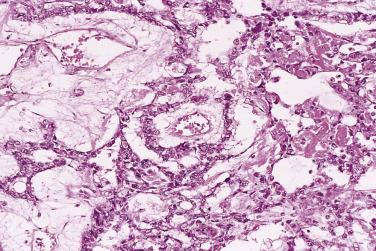

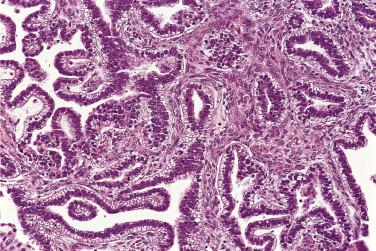
Eosinophilic, PAS-positive, diastase-resistant hyaline globules are a characteristic finding in yolk sac tumors and are most often found in the reticular and endodermal sinus patterns ( Fig. 13A.80 ). Reticular and solid areas also typically contain abundant extracellular hyaline PAS-positive material composed of laminin and type IV collagen that resembles basement membrane ultrastructurally. This material has been interpreted by some as indicative of parietal yolk sac differentiation. Small, bland, enteric glands lined by columnar and goblet cells are found in 50% of yolk sac tumors. Myxoid stroma containing spindle or stellate cells that stain with both cytokeratin and vimentin is prominent in 25% of yolk sac tumors. These stromal cells may differentiate into mesenchymal elements such as cartilage, striated muscle, and bone, which are occasionally seen in yolk sac tumors. Stromal cells are luteinized in, or adjacent to, 15% to 20% of yolk sac tumors. Syncytiotrophoblastic giant cells are present in rare cases.
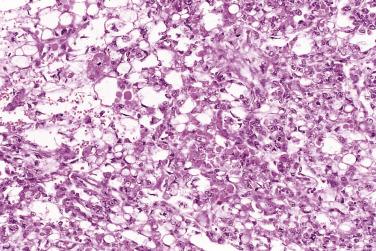
The most important immunohistochemical finding in yolk sac tumor is positive staining for α-fetoprotein. Positive staining is detected in tumor cell cytoplasm, secretory material within cysts and glands, and some hyaline bodies in more than 75% of yolk sac tumors, but staining is often weak and focal. Additional immunostains for yolk sac tumor that overcome some of the limitations of AFP include glypican-3, which stains the cytoplasm of yolk sac tumor cells in nearly all cases, and SALL4, with which there is diffuse strong nuclear staining in most yolk sac tumors. Glypican-3 is not entirely specific for yolk sac tumor and stains some cases of clear cell carcinoma. SALL4 also stains dysgerminoma and embryonal carcinoma, so it must be used in conjunction with other positive and negative markers of yolk sac tumor. OCT4, for example, is positive in dysgerminoma and embryonal carcinoma but negative in yolk sac tumor, and CD117 and CD30 are both negative in yolk sac tumor. We have found hepatocyte nuclear factor-1β to be a useful stain for yolk sac tumor. This stain is positive in clear cell carcinoma, so it is not useful in the differential diagnosis between yolk sac tumor and clear cell carcinoma. But among germ cell tumors it shows nuclear staining only in yolk sac tumor and in the nuclei of endodermal glands in teratomas. With these limitations in mind, it can be helpful in distinguishing among yolk sac tumor, dysgerminoma, and embryonal carcinoma. Immunohistochemical stains for α1-antitrypsin and placental alkaline phosphatase are positive in yolk sac tumor cells, and the extracellular hyaline material is laminin positive.
Yolk sac tumor is cytokeratin positive but, like other malignant germ cell tumors, it does not stain for epithelial membrane antigen. There is strong cytoplasmic staining with cytokeratin AE1/AE3, as compared to the membranous pattern of staining that is characteristic of embryonal carcinoma. Yolk sac tumor is cytokeratin 7 negative ; this result, together with lack of staining for epithelial membrane antigen and positive staining for AFP and SALL4, differentiates yolk sac tumor from clear cell carcinoma. Immunostains for human chorionic gonadotropin are negative except in the rare yolk sac tumors that contain syncytiotrophoblastic giant cells. Small or large zones of hepatoid differentiation show granular cytoplasmic staining with antihepatocyte antibody, although this is not a specific finding since hepatoid carcinoma and metastatic hepatocellular carcinoma are also positive. Hepatoid yolk sac tumor cells also show strong cytoplasmic staining for glypican-3 and nuclear staining for SALL4.
Embryonal carcinoma is an ovarian neoplasm that is morphologically identical to embryonal carcinoma of the testis. It occurs almost exclusively in children and young women, although there are rare reports of its occurrence in older women. The average age is about 15 years. The typical presentation is with pelvic or abdominal pain or a palpable abdominal mass. Menstrual abnormalities are common in postpubertal patients. Most patients have a positive pregnancy test or an elevated serum βhCG concentration, and about 50% of premenarcheal patients have precocious pseudopuberty.
Unilateral salpingo-oophorectomy is the appropriate surgical treatment of young patients with embryonal carcinoma limited to the ovary at diagnosis. Embryonal carcinoma is virtually never bilateral, so biopsy of the contralateral ovary is unnecessary. Treatment of advanced tumors (FIGO stage >IA) is individualized. While standard treatment for patients with advanced embryonal carcinoma is total abdominal hysterectomy, bilateral salpingo-oophorectomy, and limited debulking, young patients can be treated by unilateral salpingo-oophorectomy and limited debulking if the contralateral ovary and uterus are uninvolved.
Before effective combination chemotherapy was available, embryonal carcinoma was often rapidly fatal, with survival, even in stage I, limited to 50%. At present, patients with completely resected embryonal carcinoma are treated with postoperative cisplatin-based adjuvant chemotherapy with nearly complete success. Many patients with residual or recurrent tumors can be cured with combination chemotherapy.
Serum assays for βhCG and AFP are used to assess treatment efficacy. If serum levels of either or both markers remain elevated after treatment, or if they increase during follow-up, the patient almost certainly has recurrent or metastatic tumor.
Embryonal carcinoma is a large solid neoplasm with an average diameter of 15 to 17 cm. The cut surface is fleshy and tan or gray with small cysts and areas of hemorrhage and necrosis.
The microscopic appearance is similar to that of embryonal carcinoma of the testis. The tumor cells have large vesicular nuclei with coarse chromatin and one or two prominent nucleoli ( Fig. 13A.81 ). The nuclei are larger than those of dysgerminoma, and there is generally moderate to marked nuclear pleomorphism. Mitotic figures are numerous. The tumor cells are polygonal or columnar, and they have abundant amphophilic or clear cytoplasm. The tumor cells grow in nests and sheets or they line clefts, glands, or papillae. Most embryonal carcinomas contain syncytiotrophoblastic giant cells, present singly or in clusters; no cytotrophoblastic cells are present, so this finding is not indicative of choriocarcinoma. These cells are the source of the βhCG secreted by some embryonal carcinomas. The stroma is loose and edematous or consists of a cellular proliferation of small, primitive-appearing spindle cells.
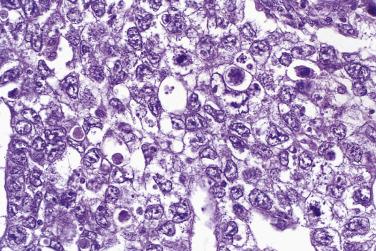
Embryonal carcinoma only rarely grows in pure form in the ovary or as an extensive component of a mixed germ cell tumor. However, it is not uncommon to find microscopic foci of embryonal carcinoma admixed with yolk sac tumor in an immature teratoma or small amounts of embryonal carcinoma in a mixed germ cell tumor, where it can also be admixed with yolk sac tumor resulting in a so-called embryoma pattern.
Immunostains for cytokeratin are positive with a membranous pattern of staining, but there is no staining for epithelial membrane antigen. Embryonal carcinoma shows positive staining for the nuclear markers OCT4, SALL4, and SOX2. When using these markers, it is important to keep in mind that OCT4 is also positive in dysgerminoma, and SALL4 is positive in dysgerminoma and yolk sac tumor. Staining for SOX2 appears to be unique to embryonal carcinoma, although not every case stains. There is membranous staining for CD30 in embryonal carcinoma, but no staining for CD117 or D2-40. Syncytiotrophoblastic giant cells and occasional large mononuclear cells resembling intermediate trophoblasts are immunoreactive for human chorionic gonadotropin.
Polyembryoma is a rare form of malignant germ cell tumor with features intermediate between embryonal carcinoma and more differentiated forms of malignant germ cell tumor; it is usually found as a component of a mixed germ cell tumor. The treatment and prognosis are similar to that of embryonal carcinoma and yolk sac tumor.
