Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
adenomatous polyposis coli protein
blood vessel epicardial substance
coxsackievirus and adenovirus receptor
Crohn’s disease
cystic fibrosis transmembrane conductance regulator
crumbs protein homolog 3
enhanced green fluorescent protein
epithelial sodium channel
extracellular signal-regulated kinase
hydrogen potassium ATPase
inflammatory bowel disease
interferon
interleukin
junctional adhesion molecule
c-Jun N-terminal kinase
lipopolysaccharide
membrane-associated guanylate kinases
mitogen-activated protein kinase
MAL and related proteins for vesicle trafficking and membrane link
myosin light chain kinase
sodium potassium ATPase
nuclear factor kappa B
sodium hydrogen exchanger
nonsteroidal anti-inflammatory drug
domain shared by postsynaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), and zonula occludens-1 protein
permeability index
sodium-glucose cotransporter
tight junction-associated MARVEL proteins
transepithelial resistance
tight junctions
tumor necrosis factor
tumor necrosis factor receptor
ulcerative colitis
zona occludin
In this chapter, we discuss the role of tight junctions (TJs) as an integral component of the intestinal epithelial barrier. The TJs, located at the boundary between apical and basolateral membrane of enterocytes, act to provide the barrier or “gate” function to paracellular permeation of water-soluble molecules. Herein, we discuss the components of the TJ complex and the major physiological regulators of intestinal TJ barrier. The TJ permeation occurs through at least two distinct permeation pathways; the pore pathway, which allows the flux of small-sized molecules (regulated in part by claudin-2), and the leak pathway, which permits the flux of large macromolecules (regulated in part by occludin and myosin light chain kinase). The mechanisms that mediate physiological (e.g., via Na + -nutrient cotransport) and pathological [e.g., inflammatory mediators such as tumor necrosis factor (TNF)-α or pathogenic bacteria] modulation of intestinal TJ barrier are detailed as it relates to intestinal epithelial homeostasis and alteration in intestinal TJ permeability. Additionally, the role of the defective intestinal TJ barrier in the pathogenesis of clinical diseases such as celiac disease and inflammatory bowel disease (IBD) are discussed. Thus, the basic components of the TJ complex, the specific TJ pathways, the physiological and pathological regulators of TJ permeability, and the clinical importance of defective intestinal TJ barrier are deliberated in this chapter.
The primary function of the gastrointestinal (GI) tract is to digest and absorb nutrients. To accomplish this, it must maintain a barrier between the luminal environment, technically a space outside the body, and the internal environment of the body; and it must selectively absorb and secrete nutrients, solutes, and water across the barrier. Separation of tissue spaces throughout the GI tract is accomplished by continuous sheets of polarized columnar epithelial cells. An exception exists in the upper two-thirds of the esophagus, which is covered by a nonkeratinizing squamous epithelium. Epithelial barriers are selective and capable of excluding potentially noxious luminal contents, such as gastric acid, colonic bacteria, and bacterial antigens, while at the same time capable of directional absorption and secretion of large volumes of solutes and water. Material can pass from one side of the epithelium to the other along one of two routes, either through the cell membranes or the space between them, referred to as the transcellular and paracellular pathways, respectively. The connection between individual epithelial cells is created by a series of intercellular junctions, the tight junction (TJ) being the most important for defining the characteristics of the paracellular barrier and its selectivity.
The specific characteristics of epithelial barriers vary widely throughout the GI tract, matched to each organ’s transport functions. However, in all cases, disruption of the barrier leads to a loss of normal transport and inflammation due to tissue damage or antigen exposure. In this chapter, we focus primarily on the role of the TJ in the intestinal barrier. We begin with the role of the TJ and paracellular pathway in normal transport. In recent years, a large number of proteins have been identified as components of the TJ and the functions of many of these proteins are being unraveled. This allows interpretation of the barrier’s physiologic properties on a stronger cellular and molecular foundation. We review the latest advances in this area. The intestinal TJ barrier is highly regulated and we review mechanisms and physiologic relevance for the GI tract. Finally, we review some of the intestinal disorders that have an associated defect in intestinal TJ barrier and the implications of TJ barrier defect in the disease pathogenesis. Since our previous version of this chapter, there have been number of important advances that better define the molecular and cellular processes that affect the TJ barrier function under both normal conditions and during pathologic states. In this latest version, we have taken a comprehensive approach to cover wide variety of topics but not all in equal depth. Although we tried to cover as much of the relevant historical advancements, core concepts, and the latest advances in the field, due to the overwhelming amount of high quality original publications in this area, it was not possible to cite all major advancements. Where appropriate, the reader is referred elsewhere for a more complete presentation, particularly of current controversies and unresolved issues.
