Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Skin is the first immune defense mechanism and functions as a barrier against microorganisms. Infections are a significant problem once open wounds compromise this barrier. According to the U.S. National Burn Repository, the four leading causes of burn morbidity are (1) pneumonia, (2) cellulitis, (3) urinary tract infections, and (4) burn wound infections. Infections are a primary factor contributing to mortality, accounting for 51% of deaths in burn patients, as discussed in both Chapters 30 and 32 , on multisystem organ failure and critical care, respectively.
A cutaneous burn is initially sterile as commensal skin flora are killed with the skin in the thermal event. Unfortunately the burn wound provides optimal bacterial growth conditions due to a reduced blood supply and a nutrient-rich environment, leading to rapid wound colonization. The 2007 American Burn Association Consensus Conference defined wound colonization as follows: (1) low concentrations of bacteria on the wound surface, (2) absence of invasive infection, and (3) less than 10 5 organisms per gram tissue. In a superficial burn, skin flora can survive in hair follicles and sebaceous glands in the same manner as keratinocytes to repopulate a physiological microbiome. However, burns are customarily colonized by pathogens from the environment, the patient's gut, or the naso-oropharyngeal tract.
A race thus exists between the patient and the pathogen to dominate the wound surface. In the log phase growth, bacteria double 2–3 times per hour; consequently a single bacterium can become 10 million in 1 day, far faster than any human cell can multiply. Therefore colonization can quickly become an infection capable of converting partial-thickness into full-thickness burns by causing vessel thrombosis and necrosis in viable tissues in the wound penumbra. Gram-positive bacteria tend to colonize an affected area first, with subsequent colonization by gram-negative bacteria. Delayed treatment risks colonization by extended-spectrum pathogens, bloodstream invasion, and the development of sepsis, all of which increase the likelihood of death.
The model of burn care advocated throughout this text is to rig this race for wound dominance in favor of the patient. Early wound excision eliminates the devitalized tissue that is the main reservoir for pathogen nourishment and habitat. Prompt autografting reestablishes skin barrier function and denies pathogens access to the host. Topical antimicrobials suppress bacterial growth and colony counts while allowing host fibroblasts and keratinocytes to proliferate and cover the wound. Assiduous washing technique allows 2-log reduction in colony counts, breaks down and removes biofilms, and purges the devitalized tissue that pathogens thrive upon. Systemic antimicrobials kill and suppress the invading pathogens accessing perfused areas of tissue. Quantitative wound culturing enables effective diagnosis and directed application of the most efficacious antimicrobial with the lowest toxicity. Coordinated critical care, therapy, and nutrition ensure that the wounds have a sufficient supply of nutrients and immune-cell–laden blood to clear pathogens, expand skin grafts, and reepithelialize wounds.
Prevention is the optimal way to minimize infection. Pathogens can be carried into or transmitted around the unit by staff, by visitors, or on equipment. Patients should have single rooms separated from other rooms by a door. Positive pressure in the rooms further aids in minimizing bacterial contamination. Patient rooms should undergo a daily cleaning in addition to deep cleaning upon patient discharge. This terminal cleaning should include washing the walls and ceiling and should be done 72 hours prior to admitting another patient to avoid the transfer of more virulent strains. Another important prevention measure is the use of contact precautions with all patients, including gowns and gloves. These items should be donned before entering and doffed prior to exiting the room, regardless of the bacteriological status of the particular patient. Routine hand hygiene before and after patient interaction is also mandatory to prevent infection. Dressing materials, supplies, devices, and equipment must not be shared between patients. Bathing, showering, tubbing, mobile diagnostic equipment, and operating facilities should be decontaminated between each patient use. Furthermore, fomites, such as ties, rings, watches, and cell phones, should be prohibited since these are possible pathogen vectors. Water and air filters ought to screen particles down to 0.2 and 0.3 µm, respectively, be changed monthly, and be cultured routinely as part of infection control monitoring. By maintaining these strict measures, transmission of infectious organisms between patients can be limited.
