Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the evaluation of diffuse and focal liver disorders, magnetic resonance imaging (MRI) has been proven to provide superior characterization of disease processes and therapy outcomes compared to other modalities in the imaging armamentarium. The particular strength of hepatic MRI is based on its unique ability to synergistically extract information on various soft tissue components in combination with assessment of functional liver status. To achieve and maintain this performance, continuous optimization of existing MR sequences as well as recognition of current limitations with the goal to establish novel acquisition sequences and processing algorithms are of paramount importance. Accurate tissue characterization through detection and quantification of imaging biomarkers specific to liver disorders represent one possible approach to redefine hepatic MRI workflows and will be the main focus of this chapter.
Traditionally, hepatic MRI sequences have been designed to either highlight locoregional tissue-inherent differences in relaxivity or, alternatively, emphasize focal contrast uptake characteristics compared to surrounding tissue; both phenomena are usually detected in a subjective fashion and reported qualitatively based on grayscale-encoded intensity levels of tissues under investigation. Novel hepatic MRI sequences that focus on detection and quantification of liver imaging biomarkers aim to assess specific pathophysiologic processes of the hepatobiliary systems and yield functional quantitative results. Although histopathologic analyses of hepatic parenchyma may yield a wide spectrum of answers regarding underlying hepatic diseases, complications from invasive sampling techniques, sampling errors, and inability to sample entire organ systems in association with associated costs limit the use of histologic sampling in many clinical scenarios, in particular if the underlying abnormality itself has a low complication or exacerbation rate. Of the existing functional MRI techniques seeking to extract reproducible and specific hepatic imaging biomarkers, this chapter focuses on MR diffusion-weighted imaging (DWI), dynamic perfusion imaging using various contrast agents, and MR relaxometry and elastography, all employed in specific disease scenarios that potentially benefit from biomarker quantitation unique to hepatic disease status or, alternatively, assessment of their longitudinal evolution over time.
Over more than 2 decades, diffusion-weighted MRI has evolved into a reliable and proven technique in the neuroradiology field, mainly for stroke detection and visualization of white matter tracts, employing diffusion tensor imaging (DTI). Despite being one of the earliest sequence techniques supplying anatomic and functional information of an organ system, hepatic diffusion-weighted MRI still faces many challenges, and its clinical usefulness remains under investigation.
In this section, basic concepts of DWI will be highlighted. Several signal models have been investigated for tissue characterization, including the apparent diffusion coefficient (ADC), intravoxel incoherent motion (IVIM), diffusion kurtosis, and stretched exponential models. The discussion will mainly focus on ADC as the model that has been explored to the greatest extent. Lastly, potential applications for hepatic tissue characterization using ADC will be discussed.
After spins experience a radiofrequency (RF) excitation pulse, they undergo dephasing because of spin-spin interactions, T2 relaxation, and effects originating from external magnetic field inhomogeneities, T2* relaxation. Furthermore, a small component of this dephasing is related to diffusion of water molecules as a direct function of random thermally induced brownian movement. This effect in particular is enhanced in DWI, which usually employs a T2-weighted sequence with an additional symmetric pair of diffusion-sensitive gradients enveloping the refocusing pulse. Water molecules in a cellular environment leading to restricted diffusion undergo an initial phase shift as a result of the first diffusion gradient, which will be completely reversed by the second diffusion gradient without residual phase shift at time of signal readout. Water molecules in a cellular environment without substantial diffusion restriction, in contrast, acquire phase information from the first gradient, but because of their inherent motion, their signal will not be completely rephased by the second gradient, leading to a signal loss at the time of the signal readout. The degree of diffusion restriction is apparent as reduced grayscale intensity of the measured signal intensity at DWI.
The sensitivity for detecting diffusion restriction using DWI sequences can be varied by changing the amplitude of the diffusion-weighted gradient, the duration of the applied gradient, and the time interval between the paired gradients. The combination of gradient characteristics is expressed as a single numeric parameter referred to as the b value. The sensitivity for detecting restrictions in diffusion is varied by changing the b value, which is measured in s/mm 2 . Most frequently, the b value is varied by altering the gradient amplitude.
