Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
adult-to-adult living donor liver transplantation study cohort
Child-Turcotte-Pugh
donation after cardiac death
extended criteria donors
endoscopic retrograde cholangiopancreatography
graft-to-recipient body weight ratio
graft volume to standard weight volume
highly active antiretroviral therapy
hepatitis C virus
human immunodeficiency virus
inferior vena cava
Model for End-Stage Liver Disease
organ procurement organization
pediatric end-stage liver disease
percutaneous transhepatic cholangiogram
primary nonfunction
portal vein thrombosis
hepatic artery thrombosis
small for size syndrome
United Network of Organ Sharing
veno-veno bypass
“Liver, brain, and heart, these sovereign thrones …” (WILLIAM SHAKESPEARE, TWELFTH NIGHT , ACT 1, SCENE 1)
The history of modern liver transplantation began in 1955, in the laboratories of Stuart Welch at Albany Medical College and Jack Cannon at the University of California, Los Angeles. Welch was the first to demonstrate the technique of auxiliary liver transplantation, experiments that he performed in dogs in which the native liver was left undisturbed and the transplanted liver was placed in a heterotopic position. It was Cannon who first demonstrated the technique of orthotopic liver transplantation in which the native liver was removed and a graft put in its place. Unfortunately, none of the dogs survived. Finally, in 1958, Francis Moore in Boston and Thomas Starzl in Denver demonstrated technical success, achieving recipient survival in canine models of orthotopic liver transplantation. However, the success was short-lived, as rejection led to graft and recipient demise within the 1st weeks after transplantation. After the technical success in canine models, Starzl attempted the first liver transplant in a human in 1963. The recipient was a 3-year-old with biliary atresia who succumbed in the operating room due to hemorrhage prior to the completion of the transplant. Throughout the ensuing year there would be six more attempts at liver transplantation in Denver, Paris, and Boston, all of which resulted in recipient mortality within 23 days of transplantation. Given the dismal results, a moratorium was imposed in 1964, which lasted for just over 3 years. In 1967, Starzl performed the first successful liver transplantation in an 18-month-old child with hepatoblastoma. She survived for 400 days before succumbing to disseminated malignancy. Although this initial success spawned more clinical activity, the 1-year survival following liver transplantation remained less than 50%, as two major hurdles stood in the way of successful liver transplantation: rejection and optimization of organ preservation.
Initial efforts at organ preservation were simplistic, with the use of chilled normal saline or lactated ringers which achieved organ preservation for a maximum of 6 hours. Gradually, it was realized that preservation solutions should contain an impermeant and/or a colloid to prevent cellular edema, strong buffering capacity to combat acidosis, an electrolyte composition to simulate either the intracellular or the extracellular milieu, and, finally, antioxidants to scavenge free radicals. In 1987, University of Wisconsin solution emerged as the leading preservation solution and allowed for cold static liver preservation for up to 18 to 24 hours.
Concomitant with efforts to optimize organ preservation were efforts to understand transplant rejection. In 1944, Peter Medawar demonstrated that allograft rejection was an immune-mediated phenomenon. More than a decade later, Sir Roy Calne demonstrated that 6-mercaptopurine and, subsequently, azathioprine, prolonged survival of kidney allografts. In 1967, the concept of induction immunosuppression—administration of an agent for a short course at the time of transplantation—emerged with the introduction of antilymphocyte globulin used in conjunction with maintenance therapy consisting of azathioprine and prednisone. This comprised the first triple drug immunosuppression regimen. It was, however, the discovery of cyclosporine in 1969 by Jean-Francois Borel that truly revolutionized organ transplantation. Derived from a fungus sample cultured from soil, it was first used by Sir Roy Calne in rodent models of heart transplantation with success. Cyclosporine was first used in humans in 1978, and Food and Drug Administration approval came in 1983. Cyclosporine achieved 1-year liver transplant survival rates of 70% and was rapidly accepted as the gold standard of maintenance immunosuppression. Six years later, tacrolimus, isolated from the culture broth of a soil sample from the Tsukuba area of Northern Japan, was introduced. Its superiority to cyclosporine was quickly demonstrated such that it supplanted cyclosporine as the mainstay of maintenance regimens, a position it retains to this day.