Diagnosis of burn infection in burn wounds can be complicated. The typical cytokine and immune cascades that create the typical presentations of infection known to all doctors are often initiated in the burn patient via the elaboration of damage-associated molecular patterns (DAMPS) and pathogen-associated molecular patterns (PAMPS) from burn wounds. This complicates diagnoses of infection and sepsis in the burn patient where clinical signs, such as elevated temperature or tachycardia, are normal components of burn pathophysiology, as discussed in Chapter 29 on hypermetabolism.
Wound culturing is a critical tool to guide the treatment of burn wounds and to determine what bacteria are colonizing the burn wound. In patients with major burns, the wound usually becomes colonized 5–7 days after injury. Since most initial infections in burn patients derive from endogenous bacteria flora, it is good clinical practice to perform initial wound cultures upon patient admission. This screening should include swabs of both sides of the groin and axilla, as well as of the nose and throat. In addition, at each burn excision, and whenever suspicion of invasive infection warrants, quantitative tissue cultures may be helpful.
Wound appearance and odor changes can help to diagnose wound colonization versus wound infection ( Fig. 11.1 ). Kwei et al. described three main methods that exist for culturing a wound: qualitative (presence or absence of growth), semi-quantitative (grading of the bacterial presence as scanty, few, moderate, or numerous), and quantitative (an absolute quantity is determined). Swabs are useful but limited since they cannot distinguish between infection and colonization and are only accurate for the region sampled. These problems are mitigated by performing multiple tissue biopsies, which can reveal significant quantitative differences in different areas. Although quantitative cultures are more expensive, bacterial counts have been shown to correlate with histological evidence of wound infection in approximately 80% of cases after biopsies are taken or after sterilization of the surface with antimicrobials. Histological examinations can be used to confirm invasive bacterial infection when counts exceed 10 5 organisms per gram of tissue. If histological evidence of invasion is present, systemic antibiotics should be administered and the wound excised.
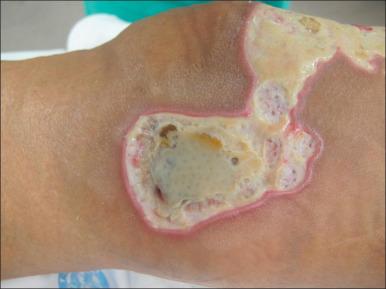
Studies by Robson demonstrated that if burn wound colony counts from biopsies or after cleaning of the surface of the wound are greater than 10 5 /g tissue, the graft survival rate is only 19%, whereas colony counts of less than 10 5 /g tissue are associated with a 94% chance of graft survival. Sensitivities to available antibiotics, both systemic and topical, should instruct therapy. In the setting of resistant organisms, antibiotic synergy testing is advised. Thus the ultimate diagnosis of burn wound infection and the guidance of antimicrobials are directed by culture data.
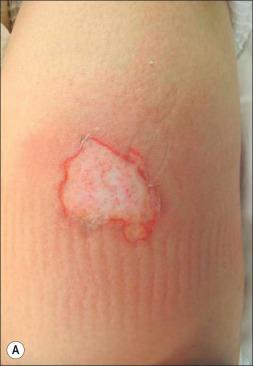
Physical examination of the patient also provides valuable insight into the infectious nature of the burn wound. Burn wound erythema is a physiologic phenomenon produced sterilely by the liberation of inflammatory mediators from tissues surrounding the burn area and must not be confused with cellulitis. Normally this erythema presents within 2–3 days of the burn injury and resolves by 1 week post burn ( Fig. 11.2 ). The best differential diagnosis comes from clinical palpation: erythema lacks significant induration or tenderness compared to an infectious process like cellulitis.
