Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An understanding of the efficacy and side-effect profile of prescription and nonprescription topical therapies is important for the successful management of the patient with a nonneoplastic condition of the external ear.
Debridement under binocular microscopy (or otoendoscopy) in the otolaryngology office is both diagnostic and therapeutic for common conditions involving the external ear.
Most ototopical therapies are safe for the management of uncomplicated infections of the outer ear, provided that the tympanic membrane is intact.
Aminoglycosides and acidifying agents should be avoided when a tympanic membrane perforation is present because of the potential risk of ototoxicity.
An emphasis on meticulous ear hygiene and avoidance of self-manipulation is crucial to avoid recurrent or chronic infections.
Systemic therapy should be considered in severe or chronic infections, or in patients who are immunocompromised.
Persistent infections should be cultured, and suspicious or diseased tissue should be biopsied to exclude the possibility of a malignancy.
Dermatologic and rheumatologic evaluations should be considered in refractory or bilateral cases of eczematoid otitis externa.
Topical therapies for external ear disorders are among the most commonly prescribed medications for pediatric and adult patients who present to an otolaryngology office. The local delivery of antibiotic and antiinflammatory medications to the external ear has several advantages over systemic therapies, including (1) ease of patient use, (2) increased concentration of drug levels in the affected region, (3) decreased systemic side effects, and (4) lower cost. The use of ototopical antibiotics in particular avoids the selection of resistant organisms in the gastrointestinal and respiratory tracts that can be seen with systemic therapy. Disadvantages include the difficulty of using topical medications in an edematous or occluded external auditory canal, hypersensitivity or allergic reactions, and a theoretical risk of injury to the inner ear when the tympanic membrane is perforated. The following chapter will provide an evidence-based discussion of topical therapies for external ear disorders based on disease categories. These categories include bacterial otitis externa (OE), fungal OE, myringitis, eczematoid OE, viral infections of the external ear, cerumen impaction, and the chronic draining ear.
Topical therapies have been used to manage external ear disorders for thousands of years. According to Myer, mixtures of red lead, tree resin, and olive oil as well as frankincense, goose grease, cream from cow's milk, crushed soda, vermilion, cumin, ass ear, hatet oil, and olive oil were used to treat chronic draining ears as early as 3500 years ago. A process known as ear candling dates back over 1000 years and was originally adopted as a method of removing cerumen from the canal. In this process, a hollow candle is lit on one end, creating a vacuum at the other end that, when inserted into the ear, draws out debris from the canal. Contemporary studies have shown that ear candling is largely ineffective. In some cases, this technique deposits hot candle wax into the canal, resulting in burns. In the 1800s, “rattlesnake oil” (turpentine, camphor, menthol, and sassafras) was marketed as a cure for otorrhea. By the early 1900s, astringents and alcohols were introduced. The acidity and high alcohol concentration made these preparations somewhat effective if given early in the course of an infection; however, they had no targeted antimicrobial activity. Only during the mid-20th century were topical antibiotics and antifungals developed to treat the most common pathogens associated with OE and media. In particular, ototopical aminoglycosides have been available for three decades and are still prescribed frequently. Newer fluoroquinolone agents are gaining in popularity as studies have shown less ototoxicity associated with their use.
The external auditory canal is lined with epithelium, providing a natural barrier against the environment. The successful delivery of topical medications requires penetration across this barrier, and the stratum corneum (the most superficial layer of the epidermis) provides the greatest resistance to the permeation of drugs. Passive diffusion allows for most of the transport across the stratum corneum, and the degree of absorption is related to the properties of the agent being used. Two routes of passive diffusion across the stratum corneum have been proposed: transappendageal and epidermal. As only 0.1% of skin area is composed of appendages such as hair follicles, sebaceous glands, etc., the epidermal route is felt to be the more important route of drug permeation. Topical medications can diffuse through the epidermis transcellularly or intercellularly. Transcellular transport occursby hydrophilic drugs, whereas intercellular diffusion occurs with lipophilic compounds. Clearly, the agent or vehicle used in a particular ototopical agent will affect its absorption and diffusion across the epidermis.