Transplantation has become the procedure of choice for a wide range of liver diseases in both adult and pediatric patients. These conditions range from acute or chronic liver disease, to metabolic/congenital conditions, and hepatic malignancy ( Table 51-1 ). Despite the varying etiologies of these diseases, the onset of decompensated liver disease is often the common final pathway that leads to liver transplantation.
| Acute Liver Injury |
|
| Chronic Liver Injury |
|
| Mass-Occupying Lesions |
|
| Metabolic Diseases |
|
| Graft Failure |
|
Table 51-2 demonstrates the characteristics for adult recipients from 2004 through 2013. Among children, cholestatic liver disease remains the most common indication, accounting for nearly half of liver transplants in 2013 (45.6%). In contrast, for adults, noncholestatic liver disease accounts for the largest proportion of liver transplants, dominated by hepatitis C cirrhosis, the most common indication for liver transplantation (29.4% in 2013). It is anticipated that over the next 1 to 2 decades hepatitis C virus (HCV) will substantially diminish, whereas nonalcoholic steatohepatitis will correspondingly escalate as an indication for liver transplantation. Indeed, the recent emergence and rapid proliferation of multiple HCV direct-acting antiviral agents, typically used in combinatorial regimens that are well-tolerated and highly efficacious, will undoubtedly exert a profound effect. Treatment and cure of chronic hepatitis C should prevent the development of cirrhosis, decompensation, and/or hepatocellular carcinoma, thereby reducing the need for transplantation. Similarly, treatment and cure of recurrent hepatitis C after liver transplantation should dramatically improve posttransplant outcomes, thereby reducing the need for retransplantation.
| Characteristic | 2003 | 2013 | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age | 18-34 | 337 | 6.6 | 335 | 5.7 |
| 35-49 | 1579 | 30.8 | 976 | 16.5 | |
| 50-64 | 2739 | 53.4 | 3644 | 61.5 | |
| 65+ | 472 | 9.2 | 966 | 16.3 | |
| Sex | Female | 1749 | 34.1 | 2020 | 34.1 |
| Male | 3378 | 65.9 | 3901 | 65.9 | |
| Race | White | 3806 | 74.2 | 4187 | 70.7 |
| Black | 439 | 8.6 | 604 | 10.2 | |
| Hispanic | 622 | 12.1 | 809 | 13.7 | |
| Asian | 212 | 4.1 | 267 | 4.5 | |
| Other/unknown | 48 | 0.9 | 54 | 0.9 | |
| Primary diagnosis | Acute hepatic necrosis | 303 | 5.9 | 233 | 3.9 |
| HCV | 1531 | 29.9 | 1482 | 25.0 | |
| Alcoholic liver disease | 901 | 17.6 | 1088 | 18.4 | |
| Cholestatic disease | 568 | 11.1 | 494 | 8.3 | |
| Malignancy | 408 | 8.0 | 1150 | 19.4 | |
| Other/unknown | 1416 | 27.6 | 1474 | 24.9 | |
| BMI (kg/m 2 ) | <18.5 | 111 | 2.2 | 126 | 2.1 |
| 18.5-25 | 1619 | 31.6 | 1678 | 28.3 | |
| 25-30 | 1842 | 35.9 | 2068 | 34.9 | |
| 30-35 | 1000 | 19.5 | 1279 | 21.6 | |
| 35+ | 536 | 10.5 | 770 | 13.0 | |
| Unknown | 19 | 0.4 | 0 | 0 | |
| Waiting time | <31 days | 1757 | 34.3 | 1777 | 30.0 |
| 31-60 days | 616 | 12.0 | 595 | 10.0 | |
| 61-90 days | 368 | 7.2 | 396 | 6.7 | |
| 3-6 months | 675 | 13.2 | 969 | 16.4 | |
| 6-12 months | 584 | 11.4 | 930 | 15.7 | |
| 1-2 years | 539 | 10.5 | 771 | 13.0 | |
| 2-3 years | 279 | 5.4 | 208 | 3.5 | |
| 3+ years | 303 | 5.9 | 273 | 4.6 | |
| Unknown | 6 | 0.1 | 2 | 0.0 | |
| Medical urgency | Status 1/1A | 306 | 6.0 | 196 | 3.3 |
| MELD 35+ | 476 | 9.3 | 1357 | 22.9 | |
| MELD 30-34 | 527 | 10.3 | 894 | 15.1 | |
| MELD 15-29 | 3026 | 59.0 | 3303 | 55.8 | |
| MELD <15 | 780 | 15.2 | 168 | 2.8 | |
| Other/unknown | 12 | 0.2 | 3 | 0.1 | |
| Procedure type | Whole liver | 4788 | 93.4 | 5645 | 95.3 |
| Partial liver | 251 | 4.9 | 204 | 3.4 | |
| Split liver | 88 | 1.7 | 72 | 1.2 | |
| Multiorgan transplant | Liver only | 4871 | 95.0 | 5390 | 91.0 |
| Liver-kidney | 235 | 4.6 | 477 | 8.1 | |
| Other | 21 | 0.4 | 54 | 0.9 | |
| Donor type | Deceased | 4873 | 95.0 | 5710 | 96.4 |
| Living | 254 | 5.0 | 211 | 3.6 | |
| Diabetes | 1013 | 19.8 | 1494 | 25.2 | |
| Portal vein thrombosis | 181 | 3.5 | 592 | 10.0 | |
