Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Access video lecture content for this chapter online at Elsevier eBooks+
Access video lecture content for this chapter online at Elsevier eBooks+
Upper extremity amputees are different from lower extremity amputees in many aspects. The patients tend to be younger, and their amputations are primarily due to trauma and tumors rather than dysvascular conditions. The patients live longer with their residual limbs, and prosthetic concerns for the two groups of patients dramatically differ. Upper extremity prostheses require more control, more movement, and more precision than those for the lower extremity. Fortunately, upper extremity prostheses have less load-bearing requirements since they do not need to support the weight of the body.
The purpose of this chapter is to familiarize the reader with necessary concepts to care for the upper extremity amputee ( Algorithm 40.1 ). Handling of soft tissues, bones, and nerves are reviewed for each of the levels of amputation. Prosthetics and prosthetic control will be a necessary accompaniment of this chapter. Recent advancements in the field, including targeted muscle reinnervation (TMR), regenerative peripheral nerve interfaces (RPNI), and direct skeletal attachment (osseointegration, or OI) will be introduced.
A key principle of prosthetic reconstruction is for the residual limb to be pain-free with stable soft tissues to allow the prosthesis to fit comfortably without causing sores, wounds, or pain generated by the weight of the device. The surgical treatment of patients with unreconstructable upper extremity conditions must consider issues unique to the amputee. The amputation must have durable soft tissues to allow for prosthetic fitting. The soft tissues cannot be overly thick or mobile, because this impedes transmission of the forces created by the residual muscles on bone. Similarly, the soft tissues cannot be overly thin, or else the slightest trauma or pressure causes breakdown, which can limit prosthesis wear.