Extremely high local concentrations (3000 mcg/mL for a 0.3% otic solution) of ototopical antibiotics can be achieved in the external canal. The minimum inhibitory concentrations (MICs) for antibiotics against common pathogens associated with external ear infections are typically less than 100, but can be in the mid-200s for resistant Pseudomonas. The local concentrations associated with ototopicals are significantly higher than the MICs for all pathogens that have been isolated from external ear infections, including resistant organisms. In contrast, the local concentration of antibiotics in the middle ear following oral administration of amoxicillin, erythromycin, azithromycin, or cefixime (given in standard adult doses) ranges from 1 to 15 mcg/mL (although it can be as high as 35 mcg/mL with IV ceftriaxone) and may not be over the MICs for the offending organisms. These levels were observed as early as 4 hours after oral administration in human subjects and were measured in middle ear fluid samples obtained using tympanocentesis. There are no studies that examine the concentrations of antibiotics given systemically in the external ear; however, it is unlikely that they would be vastly different from the concentrations observed in the middle ear.
The most commonly used prescription antiinflammatories for external ear conditions also include an antibiotic or antifungal agent, although occasionally an otolaryngologist will prescribe a topical steroid alone for chronic pruritus or eczema. Most of the current understanding of how the steroid component of these preparations is absorbed regionally through topical application can be found in the dermatologic literature. The efficacy of a particular steroid preparation is associated with the inherent potency of the compound as well as its ability to penetrate the epidermis. Lipophilic (nonpolar) preparations are transported across the stratum corneum more effectively than hydrophilic (polar) molecules. The stratum corneum can also act as a reservoir for topical steroids, allowing for a prolonged regional effect after therapy is stopped. Vehicles such as ethanol or propylene glycol increase the solubility of a topical agent to enhance permeability. The hydration of the stratum corneum can also be enhanced by using occlusive vehicles such as ointments, which also improve drug penetration.
Acute otitis externa (AOE), or “swimmer's ear” is a common disorder affecting all age groups and is a cause of significant pain and morbidity. The disease burden of AOE is significant, accounting for 2.4 million U.S. health visits (8.1 visits per 1000 population) and $0.5 billion in annual spending (MMWR, 2011). AOE is an infection of the sensitive external auditory canal skin that often arises from prolonged moisture secondary to swimming or bathing. When the skin becomes macerated from water exposure it is susceptible to bacterial invasion, which causes edema and inflammation. Copious purulent debris develops and can become trapped in the canal if the edema is significant ( Fig. 139.1 ). Additionally, trauma to the canal skin (i.e., instrumentation with cotton tip applicators) also increases the risk of AOE. Approximately 90% of AOE cases are bacterial, whereas only 10% are fungal. The most common bacterial pathogen is Pseudomonas aeruginosa , followed by Staphylococcus aureus, gram negative bacteria and anaerobes.