Microscopically, polyembryoma is composed of numerous embryoid bodies growing in a primitive embryonal stroma. The embryoid bodies resemble 13-day to 18-day embryos. They have an embryonic disc composed of tall columnar cells with hyperchromatic nuclei, similar to embryonal carcinoma cells. Immunostains for OCT4 and CD30, which are markers of embryonal carcinoma, are positive in the columnar cells. On one side of the disc is an amnionic cavity; on the other side, a yolk sac lined by AFP-positive cells. Embryoid bodies are often distorted or incomplete and can be difficult to recognize.
Pure primary ovarian choriocarcinoma of germ cell origin is extremely rare. In a review of the pathology of malignant ovarian germ cell tumors, less than 1% were choriocarcinomas, and only about 40 nongestational ovarian choriocarcinomas had been reported in the literature as of 2006. Choriocarcinoma is seen most frequently as a component of a mixed germ cell tumor.
Choriocarcinoma of the ovary occurs in children and young women. Very rarely, choriocarcinoma is found admixed with an epithelial tumor in the ovary, usually in a postmenopausal patient. The clinical presentation is with abdominal pain and abnormal vaginal bleeding. A pregnancy test is positive, and the serum βhCG is elevated. Premenarcheal children may have precocious pseudopuberty. Hemoperitoneum is sometimes found at operation.
Choriocarcinoma of the ovary is unilateral and is treated by salpingo-oophorectomy. Total abdominal hysterectomy and bilateral salpingo-oophorectomy are required only if the contralateral ovary or uterus is involved. Surgery is followed by standard germ cell combination chemotherapy with a platinum-based regimen. Favorable results have been described, even with suboptimal chemotherapy.
The chemotherapy and prognosis for patients with gestational choriocarcinoma differ from that for patients with nongestational choriocarcinoma. Those of germ cell origin are more aggressive and less chemosensitive. It is therefore important to determine whether an ovarian choriocarcinoma is of gestational or germ cell type. If the patient is premenarcheal, the choriocarcinoma is certain to be of germ cell origin. In young women of childbearing age, gestational choriocarcinoma is equally as likely as germ cell choriocarcinoma, and there are no morphologic differences between gestational choriocarcinoma and choriocarcinoma of germ cell origin. The clinical history may help in the differential diagnosis. The presence of a corpus luteum of pregnancy favors gestational choriocarcinoma, while identification of other germ cell elements in the tumor is indicative of germ cell choriocarcinoma. Choriocarcinoma can be proven to be of gestational origin if paternal alleles not present in benign maternal tissues such as fallopian tube can be identified by DNA genotyping.
Choriocarcinoma is a unilateral soft purple-red tumor with a hemorrhagic and necrotic cut surface. It ranges from 4 to 25 cm in diameter.
Microscopically, much of the tumor is hemorrhagic and necrotic. Viable tumor cells are mainly at the periphery, where cytotrophoblastic cells and syncytiotrophoblastic giant cells grow in a plexiform pattern ( Fig. 13A.82 ). Cytotrophoblastic cells have abundant clear cytoplasm and well-defined cell borders. Their nuclei are irregular and vesicular and some contain macronucleoli. Syncytiotrophoblastic giant cells have abundant vacuolated basophilic or amphophilic cytoplasm in which there are multiple hyperchromatic nuclei.
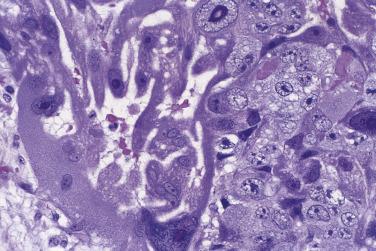
Pure choriocarcinoma of the ovary is quite rare. When it is found in the ovary, choriocarcinoma is most likely to be a component of a mixed germ cell tumor. Choriocarcinoma is present in 10% to 20% of mixed germ cell tumors. The rare ovarian choriocarcinomas found in association with an epithelial carcinoma in an older patient are presumably derived from the carcinoma and likely of somatic, not germ cell, origin.
All trophoblastic cells are cytokeratin positive; there is strong homogeneous staining of the cytoplasm of the syncytiotrophoblastic giant cells. There is strong staining for βhCG in the cytoplasm of the syncytiotrophoblastic giant cells. Based on studies of testicular choriocarcinoma, the mononuclear cytotrophoblastic cells show nuclear staining for p63.
Benign cystic teratoma (dermoid cyst) is the most common ovarian neoplasm, comprising 25% or more of all ovarian tumors. Other types of teratoma are uncommon. Most teratomas have a 46XX karyotype and appear to be derived from postmeiotic germ cells. There are numerous benign and malignant variants of teratoma of the ovary. Tumors in which one element greatly predominates are termed monodermal teratomas.
These are cystic or, rarely, solid tumors that contain various mature tissues derived from one or more of the embryonic germ layers: the ectoderm, mesoderm, and endoderm. They occur in patients of all ages. Most are found in women between 20 and 50 years of age with a peak incidence between 20 and 29 years; only about 20% are detected in postmenopausal women. Many patients are asymptomatic, and the tumors are discovered on routine examination or during evaluation or treatment for some other condition. Symptoms such as pelvic pressure or pain appear when the tumor attains a large size. The most common serious complications are torsion, which occurs in 3% to 10% of cases, and rupture, which occurs in about 1%. Torsion causes acute abdominal symptoms. Acute peritonitis or, rarely, chronic granulomatous peritonitis can be a sequela of spontaneous rupture.
A form of autoimmune encephalitis associated with antibodies against the N-methyl- d -aspartate receptor, often designated as anti-NMDAR encephalitis, occurs in rare patients with mature cystic teratomas. Antibodies against the receptor can be detected in the serum of affected patients and can be tested for to establish the diagnosis. It is proposed that patients who have teratomas develop antibodies against receptors in neural tissues in the teratoma that react with antigens in the brain, causing the encephalitis. The syndrome also occurs in patients who do not have teratomas. Patients with the syndrome develop a variety of severe neurologic and psychiatric symptoms and can die if they are not appropriately diagnosed and treated.
Mature teratoma is a benign neoplasm that can be treated conservatively. Cystectomy, which can be performed laparoscopically or at laparotomy, is adequate treatment, particularly in children and young women. Benign teratoma is bilateral in 10% to 15% of patients. Peritoneal implants composed of mature glial tissue (grade 0) occur in rare patients with mature teratomas of the ovary. Such implants do not adversely affect survival.
Benign teratomas are nearly always cystic. They range in size from only a few centimeters to large tumors weighing several kilograms; the average diameter is 7 to 8 cm. The cyst contents are liquid at body temperature but they solidify and are soft or firm at room temperature. On cross section, there is a unilocular or, less often, a multilocular cyst with a solid protuberance called the dermoid papilla in the wall. The cysts contain hair, grumous material, or oily or serous liquid. Cartilage, bone, or teeth may be found. Foci of soft gray-tan tissue resembling brain and glistening green or brown thyroid tissue are also commonly seen. Dense, solid regions in an otherwise cystic teratoma are unusual; these raise the question of immature teratoma and should be carefully sectioned for histologic study. Rare mature (benign) teratomas are completely solid and can be differentiated from an immature teratoma only by microscopic study.
Benign teratomas contain a varied mixture of ectodermal, mesodermal, and endodermal elements distributed in an organized fashion. Tissues from at least two germ layers can be identified in two-thirds of mature teratomas, and all three germ layers are represented in about a third of them. Ectodermal derivatives such as skin, hair follicles, and sebaceous and sweat glands are the most common and, when they dominate the histologic picture, the tumor is commonly referred to as a dermoid cyst ( Fig. 13A.83 ). Other ectodermal elements that are frequently present include neural tissue (usually glia), choroid plexus, peripheral nerve, and dental structures. Common endodermal tissues include digestive tract mucosa, including endocrine cells, respiratory mucosa, renal tissue, and thyroid tissue. The most frequent mesodermal derivatives are adipose tissue, smooth or striated muscle, bone or cartilage, and a loose connective tissue framework that surrounds the other elements. Male tissues such as prostate are occasionally present in a benign teratoma. Endodermal and mesodermal derivatives are most likely to be found in sections from the dermal papilla.
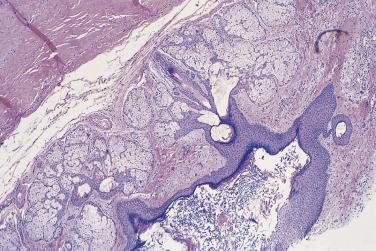
Teratomas removed from patients with NMDAR encephalitis are often small and typically contain neural tissue associated with B-lymphocytic aggregates, germinal centers, and a sprinkling of lymphocytes, as well as diminished or atypical neurons in the neural tissue.
