Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Sexual reproduction is based upon the union of an egg and a sperm. This chapter explains how the egg and sperm cells come together in the female reproductive tract so that fertilization can occur. It then outlines the complex set of interactions involved in fertilization of the egg by a sperm.
Toward the midpoint of the menstrual cycle, the mature graafian follicle, containing the egg that has been arrested in prophase of the first meiotic division, has moved to the surface of the ovary. Under the influence of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), the follicle expands dramatically. The first meiotic division is completed, and the second meiotic division proceeds until the metaphase stage, at which point the second meiotic arrest occurs. After the first meiotic division, the first polar body is expelled. By this point, the follicle bulges from the surface of the ovary. The apex of the protrusion is the stigma .
The stimulus for ovulation is the surge of LH secreted by the anterior pituitary at the midpoint of the menstrual cycle (see Figure 1.19 ). Within hours after exposure to the LH surge, the follicle reorganizes its program of gene expression from one that develops the follicle to one that produces molecules that set into gear the processes of follicular rupture and ovulation. Fundamental to this reorganization is the production of epidermal growth factor (EGF)-like ligands by the mural granulosa cells and the induction of EGF receptors on the surfaces of the cumulus cells. A reduction of cAMP levels within the oocyte (see Figure 1.11 ) stimulates the resumption and completion of the first meiotic division within the oocyte ( Figure 2.1 ). This is shortly followed by expansion of the cumulus oophorus due to the extracellular deposition of the water-binding molecule hyaluronic acid by the cumulus cells and the side-chain molecule versican by the granulosa cells. While expansion of the cumulus oophorus is taking place, further maturation of the oocyte continues.
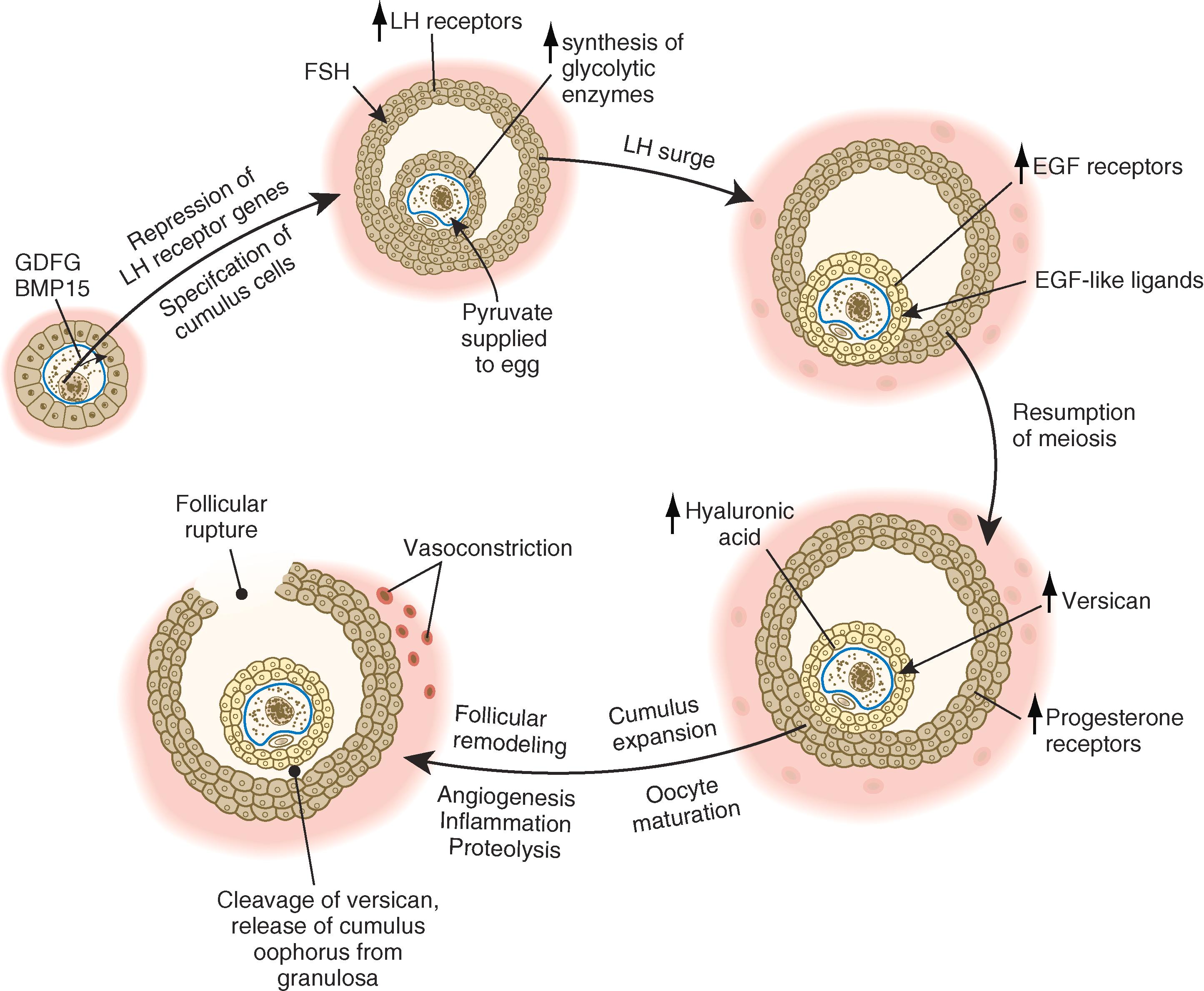