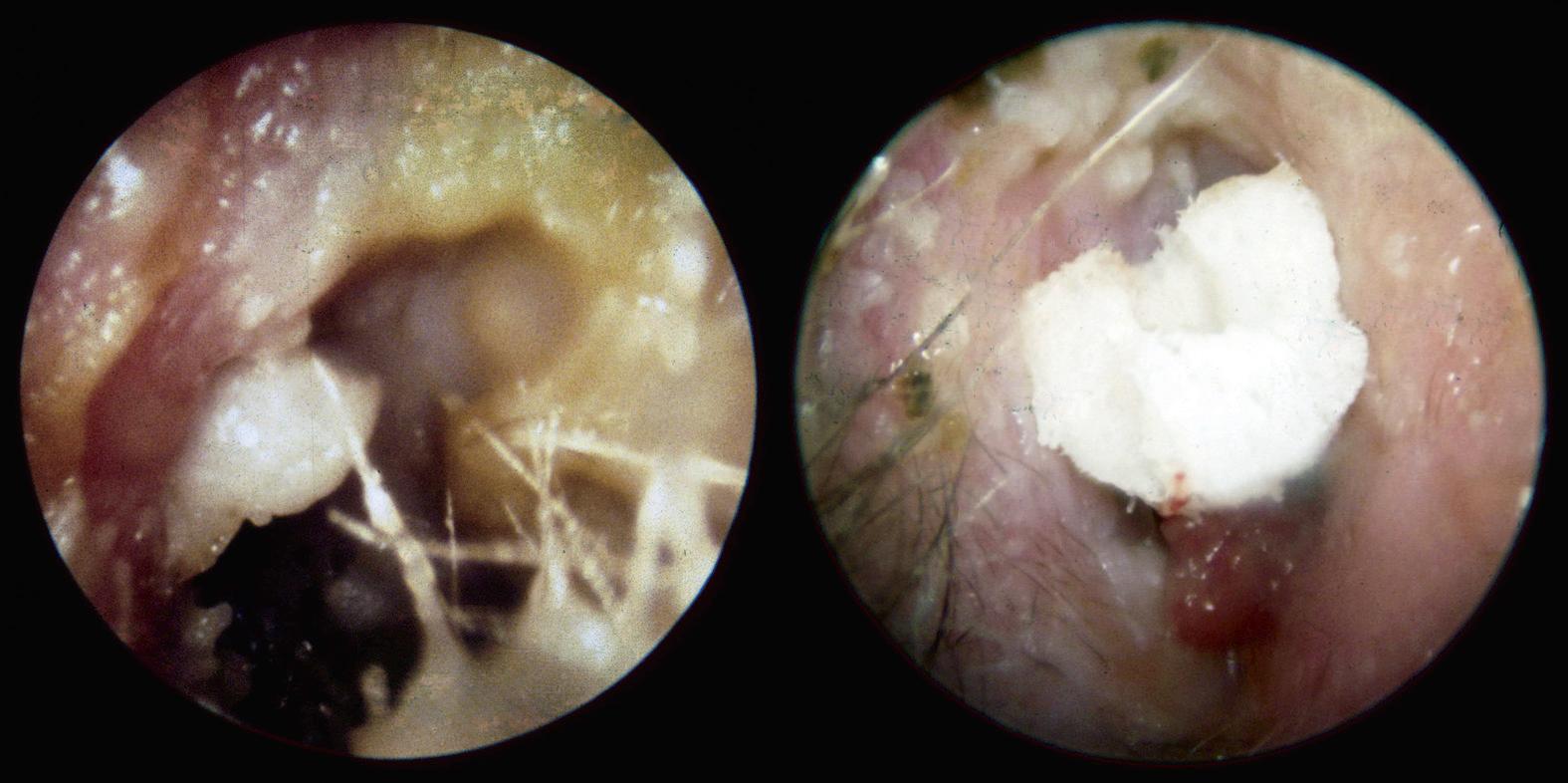
The diagnosis of diffuse AOE involves rapid onset (generally within 48 hours) in the past 3 weeks of symptoms and signs involving ear canal inflammation. Patients will present with otalgia, itching, and/or aural fullness. The physical exam will reveal pain to palpation of the tragus or pinna, edematous and erythematous canal skin, and purulent debris (see Fig. 139.1 ). The tympanic membrane is often difficult to visualize in AOE; thus, a secondary perforation cannot be excluded until after debridement or improvement of edema. Tuning forks will usually lateralize to the affected ear, even in very mild infections, and an audiogram, if performed during the acute infection, will confirm a mild conductive hearing loss. In severe infections, the patient may have cellulitis extending to the face and neck as well as cervical lymphadenopathy on the affected side. In chronic OE, patients will have minimal otalgia and will often complain of persistent otorrhea, pruritus, and muffled hearing. Physical exam may reveal canal thickening, moisture, debris, and, occasionally, granulation tissue.
Treatment of AOE often involves topical antibiotic preparations, with or without steroids. In mild acute or chronic OE, or as a preventative measure, acidifying agents may be used. Systemic antibiotics are indicated in severe or refractory cases, or in patients who are immunocompromised as discussed below. Irrespective of the therapy chosen to treat AOE, removal of debris from the external canal and evaluation of the status of the tympanic membrane is crucial prior to using any ototopicals, and oral analgesics are often needed in moderate or severe cases. Repeat debridement of the canal under binocular microscopy is essential to improve an infection that remains refractory to ototopical therapies. In cases where the canal is extremely edematous, it may be impossible to visualize the tympanic membrane. In this instance, care should be taken to avoid the use of ototopicals with potential ototoxic side effects. Significant swelling and edema also require wick placement to ensure proper delivery of ototopical antibiotics medially in the canal (see Fig. 139.1 ). The wick should be changed or removed within 3 to 5 days of its insertion. If the canal is patent, tragal massage will help deliver the medication to the medial external auditory canal, and holding the head in a dependent position for several minutes will allow for sufficient filling of the infected cavity.