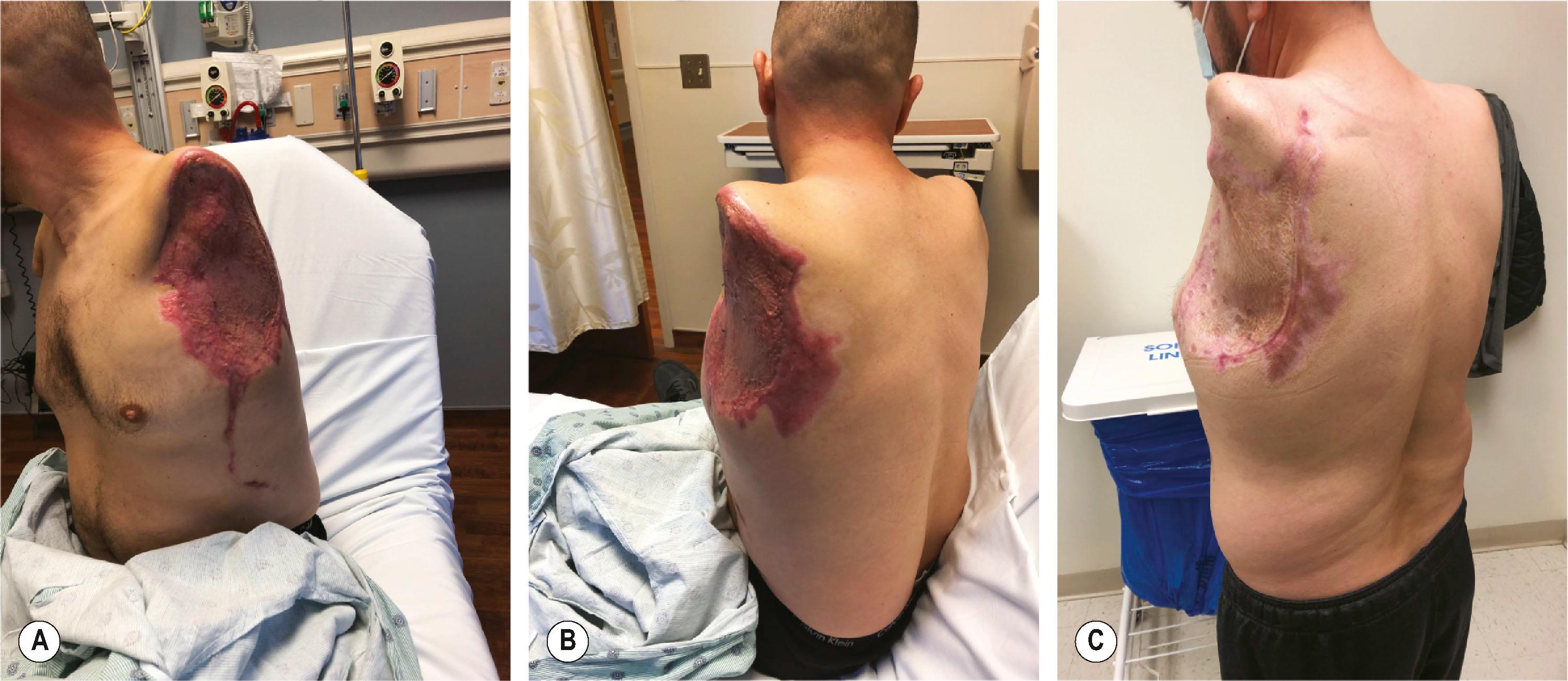
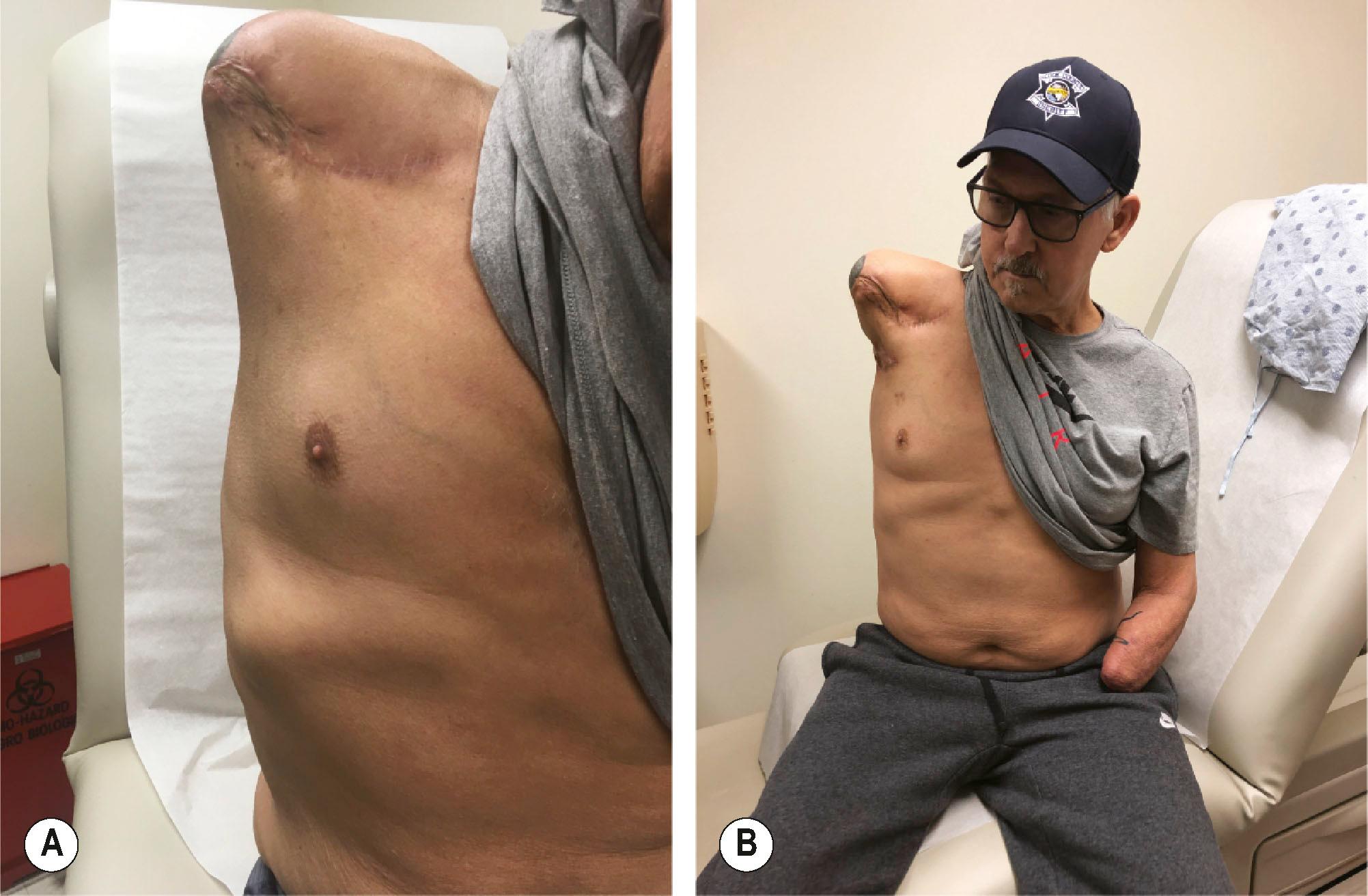
Standard plastic surgery principles involve common sense and judgment to add soft tissues where there are deficits and to remove soft tissue where there are excesses. For fat, this can involve free fat grafts to the deficient atrophied limb and liposuction or direct excision to circumferentially remove adipose tissue to improve socket fitting, as well as improve function. Functionless and excessive soft tissue can be excised to allow the prosthetic jacket to assume a smoother contour. Tissue expansion can be employed to improve soft tissues over bony prominences critical to hold the prosthesis in place ( Fig. 40.1 ). Soft-tissue procedures are valuable to improve joint mobility, as well as to deepen soft-tissue indentations to functionally lengthen a residual limb, much like a 4-flap Z-plasty of the first webspace in the hand ( Fig. 40.2 ). Excessive soft tissues can render the donning of socket liners difficult and can be removed with direct excision and/or with liposuction. Pedicled or free flaps can be used to augment tissue where there are deficits in order to avoid a more proximal amputation. The major downside of soft-tissue surgery is that amputees cannot wear their prosthetics for weeks to months, depending on the time course for swelling to resolve. However, as many of these amputees are young with many years of life expectancy to live with their residual limbs, the efforts to improve the quality of the soft tissues are often worthwhile.