The next stage leading to ovulation involves follicular remodeling. Due to significant angiogenesis, blood flow increases in the outer thecal layers of the follicular wall. With the increased blood flow, plasma proteins leak into the tissues through postcapillary venules, with resulting local edema. The edema and the release of certain pharmacologically active compounds, such as prostaglandins, histamine, vasopressin, and plasminogen activator, provide the starting point for a series of reactions that result in the production of matrix metalloproteinases —a family of proteolytic enzymes that degrade components of the extracellular matrix. The lytic action of the matrix metalloproteinases produces an inflammatory-like reaction that, along with ischemia caused by vasoconstriction of the thecal blood vessels, ultimately results in the rupture of the outer follicular wall approximately 28 to 36 hours after the LH surge ( Figure 2.2 ). Within minutes after rupture of the follicular wall, the cumulus oophorus detaches from the granulosa (due in part to cleavage of the versican side-chains in the extracellular matrix), and the egg is released from the ovary. At the time of ovulation, the egg, now a secondary oocyte, is blocked at metaphase of the second meiotic division, and it will remain at that stage until fertilized by a spermatozoon.
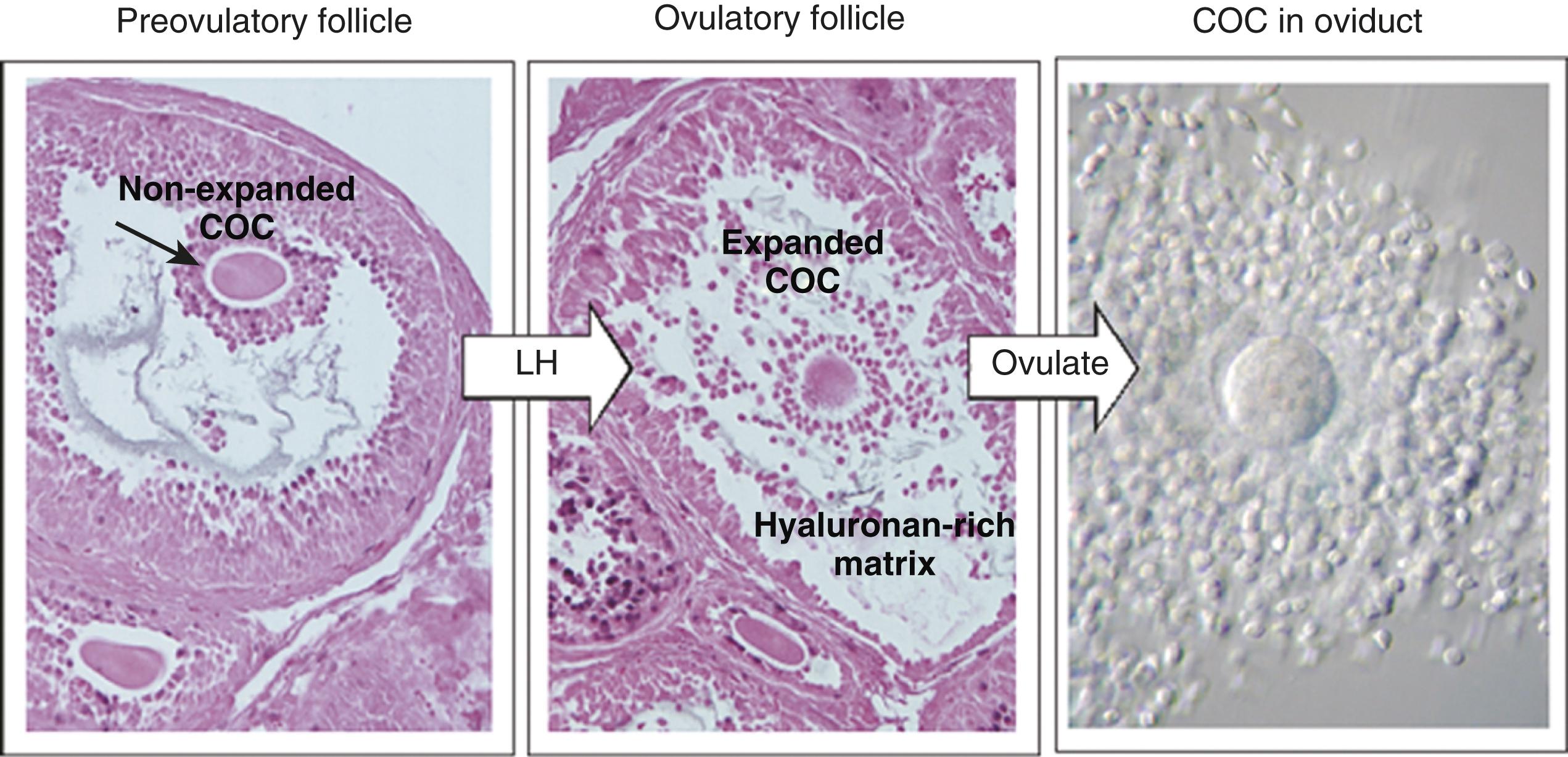
Ovulation results in the expulsion of both antral fluid and the ovum from the ovary into the peritoneal cavity. The ovum is not ovulated as a single naked cell, but as a complex consisting of (1) the ovum, (2) the zona pellucida, (3) the two- to three-cell-thick corona radiata, and (4) a sticky matrix containing surrounding cells of the cumulus oophorus. By convention, the adhering cumulus cells are designated the corona radiata after ovulation has occurred. Normally, one egg is released at ovulation. The release and fertilization of two eggs can result in fraternal twinning.
Some women experience mild to pronounced pain at the time of ovulation. Often called mittelschmerz (German for “middle pain”), this pain may accompany slight bleeding from the ruptured follicle.
The first step in egg transport is capture of the ovulated egg by the uterine tube. Shortly before ovulation, the epithelial cells of the uterine tube become more highly ciliated and smooth muscle activity in the tube and its suspensory ligament increases as the result of hormonal influences. By ovulation, the fimbriae of the uterine tube move closer to the ovary and seem to sweep rhythmically over its surface. This action, in addition to the currents set up by the cilia, efficiently captures the