Ototopical antibiotic preparations, with or without steroids, are the most common agents used to treat AOE in the otolaryngology office. These preparations achieve local tissue concentrations ∼1000 times that of systemic antibiotics, have a favorable side-effect profile, and demonstrate a lower incidence of bacterial resistance when compared to systemic antibiotics. The American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) developed a Clinical Practice Guideline in 2006 for the treatment of OE. This evidence-based report was subsequently updated in 2014 by a working group with representatives from otolaryngology–head and neck surgery, pediatrics, family medicine, infectious disease, dermatology, and consumer advocacy. The group preserved recommendations to use “topical preparations for initial therapy of diffuse, uncomplicated AOE.” Furthermore they maintained strong recommendations that “clinicians should not prescribe systemic antimicrobials as initial therapy … unless there is extension outside of the ear canal or the presence of specific host factors that would indicate a need for systemic therapy” (such as diabetes, prior radiotherapy or immune compromise). Despite consistent recommendations, systemic antimicrobials are still utilized in approximately one-third of patient visits as of 2010 after the exclusion of complicating factors and prescribing patterns did not significantly change in response to the Clinical Practice Guideline in 2006 highlighting the need to embrace evidence-based guidelines moving forward.
There are many ototopical antibiotic options available for the management of AOE. Only a few clinical studies have carefully examined the relative efficacy, safety, and cost-effectiveness among these commonly prescribed topical agents. However, a recent Cochrane review suggests that topical antimicrobials containing steroids are more effective than placebo with no major differences in efficacy between different topical formulations.
The following are commonly used preparations for the topical management of AOE:
Cortisporin otic suspension and solution (neomycin + polymyxin + hydrocortisone 1%) have both antibacterial and antiinflammatory properties. The suspension is a milky white agent and has been a popular choice for uncomplicated bacterial AOE for many years due to its efficacy, patient tolerance and low cost. The suspension and solution each contain 3.5 mg neomycin base, 10,000 units polymyxin B, and 10 mg of hydrocortisone 1% per mL. The suspension vehicle ingredients include cetyl alcohol, propylene glycol, polysorbate and water, while the solution uses cupric sulfate, glycerin, hydrochloric acid, propylene glycol, and water. The suspension has a less acidic pH (3.0 compared with 2.0 for the solution) and is therefore better tolerated. Neomycin has been in use over 40 years and is one of the oldest aminoglycosides. Pseudomonas has developed resistance to neomycin, with less than 20% of Pseudomonas retaining sensitivity. Polymyxins are cationic detergent antibiotics that disrupt the bacterial cell membrane. They work well against gram-negative rods, especially Pseudomonas. Although neomycin and polymyxin have ototoxic potential in animal studies, human data are equivocal, and the authors have used this preparation for many years in the middle ear to soak gelfoam packing without consequence. A retrospective review of type I tympanoplasty procedures in which gelatin sponges saturated in a neomycin, polymixin B, hydrocortisone preparation were used to support the graft demonstrated no associated ototoxicity as evident by no incidences of change in sensorineural hearing. Nonetheless, the manufacturer recommends that Cortisporin otic should not be used in patients with tympanic membrane perforations.
Ofloxacin (Floxin otic solution—ofloxacin 0.3% with benzalkonium chloride 0.0025%, sodium chloride 0.9%, and water, pH 6.5) is a topical fluoroquinolone commonly used in AOE. It can be used with tympanic membrane perforations and ventilation tubes as it has no known risk of ototoxicity, making ofloxacin a reasonable choice for ear surgery prophylaxis. Indeed, ofloxacin otic is FDA approved for use in patients with suppurative otitis media and perforated tympanic membranes. Like all fluoroquinolones, ofloxacin acts by inhibiting DNA synthesis and bacterial growth by binding to DNA gyrase and topoisomerases. Commonly reported side effects include pruritus and bitter taste if used with a perforated tympanic membrane. Clinical cure rates for AOE after treatment with ofloxacin have been shown to be greater than 80% in adults and greater than 95% in children.
Ciprofloxacin + hydrocortisone (Cipro HC Otic—ciprofloxacin 0.2%, hydrocortisone 1%, and benzyl alcohol as preservative) is a topical fluoroquinolone that also contains a steroid agent. It has broad-spectrum coverage, including Pseudomonas , although it has no activity against anaerobes. Ototoxicity has not been shown to be a concern with topical ciprofloxacin; thus, it is felt to be safe for use with tympanic membrane perforations. Although many physicians use Cipro HC otic in the presence of tympanic membrane perforations, the manufacturer recommends against this as the bottle is nonsterile.