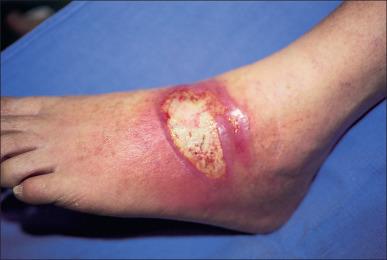
Cellulitis is a noninvasive infection of the tissues surrounding the burn wound. Cellulitis can be caused by a variety of pathogens. This infection is characterized by edema, hyperesthesia, erythema, induration, and tenderness detectable upon examination ( Fig. 11.3 ). The color of the wound contour and the odor from the wound may also raise suspicion of cellulitis. Furthermore it can have a lymphangitic component. Particular attention should be given to elderly patients and diabetic patients due to the ease and speed with which infections progress in these populations. Cellulitic burn wounds benefit from systemic antimicrobials to cover likely causative agents in addition to standard burn treatments, such as topical antimicrobials or surgical excision and grafting. Progression of cellulitis despite antibiotics must always trigger suspicions of resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA), and these organisms should be covered empirically.
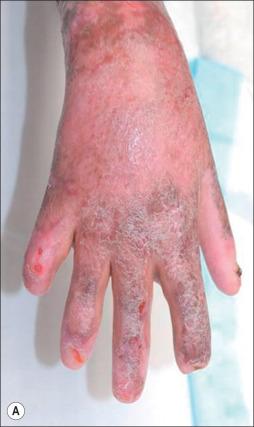
Graft ghosting (impetigo) is a wound infection that can cause late graft loss ( Fig. 11.4 ). This phenomenon can occur after spontaneous closure of a partial-thickness wound, following loss of previously adherent graft sites, and in skin donor sites. Characterized by multiple small abscesses, graft ghosting can lead to the complete destruction of the healed wound. The culprit typically responsible for this condition is S. aureus , particularly MRSA. The diagnosis is essentially clinical and can be confirmed by culturing. Treatment requires regular dressing changes, débridement of abscesses, local disinfection, and application of topical antimicrobials, such as mupirocin.
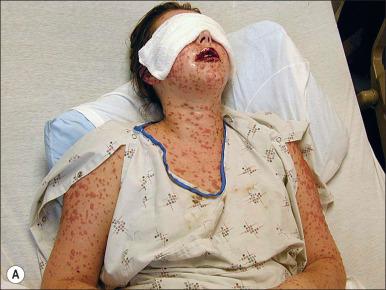
Toxic shock syndrome (TSS) is a complication of severe soft tissue infections (SSTI), which occurs predominately in small burns. This syndrome results from colonization with TSS toxin-1–producing S. aureus . This disease is primarily seen in young children with burns covering less than 10% of the total body surface area (TBSA) and that would otherwise be expected to heal without problems. The incidence is approximately 2.6% with a mean age of 2 years. TSS is clinically characterized by a prodromal period lasting 1–2 days with pyrexia, diarrhea, vomiting, and malaise. While a rash is often present, at this stage, the burn appears clean ( Fig. 11.5 ). Shock subsequently develops in untreated cases, but determining the cause of shock to be TSS can be complicated in this early phase of the patient's presentation when a litany of potential and more common etiologies of shock exist. Once shock has developed, mortality can be as high as 50%. Awareness and aggressive action are the principal safeguards against the development and progression of TSS. A possible diagnosis of TSS should be considered in small burns where the patient is unexpectedly in shock. Because MRSA has emerged as the most commonly identified cause of SSTI, initiation of empiric anti-MRSA antimicrobials is warranted in all cases of suspected TSS.
Invasive wound infections manifest clinically with wound color changes, exudate, and odor. Within a short time, partial-thickness burns progress to full-thickness necrosis and begin expanding into unburned tissues. The 2007 American Burn Association Consensus Conference defined invasive infections as follows: “the presence of pathogens in a burn wound at concentrations sufficient in conjunction with depth, surface area involved, and age of patient, to cause suppurative separation of eschar or graft loss, invasion of adjacent unburned tissue or cause the systemic response of sepsis syndrome.” Goal-standard diagnosis is made with histologic examination; however, clinical exam and quantitative cultures usually suffice ( Fig. 11.6 ). It should be noted that sepsis does not always develop during invasive infections ( Fig. 11.7 ). Treatment must be immediate and include aggressive surgical intervention augmented by the administration of systemic and topical antimicrobials. If no culture results are initially available, a broad-spectrum empirical therapy against fungi, drug-resistant gram-positive and -negative organisms should be initiated until culture data become available. Surgical extirpation must be aggressive and encompass excision of all necrotic and infected tissue, including fascia and muscle when warranted. Definitive wound coverage is not always indicated in this extirpative operation as dressing changes and hydrotherapy may be needed to further decrease heavy bacterial loads. In cases where tissue has already been excised or there is a life-threatening infection, limb amputation may be indicated. Topical antimicrobials and assiduous washing technique are indicated after extirpation to suppress microbial growth. However, the optimal methodology is to prevent infection, with swift action being taken should an infection arise.