ovulated egg complex. Experimental studies on rabbits have shown that the bulk provided by the cellular coverings of the ovulated egg is important in facilitating the egg’s capture and transport by the uterine tube. Denuded ova or inert objects of that size are not so readily transported. Capture of the egg by the uterine tube also involves an adhesive interaction between the egg complex and the ciliary surface of the tube.
Even without these types of natural adaptations, the ability of the uterine tubes to capture eggs is remarkable. If the fimbriated end of the tube has been removed, egg capture occurs remarkably often, and pregnancies have even occurred in women who have had one ovary and the contralateral uterine tube removed. In such cases, the ovulated egg would have to travel free in the pelvic cavity for a considerable distance before entering the ostium of the uterine tube on the other side.
When inside the uterine tube, the egg is transported toward the uterus, mainly as the result of contractions of the smooth musculature of the tubal wall. Although the cilia lining the tubal mucosa also plays a role in egg transport, their action is not obligatory because women with immotile cilia syndrome are often fertile.
While in the uterine tube, the egg is bathed in tubal fluid , which is a combination of secretion by the tubal epithelial cells and transudate from capillaries just below the epithelium. Tubal fluid is a complex mixture of molecules consisting of energy substrates, prostaglandins, estrogen and progesterone, and a variety of proteins and growth factors. One important protein, oviductin (oviduct-specific glycoprotein1), plays several different roles, ranging from modifying the zona pellucida to affecting sperm transport within the uterine tube.
Tubal transport of the egg usually takes 3 to 4 days, whether or not fertilization occurs (see Figure 2.3 ). Egg transport typically occurs in two phases: slow transport in the ampulla (approximately 72 hours) and a more rapid phase (8 hours) during which the egg or embryo passes through the isthmus and into the uterus (see p. 67). By a poorly understood mechanism, possibly local edema or reduced muscular activity, the egg is temporarily prevented from entering the isthmic portion of the tube, but under the influence of progesterone, the uterotubal junction relaxes and permits entry of the ovum.