Ciprofloxacin + dexamethasone (Ciprodex—ciprofloxacin 0.3%, dexamethasone 0.1%) also combines ciprofloxacin with a steroid for antiinflammatory properties. It is safe for use with tympanic membrane perforations and is FDA-approved for use in patients with patent tympanostomy tubes. Ciprodex has been shown in randomized clinical trials to be more effective at resolving AOE than neomycin/polymyxin/hydrocortisone, and has also been shown in a large randomized blinded study to be superior to ofloxacin in treating acute otitis media (AOM) with otorrhea through tympanostomy tubes. There is no study directly comparing Ciprodex with Cipro HC otic suspension in humans, although Sobol et al. demonstrated in an animal model that Ciprodex was superior to Cipro HC at treating granulation tissue in the external and middle ear.
Tobramycin + dexamethasone (Tobradex—tobramycin 0.3% and dexamethasone 0.1% and benzalkonium chloride 0.01% as a preservative) is an ophthalmic preparation with both antibacterial and antiinflammatory activity. Tobramycin is an aminoglycoside that binds to the 30S and 50S ribosomal subunits leading to inhibition of bacterial protein synthesis a defective bacterial cell membrane. It should be avoided in patients with tympanic membrane perforations or tympanostomy tubes due to possible ototoxicity.
Topical gentamicin (Garamycin Ophthalmic—gentamicin 0.3%, pH 7) is another ophthalmic preparation that can be used in AOE, especially when additional gram negative coverage is needed. Again, it should be avoided with tympanic membrane perforations due to concerns for ototoxicity.
Roland and colleagues performed two randomized multicenter studies comparing ototopical antibiotics. The clinical efficacy of one week of ciprofloxacin + dexamethasone (Ciprodex) or neomycin + polymyxin B + hydrocortisone (Cortisporin) was studied in 468 adults and children with AOE and intact tympanic membranes. Ciprofloxacin + dexamethasone showed higher bacterial eradication rates and more rapid symptom improvement compared with neomycin + polymyxin B + hydrocortisone. This was followed by a study investigating the efficacy of ciprofloxacin hydrocortisone (Cipro HC) compared to that of Cortisporin and systemic amoxicillin. Cipro HC was found to be clinically equivalent for the treatment of adults and children with OE. Van Balen performed a randomized clinical trial of ototopical therapies that compared acetic acid alone, acetic acid + corticosteroid, and antibiotic + corticosteroid for AOE. In 213 adults with AOE, patients who received preparations (either acetic acid or antibiotics) that included steroids had significantly higher cure rates compared with those who received acetic acid alone. The acetic acid alone group also had higher recurrence rates of OE. Schwartz performed a randomized multicenter blinded study in which patients with AOE received either ototopical ofloxacin administered once daily or neomycin/polymyxin/hydrocortisone administered four times daily. In 278 pediatric patients with pseudomonal OE, both agents were equally effective at eradicating disease and had similar safety profiles. Given the decreased ototoxic potential of ofloxacin and the easier dosing schedule, the authors concluded that ofloxacin might be a better first-line agent. Simpson et al. reached the same conclusion in a meta-analysis of the literature concerning ofloxacin use for AOE. Myer found that when compared to aminoglycosides, ototopical fluoroquinolones have an improved safety profile, broad antimicrobial spectrum, lower cost, and more convenient dosing schedule that is tolerated well by most patients. Rosenfeld and colleagues found 18 studies concerning ototopical therapy for AOE that compared any of the following groups: antimicrobial vs. placebo, antiseptic vs. antimicrobial, fluoroquinolone antibiotic vs. antibiotic, steroid-antimicrobial vs. antimicrobial, or antimicrobial-steroid vs. steroid. Clinical cure rates were between 65% and 80% within 10 days of therapy with all of the above ototopical antibiotics, and there was no statistical difference in clinical cure rates among any of the treatment groups. Fluoroquinolones did have an 8% higher bacteriologic cure rate compared to nonquinolone ototopicals; however, the clinical cure rate and rates of adverse side effects were the same as those seen with the other preparations. The addition of a steroid to fluoroquinolone agents decreases the symptomatic period by approximately 0.8 days. However, as discussed below, steroids do have a small risk of causing a hypersensitivity reaction.
