Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Extraadrenal paraganglia have a centripetal and roughly symmetric distribution on either side of the midline, extending from the middle ear region and base of the skull to the pelvic floor. The sympathoadrenal neuroendocrine system is an integrated complex composed of the sympathetic nervous system, with postganglionic neurons mediating effector responses via the neurotransmitter norepinephrine, and the adrenal medullae, which synthesize and secrete the hormones epinephrine and, in lesser amounts, norepinephrine. These paraganglia mediate rapid adaptations to changes in the environment by a combination of neural (norepinephrine release from postganglionic sympathetic neurons) and hormonal effects, with the latter due to secretion of catecholamines (mainly epinephrine) from the adrenal medullae. The strategic location of head and neck paraganglia, which are more closely aligned with the parasympathetic nervous system, makes some of them more suited for having a chemoreceptor role in reflex changes in respiratory and cardiovascular function in response to alterations in the composition of arterial blood.
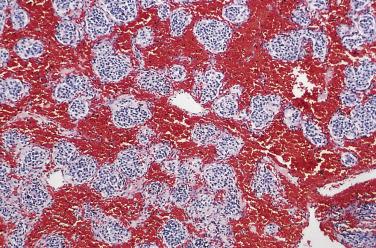
Paraganglia of the head and neck are closely aligned with the parasympathetic nervous system and are often juxtaposed with branchial arch mesodermal derivatives ( Fig. 28.1 ). The largest, most compact collection of paraganglia is the carotid body, which is located on the medial aspect of the carotid bifurcation on each side of the neck ( Fig. 28.2 ). The average combined weight of the carotid bodies in adults is a little over 12 mg. Each carotid body is composed of lobules that are compact at low magnification ( Fig. 28.3A ) and at higher power (see Fig. 28.3B ) contain chief (or glomus type I) cells and sustentacular (glomus type II) cells. Immunohistochemistry (IHC) for chromogranin A and synaptophysin clearly delineates the chief cells in small clusters or short cords (see Fig. 28.3C ). Immunostaining for S-100 protein demonstrates the sustentacular cells at the periphery of cords and clusters of chief cells (see Fig. 28.3D ) but also stains Schwann cells within the carotid body and other paraganglia.
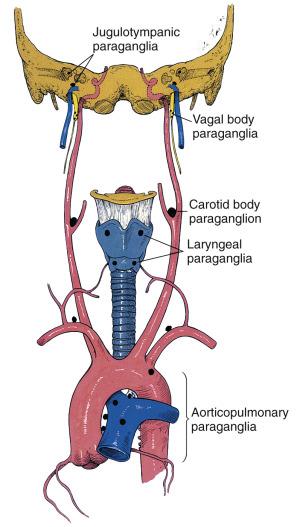
Other head and neck paraganglia are located at or near the skull base such as jugulotympanic paraganglia (promontory of middle ear, adventitia of jugular bulb, facial canal) and vagal paraganglia located in the rostral vagus nerve in close association with the ganglion nodosum. Other paraganglia are located in soft tissues of the larynx (superior and inferior laryngeal paraganglia), adjacent to the thyroid gland and at the base of the heart in relation to the great vessels (aorticopulmonary paraganglia). These paraganglia are microscopic, about the size of a carotid body lobule or smaller subunit. Experimental studies in animals have demonstrated a chemoreceptor role for carotid bodies and some paraganglia near the base of the heart. The carotid body has been most intensely studied given its size and predictable anatomic location in humans and in experimental animals. Historically therapeutic glomectomy for chronic bronchial asthma had provided an opportunity to study physiologic effects of carotid body ablation in humans, but this surgical procedure has been largely abandoned. Other settings providing an opportunity for study of carotid body function include patients undergoing unilateral or bilateral resection of carotid body paraganglioma (CBP) or carotid body resection as part of an elective neck dissection, all procedures done under normoxic and normocarbic mechanical ventilation. The carotid body paraganglion had also been referred to historically as the ganglion minutum. Some of the physiologic effects studied include changes in ventilation, alterations in arterial pO 2 , pCO 2 , pH, and blood pressure, as well as monitoring counterregulatory response to hypoglycemia and hypoxia-induced oxidative stress in the adrenal medulla.