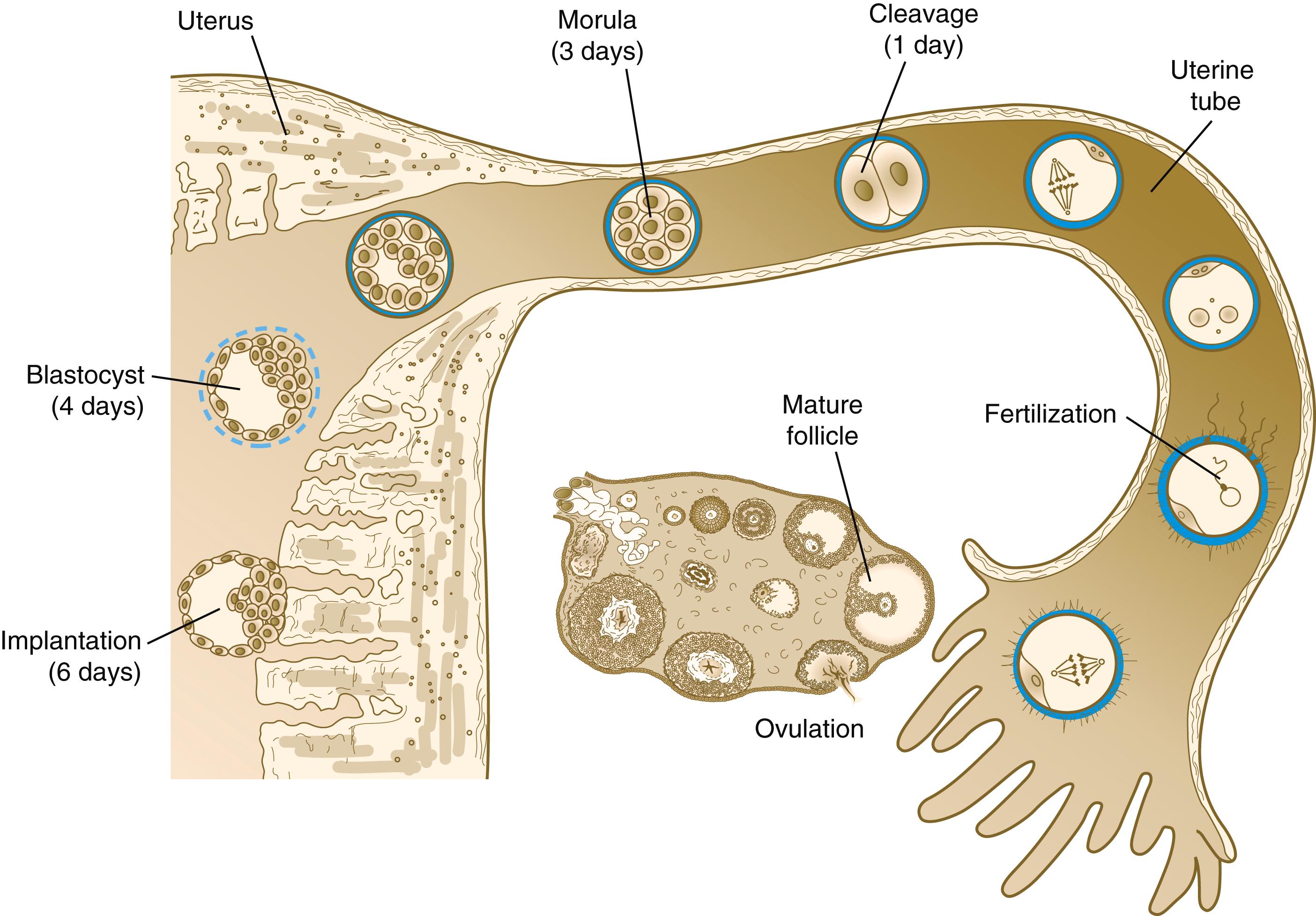
By roughly 80 hours after ovulation, the ovulated egg or embryo has passed from the uterine tube into the uterus. If fertilization has not occurred, the egg degenerates and is phagocytized. (Implantation of the embryo is discussed in Chapter 4 .)
Sperm transport occurs in both the male reproductive tract and the female reproductive tract. In the male reproductive tract, transport of spermatozoa is closely connected with their structural and functional maturation, whereas in the female reproductive tract, it is important for spermatozoa to pass to the upper uterine tube where they can meet the ovulated egg.
After spermiogenesis in the seminiferous tubules, the spermatozoa are morphologically mature but are nonmotile and incapable of fertilizing an egg ( Figure 2.4 ). Spermatozoa are passively transported via testicular fluid from the seminiferous tubules to the caput (head) of the epididymis through the rete testis and the efferent ductules. They are propelled by fluid pressure generated in the seminiferous tubules and through contractions of myoid cells that lie outside the seminiferous tubules. Their progress is then assisted by smooth muscle contractions and ciliary currents in the efferent ductules. Spermatozoa spend approximately 12 days in the highly convoluted duct of the epididymis, which measures 6 m in the human, during which they undergo biochemical maturation ( Box 2.1 ). This period of maturation is associated with changes in the glycoproteins in the plasma membrane of the sperm head. By the time the spermatozoa have reached the cauda (tail) of the epididymis, they are capable of fertilizing an egg.