Although less commonly recommended by otolaryngologists, some studies advocate using only steroid preparations without antibiotics. Tsikoudas et al. performed a randomized double-blinded study of 39 patients with AOE who were treated with either steroid and aminoglycoside or the same steroid alone. They found no additional benefit of the added aminoglycoside. Similarly, Emgård et al. studied 51 patients with AOE in an open randomized parallel-group trial that compared steroid drops alone (0.05% solution of betamethasone dipropionate) to ear drops containing a steroid with an antibiotic (hydrocortisone with oxytetracycline hydrochloride and polymyxin B). They found that the steroid alone had a higher clinical cure rate than the antibiotic and steroid together. These data are in contrast to a more recent study showing superiority of the antibiotic-steroid combination betamethasone sodium phosphate 0.1% with neomycin sulphate 0.5% (Vista-Methasone N) when compared to betamethasone sodium phosphate 0.1% (Vista-Methasone). Similarly, Lorente et al. evaluated the clinical efficacy of ciprofloxacin + fluocinolone acetonide versus ciprofloxacin suspension alone in the treatment of diffuse OE in a double blind study involving 590 patients. Clinical cure rate and symptom improvement was higher with the steroid combination versus antibiotic drops alone.
Weber found that overall there is Grade B evidence (defined by the U.S. Preventative Services Taskforce as follows: At least fair scientific evidence suggests that the benefits of the clinical service outweighs the potential risks) that no significant antibiotic resistance develops from the use of ototopical antibiotic therapy. Cantrell and colleagues examined the susceptibility of isolates from AOE to neomycin/polymyxin and ofloxacin. The MICs of each antimicrobial drug for the major pathogens (Pseudomonas and Staphylococcus aureus ), bacterial eradication, and clinical efficacy were studied from 1995 to 1996 and from 1999 to 2000. The data from 1999 to 2000 showed that the MICs for all pathogens increased above the breakpoint for polymyxin B. In contrast, the MICs of all isolates for ofloxacin remained similar between the two time periods, indicating that resistance developed to neomycin/polymyxin but not to ofloxacin. Wai et al. notes that minimal resistance has been documented against ofloxacin since its initial use in the 1980s, as only two strains of Pseudomonas have been shown to have slight resistance to ofloxacin. Due to extremely high local concentrations achieved with topical therapies that far exceed even the highest MICs for resistant Pseudomonas , it is unlikely that resistance will be a large factor in the choice of ototopical antibiotics.
Acidifying agents may be used in mild acute or chronic cases where there is minimal otalgia, but their main utility is in the preventative care of patients who are prone to developing recurrent acute or chronic OE (swimmers, hearing aid users, etc.). Alkaline pH has been shown to be a risk factor for the development of acute and chronic OE with loss of acidity proportional to the degree of OE, and thus restoring the natural acidity of the external canal can inhibit the growth of bacteria responsible for OE. Unfortunately, the acidic pH of these preparations may limit patient compliance due to pain and local irritation. These agents are contraindicated if a tympanic membrane perforation or tympanostomy tube is present, due to possible ototoxicity.
The following are commonly used acidifying solutions for mild OE, chronic OE, or preventive care of the external canal:
Alcohol-vinegar solution: ![]() alcohol, one-fourth white vinegar and one-fourth distilled water. This is inexpensive, easy to prepare at home and can be as effective as prescription agents in this category. Typically, several drops (4 to 5), which can easily be delivered with a syringe, are used in the affected ear two to four times daily until symptoms resolve. It is contraindicated in patients with tympanic membrane perforations or ventilation tubes, or if the patient has a hypersensitivity to any of the components. This preparation can be used safely as an irrigation solution if copious debris is present in the canal and there is no tympanic membrane perforation.
alcohol, one-fourth white vinegar and one-fourth distilled water. This is inexpensive, easy to prepare at home and can be as effective as prescription agents in this category. Typically, several drops (4 to 5), which can easily be delivered with a syringe, are used in the affected ear two to four times daily until symptoms resolve. It is contraindicated in patients with tympanic membrane perforations or ventilation tubes, or if the patient has a hypersensitivity to any of the components. This preparation can be used safely as an irrigation solution if copious debris is present in the canal and there is no tympanic membrane perforation.