There are four main categories of prostheses, defined by the method used to control the device. There is a rough correlation between prosthetic type and the level of amputation. Selection of a prosthesis also depends on the functional requirements of the patient and the level of financial support available to the patient for the device. Prostheses are compared based on issues of weight, durability, speed and accuracy of motion, degrees of freedom of movement, battery life for externally powered devices, and aesthetics.
Passive prostheses are the lightest-weight devices because they contain no motors and few mechanical systems. Used predominantly for finger and distal hand amputations, passive prostheses do not permit any motion, and they are relatively soft and fragile. The primary goal of a passive device is aesthetic so that the upper extremity injury does not garner attention. Some patients use these passive devices to help with holding down objects for manipulation by their non-injured hand. Passive devices can also fill out and support clothing.
An aesthetic prosthesis assists patients with addressing issues of body image and self-esteem, with possible functional gains due to a newfound social openness and ability to expose the limb ( Fig. 40.3 ). Most of these prostheses are custom-made and require a high level of expertise and artistry on the part of the prosthetist. More recently, 3D printing has made these devices more accessible and less costly. Because intact skin is used to secure the prosthetics in place, some amount of sensory feedback of the residual limb will be lost, which is one drawback to these devices. Osseointegration or direct skeletal attachment of passive prostheses may solve some of these attachment issues and is described below. A well-attached passive prosthesis has the potential to improve function by simply increasing the functional length of amputated digits.

As the name implies, a body-powered prosthesis is moved by the remaining muscles in the individual's body. Typically, a harness with a strap that lies over the lower third of the scapula connects to a cable that operates the terminal device, which is sometimes a hook. The Ballif arm of 1812 was one of the first to provide body-powered active control of the terminal prosthetic "hand", as well as to utilize softer materials for the socket. Rather than locking prosthetic fingers into place for a static grip, the Ballif arm utilized cables attached to the shoulder and arm to allow opening and closing of the prosthetic hand. The Civil War created a large number of amputees in the United States, leading to companies such as the A. A. Marks Company (1853) of New York and the J. E. Hanger Company (1861), which is still in existence today.
The benefits of body-powered prostheses are that they are relatively lightweight and durable; can be made to be waterproof; and can provide feedback to the user based on the tension in the control cable. Similar to aesthetic devices, 3D printing has lowered the cost of these devices and allowed them to be more "custom" for improved fitting. Body-powered prostheses work best in transradial amputees with good elbow and shoulder function who will use their residual limb for forceful activity. The disadvantages are that they require harnessing, and the user must have the strength and range of motion to pull the cable sufficiently to make the device work in all positions.
An externally powered prosthesis is powered by batteries contained within the system. The device can be controlled with various inputs, including electromyographic (EMG) signals, force-sensing resistors, pull switches, and push switches. The most prevalent type of externally powered device is a myoelectric prosthesis control scheme which uses EMG signals from two antagonist muscle contractions to operate two directions of movement. With a simple two-site direct control system, for example, wrist extensor EMG signals control opening of the hand/fingers, while wrist flexor EMG signals control closing.