Progressive increase in motility
Increased ability to fertilize ovum
Maturation of acrosome
Molecular reorganization of plasma membrane
Acquisition of receptors for proteins of zona pellucida
Increased disulfide bonding between nucleoproteins
Regionalization of surface glycosidic residues
Accumulation of mannosylated residues on the perioacrosomal plasma membrane
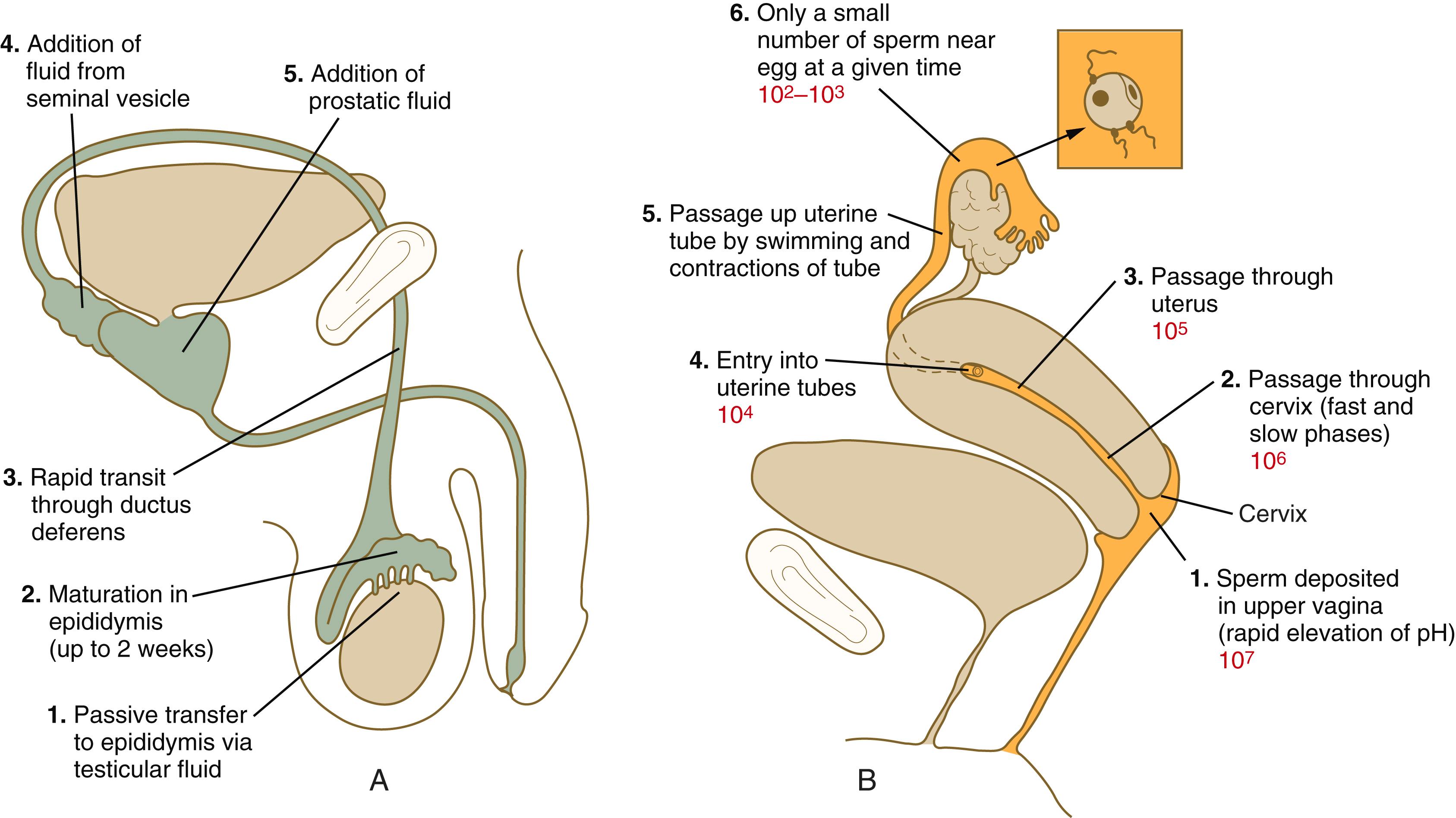
On ejaculation, the spermatozoa rapidly pass through the ductus deferens and are mixed with fluid secretions from the seminal vesicles and prostate gland . Prostatic fluid is rich in citric acid, acid phosphatase, zinc, and magnesium ions, whereas fluid of the seminal vesicle is rich in fructose (the principal energy source of spermatozoa) and prostaglandins. The 2 to 6 mL of ejaculate ( semen , or seminal fluid ) typically consists of 40 to 250 million spermatozoa mixed with alkaline fluid from the seminal vesicles (approximately 70% of the total) and acid secretion (pH, 6.5) from the prostate (approximately 20% of the total). The pH of normal semen ranges from 7.2 to 7.8. Despite the numerous spermatozoa (>100 million) normally present in an ejaculate, a number as small as 25 million spermatozoa per ejaculate may be compatible with fertility.