| All Recipients | 5127 | 100.0 | 5921 | 100.0 | |
Table 51-3 lists the absolute and relative contraindications to liver transplantation. Among the relative contraindications to liver transplantation, special note should be made of human immunodeficiency virus (HIV) infection. The advent of highly active antiretroviral therapy (HAART) has dramatically improved the prognosis of HIV infection. Common criteria for HIV-positive patients to qualify for liver transplantation include a viable plan for viral suppression after transplant, particularly for those unable to tolerate HAART prior to transplant, a CD4+ T cell count of more than 100 cells/µL for 6 months, and of the absence of opportunistic infections and HIV-related neoplasms. In a prospective series, 1-year and 3-year patient survival were 91% and 64%, respectively; 1-year and 3-year liver graft survival were 82% and 64%, respectively. Although equivalent posttransplant survival has been demonstrated between hepatitis B virus (HBV) monoinfected and HBV/HIV coinfected patients, posttransplant survival and graft survival rates are lower in HCV/HIV coinfected patients compared with the HCV monoinfected population. Advances in HCV antiviral therapy, combined with the pretransplant identification of the subset of HCV/HIV coinfected candidates with a favorable posttransplant prognosis, may improve results in this distinctly difficult population. In addition, with reported success transplanting kidneys from HIV-positive deceased donors into HIV-positive recipients in South Africa, and recent passage of the HIV Organ Policy Equity (HOPE) Act allowing for the eventual use of HIV-positive donors in the United States, the HIV-positive candidate on a waitlist may soon have expedited avenues to deceased donors, thus allowing for transplantation at an earlier point in their disease process with the potential to further improve pretransplant and posttransplant outcomes.
| Absolute |
|
| Relative |
|
Obesity is another relative contraindication that deserves special mention, particularly as its prevalence is steeply increasing in the United States. Many transplant centers have body mass index (BMI) limitations to qualify for transplantation, although there is certainly no uniformity in practice. Obese recipients pose a substantial technical challenge as reflected by the increased frequency of postoperative complications. Although a previous report suggested that severe obesity (BMI >40 kg/m 2 ) was a significant predictor of death after liver transplantation, another study failed to identify any association between a corrected BMI (one that accounts for the extent of ascites) and either patient or graft survival. One proposed approach to address pretransplant obesity has been the utilization of laparoscopic bariatric surgery either prior to or during liver transplantation. Bariatric surgery prior to transplantation has been reported not only to be safe and well tolerated, but effective to facilitate weight loss and thereby improve transplant candidacy by facilitating weight loss. Moreover, for patients with a BMI greater than 35, laparoscopic sleeve gastrectomy has been safely performed at the time of transplantation, resulting in effective weight reduction and mitigation of posttransplant metabolic complications such as diabetes mellitus and allograft steatosis, compared with those obese recipients who underwent liver transplantation alone. Although these results are promising, especially in the context of an expanding population of obese candidates on waitlists, larger studies will be needed to assess the safety, feasibility, and efficacy of bariatric surgery either prior to or during transplantation for morbidly obese patients with cirrhosis.
Patients with end-stage liver disease (ELD) who are referred for liver transplantation undergo an extensive evaluation either as an outpatient or as an inpatient. This topic is reviewed in great detail in Chapter 50 .