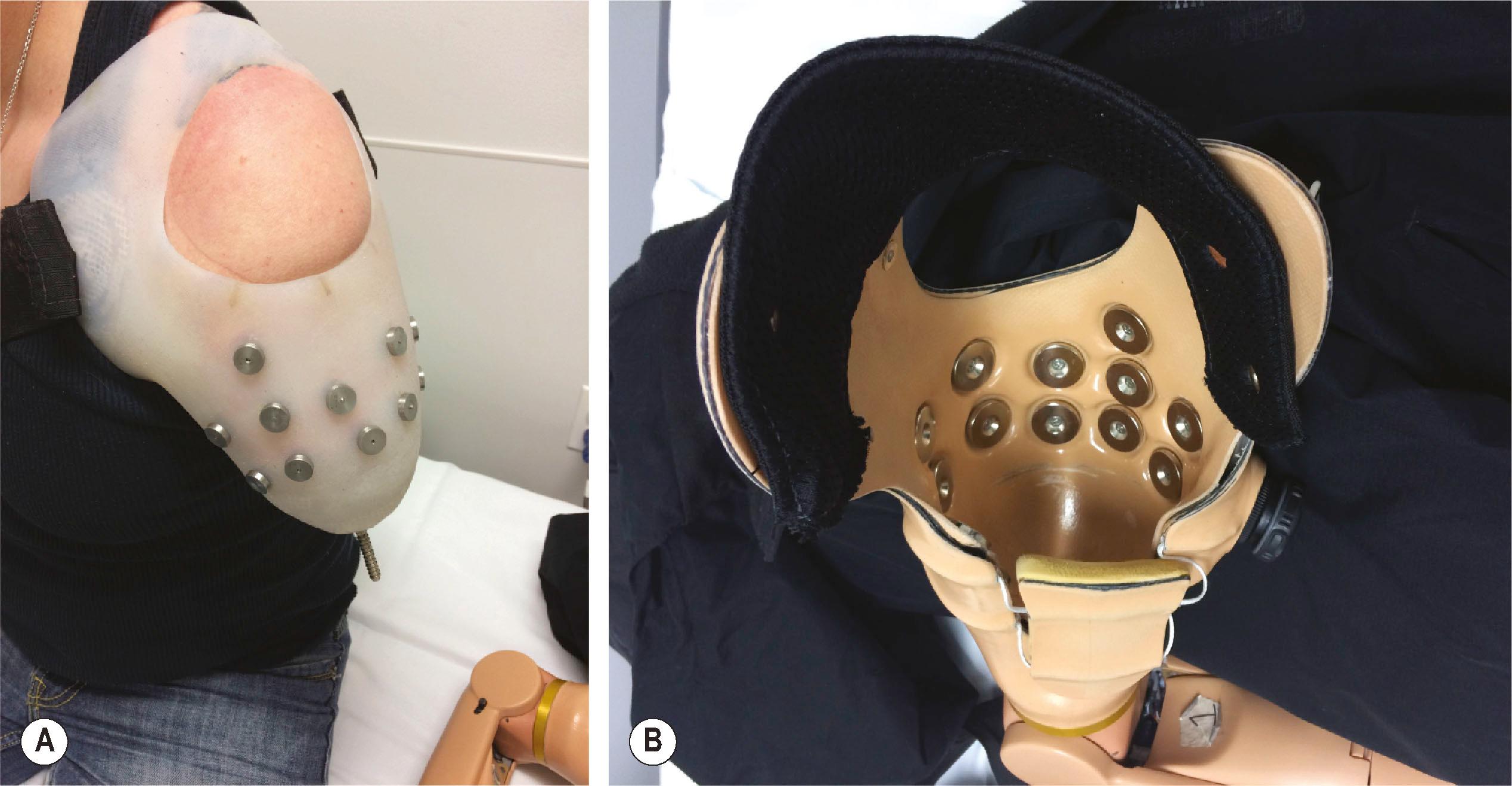
The benefits of myoelectric prostheses are that they require less harnessing than body-powered systems; they can often be operated in more planes of movement; and because there are no cables and straps on the outside of the device, they are aesthetically more appealing than body-powered prosthetics. While some patients prefer an outer covering of their device to appear like skin, others welcome a futuristic appearance with digits made of metal, along with visible joints and articulations. The disadvantages of myoelectric devices are that the batteries and motors make them heavier than body-powered systems; they can be water-resistant but not waterproof; they need to be charged daily; they require more maintenance than body-powered devices; and they are relatively more expensive. In addition, myoelectric prostheses require that the electrode sensors that record signals from the muscles to control the device maintain contact with the skin. Thus, they require an intimate fit that may be uncomfortable or not tolerated by fragile skin or by scar tissue. Excessive sweating tends to decrease contact reliability. Additionally, in order for surface sensors to accurately record EMG signals, there must be a very tight socket fit ( Fig. 40.4 ). When planning for a myoelectric prosthesis, the removal of fat surgically can be advantageous to improve signal detection. The removal of subcutaneous fat decreases the resistance between the underlying muscle and skin, thereby increasing the amplitude of the signal and decreasing cross-talk. Skin grafts used as part of the closure of the residual limb can be advantageous, as the EMG signal is more easily detected due to the lack of subcutaneous fat.
Implantable EMG sensors that transmit a signal to the myoelectric device will avoid these problems of transcutaneous signal detection and are the focus of much research. However, thin wires that exit the skin to provide direct signaling capabilities can break, dislodge, and/or become infected. EMG information can be brought out through the abutment of an osseointegrated prosthesis, but these systems (like the transcutaneous thin wires) are all experimental and are not available for commercial use. Direct recording and stimulation of the cerebral cortex has been performed in monkeys in order to drive peripheral movement. This brain–machine interface, if successful, could have wide applicability not only for amputees, but also for patients with spinal cord injuries.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here