Carotid body enlargement and hyperplasia have been reported in humans living at high altitude presumably as a compensatory response to chronic hypoxemia and it has also been observed in some patients with chronic hypoxemia under normobaric conditions. Similar changes have been noted in vagal and aorticopulmonary paraganglia.
Paragangliomas (PGLs) of the head and neck region are referred to here as HNPGLs. These are rare tumors with carotid body paraganglioma being most common in a number of clinical series. CBPs have a reported incidence of between 0.06 and 3.3 per 100,000 population. Jugulotympanic paragangliomas (JTPs) of the skull base and middle ear are the next most common, but the proportion of tumors may depend upon the relative volume of middle ear operative procedures at any particular institution. HNPGLs are typically nonfunctional, but rare examples have been reported with excess catecholamine secretion. Malignancy has been reported in 2% to 19% of HNPGLs depending upon anatomic localization in the head and neck region.
CBPs usually affect adults, most often in the fifth decade of life, and in most series the sex incidence is nearly equal, although some have reported an overwhelming preponderance of female patients. Rarely the tumor can affect the very young as evidenced by a massive CBP reported in a 3-year-old patient. CBP has also been referred to as chemodectoma ( chemeia, infusion; deschesthai, to receive; oma, tumor), but this term may not be appropriate because none of the HNPGLs has been shown to have a chemoreceptor function. CBP usually presents as a painless, slow-growing mass near the angle of the mandible. This is the most common location for tumors of this type in the head and neck region. Depending on their anatomic location these tumors may cause important problems in differential diagnosis. An increased incidence of CBPs has been reported at high altitude, which has been associated with chronic hypoxemia.
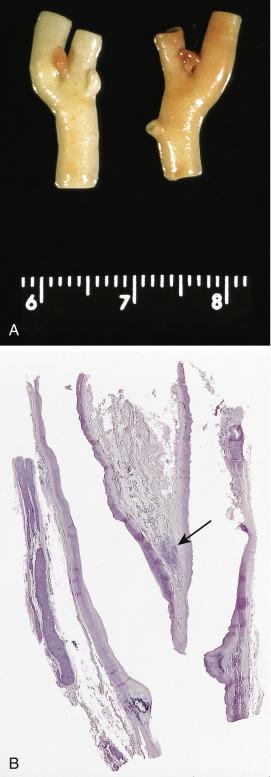
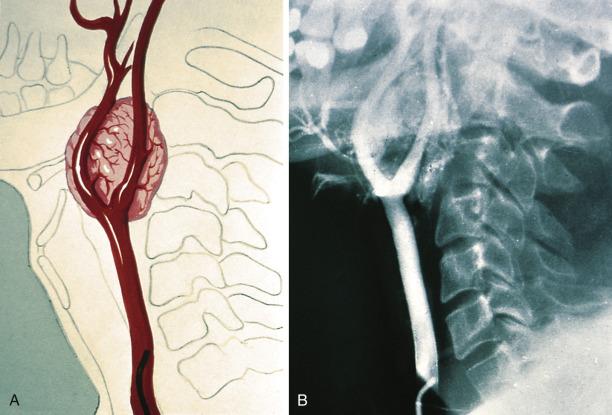
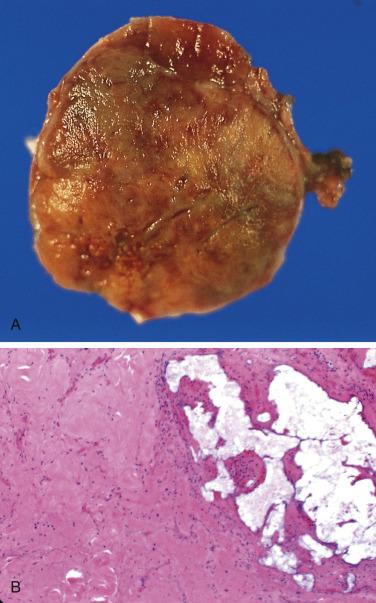
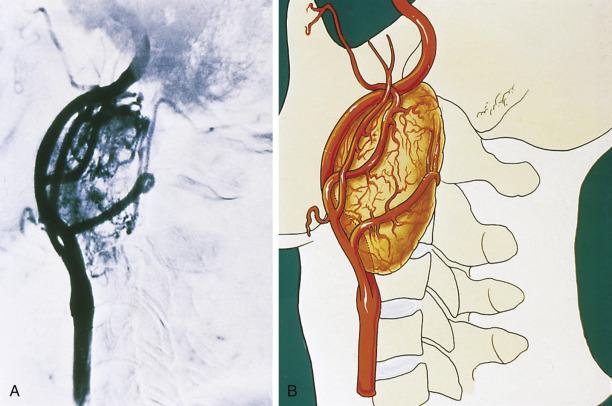
The average diameter of CBP in studies from Memorial Hospital in New York City was 3.8 cm (range 1.8–8.5 cm). Selective angiography or magnetic resonance angiography can be instrumental in demonstrating the vascular supply and precise location of CBP and other HNPGLs ( Fig. 28.4 ). This procedure can also provide a means of therapeutic embolization of CBP or other head and neck paragangliomas prior to operation. The tumors are typically solitary, unilateral, and tan to red-brown on cross section ( Fig. 28.5 ). Patients with hereditary/familial tumors have an increased incidence of bilateral or multicentric tumors which can be synchronous or metachronous. Some tumors are markedly hemorrhagic and, when large, may show areas of degenerative change with fibrosis and some cystic alteration.