In the female reproductive tract, sperm transport begins in the upper vagina and ends in the ampulla of the uterine tube, where the spermatozoa make contact with the ovulated egg. During copulation, the seminal fluid is normally deposited in the upper vagina (see Figure 2.4 ), where its composition and buffering capacity immediately protect the spermatozoa from the acid fluid found in the upper vaginal area. The acidic vaginal fluid normally serves a bactericidal function in protecting the cervical canal from pathogenic organisms. After approximately 10 seconds, the pH of the upper vagina is increased from 4.3 to as much as 7.2. The buffering effect lasts only a few minutes in humans, but it is enough time for the spermatozoa to approach the cervix in an environment (pH 6.0 to 6.5) optimal for sperm motility. Within a minute after entry into the vagina, human semen coagulates into a loose gel. The coagulum protects the spermatozoa from the harsh vaginal microenvironment and may also hold the sperm close to the cervical os. A major factor in generating the seminal gel is the presence of semenogelin proteins contributed by the seminal vesicles. About 30 to 60 minutes later, the gel becomes degraded through the actions of prostate-specific antigen (PSA), which has serine protease activity and is secreted by the prostate gland.
The next barriers that the sperm cells must overcome are the cervical canal and the cervical mucus that blocks it. Swimming movements by the spermatozoa are important for the penetration of the cervical mucus.
The composition and viscosity of cervical mucus vary considerably throughout the menstrual cycle. Composed of cervical mucin (a glycoprotein with a high carbohydrate composition) and soluble components, cervical mucus is not readily penetrable. Between days 9 and 16 of the cycle, however, its water content increases, and this change facilitates the passage of sperm through the cervix around the time of ovulation; such mucus is sometimes called E mucus . After ovulation, under the influence of progesterone, the production of watery cervical mucus ceases, and a new type of sticky mucus, which has a much-decreased water content, is produced. This progestational mucus, sometimes called G mucus , is almost completely resistant to sperm penetration. A highly effective method of natural family planning makes use of the properties of cervical mucus.
Another barrier facing spermatozoa entering the cervix is an immunological one. In addition to its low pH, the vagina and also the cervix use a variety of immunological strategies to prevent pathogens from entering the upper reaches of the female reproductive tract. These include cells (mainly neutrophils and macrophages), immunoglobulins (mainly IgA and IgG), and complement proteins to protect against invaders. Although spermatozoa do stimulate some of these defenses, they are able to avoid most of them in their transit into and through the cervix. Nevertheless, after 24 hours, phagocytosis of spermatozoa seriously reduces the number of viable spermatozoa that could potentially fertilize an egg.
Some studies have suggested the presence of cervical crypts, which can potentially hold viable sperm for several days. Others have shown the presence of small cervical grooves or canals that could guide spermatozoa in their transit through the cervical canal. Linear strands of cervical mucus may also play a role in the cervical transport of spermatozoa.
There are two main modes of sperm transport through the cervix. One is a phase of initial rapid transport, by which some spermatozoa can reach the uterine tubes within 5 to 20 minutes of ejaculation. Such rapid transport relies more on muscular movements of the female reproductive tract than on the motility of the spermatozoa themselves. These early arriving sperm, however, appear not to be as capable of fertilizing an egg as do those that have spent more time in the female reproductive tract. The second, slow phase of sperm transport involves the swimming of spermatozoa through the cervical mucus (traveling at a rate of 2 to 3 mm/h), their storage in cervical crypts, and their final passage through the cervical canal as much as 2 to 4 days later.
Many questions still exist about how sperm cells traverse the uterine cavity. According to some calculations, swimming movements alone could drive spermatozoa across the uterine cavity in slightly more than 10 minutes, but contractions of the uterine musculature play an important role. Late in the follicular phase of the menstrual cycle, cranially directed waves of smooth muscle contractions in the uterine wall increase in intensity, and these can propel spermatozoa toward the uterotubal junction.