An individual element is so dominant in some tumors that classification as a monodermal teratoma is appropriate (e.g., as struma ovarii when thyroid tissue constitutes >50% of the tumor). Cystic spaces lined by flattened epithelium or granulation tissue and surrounded by multinucleated giant cells and lipophages are present in some teratomas. The foreign body granulomatous reaction is caused by disruption of the epithelium with liberation of the cyst contents into the surrounding tissue.
Benign or malignant tumors that arise in a benign teratoma are rare. Malignant transformation occurs in only 1% to 3% of benign cystic teratomas.
Secondary malignancies typically occur in postmenopausal women, but they can be found in patients of any age. Presenting complaints are nonspecific and include abdominal or pelvic pain, abdominal distention, or a palpable mass. Tumors are confined to the ovary (stage I) in 50% to 75% of patients, and those patients have a favorable prognosis. The outcome is unfavorable in women with more advanced tumors, as most do not respond to chemotherapy or radiotherapy and die within 1 to 2 years of diagnosis.
A secondary neoplasm typically forms a nodule or a papillary growth in the cyst lining, but sometimes there is only a thickening or induration of the cyst wall. In some instances, the secondary neoplasm is detected only by microscopic examination. Secondary neoplasms are usually unilateral, but the contralateral ovary may contain a benign cystic teratoma.
Nevi, sebaceous adenomas and other cutaneous adnexal neoplasms, benign salivary gland type tumors, meningiomas, glomus tumors, and hemangiomatous vascular proliferations are among the benign neoplasms that arise in benign cystic teratoma. In situ malignant tumors that have been reported include squamous cell carcinoma in situ and Paget disease.
Invasive squamous cell carcinoma comprises about 85% of secondary malignancies arising in benign cystic teratoma ( Fig. 13A.84 ). Most are moderately to poorly differentiated, and they are sometimes associated with squamous cell carcinoma in situ. Other types of malignancy that have been reported to arise in a benign teratoma include various types of cutaneous carcinoma, such as basal cell carcinoma or sebaceous carcinoma, melanoma, adenocarcinoma, various types of sarcoma, and other rare tumor types.
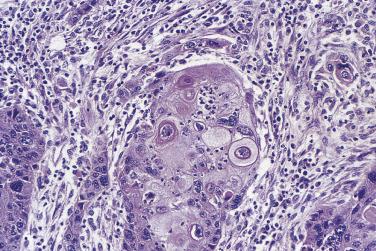
Occasional mucinous epithelial tumors arise in a teratoma. Any type of mucinous tumor can arise in a teratoma; cystadenomas are most common, but borderline tumors and carcinomas also occur. Occasional patients with mucinous tumors arising in a teratoma develop pseudomyxoma peritonei, which is presumably of ovarian origin in this setting.
Immature teratoma is one of most common malignant germ cell tumors of the ovary, representing 20% to 35% of such tumors at major cancer centers and in the US population as a whole.
Immature teratoma occurs predominantly in children and young women. The average patient is about 20 years old, and few are younger than 7 or older than 40. Immature teratomas are exceptional in older or postmenopausal women. The clinical presentation is nonspecific. Patients complain of pelvic or abdominal pain, abdominal swelling, or a palpable abdominal mass. A few have acute abdominal symptoms caused by torsion or rupture of the tumor. Rare patients have serious neurologic or psychiatric symptoms caused by a paraneoplastic syndrome (anti-NMDAR encephalitis) associated with the teratoma. Serum AFP can be elevated in patients with pure immature teratoma, and there is often modest elevation of the tumor marker CA125. Most (50%–80%) patients have localized tumors (stage I) at diagnosis. Bilaterality is exceptional, although metastases can involve the contralateral ovary in patients with advanced disease. Immature teratoma spreads mainly by implantation on the pelvic and abdominal peritoneum and the omentum. There is a benign cystic teratoma in the contralateral ovary in 10% to 15% of cases.
Patients with localized (stage IA) tumors are treated by unilateral salpingo-oophorectomy. A few patients have been treated successfully by cystectomy, sometimes followed by chemotherapy. More advanced tumors can be treated by unilateral salpingo-oophorectomy and excision of extraovarian tumor. If preservation of fertility is not an issue or the contralateral ovary or uterus is involved, the treatment is hysterectomy and bilateral salpingo-oophorectomy.
Patients with stage IA grade 1 immature teratoma have an excellent prognosis and are treated by surgery alone. Those who have advanced disease usually receive postoperative chemotherapy. The issue of adjuvant chemotherapy for patients with stage IA grade 2 and grade 3 tumors is controversial, with some authors advocating adjuvant chemotherapy and others advocating observation only, with chemotherapy reserved for patients who develop a recurrence. The prognosis appears to be more favorable and unrelated to tumor grade in children. In the absence of metastases containing immature tissue or other malignant germ cell elements such as yolk sac tumor, pediatric oncologists frequently withhold chemotherapy regardless of the tumor grade. Cisplatin-containing regimens such as BEP (cisplatin, etoposide, and bleomycin) are highly effective forms of adjuvant chemotherapy for patients with no residual tumor after surgery, with survival rates of 90% to 100%. The prognosis is somewhat less favorable for patients with residual gross tumor or recurrent immature teratoma, but in recent years the overall survival for patients with immature teratomas has approached 95%.
In patients with extraovarian tumor spread, the microscopic appearance of the metastases is of prognostic importance. Some peritoneal implants or lymph node deposits contain only mature tissues, usually predominantly glial cells ( Fig. 13A.85 ), and do not adversely affect the prognosis. Second-look operations performed after chemotherapy in patients with incompletely resected immature teratoma can reveal residual immature teratoma, no residual tumor, small glial implants, or bulky nodules of mature teratoma. These bulky nodules are resected to avoid adhesions or compression of adjacent organs and to forestall development of the growing teratoma syndrome. On rare occasions a secondary malignancy may arise in long-standing incompletely resected low-grade teratoma implants.
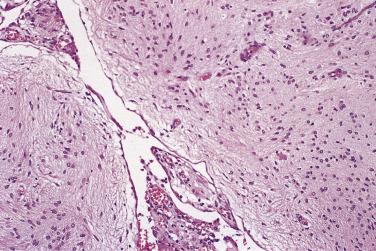
Immature teratoma is a predominantly solid unilateral tumor that averages 18 cm in diameter. The solid component is gray or brown and can be soft or hard and gritty. Scattered small cysts are typically seen on the cut surface. In about a quarter of these tumors one or more large cysts contain keratinous debris or hair and resemble a dermoid.
Tissues derived from all three germ cell layers are present, and a mixture of mature and immature elements is found in most tumors. These usually have a haphazard distribution and often lack the organized growth patterns seen in benign cystic teratoma. Ectodermal and mesodermal derivatives typically predominate among the immature elements. Immature neuroectodermal elements are the easiest immature tissues to recognize and quantitate. These include sheets of mitotically active immature neuroepithelial cells, tubules lined by columnar embryonal cells with stratified hyperchromatic nuclei, sheets and nests of neuroblasts containing anuclear fibrillary zones and Homer Wright rosettes, mitotically active immature glia, and primitive retina with melanin pigmentation ( Figs. 13A.86 and 13A.87 ). Florid benign vascular proliferations are seen occasionally in association with the neural elements of an immature teratoma. Immature mesenchymal stroma is hypercellular and composed of small spindle cells with dark nuclei ( Fig. 13A.88 ). Mitotic figures are usually present. Cartilage is often present in immature teratomas, and it can be difficult to determine whether it is immature. When there are numerous lacunae containing midsize chondrocytes with round vesicular nuclei and the foci of chondroid differentiation are surrounded by immature small round mesenchymal cells, it is appropriate to interpret the cartilage as an immature mesenchymal element. Immature endodermal tissues are less common and are rarely seen in the absence of immature ectodermal derivatives. The types of immature endodermal structures that can be seen include primitive glands lined by columnar cells with subnuclear and supranuclear vacuoles, partially differentiated stratified columnar intestinal epithelium with goblet cells, and islands of fetal liver tissue. Immature renal (metanephrogenic) structures such as embryonal-appearing glomeruli are occasionally present.