In 1964 C. Garner Child and Jeremiah Turcotte of the University of Michigan proposed a scoring system that stratified the severity of end-stage liver disease. Their scoring system, the first of its kind, relied on five clinical parameters: total bilirubin, serum albumin, nutritional status, extent of ascites, and degree of hepatic encephalopathy. The Child-Turcotte scoring system was developed to preoperatively standardize the severity of chronic liver disease using a reliable set of clinical criteria, which allowed for the prediction of postoperative outcome. The scoring system was modified by Pugh in 1972, to allow for consideration of the bleeding tendency in patients with portal hypertension. The Child-Turcotte-Pugh (CTP) score replaced the criterion of nutritional status with prothrombin time. Lacking any other standardized method, the CTP score, combined with waiting time on the transplant list, was used to stratify liver transplant candidates for organ allocation. However, the CTP score required the subjective assessment of ascites and severity of encephalopathy. Dissatisfaction with the subjective components of the CTP score helped prompt the development of a completely objective scoring system for organ allocation.
The Model for End-Stage Liver Disease (MELD) score was originally developed to predict 3-month survival in patients undergoing transjugular intrahepatic portosystemic shunt. Based upon the patient's total serum bilirubin, serum creatinine, and the international normalized ratio (INR), the MELD score did not rely on the subjective assessment of ascites and encephalopathy severity inherent to the CTP score. The MELD score also proved to be superior to the CTP score in predicting transplant waitlist survival. In February 2002 the United Network of Organ Sharing (UNOS) adopted the MELD allocation system, and in doing so gave priority to the sickest patients based upon objective parameters, deemphasizing the importance of waitlist time. In 2008, Kim et al. demonstrated that a composite measure of serum sodium concentration and MELD, MELD-Na, was superior to MELD alone to predict waitlist mortality. MELD-Na allocation, approved in 2014 and implemented nationally in 2016, is expected to alter the score of approximately one third of the candidates on a waitlist. It should be noted that patients under the age of 12 years are prioritized on the waitlist according to the Pediatric End-Stage Liver Disease (PELD) score which is based on four clinical parameters: total bilirubin, INR, albumin, and extent of growth failure.
Although the MELD score has facilitated objective prioritization of candidates on a waitlist, geographic disparities in access to deceased donor livers exist because organ allocation and distribution for candidates with chronic liver disease predominantly occurs at the local level, followed by the regional and then national levels. The inequity may be best evidenced by the significant regional differences in the median MELD at which candidates achieve transplantation. Transplant centers in regions characterized by high, compared with low, median MELD scores exhibit more aggressive deceased donor organ utilization practices and more vigorous living donor transplant programs as strategies to meet need. Moreover, subsets of candidates, typically those with financial means, relocate to regions of relatively high organ supply to achieve transplantation.
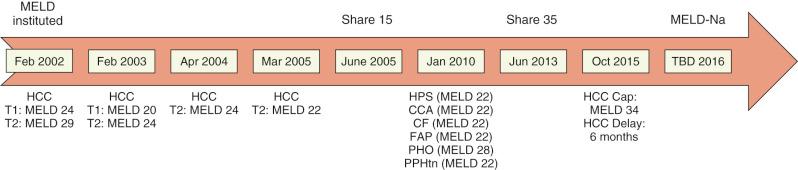
Recently, the U.S. Health Resources and Services Administration charged the United Network for Organ Sharing (UNOS) to reconsider MELD allocation with the specific aim of mitigating geographic disparities in access to deceased donor livers. As a first step, in June 2013, “Share 35” allocation was implemented. Whereas prior to Share 35 implementation local candidates retained priority for liver grafts with the exception of Status 1 listed individuals, the new policy stipulates that liver grafts are first allocated to regional candidates with MELD scores greater than or equal to 35, ahead of local candidates with MELD less than 35. Analysis of the impact of Share 35, comparing the year immediately preceding to the year immediately following, has shown an increase in the overall number of transplants performed, increased frequency of regional sharing, increased distance of organ travel, no change in cold ischemia time, reduction in waitlist mortality, and no change in posttransplant outcomes. Currently, the UNOS Liver and Intestine Transplant Committee is considering more significant policy revisions by newly defining liver distribution units through mathematical modeling and optimization to further reduce waitlist mortality and geographic disparity. Fig. 51-1 documents the major changes in MELD allocation policies since its inception in 2002.