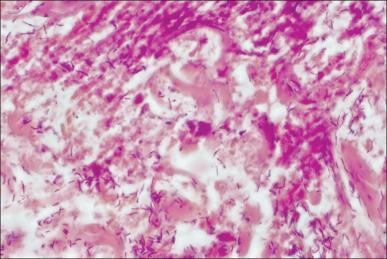
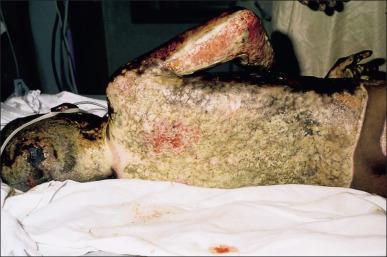
Sepsis and septic shock are complicated diagnoses in burn patients because large burns create a systemic inflammatory response syndrome (SIRS) and hypermetabolism of their own, as discussed in Chapter 8 on the etiology of shock. This hypermetabolism is a natural part of the body's compensatory mechanism to burn injury and can last for up to a year following the insult, making it difficult to fit shock in burn patients into the definitions of sepsis and septic shock established by the Society of Critical Care Medicine. Patients with high concentrations of bacteria in the burn wounds in conjunction with delayed admission to a burn center or delayed removal of burned tissues are at the greatest risk of developing sepsis. Rapid and complete closure of deep burns is the best defense against this condition. An additional factor influencing the development of sepsis and increasing mortality during hospitalization in patients with equal burn sizes is decreased lean body mass. The identification of parameters associated with sepsis in burn patients is extremely important and ongoing. The American Burn Association Consensus Conference in 2007 provided criteria ( Box 11.1 ). These criteria are useful markers and indicators for sepsis but are by no means gospel. A skilled physician must take these factors into account along with changes in the patient's clinical condition over time to make a presumptive diagnosis of sepsis. Aggressive treatment should be initiated and de-escalated based upon definitive diagnosis and patient response.
“Sepsis is a change in the burn patient that triggers the concern for infection. It is a presumptive diagnosis where antibiotics are usually started and a search for a cause of infection should be initiated. While there is need for clinical interpretation, the diagnosis needs to be tied to the discovery of an infection (defined below). The definition is age-dependent with adjustments necessary for children.
The trigger includes at least three of the following of sepsis:
Temperature >39mpera<36.5er
Progressive tachycardia
Adults >110 bpm
Children >2 SD above age-specific norms (85% age-adjusted max heart rate)
Progressive tachypnea
Adults >25 bpm not ventilated
Minute ventilation >12 L/min ventilated
Children >2 SD above age-specific norms (85% age-adjusted max respiratory rate)
Thrombocytopenia (will not apply until 3 days after initial resuscitation)
Adults <100,000/mcl
Children <2 SD below age-specific norms
Hyperglycemia (in the absence of pre-existing diabetes mellitus)
Untreated plasma glucose >200 mg/dL or equivalent mM/L
Insulin resistance – examples include
>7 units of insulin/h intravenous drip (adults)
Resistance to insulin (>25% increase in insulin requirements over 24 hours)
Inability to continue enteral feedings >24 hours
Abdominal distension
Enteral feeding intolerance (residual >150 mL/h in children or 2ce (residual er 24 hours
Uncontrollable diarrhea (>2,500 mL/d for adults or >400 mL/d in children)
In addition, it is required that a documented infection (defined below) is identified
Culture positive infection, or
Pathologic tissue source identified, or
Clinical response to antimicrobials.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here