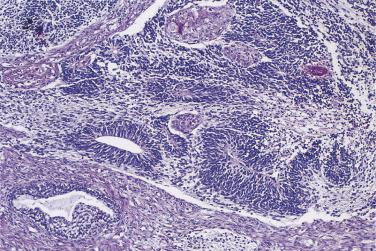
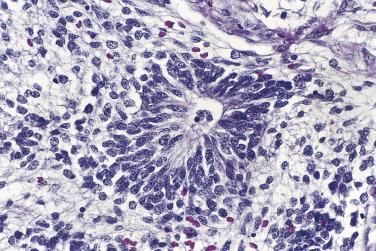
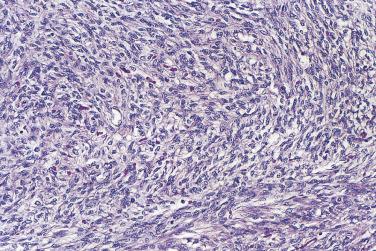
Microscopic foci of yolk sac tumor are seen occasionally in an immature teratoma, and as long as there are only a few of them (≤3) and they are less than 3 mm in diameter, they do not appear to affect the prognosis. Embryonal carcinoma glands are sometimes mixed with yolk sac tumor in these foci; it is unknown whether the presence of embryonal carcinoma alters the prognosis in such cases.
Ovarian teratomas are graded using a system that rates them from grade 0 for a neoplasm composed entirely of mature tissues to grade 3 for a neoplasm containing abundant immature tissue ( Table 13A.5 ). In a grade 1 immature teratoma, immature neuroepithelium is limited to one low power field per slide. Grade 2 immature teratomas have immature neuroepithelium in two or three low power fields on any slide. A grade 3 immature teratoma contains immature neuroepithelium in four or more low power fields on any slide. An immature teratoma can be associated with grade 0 extraovarian tumor deposits, but the primary ovarian tumor must, by definition, be grade 1, 2, or 3.
| Grade | Immature Tissue | Amount Neuroepithelium |
|---|---|---|
| 0 a | None a | None a |
| 1 | + | Rare, not >1 lpf/slide |
| 2 | ++ | Common, not >3 lpf/slide |
| 3 | +++ | Prominent, ≥4 lpf/slide |
a Grade 0 is only possible in extraovarian deposits, the ovarian primary site must be grade 1, 2, or 3.
Analysis of ovarian teratomas for the presence of an isochromosome 12p (i12p) revealed that mature teratomas and pure immature teratomas lack an i12p, while immature teratomas that are components of mixed germ cell tumors generally have an i12p. This suggests a variable pathogenesis for ovarian immature teratomas, depending on whether they are pure (likely derived from postmeiotic germ cells) or mixed with other elements such as yolk sac tumor or embryonal carcinoma (derived by differentiation of a primitive germ cell tumor).
Immunohistochemistry plays a limited role in the diagnosis of immature teratoma. A stain for glial fibrillary acidic protein (GFAP), which stains both glial fibrils and cell bodies, can help identify glial differentiation. Primitive neuroepithelial cells do not stain with GFAP, although in some tumors they are stained with antibodies for neurofilaments or neuron-specific enolase (NSE). Intestinal and respiratory epithelia contain argyrophilic cells that react with antibodies to a variety of neurohormonal peptides. AFP stains immature endodermal structures such as isolated yolk sac–like vesicles, immature glandular and intestinal epithelium, and liver. Immunostains can also help to confirm the presence of certain immature elements. For example, primitive neuroepithelium stains for CD99 and bcl2, and immature cartilage stains for CD34 and bcl2.
The nature of grade 0 extraovarian tumor deposits is not entirely clear. While they have traditionally been viewed as a pattern of spread from the ovarian tumor, some molecular studies of glial implants have shown that they are genetically different from the ovarian tumor but have the same genetic pattern as the patient's normal tissues. This raises the possibility that in some cases substances produced by the tumor result in metaplastic transformation of peritoneal or subperitoneal tissues into glia. Other studies dispute a maternal origin in all cases.
Rare ovarian tumors consist of neuroectodermal cells growing in patterns reminiscent of various tumors of the central nervous system (CNS). Such tumors are frequently associated with and appear to arise from teratomas. Tumors of this type also occur in the testis where they are often associated with teratoma. The diagnosis of a neuroectodermal tumor of the testis is restricted to tumors in which the neuroectodermal component measures at least 1 cm in diameter. This size criterion can be applied to ovarian tumors as well, although in ovarian tumors the neuroectodermal component generally comprises most or all of a large neoplasm, overgrowing the underlying teratoma. There are three main patterns.
Most primitive neuroectodermal tumors (PNETs) resemble embryonal tumors of the central nervous system and consist of nests and sheets of small cells with hyperchromatic mitotically active nuclei ( Fig. 13A.89 ). Some cells have fibrillary cytoplasm and some tumors contain rosettes with central lumina, neuropil, neuroblastic rosettes, or foci of glial differentiation. Areas within tumors of this type resemble tumors that were formerly called medulloepithelioma, ependymoblastoma, or neuroblastoma. The classification of embryonal tumors of the CNS has undergone considerable modification recently, with many new and combined categories, and immunohistochemical and molecular studies are often required for accurate classification. Some authors have recently recommended that ovarian lesions of this type be classified according to the established CNS criteria. Occasional ovarian PNETs exhibit the histologic and molecular features of a tumor in the Ewing sarcoma/peripheral PNET category, and some most resemble a peripheral neuroblastoma.
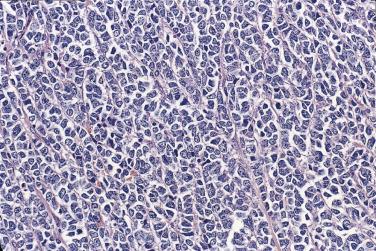
A second type of neuroectodermal tumor shows extensive glial differentiation and resembles a glioblastoma multiforme or some other type of glial neoplasm. These tumors show positive immunohistochemical staining for glial fibrillary acidic protein, but unlike CNS astrocytic tumors they lack staining for IDH1, and IDH mutations are not present.
The third kind of neuroectodermal tumor resembles an ependymoma. Round or columnar cells with fibrillary cytoplasm form perivascular pseudorosettes and true ependymal rosettes with central lumina. In some tumors the ependymal cells line glands, cysts, or papillae, which can result in misdiagnosis as an epithelial tumor. Like CNS ependymomas, those of the ovary show diffuse expression of glial fibrillary acidic protein, but in contrast to CNS ependymomas they typically show positive staining for high molecular weight keratin, cytokeratins 7, 8, and 18, and estrogen and progesterone receptors. Dotlike staining for EMA is seen in some tumors. This unusual immunophenotype can add to the diagnostic problem if the pathologist is not aware of it.
Primitive neuroectodermal tumors and those resembling glioblastoma multiforme often contain teratomatous elements, but usually none are found in an ependymoma. Primitive neuroectodermal tumors and malignant glial tumors have an unfavorable prognosis if they have spread beyond the ovary, but ependymoma appears to be an indolent tumor, even when metastases are present. These tumors are generally classified as monodermal teratomas, although the primitive ones might also be viewed as variants of immature teratoma.
Struma ovarii is a teratoma in which thyroid tissue predominates. More than 50% of the tumor should consist of thyroid tissue before it is designated as a struma ovarii. Using this definition, 1% to 3% of benign ovarian teratomas are strumas.
Struma ovarii occurs mainly in women older than 40 years. It is usually an incidental finding or it is discovered in a patient with nonspecific symptoms such as abdominal or pelvic pain or abdominal distention. Rare patients have ascites or ascites and hydrothorax (pseudo-Meigs syndrome). Occasional patients have hormonally mediated symptoms such as abnormal vaginal bleeding. Symptoms of hyperthyroidism occur in fewer than 10% of patients with struma ovarii, and some of these women have been reported to have enlarged thyroid glands, raising questions about the source of the excess thyroid hormone.
Struma ovarii is benign in most instances, and cystectomy or unilateral salpingo-oophorectomy is adequate treatment. Tumors diagnosed as malignant struma ovarii on histologic grounds are rarely clinically malignant and do not always require radical treatment. Biologically malignant strumas have frequently spread beyond the ovary at the time of diagnosis. Malignant strumas metastasize locally to the peritoneum or omentum or to such distant sites as lymph nodes, liver, bone, or lung. These appear best treated by hysterectomy and bilateral salpingo-oophorectomy, thyroidectomy, and administration of radioactive iodine.
Struma ovarii is a circumscribed neoplasm that ranges in size from a small nodule, only 1 or 2 cm in diameter, to a large mass more than 10 cm in diameter. The average size is 5 to 10 cm. On cross section, struma ovarii is red, green, or tan with a glairy, meaty appearance. Small cysts are commonly present, and occasional tumors are largely or totally cystic. Strumas smaller than 4 cm rarely metastasize. Above that size, increasing size correlates with an increased risk of malignancy; the average diameter of malignant strumas is 13 to 14 cm. Any ovarian tumor greater than 5 cm in diameter in which there is a predominance of thyroid tissue should be considered to be at least of uncertain malignant potential.