Acetic acid in aluminum acetate drops (Domeboro), also known as Burow solution, may be used in mild cases of OE to help acidify and dry the canal. Five drops are applied to the affected ear two to four times daily. This should not be used if a tympanic membrane perforation or ventilation tube is present. In a study comparing in vitro antimicrobial activity of the antiseptic solutions Burow solution, acetic acid + vinegar + water, and boric acid, Burow solution was found to have the highest antimicrobial activity against methicillin-resistant and sensitive Staphylococcus aureus and quinolone-resistant and sensitive Pseudomonas aeruginosa , the two most common pathogens in OE. At 5 minutes, 100% and 50% Burow solution, along with the acetic acid + vinegar + water solution, had completely inhibited quinolone-resistant and sensitive pseudomonal survival. Furthermore, the time required to inactivate 90% of the MRSA microorganism population was lower for the 100% and 50% Burow solution compared with the other agents.
Propylene glycol and acetic acid otic solution (VoSol) and hydrocortisone 1% + propylene glycol + acetic acid otic solution (VoSol HC) also work well in superficial infections. The acetic acid has antibacterial properties, whereas the hydrocortisone helps reduce inflammation and pruritus. Both of these preparations are quite viscous and have a pH of approximately 3. Two to four drops are applied two to four times daily. This should not be used in the setting of a tympanic membrane perforation or ventilation tube due to concern for ototoxicity.
With so few trials examining and comparing the many different ototopical formulations, the decision regarding which one to choose for AOE is often left up to the personal clinical experience and opinion of the treating physician. In addition to efficacy, the concern of ototoxicity when a tympanic membrane perforation or ventilation tube is present, patient tolerance and dosage schedule, cost and hypersensitivity concerns may help guide the choice of topical antibiotic therapy for AOE.
When initial therapy fails to treat AOE, the physician should consider not only the possibilities of improper administration or ineffectiveness of the ototopical, but also other possible diagnoses such as malignant OE, contact dermatitis, and malignancy ( Box 139.1 ).
Self-instrumentation trauma
Malignant external otitis
Contact dermatitis
Failure to adhere to preventive measures (such as avoidance of water exposure)
Improper administration of ototopical therapy
Immunosuppression: diabetes, prior radiotherapy
Inadequate penetration of ototopical therapy due to copious debris or thickened canal skin
Misdiagnosis: canal cholesteatoma or keratosis obturans, autoimmune condition, mycobacterial infection, malignancy
Resistance of involved organism to ototopical therapy choice
Restrictive use of oral antibiotics for AOE is important given increasing resistance among common pathogens. The AAO-HNSF recommends that adding a systemic antibiotic may be beneficial in patients with OE and a history of prior radiation therapy, diabetes, or immunocompromised state. Additionally, patients with concomitant parotitis or cellulitis extending to the auricle, face, or neck, and patients with concurrent otitis media without tympanic membrane perforation or tympanostomy tube should receive oral antibiotics. Finally, patients who fail to respond to a full course of ototopicals may benefit from oral antibiotics. It may be prudent to obtain a culture prior to starting antibiotics in patients with refractory OE or prior to starting topical therapy in patients with underlying immune compromise.
The AAO-HNSF strongly recommends adequate pain management for acute OE. Pain may be severe and the intensity may be underappreciated therefore ongoing assessment of severity of discomfort is important. Mild to moderate pain may respond to acetaminophen or a nonsteroidal antiinflammatory medication given alone or in combination with an opioid. Nonsteroidal antiinflammatory drugs have been shown to significantly reduce pain in the acute phase. Although topical agents are preferred in the primary treatment of OE, oral medications are preferred for pain management.
In 2015, the U.S. Food and Drug Administration (FDA) announced plans for enforcement action against companies manufacturing and distributing unapproved otic products. In particular, topical preparations for otalgia containing combinations of the following: benzocaine and/or chloroxylenol with or without antipyrine, hydrocortisone, zinc acetate, or pramocaine are not approved by the FDA for safety, effectiveness, and quality. One such medication, Auralgan (benzocaine, antipyrene + dehydrated glycerin) was an over-the-counter ototopical drug used to relieve otalgia related to AOM and OE. Mujica-Mota and colleagues assessed the cytotoxicity of Auralgan on auditory cells in vitro. They demonstrated cytotoxic effects in cultured auditory hair cells and ototoxic effects after intratympanic administration in an animal model. Although not recommended for use in the presence of tympanic membrane perforation, should this topical agent enter the middle ear cavity there is potential for ototoxic effects.