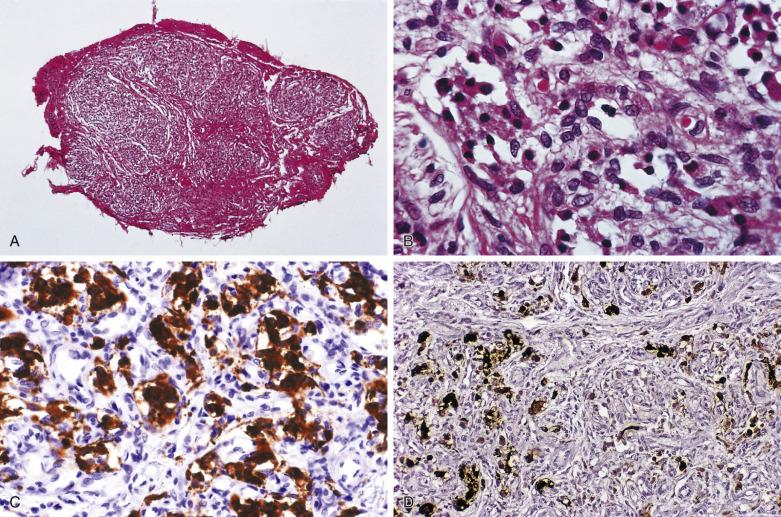
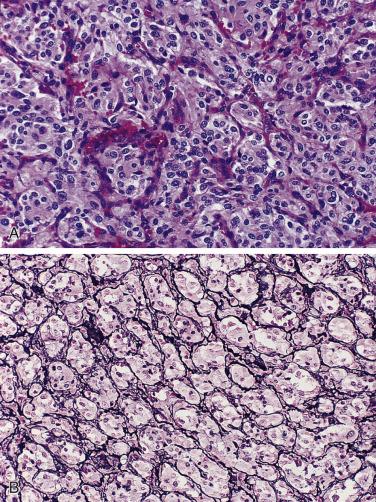
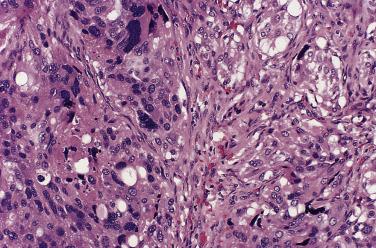
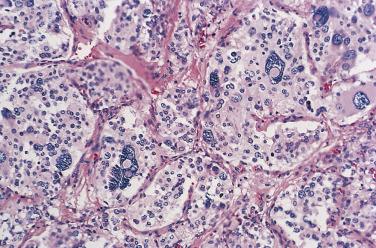
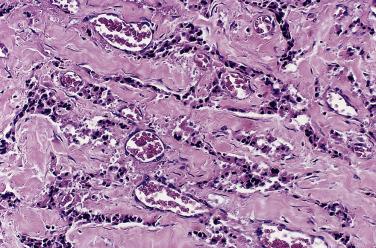
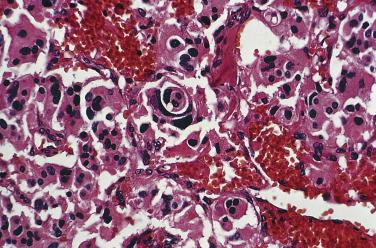
The characteristic microscopic pattern is alveolar, with formation of Zellballen or cell nests ( Fig. 28.6A ), which can be accentuated by staining for reticulin (see Fig. 28.6B ). This arrangement of neoplastic cells vaguely mimics the organoid pattern of chief cells seen in the normal head and neck paraganglia (see Fig. 28.3B and C ). In some tumors the clusters of neoplastic chief cells can be large with central degenerative change ( Fig. 28.7 ). Problems can arise in correct diagnosis because of small or nonrepresentative tissue sampling or crush artifact. This is often the case in JTPs because of the space limitations in the middle ear and skull base. Irregular cords and bands of fibrosis in sclerosing PGLs may impart an infiltrative appearance that can be misleading for malignancy. Nuclear pleomorphism is a prominent feature in some tumors, and one may see nuclear pseudoinclusions similar to pheochromocytomas ( Fig. 28.8 ). Nuclear pleomorphism and hyperchromasia are not reliable features for a diagnosis of malignancy. Mitotic figures are generally scarce. Only a few tumors have been studied by DNA flow cytometric analysis, but DNA ploidy status does not appear to be a reliable indicator of malignancy. Criteria for malignancy are discussed later. Congestion or hemorrhage can accentuate the alveolar or nesting pattern with separation of nests of neoplastic cells ( Fig. 28.9 ). A variety of stromal alterations such as old or recent hemorrhage, fibrosis, and vascular sclerosis ( Fig. 28.10 ) can be present. In some cases, the stromal changes, such as prominent sclerosis, may cause difficulty in diagnosis, but usually some portion of the tumor has a more well-preserved organoid pattern. A minority of tumors contain cells having marked oncocytic features with abundant deeply eosinophilic cytoplasm; a cell-embracing appearance has also been described with interdigitation of cytoplasmic processes as one may see in the normal paraganglia ( Fig. 28.11 ). Rarely, a spindled pattern is present, but other more diagnostic areas are usually seen.