Struma ovarii is composed of follicles filled with eosinophilic colloid and lined by cuboidal or columnar cells with uniform round nuclei ( Fig. 13A.90 ). Degenerative changes such as fibrosis, calcification, and aggregates of hemosiderin-laden macrophages may be present. Cystic variants of struma ovarii may contain few follicles and can be difficult to recognize. Other growth patterns that can cause diagnostic problems include tumors composed of clear, oxyphilic, or signet ring cells, those with cordlike arrangements of tumor cells, and hypercellular tumors in which the cells grow in microfollicular, trabecular, or solid patterns similar to those seen in a follicular adenoma of the thyroid. Hypercellular variants of struma ovarii can be designated as proliferative struma ovarii.
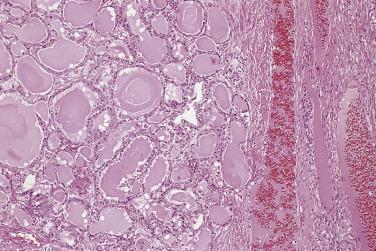
Other teratomatous elements can be found in many cases if they are carefully searched for. Luteinized ovarian stromal cells are present around some examples of struma ovarii. These cells, which are the likely source of the hormones that cause endocrinologic symptoms in some patients, can be highlighted with an immunostain for inhibin or calretinin.
Any type of thyroid cancer can arise in struma ovarii. Papillary carcinoma is most common and has the same histologic appearance as papillary carcinoma of the thyroid gland. It grows in an infiltrative manner, usually contains well-developed papillae, and is composed of cells with large, crowded, grooved nuclei with small nucleoli. Some nuclei are clear and others contain intranuclear cytoplasmic inclusions. The cell cytoplasm is moderate or abundant and appears dense. The colloid within the follicles often has a solid, hypereosinophilic appearance. Follicular variants of papillary carcinoma have been described in struma ovarii, as in the thyroid. Follicular carcinoma is uncommon. It is a hypercellular neoplasm in which the tumor cells grow in a microfollicular, trabecular, or solid pattern. It is difficult to differentiate follicular carcinoma from proliferative struma ovarii, but nuclear atypia, frequent mitotic figures, and, especially an invasive growth pattern or vascular invasion favor follicular carcinoma. Some cases of follicular carcinoma are recognized only because metastases are present at the time of initial surgery or subsequently. Strumosis was a term used in the past for a condition characterized by the finding of peritoneal and omental implants of well-differentiated thyroid tissue in a patient with a histologically benign struma ovarii. It is now thought to represent metastasis from a highly differentiated follicular carcinoma arising in struma ovarii. The ovarian tumor is so well differentiated that it cannot be recognized as a follicular carcinoma unless extraovarian spread is present or subsequently develops. Highly differentiated variants of papillary carcinoma also occur and are typically not recognized until extraovarian metastases become evident.
Many cases of malignant struma ovarii have been designated as carcinoma on histologic grounds alone and never spread beyond the ovary. Clinically malignant examples of struma ovarii that have metastasized are nevertheless well documented, and biologically malignant follicular tumors can be particularly difficult to identify based on their histology alone.
BRAF mutations are sometimes present in malignant strumas, as they are in thyroid carcinomas, and the presence of such a mutation can be diagnostically helpful. A BRAF V600E mutation is present in about 30% of papillary carcinomas. It appears to occur only in cases with the classic papillary growth pattern, however, and it has not been demonstrated in examples of the follicular variant of papillary carcinoma that have been tested.
Immunostains for thyroglobulin are positive in the colloid and in the cytoplasm of the follicular cells, and immunostains for TTF1 are positive in the tumor cell nuclei. Papillary carcinomas arising in struma ovarii usually show positive staining for CK19 and HBME1. Immunohistochemistry sometimes helps confirm the diagnosis in difficult cases. A potential pitfall is that struma ovarii and tumors arising in it are PAX8 positive, which can result in confusion with an ovarian epithelial tumor, most of which are also PAX8 positive.
Carcinoid tumors are uncommon ovarian neoplasms that are classified as monodermal teratomas since many are associated with other teratomatous elements. A pure ovarian carcinoid has most likely overgrown the teratoma in which it originated, but other possible sources include neuroendocrine cells in a mucinous tumor or the mucinous component of a heterologous Sertoli-Leydig cell tumor, ovarian endocrine cells, or nonendocrine ovarian cells by neometaplasia. When no associated teratomatous elements are detected the possibility of a metastatic carcinoid must be considered.
Most patients are perimenopausal or postmenopausal women who have symptoms such as pelvic or abdominal pain, abdominal enlargement, menstrual irregularities, or abnormal vaginal bleeding. Of women with ovarian carcinoid tumors, 25% to 30% have the carcinoid syndrome. Most have large insular carcinoid tumors; the syndrome rarely occurs with other types of carcinoid. Typical symptoms of the carcinoid syndrome include facial flushing, diarrhea, bronchospasm, hypertension, and edema secondary to carcinoid heart disease. Some women with trabecular or strumal carcinoids develop severe chronic constipation, caused by tumor secretion of the intestinal hormone peptide YY. Rare patients with a strumal carcinoid are hyperthyroid.
Carcinoid tumors are usually unilateral and confined to the ovary at the time of diagnosis. There is a benign cystic teratoma in the contralateral ovary in 10% to 15% of patients. The standard treatment is hysterectomy and bilateral salpingo-oophorectomy, but unilateral salpingo-oophorectomy is adequate treatment for a young woman. Symptoms usually abate rapidly once the tumor is removed, although patients with carcinoid heart disease can have progressive cardiac disease, despite the complete removal of the carcinoid tumor. The prognosis is generally favorable. Metastases and tumor-related deaths are rare, and deaths occur mainly in patients with extraovarian spread at diagnosis.
The ovary is commonly involved by metastases from carcinoids of the gastrointestinal tract, usually the small intestine, or the appendix. These can cause the same symptoms as primary ovarian carcinoids, including the carcinoid syndrome. Clues that a carcinoid might be metastatic include bilaterality, multinodularity, and the presence of peritoneal metastases.
Carcinoid tumors are unilateral, firm, and tan or yellow solid tumors. They range from microscopic to 8 to 10 cm in diameter and often appear to arise in the wall of a benign cystic teratoma or a mucinous tumor. Patients with the carcinoid syndrome tend to have larger tumors.
Ovarian carcinoids consist completely or in part of round, cuboidal or columnar neuroendocrine cells with uniform round or oval nuclei, coarse chromatin, and small nucleoli ( Fig. 13A.91 ). The cytoplasm is moderate in amount and varies from clear to eosinophilic. The cytoplasm typically appears granular in H&E-stained sections. The granules can usually be stained with argentaffin or argyrophil, although, these days, immunohistochemical stains for neuroendocrine markers are generally used to confirm the diagnosis. Four histologic types of carcinoid tumor occur in the ovary; mixtures of these types are present in some tumors. Insular carcinoids are most common. In these tumors, the cells grow in nests, sheets, or islands, or cells line small tubular acini. The microscopic appearance is similar to that of a midgut carcinoid. The trabecular carcinoid is composed of tall columnar cells with central nuclei and granular eosinophilic cytoplasm. The tumor cells grow in cords, ribbons, or trabecula, a pattern reminiscent of that seen in foregut and hindgut carcinoids. The strumal carcinoid is a mixed tumor that contains both carcinoid and strumal (thyroid) elements. The carcinoid component usually has a trabecular pattern, although foci of insular or mucinous growth are present in some examples. The strumal component consists of thyroid-type follicles filled with colloid and lined by columnar follicular cells. There are calcium oxalate crystals in the colloid in about 50% of cases. In the regions where the two elements merge, the carcinoid cells grow between and into the follicles, where they appear to undermine and replace the follicular cells. Thus some follicles are lined by thyroid cells, some by a mixture of thyroid and carcinoid cells, and some by carcinoid cells. Amyloid is present in rare examples of strumal carcinoid. Finally, rare cases of primary mucinous carcinoid occur in the ovary. In mucinous carcinoid, round or tubular glands lined by columnar or cuboidal cells and goblet cells infiltrate the stroma or float in pools of mucin. Atypical variants of mucinous carcinoid exhibit a greater degree of glandular crowding with cribriform or microcystic growth and increased nuclear atypia. Mucinous carcinoids can be mixed with carcinoma, and the mixed forms are the type of mucinous carcinoid most likely to spread beyond the ovary.