Increasing evidence exists that the ovary containing the dominant follicle that will be ovulated influences the uterine tube on that side to serve as a preferential passageway for sperm. A complex ovarian circulatory pathway provides a pathway for carrying hormones emanating from the dominant ovary to the same side of the uterus. In addition, the temperature of the uterine tube on that side has been shown to be 1.6°C higher than that of the contralateral tube. These influences result in a greater patency of the ipsilateral uterotubular junction than the one leading from the tube that will not carry the ovulated egg. According to some estimates, only several hundred spermatozoa enter the uterine tubes, and most enter the tube containing the ovulated egg.
Viable sperm use swimming to enter the uterotubal junction, but still poorly defined molecular interactions also appear to play an important role. One such molecule is ADAM3 (a disintegrin and metalloprotease 3), which is located in the plasma membrane of the sperm and interacts with the epithelial cells at the uterotubal junction. Another is binder of sperm (BSM), which is secreted by the seminal vesicles and binds to sperm and then later facilitates the binding of sperm to the oviductal epithelium.
Once inside the uterine tube, the spermatozoa collect in the isthmus and bind to glycans on the tubal epithelium for approximately 24 hours. During this time, they maintain or even increase their fertilizing capacity and are influenced by secretions of the tube to undergo the capacitation reaction. One phase of capacitation is removal of cholesterol from the surface of the sperm. Cholesterol is a component of semen and acts to inhibit premature capacitation. The next phase of capacitation consists of removal of many of the glycoproteins that were deposited on the surface of the spermatozoa during their tenure in the epididymis. Capacitation is required for spermatozoa to be able to fertilize an egg (specifically, to undergo the acrosome reaction; see p. 29). After the capacitation reaction, the spermatozoa undergo a period of hyperactivity and detach from the tubal epithelium. Hyperactivation helps the spermatozoa break free of the bonds that held them to the tubal epithelium. It also assists the sperm in penetrating isthmic mucus and the corona radiata and the zona pellucida, which surround the ovum. Only small numbers of sperm are released at a time. This may reduce the chances of polyspermy (see p.31).
On their release from the isthmus, the spermatozoa make their way up the tube through a combination of muscular movements of the tube and swimming movements. Capacitated sperm use rheotaxis (swimming against a current) as a major mechanism for ascending the uterine tube.
The simultaneous transport of an egg down and spermatozoa up the tube is currently explained on the basis of peristaltic contractions of the uterine tube muscles. These contractions subdivide the tube into compartments. Within a given compartment, the gametes are caught up in churning movements that during 1 or 2 days bring the egg and spermatozoa together. Fertilization of the egg normally occurs in the ampullary portion (upper third) of the uterine tube. Estimates suggest that spermatozoa retain their function in the female reproductive tract for approximately 80 hours.
Some spermatozoa actually pass through the fimbriated end of the uterine tube and make their way into the peritoneal cavity. This has been demonstrated clinically by ectopic pregnancies (see p. 72) in uterine tubes that have had complete blockages close to the uterus. In these cases, fertilization likely occurred through sperm passing through the contralateral patent uterine tube and into the peritoneal cavity. From there, they would have been picked up by the ampullary end of the blocked tube and carried down to the ovulated egg, where fertilization occurred.
After years of debate concerning the possibility that mammalian spermatozoa may be guided to the egg through chemical attractants, more recent research suggests that this could be the case. Mammalian spermatozoa have been found to possess taste (umami, glutamate) as well as odorant receptors of the same family as olfactory receptors in the nose, and they can respond behaviorally to chemically defined odorants. Human spermatozoa also respond to cumulus-derived progesterone and to yet undefined chemoattractants emanating from follicular fluid and cumulus cells. Human spermatozoa are also known to respond to a temperature gradient, and studies on rabbits have shown that the site of sperm storage in the oviduct is cooler than that farther up the tube where fertilization occurs. It seems that only capacitated spermatozoa have the capability of responding to chemical or thermal stimuli. Because many of the sperm cells that enter the uterine tube fail to become capacitated, these spermatozoa are less likely to find their way to the egg. Spermatozoa that remain in the female reproductive tract are phagocytized by macrophages that enter the lumen of the reproductive tract through the epithelium. See Clinical Correlation 2.1 for contraception strategies.
Over the years, many methods have been developed for preventing conception. To date, almost all have been designed for women. Other than sterilization and the use of condoms, male-oriented contraceptive techniques have proved to be more difficult to develop. A variety of strategies have been used to prevent conception.
One long-used strategy depends upon preventing access between sperm and egg. Permanent examples of this strategy are sterilization effected by interrupting the channel of the vas deferens (vasectomy) or the uterine tube (tubal ligation). Reversal is possible, but difficult, and the results are not consistently positive.
Temporary strategies in this category consist of male and female condoms or the use of diaphragms or cervical caps in women. The latter two techniques involve placing barriers over the cervical opening. To function properly, they must be fitted by a physician. A similar technique is the birth control sponge. This device is also placed across the cervix, but in addition to providing a mechanical barrier, the sponge contains a spermicide designed to kill any sperm that might penetrate the barrier. All of these techniques have significant failure rates.