In general, DWI is performed as a spin echo pulse sequence with echo-planar imaging (EPI) readout, with the additional set of diffusion gradients mentioned before. The main advantage of EPI sequences remains the combination of relatively short acquisition times and high signal-to-noise ratios (SNRs). EPI is, however, limited by spatial resolution and its high sensitivity to susceptibility variations. In particular, stronger magnetic fields may lead to pronounced susceptibility artifacts, inhomogeneities in the base magnetic field (B0), that result in shorter T2* relaxation times, as well as amplified inhomogeneities in the B1 transmit fields, with a higher characteristic frequency spectrum compared to less intense magnetic field strength. Mainly to improve image quality by increasing spatial resolution and decreasing the impact of susceptibility variations, improvements in DWI rely on implementation of parallel imaging techniques, half-Fourier acquisitions of single-shot turbo-spin echo (HASTE) sequence designs, and radial k-space acquisitions through periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER). Hepatic DWI can be performed as a free-breathing or breathhold technique, but the latter is usually limited by a maximum possible b value of less than 500 s/mm 2 if a breathheld acquisition is desired. Additional cardiac gating, in particular, improves left hepatic lobe lesion characterization. For liver imaging, respiratory gating techniques and short echo times may be used because the liver undergoes considerable translation, rotational motion, and deformation during the respirational cycle.
Owing to the T2 weighting of DWI sequences, certain focal hepatic lesions such as hemangiomas or cysts, which appear intrinsically hyperintense on standard T2-weighted imaging sequences, may also appear hyperintense on DWI. The hyperintense signal on DWI may, however, not be a sign of restricted diffusion but rather represent the strong contribution of the T2 hyperintensity bleeding into the DWI sequence, because all signal intensities on DWI images are composed of mixed contributions from the intrinsic T2 properties of the tissue being examined, as well as spin density detected after defocusing and refocusing of the diffusion gradients. This phenomenon is known as the T2 shine-through effect. To differentiate T2 shine-through effects from true areas of restricted diffusion, apparent diffusion coefficient (ADC) maps can be calculated to remove signal dependence on underlying tissue T2. To be able to calculate ADC maps for each pixel in a DWI image series, at least two DWI datasets with differing b values need to be obtained. The ADC map represents grayscale-encoded ratios of logarithmized differences in signal intensities over the differences of b values of at least two sequential DWI sequences measured in mm 2 /s. Areas of restricted diffusion demonstrate hyperintense voxels on DWI, with corresponding hypointense voxels on the ADC map ( Fig. 7-1 ).
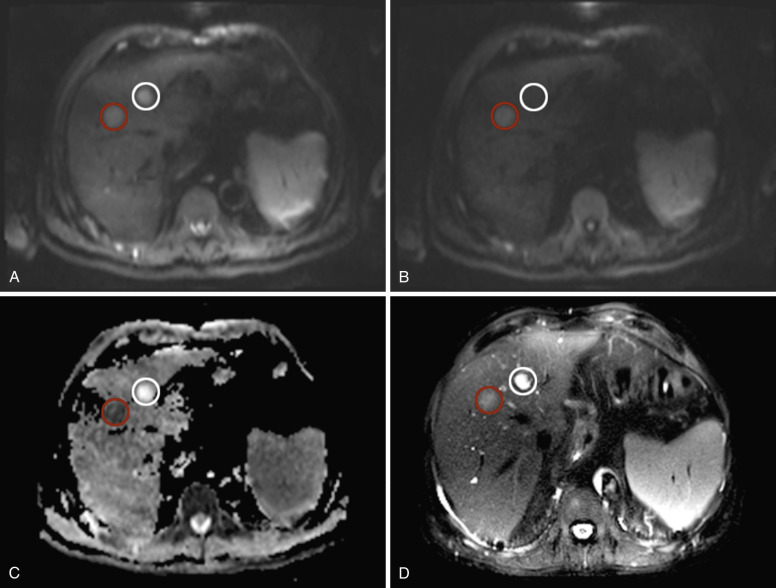
In particular for low b values, the effects of microscopic perfusion and diffusion are contributing to the grayscale-encoded intensity of individual voxels. Intravoxel incoherent motion (IVIM) analyses seek to separate capillary microcirculation from molecular diffusion. IVIM uses multiple b values, and by employing theoretic models/curve fitting, allows separation of perfusion from diffusion effects throughout the entire b-value spectrum. DWI-derived parameters such as free molecular-based (D) and perfusion-related (D*, f) diffusion parameters can be subsequently calculated. IVIM-DWI imaging is especially beneficial for patients with focal lesions for whom tissue vascularity and cellular treatment response need to be assessed individually. IVIM-DWI imaging allows differentiation among viable tumor tissue and tumor necrosis, as well as developing fibrosis, and can potentially be used as a surrogate marker of tumor response to treatment.