Selective embolization of tumor prior to surgery can cause significant ischemic necrosis of CBP and other HNPGLs; this procedure may decrease blood loss and reduce operative time. Preoperative embolization has usually been done using a transarterial route focusing mainly on smaller branches of the external carotid artery, but due to multiplicity, small caliber, and highly tortuous arterial feeders this endovascular intervention can be complex and time consuming. Recently direct intratumoral injection of an embolic agent has been shown to be an effective and safe procedure for tumor devascularization.
Depending on their size and anatomic location, PGLs can cause a variety of signs and symptoms, but their morphology alone is not significantly different from that of CBP. JTPs can be divided into jugular paraganglioma (JP), involving the lateral temporal bone at the base of the skull, and tympanic paraganglioma (TP), usually located in the middle ear cavity along the course of Jacobson nerve. TP can appear as a small tumor on the promontory of the middle ear or it can be a much larger tumor engulfing middle ear ossicles and bulging or protruding through the tympanic membrane. JPs can also vary in size and growth pattern with presentation as a mass in the skull base, the middle cranial cavity, or the jugular bulb. Occasionally the tumor can grow as a sausagelike mass into the lumen of the internal jugular vein. These tumors can cause a myriad of symptoms, including tinnitus, hearing loss, and jugular foramen syndrome due to compression of cranial nerves.
Vagal paraganglioma (VP) arises higher in the neck from small collections of paraganglia at or just below the ganglion nodosum of the vagus nerve. These paraganglia have been referred to as vagal body paraganglia, even though they are anatomically dispersed microscopic structures. In patients with VP and JTP there is a predilection for women. VPs usually manifest in the fifth or sixth decade of life, and similar to CBP patients may present with multicentric PGLs, primarily in the setting of hereditary/familial PGLs. In addition an increased frequency of malignancy appears to exist in VPs over other cervical-cephalic PGLs. VPs do not primarily involve the carotid bifurcation ( Fig. 28.12 ). VPs may cause irregular expansion of the cephalic portion of the vagus nerve and involve the base of the skull. Another endocrine structure that may be present within the rostral vagus nerve is ectopic parathyroid tissue, which may be associated with hyperparathyroidism due to an adenoma or rarely hyperplasia. Two exceptionally rare cases of primary parathyroid paraganglioma have been reported. Other anatomic locations for PGLs in the head and neck region include larynx, thyroid, nasal cavity, orbit, mediastinum, and base of the heart (aorticopulmonary PGLs and cardiac PGLs).