The term “epithelial barrier function” is often used to describe all the mechanisms contributing to homeostasis of the epithelial barrier. The single layer of continuous epithelial cells and their intercellular junctions constitute the intrinsic elements of the barrier. The magnitude of this barrier is most often measured as the transepithelial electrical resistance (TER) and the permeability to paracellular markers, such as mannitol and inulin. TER correlates with the ability to separate ionic charge across the epithelia, reflected in either a transepithelial electrical potential difference or the current that creates the potential, measured experimentally as the short circuit current ( I sc ). Extrinsic elements include the innate and acquired mucosal immune system, protective secretion of mucus, bicarbonate, IgA and antimicrobial peptides as well as mechanism of epithelial repair or restitution. The contribution of each element varies along the GI tract, with mucus secretion being the most constant along the entire length from mouth to anus. Our goal in this chapter is to focus predominantly on the intrinsic barrier of epithelial cells and TJs in health and disease.
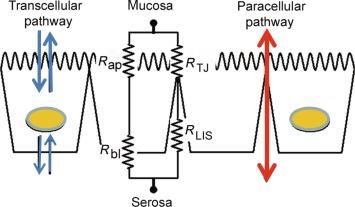
Throughout the GI tract, transport of electrolytes, solutes, and water across epithelia occurs across both a transcellular and paracellular pathways ( Fig. 25.1 ). The transcellular route for hydrophilic molecules, for example, Na + , Cl − , and glucose, is governed by the profile of membrane pumps, carrier, and channels expressed in a particular cell type. The passive movement across the lipid component of the membrane is very limited for charged and hydrophilic molecules. For example, the electrical resistance across model lipid membrane bilayers is in the range of 10 6 –10 9 ohm × cm 2 whereas the resistance across real membranes in the GI tract is 3–4 orders of magnitude less, reflecting facilitated conductance through protein-based channels ( Table 25.1 ). The profile of conductance proteins differs among epithelia explaining their unique functions. Individual transporters also show a polarized distribution to either the apical or basolateral membrane surface as the basis for directional transport. For example, the apical H + -K + ATPase of gastric parietal cells is responsible for secreting hydrochloric acid within the stomach. Na + -dependent bile acid transporters are positioned on the hepatocyte’s sinusoidal surface and the apical-luminal surface in the ileum to produce the enterohepatic circulation of bile salts. The cystic fibrosis transmembrane regulator (CFTR), a chloride channel, is positioned on the apical surface of biliary, pancreatic, and intestinal surfaces to bring about luminal Cl − secretion which is followed by Na + and water secretion.
| Epithelium a | Species | R cell b | R paracellular | P Na /P Cl c |
|---|---|---|---|---|
| Proximal tubule | Dog | – | 6–7 | 1.4 |
| Gallbladder | Rabbit | 229 | 21 | 3.3 |
| Duodenum | Rat | – | 98 | – |
| Jejunum | Rat | 67 | 51 | 10.0 |
| Ileum | Rabbit | 115 | 100 | 2.5 |
| Distal colon | Rabbit | 730 | 385 | 0.6 |
| Mouse surface | 132 | 3,200 | – | |
| Crypt | 429 | – | – | |
| Gastric fundus | Necturus | 2,826 | 10,573 | – |
| Urinary bladder | Rabbit | 160,000 | 300,000 | – |
| Cell lines d | ||||
| Caco-2 | Human colon | 125–250 | – | 3.0 |
| LLC-PK 1 | Pig prox. tubule | 100 | – | 0.6 |
| MDCK | Dog | 60–4000 | – | 10.0 |
a All values can be found in Powell.
b Electrical resistance values in ohms × cm 2 .
c Permeability ratio of Na + versus Cl − . P Na /P Cl in free solute is 0.66. Paracellular pathways with ratios above this value are more permeable for Na + than Cl − , i.e., cation-selective.
d Values for cell lines are the personal observations of Dr. C. Van Itallie.