The pathophysiologic features of many tumor tissues, such as distinct cytologic patterns and unique forms of clustering, are linked to tumor grading and resultant aggressiveness. These patterns and clusters affect molecular diffusion properties. The basic hypothesis of DWI is free and unrestricted gaussian diffusion of water protons; complex biologic tissues, however, often show a non-gaussian distribution of the water displacement profile when compartmentalization and/or tissue barriers are present. Therefore the measurement of a non-gaussian diffusion distribution (i.e., kurtosis) may allow quantitation of diffusional heterogeneity and improve characterization of water diffusion properties in the tumoral microenvironment.
Potential clinical use of DWI focuses on a wide range of hepatic disease scenarios, owing to the fact that by now DWI sequence sets have been routinely incorporated into standard MR liver imaging protocols. Diffuse liver disease generally is associated with lower ADC values than normal liver parenchyma. Mechanisms leading to diffusion restriction differ, however, based on the underlying cellular pathodynamic environment. In the disease evolution ranging from early hepatic fibrosis to end-stage cirrhosis, progressive deposition of collagen with lower unbound water content compared to normal hepatic parenchyma into the extracellular space occurs in combination with a decrease in hepatic perfusion. The resultant reduction in ADC values allows differentiation of normal liver parenchyma from end-stage fibrosis. However, ADC values seem to have a limited capability to distinguish early stages of fibrosis from advanced disease. In hepatic steatosis, on the other hand, the reduced ADC values result from reduced diffusivity of water protons associated with intra- and extracellular fat. Significant ADC reduction has been observed in patients with fatty infiltration that show hepatic fat fractions of greater than 5%. Lastly, in patients with diffuse iron deposition, as seen in hemochromatosis, the local susceptibility artifacts induced by high parenchymal iron concentration results directly in decreased ADC values.
For all types of diffuse hepatic disease, the longitudinal evolution of ADC parameters over time appears more meaningful than defining disease-specific thresholds.
In the detection of focal liver lesions, increased lesional conspicuity can be achieved through enhanced lesion-to-background tissue contrast by employing smaller diffusion-weighting values (b < 50-150 s/mm 2 ), essentially nulling the intrahepatic vascular signal and resulting in black blood images. Lesion characterization requires higher b values (b > 500 s/mm 2 ) to differentiate between underlying cystic or solid nature. Whereas a differentiation between cystic and solid focal lesions usually does not represent a diagnostic dilemma, differentiation between differing solid hepatic lesions is a greater diagnostic challenge owing to substantial overlap in morphologic appearances. Generally it has been shown that benign lesions show a higher ADC value than malignant ones; it seems that a threshold value of 1.7 × 10 −3 mm 2 /s shows a fairly high sensitivity and specificity for differentiating benign from malignant disease. Metastatic disease from mucinous primaries (e.g., ovarian or colorectal cancers) can, however, mimic benign lesions. Therefore quantitative ADC measurements performed for lesion characterization should always be used in concert with other quantitative markers as well as clinical background and temporal context. It has to be emphasized that none of the described biomarkers in this review article appear to be robust enough to allow deducing diagnoses without placing the extracted biomarker values into the appropriate clinical settings. Additionally, acquisition of serial b values can highlight changes in grayscale-encoded diffusivity, depending on diffusion weighting. Focal liver lesions generally appear hyperintense on b0 images, but benign lesions transition to iso- or hypointensity on b800 DWI, in contrast to malignant lesions, which remain hyperintense.