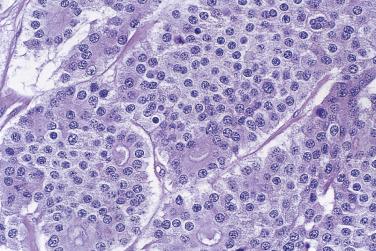
Luteinized stromal cells are noted at the periphery of some carcinoids. Benign teratomatous elements are often detected adjacent to an ovarian carcinoid. Their identification is important evidence that the carcinoid is primary in the ovary. The possibility that an ovarian carcinoid tumor is metastatic should always be considered. Metastatic carcinoid tumors are usually multinodular and bilateral and are not associated with a teratoma. An extraovarian primary can often be demonstrated by appropriate clinical studies.
Chromogranin and synaptophysin are the immunohistochemical stains that are most useful for confirming a diagnosis of carcinoid. Staining for these substances has replaced the use of silver stains. Strumal carcinoid is unique. The thyroid component shows positive staining for thyroglobulin and TTF1, while the carcinoid elements of the tumor are chromogranin and synaptophysin positive. Carcinoids are cytokeratin positive, but they do not stain for CK7, which helps differentiate them from surface epithelial tumors.
Trabecular and strumal carcinoids typically stain for prostate-specific acid phosphatase (PSAP), as do hindgut carcinoids, but not for prostate-specific antigen (PSA). A variety of peptide hormones can be detected in carcinoid cells, including serotonin, gastrin, pancreatic polypeptide (PP), vasoactive intestinal polypeptide (VIP), insulin, glucagon, substance P, adrenocorticotropic hormone, and somatostatin. Trabecular and strumal carcinoids from patients with chronic constipation typically stain for protein YY.
It is worth noting that many primary ovarian insular carcinoids show positive nuclear staining for CDX2. CDX2 therefore cannot be used to differentiate between a primary carcinoid and one metastatic from the intestine.
Malignant mixed germ cell tumors contain a mixture of the various pure types of germ cell tumors. They comprise 5% to 20% of all malignant germ cell tumors. A malignant germ cell tumor that contains one malignant germ cell component along with benign teratomatous elements does not fall into this category.
Mixed germ cell tumors occur in children and young women. The average age is 16 years. The usual presentation is with abdominal pain or swelling or a palpable abdominal mass. A few patients have acute abdominal symptoms. About a third of premenarcheal children with mixed germ cell tumors have precocious pseudopuberty, while postmenarcheal children and adults often have amenorrhea or abnormal vaginal bleeding. The results of serum marker studies depend on which germ cell elements are present. About 50% of patients have a positive pregnancy test or an elevated serum βhCG. Serum AFP is elevated in 50% of patients. About two-thirds of patients have stage I tumors at diagnosis.
Encapsulated unilateral tumors (stage IA) are treated by salpingo-oophorectomy. More advanced tumors are treated by total abdominal hysterectomy and bilateral salpingo-oophorectomy, or, if conservation of fertility is important and the uterus and contralateral ovary are uninvolved, by unilateral salpingo-oophorectomy and limited debulking. More than half of stage I tumors treated by surgery alone recur. Chemotherapy is therefore administered to most patients except those with stage IA tumors that contain only dysgerminoma and grade 1 immature teratoma. An alternative is to closely follow the patient and administer chemotherapy only if a recurrence develops. Most stage I patients treated with adjuvant chemotherapy are cured. Those with more advanced tumors had a survival rate of only about 50% in the prechemotherapy era, but with current chemotherapy a substantial proportion of patients with advanced disease are cured. In early reports it appeared that the prognosis depended on the size of the tumor, the types and amounts of the various germ cell components present, and the stage, but modern combination chemotherapy has essentially eliminated the prognostic differences among the various tumor types, and stage is currently the most important prognostic factor.
Mixed germ cell tumors tend to be large, with an average diameter of 15 cm. Their gross appearance depends on which elements are present. Dysgerminoma is fleshy and gray, tan, or white. Yolk sac tumor varies in color, contains small cysts, and often has areas of necrosis. Immature teratoma is white or tan and may contain cysts and firm cartilaginous or bony foci. Areas of choriocarcinoma are hemorrhagic and necrotic. Mixed germ cell tumors are ordinarily unilateral, but when dysgerminoma is present they can rarely be bilateral.
There are two malignant elements in 80% of mixed germ cell tumors ( Fig. 13A.92 ), three in 15% of them and four or more in the remainder. The elements can be mixed together or they can grow in separate but contiguous foci. Dysgerminoma is the most frequent element, followed by yolk sac tumor and immature teratoma. Embryonal carcinoma, choriocarcinoma, and polyembryoma are less common. Choriocarcinoma and polyembryoma are rarely found in the ovary except as a component of a mixed germ cell tumor.
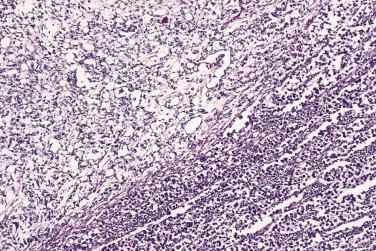
Gonadoblastoma is a rare tumor that contains an admixture of germ cells and sex cord cells and arises almost exclusively in abnormal gonads. It can be viewed as a form of carcinoma in situ that can give rise to a malignant germ cell tumor, usually a dysgerminoma.
Gonadoblastomas have been detected in patients of all ages, but they are found mainly in the young. The average age at diagnosis is 18 years; 80% of gonadoblastomas are detected before the age of 20 years, and they may be found in young children. Occasional tumors are incidental findings or are found when adnexal calcifications are noted on abdominal or pelvic x-rays. Most gonadoblastomas are identified when a patient is evaluated for primary or secondary amenorrhea or for an abnormally formed genital tract. Most patients are phenotypic females, but gonadoblastoma also occurs in phenotypic males. Phenotypic females have a normal or short vagina and a small cervix. The uterus is small in 75% of patients, and the fallopian tubes are small or rudimentary in 35% of them. Most patients are mildly virilized. The most common karyotypes are 46XY and 45X/46XY. A Y chromosome or a Y chromosome fragment is present in more than 90% of patients. In some, Y chromosome material cannot be detected by routine karyotypic analysis, but it is identified using more sensitive molecular techniques. In situ hybridization studies performed on gonadoblastomas in patients with a mosaic karyotype indicate that the gonadoblastoma is derived from cells with a Y chromosome. There is a region on the long arm of the Y chromosome near the centromere, termed the GBY region ( g onado b lastoma locus on the Y chromosome), that contains a gene associated with susceptibility to gonadoblastoma in patients with dysgenetic gonads; the most likely candidate gene is testis-specific protein Y-encoded (TSPY) .
Gonadoblastoma is benign unless it is overgrown by a germinoma or some other type of malignant germ cell tumor. The treatment is gonadectomy since the gonads are abnormal and are not capable of normal reproductive function. Gonadoblastoma is often bilateral, so bilateral gonadectomy is necessary to prevent virilization or evolution of a malignant germ cell tumor. The risk that a gonadoblastoma or a malignant germ cell tumor will originate in the abnormal gonads of a patient with a Y chromosome is about 25%. Most malignant tumors arising in a gonadoblastoma are dysgerminomas, but about 10% are yolk sac tumors or embryonal carcinomas.
Gonadoblastoma arises in abnormal gonads, including streak gonads, indeterminate gonads, and dysgenetic testes. It is typically small, ranging from microscopic to 2 to 3 cm in diameter. More than 40% are bilateral. The cut surface is tan or white and often contains gritty calcified areas.
Nests of germ cells and sex cord cells are surrounded by fibrous stroma ( Fig. 13A.93 ). Two types of germ cells have been described in gonadoblastoma. Some are immature, with a high nucleus to cytoplasm ratio, coarse chromatin, prominent nucleoli in some nuclei, and scanty cytoplasm, while others are more mature, with smaller, denser nuclei and more abundant clear cytoplasm. Sex cord cells surround the germ cells. These are difficult to classify and show overlapping features between granulosa cells and Sertoli cells. They surround germ cells and hyaline cylinders of eosinophilic basement membrane–like material or palisade at the periphery of gonadoblastoma cell nests. Additional patterns of gonadoblastoma have recently been described under the name dissecting gonadoblastoma . In this variant are small cordlike arrangements of germ cells and sex cord cells that have an infiltrative appearance or expanded sheets and nests of germ cells with often inconspicuous sex cord cells intermixed with the germ cells or scattered at the periphery of the nests. The significance of the dissecting patterns is that they represent forms of gonadoblastoma that could easily be mistaken for invasive germinoma. The stroma surrounding a gonadoblastoma frequently contains luteinized or Leydig-like cells in postpubertal patients. The stroma may contain microcalcifications of variable size.