Primary transcellular transport is “active,” powered by ATP hydrolysis to move ions against an electrical or concentration gradient. The prime example is the ubiquitously expressed Na + -K + -ATPase, which moves three Na + ions out the basolateral surface in exchange for two K + ions, with the net effect being to generate an inwardly directed Na + and outwardly directed K + gradient and negative intracellular electrical potential. The high membrane conductance for K + and its exit from the cell further enhances the intracellular negative electrical potential. These electrical and chemical gradients are then used in “secondary” active transport to couple energetically unfavorable uphill movement of nutrients, such as glucose or amino acids, to the downhill movement of Na + through, for example, the Na + -coupled glucose cotransporter (SGLT-1) of the jejunum. As a final generalization, the characteristics of transcellular transport are highly regulated by short-term signals, for example, hormone-stimulated bicarbonate secretion from pancreatic ducts, and long-term transcriptional control, for example, aldosterone-stimulated expression of the Na + -K + -ATPase or electrogenic sodium channel ENAC. We outline these features of transcellular transport before proceeding with a detailed discussion of the paracellular pathway, to highlight the sharp distinction to paracellular transport, which is passive, nonrectifying and does not appear to be as highly regulated at least by physiologic stimuli. The transcellular and paracellular transport can be coordinated via intracellular signaling and actin cytoskeleton to enhance the total transport.
The paracellular barrier to material movement coincides with continuous cell-cell contacts located at the apical end of their lateral surfaces ( Figs. 25.2 and 25.3 ). The earliest histological description of what we now refer to as the “apical junction complex” comes from the late 19th century. When sections of small intestine were stained with vital dyes a distinct intercellular density was observed between cells at the apical end of the lateral space. The English literature referred to this as the terminal bar ; other names reveal an assumed role in intercellular adhesion, e.g., “Schlussleiten,” and “bandelettes de fermeture.” The first speculation about a barrier function is attributed to Bonnet. After examining several different GI tissues obtained from an executed man, he concluded that the terminal bar was a general feature of all epithelia and might play a role in segregating the distinct fluid compositions found in different regions of the GI tract.
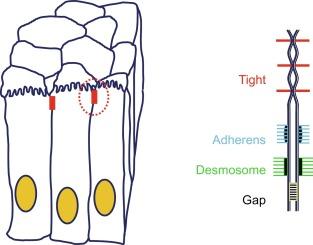
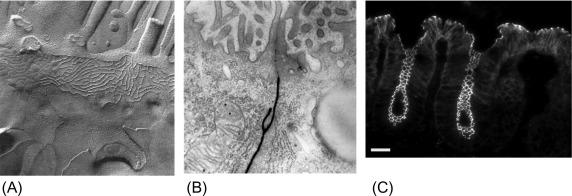
With the first ultrastructural images of intestinal epithelia in 1963, the apical junction complex was revealed as a set of morphologically distinct junction types ( Fig. 25.2 ). Each of these functions in cell-cell adhesion and signal transduction and provide links to the cytoskeleton. The TJ is invariably the most apical. It appears along the apical to basal axis, in transverse sections, as a series of close cell-to-cell contacts, or “kisses.” In freeze fracture images ( Fig. 25.3 A), the contacts are revealed as continuous rows of transmembrane protein particles. Actin filaments terminate on the plasma membrane directly at the contacts and participate in the regulation of the TJ barrier, , and are known to bind the peripheral membrane scaffolding proteins ZO-1 and cingulin ( Table 25.2 ). Below this is the adherens junction, location of the intercellular adhesion molecule cadherin and its cytoplasmic binding partner beta-catenin and extensive attachments to a ring of peri-junctional actin filaments. The importance of cadherin in adhesion and maintaining the differentiated cell phenotype are underscored by its frequent mutation as a final step facilitating metastasis of colon cancer. Beta-catenin has a second role in the nucleus where it signals cell growth and adenoma formation unless degraded by interacting with the adenomatous polyposis coli protein (APC). Human mutations in APC leave beta-catenin free to signal cell growth and transformation into adenomatous polyps. Below the adherens junctions are desmosomes, whose transmembrane proteins, while homologous to cadherin, are linked to intermediate filaments not actin. Desmosomes serve to protect the alimentary epithelia from shear-induced damage. Gap junctions that are made up of intercellular channels formed by six subunits of connexins that are bundled together in homomeric or heteromeric fashion and allow transfer of small metabolites and second messengers like Ca ++ and IP3, between adjacent cells for coordination of epithelial functions like secretion and exocytosis.