For hepatocellular carcinomas (HCCs), histopathologic grading is one of the most important prognostic indicators and of paramount importance to determine treatment. It has been shown that threshold ADC values allowed differentiation of poorly differentiated tumors from moderate to poorly differentiated HCCs when a cutoff of 0.972 × 10 −3 mm 2 /s was used. In general, lower mean ADC values indicate less differentiated and thereby worse histopathologic grades compared to well-differentiated HCCs. In addition, it is conceivable that cardiac motion artifacts may affect extraction of reproducible and reliable ADC values, in particular in the left hepatic lobe.
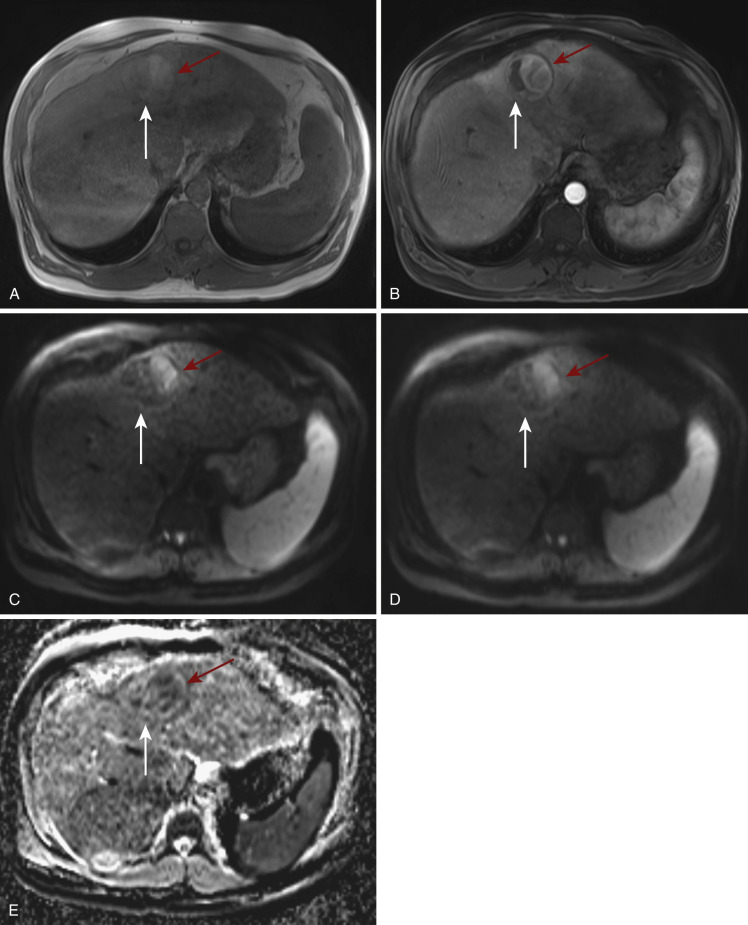
Finally, DWI can, as part of the quantitative imaging arsenal, also be used in assessment of treatment response and disease prognostication. Although anatomic descriptive methods such as tumor size and enhancement patterns are not able to document evidence of treatment response or lack thereof for several weeks or months following treatment—in particular if novel cytostatic therapeutic agents are used—DWI may potentially provide an earlier assessment. Within hours of systemic chemotherapy, malignant tumors respond with an increase in cellular size, resulting in a reduction in ADC value as the intracellular/extracellular volume ratio per voxel increases. Eventually, cellular necrosis and a resultant increase in ADC are observed. This pattern of ADC change over time has been particularly documented in patients showing at least partial response to treatment. In contrast, focal hepatic metastases, which either show high ADC values before treatment or lack of ADC value increase early following treatment, may be characterized by a more adverse response to chemotherapy. For patients receiving focal therapy such as transcatheter arterial chemoembolization or radiofrequency ablation, analogously lower ADC values on posttreatment DWI correlated with tumor recurrence and thereby allowed differentiation of viable from necrotic portions of the treated lesion. Because pretreatment ADC values are composed of variable combinations of diffusion and perfusion effects, quantitated IVIM might be helpful in predicting the effectiveness of transcatheter arterial chemoembolization prior to treatment ( Fig. 7-2 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here