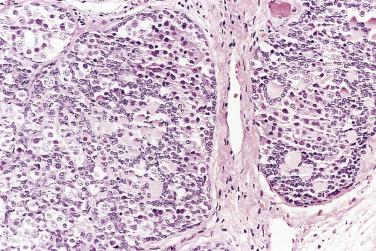
Rare examples of another type of combined germ cell–sex cord–stromal tumor have been described ( Fig. 13A.94 ). These occur in genotypically normal females. The germ cells and stromal cells are randomly admixed; the absence of discrete cell nests containing germ cells, sex cord cells, and hyaline cores differentiates them from gonadoblastoma. Since this tumor arises in a normal gonad, the most appropriate therapy in a young woman is by unilateral salpingo-oophorectomy, not bilateral gonadectomy. Microscopic gonadoblastoma-like lesions occur in fetal and infant ovaries in the absence of genetic abnormalities. They are often found in the walls of follicular cysts and do not appear to have any relationship with a true gonadoblastoma.

FISH testing typically reveals the presence of a Y chromosome or Y chromosome material in gonadoblastomas. Tests with probes for the TSPY1 region revealed the presence of TSPY1 in all gonadoblastomas from intersex patients. A gonadoblastoma in a 46XX female revealed no TSPY1 signal confirming that rare gonadoblastomas arise in apparently normal gonads. TSPY can also be detected by reverse transcriptase polymerase chain reaction (RT-PCR).
The germ cells in gonadoblastoma have the same immunophenotype as dysgerminoma cells. Most tumor cells show membranous and cytoplasmic staining for placental alkaline phosphatase, CD117 (c-kit), and D2-40 and nuclear staining for OCT4, SALL4, and NANOG. They are also TSPY positive. The sex cord cells show positive cytoplasmic staining for inhibin, vimentin, and cytokeratin and positive nuclear staining for SF1, FOXL2, and WT1. The hyaline material reacts with antilaminin antibodies. Luteinized cells in the stroma between gonadoblastoma nests stain strongly for inhibin and calretinin.
Small cell carcinoma is a biologically aggressive neoplasm that occurs over a wide age range, from childhood to 55 years of age, but it is predominantly a tumor of young women, with an average patient age of 24 years. Rare cases appear to be familial. The clinical presentation is typically with nonspecific symptoms such as abdominal distention, abdominal or pelvic pain, nausea or vomiting, or a palpable abdominal mass. About two-thirds of patients have hypercalcemia, but the hypercalcemia is generally asymptomatic. Serum levels of parathyroid hormone are not elevated. Small cell carcinoma is usually unilateral, and about 50% of patients have localized disease at diagnosis. Bilateral tumors occur mainly in patients with widespread metastases, and in such cases probably represent metastatic spread to the contralateral ovary. Small cell carcinoma is aggressive, with a high mortality rate even when the tumor is limited to the ovary at diagnosis. Women with localized tumors may have better survival when treated with hysterectomy and bilateral salpingo-oophorectomy, so that has been the standard surgical treatment. Women with germline mutations of SMARCA4 , the gene that is linked to small cell carcinoma, are at risk for developing a second primary tumor in the contralateral ovary if it is not removed. In the largest reported series, 33% of patients with stage Ia tumors were alive with no evidence of disease at last follow-up, compared with 10% survival in stage Ic and only 6.5% survival in stages II, III, and IV. Until recently successful adjuvant chemotherapy or radiation therapy protocols were lacking, and patients with advanced or recurrent disease had limited responses to radiotherapy or chemotherapy. Lately there have been reports of improved survival with postoperative adjuvant chemotherapy or chemotherapy and radiation therapy, with the most favorable results obtained in patients with stage I tumors, but this is still a lethal tumor in a majority of cases.
Small cell carcinoma is a solid, nodular, grey or tan tumor that ranges from 6 to 27 cm, with an average diameter of 15 cm. On cross section, small cysts and areas of hemorrhage and necrosis are present in some tumors.
The typical appearance is one of uniform small cells growing in diffuse sheets, occasionally punctuated by irregular follicle-like structures filled with lightly eosinophilic material ( Fig. 13A.95 ). In some tumors, the cells grow in nests and cords. The tumor cells are round or spindled, with scanty cytoplasm and hyperchromatic round, oval, or fusiform nuclei with finely granular chromatin and inconspicuous or absent nucleoli ( Fig. 13A.96 ). Mitotic figures are numerous and typically exceed 20 per 10 hpf. Flow cytometric studies reveal that the tumor cells are DNA diploid. Larger cells with abundant eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli are present in about 50% of cases. When they predominate, the tumor is designated as the large cell variant of small cell carcinoma. There are glands lined by benign or malignant mucinous epithelium in 12% of small cell carcinomas.

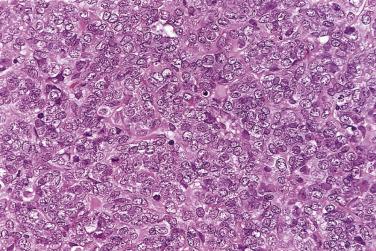
Immunostains for low molecular weight cytokeratin and epithelial membrane antigen generally show patchy positive staining. Most small cell carcinomas show moderate positive nuclear staining for p53 protein, with staining limited to 40% to 50% of tumor cell nuclei. There is usually diffuse strong nuclear staining for WT1. Nuclear and cytoplasmic staining for calretinin and patchy membrane staining for CD10 have been reported. A minority of tumors show staining for neuroendocrine markers such as CD56, chromogranin, or synaptophysin; staining for CD99, desmin, inhibin, and TTF1 is negative. Despite the absence of parathyroid hormone in the serum, occasional tumors stain for parathyroid hormone, and immunoreactivity for parathyroid hormone-related protein has been reported. Negative staining for inhibin and FOXL2 and positive staining for EMA helps in the differential diagnosis with juvenile granulosa cell tumor, which is inhibin and FOXL2 positive and usually EMA negative. It is important to note that both small cell carcinoma and juvenile granulosa cell tumor can show positive staining for calretinin.
Small cell carcinoma has the ultrastructural features of an epithelial tumor. A discontinuous basal lamina surrounds groups of tumor cells, and there are desmosome-like junctions between adjacent cells. Rare tubules with lumina are present, lined by cells with a microvillous surface. The most characteristic ultrastructural feature is prominent dilated cisterns of rough endoplasmic reticulum filled with amorphous, moderately electron-dense material. Dense core neurosecretory granules have been observed by some investigators but not by others.
Molecular genetic studies have revealed that small cell carcinoma of hypercalcemic type is characterized by germline or somatic mutations in the SMARCA4 gene, a component of the SWI/SNF complex. These mutations result in loss of BRG1, which is the protein product of the SMARCA4 gene. This loss can be detected immunohistochemically, providing a sensitive and specific test that is very helpful in establishing the histologic diagnosis of small cell carcinoma. BRM, the protein product of SMARCA2 , another gene in the SWI/SNF complex, is also almost always lost in small cell carcinomas, but this appears to be due to absence of mRNA expression, and mutations of SMARCA2 have not been demonstrated. The combined loss of BRG1 and BRM provides, for the first time, a specific immunohistochemical test that can confirm a histologic diagnosis of small cell carcinoma.
Small cell carcinoma has been designated as a type of sex cord–stromal tumor, a neuroendocrine tumor of germ cell origin, a germ cell tumor related to yolk sac tumor, and an epithelial tumor, but its lineage is currently uncertain. Recently, the finding of a mutation in a SWI/SNF complex gene has prompted the suggestion of a relationship to a malignant rhabdoid tumor, which is characterized by mutation of another gene in the SWI/SNF complex and has a somewhat similar histology.
Desmoplastic small round cell tumor (DSRCT) is a highly malignant intraabdominal tumor that occurs mainly in young males. The tumor also occurs occasionally in females, and rare examples present clinically as primary ovarian tumors. There is invariably extensive intraabdominal tumor growth at diagnosis and, despite aggressive therapy, most patients die of tumor. Patients treated with multimodality therapy, including surgical resection, chemotherapy, and radiation therapy, appear to have the most favorable prognosis.
The tumors are solid and gray, tan, or white, with areas of necrosis. Microscopically, the tumor cells are small, with uniform, round, hyperchromatic nuclei, inconspicuous nucleoli, and scanty to modest amounts of cytoplasm. The tumor cells grow in sheets and nests separated by sclerotic fibrous stroma ( Fig. 13A.97 ).