Symptoms such as persistent otorrhea, aural fullness, muffled hearing, and pruritus lasting longer than 3 months may indicate chronic OE. This can be the result of inadequately treated infectious OE, but noninfectious causes should also be considered and are discussed in the section on eczematoid OE below. In chronic OE, topical antibiotic therapies may not be as effective in resolving long-standing inflammation. Recent evidence suggests a role for biofilms in the pathogenesis of chronic bacterial OE with biofilms present in over 90% of cases, which may contribute to the difficult nature of these cases. In many cases, inadequate ear hygiene or manipulation with cotton-tip applicators may contribute to chronic bacterial infection and should be addressed by the otolaryngologist. Daily irrigation with an acidifying or dehydrating agent and use of a hair dryer on a low, cool setting after water exposure will help to optimize daily ear hygiene. Dry powder preparations as discussed below are a reasonable alternative to topical drops, as continued moisture from long-term ototopical use can contribute to maceration of the external canal skin and chronic infection. Cultures of the canal should be obtained to ensure sensitivity of the organisms to the chosen ototopical antibiotic.
Steroid creams and local steroid injections have been used with topical antibiotics to improve the efficacy of treatment in chronic OE. Stuck et al. examined thirteen patients with refractory OE despite treatment with topical steroids and antibiotics and injected the ear canal skin with triamcinolone acetonide. The majority of these patients reported a complete resolution of symptoms. In such cases, chronic OE may not be the result of persistent bacterial infection, and it is important to consider other etiologies such as contact dermatitis and eczema, or an underlying canal cholesteatoma, keratosis obturans, or malignancy. In addition, it may be difficult to distinguish a failure of initial therapy from hypersensitivity reactions to the ototopical agent, as the signs and symptoms are often similar. It is therefore important to have a high index of suspicion for possible drug reactions, especially when using preparations with higher rates of sensitization.
Other less common approaches include silver nitrate gel, which shows some efficacy in patients with refractory OE or otomycosis. In rare cases, persistent foci of granulation tissue have been treated with small fragments of silver nitrate placed in the external canal with the binocular microscope. This approach should be avoided in previously operated ears or in canal wall down cavities in the event that an unrecognized dehiscent facial nerve is found in the region of granulation tissue.
Poorly managed or refractory chronic OE can lead to canal skin thickening, scarring, canal stenosis, and blunting of the external canal/tympanic membrane with associated conductive hearing loss. Some physicians refer to this process as “stenosing otitis externa” in which the external canal continues to be moist despite extensive medical therapy. The canal gradually narrows from medial to lateral, and in the end stage it is dry with minimal to no drainage or irritation. Tacrolimus, a nonsteroidal immunosuppressant, may be considered in recalcitrant cases when other therapies are unsuccessful. In these cases, canalplasty and tympanoplasty can be helpful to remove scarred or diseased tissue once the disease has reached its noninflammatory endpoint.
In patients with OE not responsive to maximal medical therapy, including failed topical and systemic antibiotics, repeat cultures and a biopsy of the diseased ear canal should be considered and an autoimmune workup initiated. A biopsy should be performed if persistent granulation tissue or ulcerative lesions in the external auditory canal are visualized (especially if there is pain) and in patients who are immunocompromised. High-resolution temporal bone CT scans are helpful to determine bony involvement. Aggressive fungal infections, malignancy, and tuberculosis can masquerade as recurrent acute or chronic OE by presenting as an inflamed and draining ear.
Malignant OE is an osteomyelitis that originates in the ear canal and extends to the surrounding bone. This aggressive bacterial infection spreads through the fissures of Santorini and the bony-cartilaginous junction to involve the temporal bone, skull base, and surrounding cranial nerves. Malignant OE is most commonly caused by Pseudomonas aeruginosa; however, nonpseudomonal cases have been reported, most notably those secondary to methicillin-resistant Staphylococcus aureus .
Elderly, diabetic, or immunocompromised patients are most commonly affected. Patients with malignant OE may complain of progressive nocturnal pain, aural fullness, fever, and otorrhea. The exam can reveal proptosis of the auricle, canal skin necrosis, granulation at the osseocartilaginous junction, cranial nerve involvement, vertigo, or meningeal signs. The facial nerve is the most commonly affected cranial nerve in malignant OE, but cranial nerves IX, X, XI, and XII can be affected if the disease progresses along the skull base. Cranial nerves V and VI can be affected if the disease extends to the petrous apex. Technetium Tc 99 scan can determine the presence of bony involvement with malignant OE, and high-resolution temporal bone CT scans are being used increasingly to assess bony erosion. A high index of clinical suspicion must be maintained because a delay in diagnosis commonly occurs in cases of malignant OE, particularly when risk factors such as diabetes are present.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here