A final strategy in this category of birth control involves behavior. One method (coitus interruptus) consists of removing the penis from the vagina before ejaculation. The other (rhythm method) makes use of physiological indicators, e.g., basal body temperature, to determine the time of ovulation and to abstain from intercourse during the critical period. These are among the least successful methods of contraception.
Hormonal means of contraception have centered primarily on women, with the focus being the prevention of ovulation. Despite considerable research on male contraception, progress has been limited, although some clinical trials of potential male contraceptives are in progress.
The first widely used strategy in hormonal contraception in females was the contraceptive pill, which debuted in the 1960s. The general principle of “The Pill” was to increase blood levels of female reproductive hormones (first estrogens plus progesterone and later progestins only) on a graded daily basis to reduce or prevent the release of gonadotropic hormones from the anterior pituitary. This interferes with follicular development and ovulation and also affects the consistency of cervical mucus. Subsequent developments in the technology have used patches, implants, or injections to accomplish the same purpose. Used properly, these methods are highly effective. Intramuscular injections of progesterone compounds can be effective for up to 3 months, and subdermal implants of synthetic progestins produce effective but reversible contraceptive protection for up to several years.
A variety of emergency contraceptives are designed to be taken after intercourse. Some, such as Plan B, make use of high doses of hormones to prevent ovulation or to antagonize the effects of progesterone. Another, RU 486 (mifepristone), may prevent implantation or, if used later in pregnancy, cause an abortion by inducing shedding of the uterine lining.
Normal lactation reduces fertility through the normal hormonal environment found in a lactating woman, which inhibits ovulation. Some women prolong the period of lactation to reduce their chances of becoming pregnant in the early months after childbirth.
For over a century, a variety of different devices have been inserted into the uterus or cervix in attempts to interfere with fertilization. After many false starts and issues with infection or puncture of the uterus, intrauterine devices (IUDs) are now a standard part of the contraceptive repertoire. How they function is not always certain, but those containing copper elements are likely effective because of the spermicidal effects of copper ions. Other types of IUDs contain progestins and act through hormonal means. One advantage of IUDs is that upon removal of the devices fertility is rapidly restored.
While the ovulated egg is passing through the uterine tubes, the ruptured follicle from which it arose undergoes a series of striking changes that are essential for the progression of events leading to and supporting pregnancy (see Figure 1.8 ). Soon after ovulation, the basement membrane that separates the granulosa cells from the theca interna breaks down, thus allowing thecal blood vessels to grow into the cavity of the ruptured follicle. The granulosa cells simultaneously undergo a series of major changes in form and function ( luteinization ). Within 30 to 40 hours of the LH surge, these cells, now called granulosa lutein cells , begin secreting increasing amounts of progesterone along with some estrogen. This pattern of secretion provides the hormonal basis for the changes in the female reproductive tissues during the last half of the menstrual cycle. During this period, the follicle continues to enlarge. Because of its yellow color, it is known as the corpus luteum . The granulosa lutein cells are terminally differentiated. They have stopped dividing, but they continue to secrete progesterone for 10 days.
In the absence of fertilization and a hormonal stimulus provided by the early embryo, the corpus luteum begins to deteriorate ( luteolysis ) late in the menstrual cycle. Luteolysis seems to involve both the preprogramming of the luteal cells to apoptosis (cell death) and uterine luteolytic factors , such as prostaglandin F2 . Regression of the corpus luteum and the accompanying reduction in progesterone production cause the hormonal withdrawal that results in the degenerative changes of the endometrial tissue during the last days of the menstrual cycle.
During the regression of the corpus luteum, the granulosa lutein cells degenerate and are replaced with collagenous scar tissue. Because of its white color, the former corpus luteum now becomes known as the corpus albicans (“white body”).
If fertilization occurs, production of the protein hormone chorionic gonadotropin by the future placental tissues maintains the corpus luteum in a functional condition and causes an increase in its size and hormone production. Because the granulosa lutein cells are unable to divide and cease producing progesterone after 10 days, the large corpus luteum of pregnancy is composed principally of theca lutein cells. The corpus luteum of pregnancy remains functional for the first few months of pregnancy. After the second month, the placenta produces enough estrogens and progesterone to maintain pregnancy on its own. At this point, the ovaries can be removed, and pregnancy would continue.
Fertilization is a series of processes rather than a single event. Viewed in the broadest sense, these processes begin when spermatozoa start to penetrate the corona radiata that surrounds the egg and end with the intermingling of the maternal and paternal chromosomes after the spermatozoon has entered the egg.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here