Early results for the treatment of hepatocellular cancer (HCC) by liver transplantation were unfavorable, consisting of poor survival rates and a high incidence of posttransplantation recurrence. In 1996, Mazzaferro et al. reported results that have led to the codification of the “Milan criteria”, thereby defining the subset of patients with unresectable HCC for whom liver transplantation is the appropriate treatment. Transplantation within the Milan criteria (single tumor less than or equal to 5 cm in diameter, or two to three tumors less than or equal to 3 cm in diameter) resulted in overall and recurrence-free survival rates of 85% and 92%, respectively, at 4 years. Therefore, HCC with tumor burden within the Milan criteria gained wide acceptance as a legitimate indication for liver transplantation.
As patients with HCC often have preserved hepatic function, their calculated MELD score predicted a low risk of death from their liver disease alone. To provide HCC candidates access to liver transplantation prior to HCC progression beyond the Milan criteria, MELD exception points were awarded to this subset of liver transplant candidates. Under the initial HCC-adjusted MELD scheme of organ allocation, patients with Stage 1 HCC (one lesion <2 cm) were given a MELD score of 24, corresponding to an expected 15% 3-month dropout rate due to tumor progression beyond the Milan criteria. Those with Stage 2 HCC (one lesion 2 to 5 cm, two to three nodules all ≤3 cm) were assigned a MELD score of 29, which reflected an expected 30% 3-month dropout rate. For every 3 months on the waiting list, HCC candidates who remained within the Milan criteria were awarded additional exception points equivalent to a 10% increase in mortality risk. However, soon after its implementation, there was evidence that HCC was overvalued in this original MELD allocation, prompting two subsequent adjustments in the attribution of MELD points for HCC patients in April 2003 and again in January 2004. Stage 1 HCC candidates no longer received any MELD exception points, and Stage 2 HCC candidates were awarded a MELD score of 22.
Despite these downward adjustments in HCC exception points, mounting evidence showed that HCC candidates, compared with the non-HCC candidates, enjoyed a substantial transplant advantage irrespective of geographic location. Indeed, half of the HCC candidates on the national waitlist achieved liver transplant at a MELD score of 22, within 3 months of listing. To address the disparity in access to deceased donor livers between HCC and non-HCC candidates, the following two strategies were implemented in October 2015: (a) “HCC Delay”—instead of being listed with a MELD score of 22, candidates with HCC exception applications are registered with their calculated MELD score for the first 6 months. After this 6-month waiting period, candidates who continue to meet HCC listing criteria are awarded 28 exception points. (b) “HCC Cap”—HCC exception scores are capped at a MELD of 34 in light of the current Share 35 allocation policy.
Simultaneous to considering mechanisms to devalue HCC within the MELD economy, there are discussions to consider expansion of tumor criteria acceptable for transplantation beyond the current limits of the Milan criteria. In 2001, Yao et al. at UCSF proposed expansion of the Milan criteria for HCC liver transplant candidates. Termed the UCSF criteria, these patients with HCC had a single tumor less than or equal to 6.5 cm in diameter, or two to three tumors none exceeding 4.5 cm in diameter and whose sum of tumor diameters did not exceed 8 cm. This cohort that underwent liver transplantation following downstaging with locoregional therapy to within Milan Criteria achieved 1- and 5-year survival rates of 90% and 75%, respectively, thus demonstrating equivalent rates of long-term survival when compared with candidates presenting within the Milan criteria.
Indeed, with the success of HCC downstaging protocols, and continued advances in locoregional therapy, the upper boundaries of HCC tumor burden suitable for liver transplantation have come into question. Aside from gross evidence of macrovascular invasion, solely relying on tumor number/size has proved to be insufficient in a “one size fits all” system of granting HCC exception points. Consideration of AFP levels in conjunction with tumor size/volume, better identify HCC candidates best suited for transplant by excluding those candidates at highest risk for posttransplant recurrence. Data also suggests that waiting time serves as an effective, if not archaic, marker for tumor biology. HCC recipients emanating from UNOS regions with relatively longer waitlist times, compared with those with shorter waitlist times, have demonstrated improved long term posttransplant survival and a reduced risk for posttransplant HCC recurrence. Finally, an emerging theme in the literature is the prognostic utility of a candidate's individual response to locoregional therapy. Although traditionally utilized to reduce tumor burden with the aim of preventing waitlist dropout, achieving transplant eligibility (downstaging of patients who are outside of Milan criteria to within Milan criteria), and/or reducing posttransplant HCC recurrence, there is growing evidence that the tumor response to locoregional therapy—much like that of time on the waitlist—may allow for enhanced identification of candidates ideally suited for liver transplantation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here