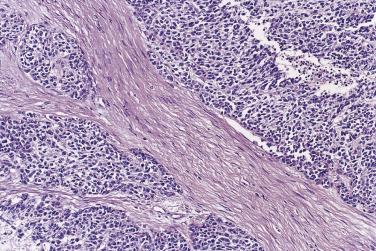
DSRCT has a distinctive polyphenotypic pattern of immunoreactivity. The tumor cells typically show positive staining for cytokeratin, epithelial membrane antigen, neuron-specific enolase, desmin with a dotlike staining pattern, WT1 (but only with antibodies to the C-terminal end of the protein), and occasionally CD99. Rarely, staining for keratin and EMA is absent, and stains for other neuroendocrine and muscle markers are usually negative. The cell of origin is unknown, but the tumor cells exhibit a translocation, usually t(11;22) (p13;q12), in which the Ewing sarcoma and Wilms tumor genes are fused ( EWSR1-WT1 ). FISH testing with EWSR1 breakapart probes and RT-PCR testing that is specific for the EWSR1-WT1 fusion are very helpful in establishing the diagnosis. Rare solid variants of DSRCT without desmoplastic stroma have been identified, and these could potentially be misdiagnosed as another type of small cell neoplasm, such as Ewing sarcoma, if RT-PCR for the specific translocation present in DSRCT is not performed.
The female adnexal tumor of probable wolffian origin (FATWO) is a distinctive tumor that usually occurs in the broad ligament or mesosalpinx, but rare examples appear to arise in the ovary, where they often appear to originate in the hilar region when they are small enough to localize. The proposed origin is from mesonephric (wolffian) remnants. The tumor occurs mainly in middle-aged women. Some tumors are incidental findings in asymptomatic women, but when the tumor is large the symptoms are those of a pelvic mass. Most of these neoplasms are clinically benign, but occasional malignant FATWOs have been reported. In the largest study, 2 of 11 patients with ovarian FATWOs exhibited malignant behavior.
The tumors are solid and gray, tan, or yellow. They range from 2 to 20 cm in diameter, with an average of 11 to 12 cm. The microscopic picture is one of uniform, small to midsize epithelial cells growing in diffuse, trabecular, tubular, and microcystic or sievelike patterns ( Fig. 13A.98 ). Usually, mitotic figures are rare, and there is little cytologic atypia. Features that suggest the possibility of malignant behavior include increased mitotic activity, cytologic atypia, overgrowth of spindle cells, or lymphovascular space invasion. Prominent peritubular basement membranes are characteristic, and there is a variable amount of fibrous stroma.
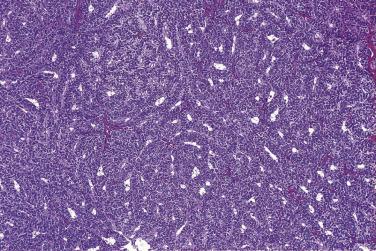
The tumor cells are immunoreactive for vimentin, cytokeratin, androgen receptor, inhibin, calretinin, and FOXL2, but they are epithelial membrane antigen negative. In one study, all FATWOs tested lacked staining for PAX8, PAX2, GATA3, and SF1, so the latter marker might be helpful in differentiating between a FATWO and a sex cord–stromal tumor. Sequencing of a few cases revealed no recurrent mutations and provided no insights into the pathogenesis; none of the tested tumors had a FOXL2 mutation. Rare variants of endometrioid carcinoma are morphologically reminiscent of a FATWO, but these show positive staining for EMA and PAX8, which are reportedly absent in FATWOs, and they lack staining for sex cord–stromal markers, which tend to be positive in FATWOs.
Adenomatoid tumors are most common in the fallopian tube and myometrium, but rare cases have been reported in or immediately adjacent to the hilum of the ovary. Some are microscopic, but others are firm gray or tan nodules, typically less than 2 cm in diameter. The microscopic appearance is similar to that of adenomatoid tumors at other sites. They are benign neoplasms of mesothelial derivation and are circumscribed but not encapsulated. They consist of tubules, trabecula and cysts lined by cuboidal cells with uniform round nuclei, and eosinophilic cytoplasm surrounded by a variable amount of fibrous or fibromuscular stroma. Lymphoid aggregates are sometimes present in or adjacent to the tumor. Cytoplasmic strands that bridge tubules (i.e., threadlike bridging strands) are a characteristic finding. An unusual oxyphilic variant of ovarian adenomatoid tumor had cytoplasmic vacuoles that resulted in a signet ring cell appearance. Immunostains for cytokeratin, calretinin, and D2-40, and usually WT1, are positive. Our group recently discovered that adenomatoid tumors are characterized by mutations in the TRAF7 gene, which encodes an E3 ubiquitin ligase.
Metastatic tumors involve the ovaries more often than any other site in the female genital tract. Ovarian metastases comprise 5% to 15% of all malignant ovarian tumors. Possible metastatic pathways include retrograde lymphatic spread, transtubal and transperitoneal dissemination, and hematogenous metastasis. Based on the pathology of the involved ovaries and on clinical and operative findings, lymphatic and transperitoneal spread account for most ovarian metastases, particularly from primary sites within the abdomen. Adenocarcinomas of the breast, large intestine, endometrium, appendix, and stomach are the most common primary sites, but a wide variety of malignant tumors occasionally give rise to ovarian metastases. These include cancers of the cervix, appendix, small intestine, pancreas, bile duct and gallbladder, liver, kidney, urinary tract and lung, as well as melanoma, malignant lymphoma, and various types of soft tissue and gastrointestinal sarcomas. It is often difficult to differentiate metastatic endometrial adenocarcinoma in the ovary from synchronous primary endometrioid adenocarcinomas of the endometrium and ovary. This problem is discussed in the section on endometrioid adenocarcinoma.
Clinically significant ovarian metastases most often occur in women with a history of an extraovarian cancer, usually of the colon or rectum. The average time between surgery for the primary tumor and detection of the ovarian metastases is about 2 years, but the length of the interval varies and ranges from a few months to more than 5 years. Some patients have no history of a primary extraovarian tumor. They present with symptoms of a pelvic tumor, such as pelvic or abdominal pain, gastrointestinal or urinary disturbances, abdominal distention, or abnormal uterine bleeding, and are often thought to have primary ovarian cancer. In most of these patients, a locally advanced gastrointestinal primary cancer is discovered concurrently with the ovarian metastases but, in rare instances, the primary site is discovered only months or years after the ovarian tumors are removed. When a woman with a history of colorectal cancer develops a new pelvic mass the most likely diagnosis is metastatic adenocarcinoma, but benign or primary malignant ovarian tumors are discovered in a significant percentage of cases (26% benign and 17% primary ovarian cancers in one study). Ovarian metastases develop in 2% to 13% of women with colorectal carcinoma, but prophylactic oophorectomy has not been shown to improve survival, and its efficacy is at present unclear. The prognosis is poor for women with ovarian metastases, but resection appears to lengthen survival and relieve symptoms. Some investigators have found that ovarian metastases are more likely to develop in premenopausal women, but this finding has not been confirmed by others.
Metastatic tumors in the ovary vary in appearance depending on the primary site. Metastatic colorectal cancer is bilateral in 50% to 70% of cases. The ovarian tumors average 10 to 11 cm in diameter, have a smooth surface, and tend to be cystic or solid and cystic. The cysts are unilocular or multilocular and are filled with mucin. In one study, 90% of mucinous carcinomas were classified correctly as primary or metastatic by assuming that all bilateral mucinous tumors or mucinous tumors smaller than 10 cm were metastatic, and all unilateral mucinous tumors larger than 10 cm were primary. The criteria were subsequently modified by increasing the size cutoff to 13 cm, resulting in improved performance of the algorithm. Metastatic colorectal and cervical adenocarcinomas proved to be the most difficult to correctly identify.
Metastatic stomach cancer usually has the appearance of a Krukenberg tumor. The ovaries tend to retain their shape but are symmetrically or asymmetrically enlarged. They are firm and have areas of nodularity on the surface. The cut surface is gray, tan, or white and edematous and honeycombed with small mucinous cysts. Most other types of metastatic tumors tend to be solid and grow as multiple nodules in the cortex and medulla, often with implants on the serosa.
General microscopic features that raise the possibility that an ovarian tumor might be metastatic include bilaterality, a multinodular growth pattern, implants on the surface of the ovary or just beneath it, numerous emboli of metastatic carcinoma in lymphatic spaces, especially in the hilum and mesovarium, a desmoplastic or unusually fibrous or myxoid stromal reaction, an unusual microscopic pattern for a primary ovarian tumor, such as a signet ring cell appearance or a linear pattern of invasion, and a higher nuclear grade than would be expected for the architectural pattern (e.g., high-grade nuclei in a purely glandular growth). Luteinization of the stroma occurs around some metastatic tumors, but it also occurs around primary ovarian tumors, and does not necessarily imply that a tumor is metastatic. The luteinized cells occasionally secrete sufficient amounts of estrogen or androgen to cause clinical symptoms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here