There have been considerable advances in identifying genetic markers for increased susceptibility for hereditary/familial PCC-PGL. In the past, PCC had been referred to as the 10% tumor (10% familial, 10% malignant, 10% extraadrenal), but this broad generalization no longer applies to the familial aspect of these tumors. Since 1990 at least 14 susceptibility genes have been identified, 10 of which have been validated in large studies ( Table 28.1 ). These genes currently form the molecular basis for hereditary PCC-PGL syndromes; thus in lieu of the “10% tumor,” the “10 gene tumor” has been proposed. Additional genes will probably be tested in future validation studies. PCC/PGL is a highly heritable form of human neoplasia with about 40% of cases associated with familial syndromes involving autosomal dominant mutations, among which those encoding the SDH enzymes are most common. Six different hereditary autosomal dominant diseases can be suspected clinically—neurofibromatosis type 1 (NF1), multiple endocrine neoplasia (MEN) type 2, von Hippel-Lindau disease, renal cell carcinoma with SDHB mutation, Carney triad (PGL, gastric stromal tumor, and pulmonary chondroma), and Carney-Stratakis dyad (PGL and gastric stromal tumor). The possibility of hereditary PCC-PGL syndrome should be considered in individuals with a family history of such tumors (or the presence of syndromic features) or having tumors with some of the following features: (1) multiple tumors (i.e., >1 PGL or PCC, including bilateral PCC), (2) multifocal tumors in different anatomic sites (synchronous or metachronous), (3) malignant tumors, and (4) early onset (e.g., <45 years). Germline mutations have also been reported in 24% of patients who presented with nonsyndromic apparently sporadic PCCs; younger age, multifocal tumors, and extraadrenal PGLs were significantly associated with the presence of a mutation.
| Mutation | Frequency |
|---|---|
| SDHB (PGL 4) | 10.3% |
| SDHD (PGL 1) | 8.9% |
| VHL | 7.3% |
| RET | 6.3% |
| NF1 | 3.3% |
| SDHC (PGL 3) | 1.0% |
| SDHA (PGL 5) | <2% |
| SDHAF2 (PGL 2) | <2% |
| TMEM127 | <2% |
| MAX | <2% |
Genotyping of the main PCC-PGL susceptibility genes ( SDHB, SDHD, VHL , and RET ) has been done in eight studies each having more than 200 patients. The total cohort of patients was 3694, with 1250 individuals harboring germline mutations (33.8%) ; thus at least a third of all patients with PCC-PGL have disease-causing genetic mutations. Patients with mutations in the succinate dehydrogenase gene encoding subunit B (SDHB) of the mitochondrial respiratory chain complex II are at increased risk for PCCs and less so for HNPGLs (including skull base). A notable clinical feature however is the increased incidence of malignant tumors in 40% or more of affected individuals. The SDHD mutation carriers are at increased risk for developing both HNPGLs and PCCs, but head and neck tumors are more frequent and there is an increased incidence of multifocal tumors.
Von Hippel-Lindau (VHL) disease is caused by heterozygous mutations in the VHL tumor suppressor gene and has been divided into type 1 and type 2 based on genotypic associations ; PCCs occur only in type 2 disease accounting for about 10% of all VHL disease cases. In contrast to MEN type 2 syndrome, adrenal medullary hyperplasia is not reported to be associated with VHL-related PCC. Five of the susceptibility genes in Table 28.1 were detected in 2% or fewer of the cases, including two of the more recently reported genes TMEM127 and MAX (MYC-associated factor X). Another recently described gene is HIF2A (hypoxia-inducible factor 2 alpha); this has been associated with a newly described Pacak-Zhuang syndrome of PGL, somatostatinoma, and polycythemia.
A task force organized to formulate clinical practice guidelines for PCC-PGL recommended that genetic testing should be considered in each patient, but this was not meant to imply that genetic testing should be done in each patient. In view of financial costs and possible negative impact, genetic testing has limited incremental value in patients with unilateral PCC, no syndromic or malignant features, and no positive family history. A decisional algorithm for genetic testing has been proposed in patients with a documented PCC-PGL. Evidence reviewed in the literature apparently justifies SDHB genetic testing in patients with malignant PCC-PGL.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here