| Category | Protein | Function |
|---|---|---|
| Transmembrane | Claudin(s) | Barrier and pore selectivity |
| TAMPs | Signaling scaffold, adhesion, barrier regulation | |
| JAM(s) | Many | |
| CAR | Coxsackie virus receptor | |
| PDZ-Scaffolding | ZO-1 | MAGUK, binds occludin, claudin, ZAK, JAM, ZAK, actin, ASIP, ZONAB |
| ZO-2 | Binds ZO-1, actin, claudins, fos, jun, CEBP | |
| ZO-3 | Binds ZO-1, actin, claudins | |
| MUPP-1 | 13 PDZs and binds claudins | |
| Polarity | ASIP/PAR-3 | Atypical PKC binding protein |
| PAR-6 | Cdc42–Par6–Par3–aPKC interaction required for polarity and junction formation | |
| PAT-J | AKA Discs-lost | |
| Pals-1 Crb3 Scribble |
aka Crumbs polarity protein Cell polarity complex component Regulates assembly of tight junctions |
|
| Kinases | ZAK | Binds and phosphorylates zo-1 |
| aPKC | Binds polarity proteins par-3, par-6, interaction required for junction assembly | |
| src | Occludin phosphorylation blocks zo-1 binding | |
| yes | Binds occludin | |
| Phosphatases | PTEN | Tumor suppressor binds MAGI-2 and 3 |
| PP2A | binds aPKC, disassembles junction | |
| Transcription factors | ZONAB | ErbB-2 activator |
| HuASH1 | Drosophila ash1 homolog | |
| CEBP | ||
| Fos, Jun | ||
| GTP-binding proteins | Rab 3B | Mutants inhibit ldlr delivery |
| Rab 13 | Mutants inhibit claudin-1 delivery | |
| Gαi2 | Binds sh3 of zo-1 | |
| AF6 | Binds to Ras, ZO-1 and actin | |
| GEF-H1 | Guanine nucleotide exchange factor influences permeability | |
| Vesicle targeting | Sec6/8 | Exocyst complex |
| VAP33 | Binds occludin and v-snares | |
| Other | Cingulin Blood vessel Epicardial Substance (Bves) |
Binds ZO-MAGUKs, JAM-1, actin Regulates cell adhesion |
Viewed by freeze-fracture election microscopy ( Fig. 25.3 A), the TJ barrier coincides with a network of transmembrane strands. In unfixed tissue, the strands often appear as rows of individual particles, now known to be a family of transmembrane adhesion molecules claudins. The Latin root, claudere , means to close. Rows of claudins from each cell meet in the intercellular space forming adhesive contacts and a semipermeable seal. The complexity (number and cross-linking) of strands differs among various tissues. It was long thought that the number of strands correlated with the resistance of the barrier. Consistent with this, in the small intestine, the complexity of strands increases at the crypt-to-villus transition. Since discovery of the barrier forming proteins, this structure-function correlation has been called into question; the molecular species of claudin in a particular junction appear be an important determinant. However, composition of other transmembrane TJ proteins is also likely to be important in the regulation and development of TJ barrier function. The specific proteins that affect paracellular flux of solutes of varying size need continuing clarification.
Early electron microscopic studies ( Fig. 25.3 B) interpreted the close membrane apposition of adjacent cells at TJ contact points as membrane fusion, and even suggested convergence of the outer leaflets of the lipid bilayer. Supported by studies showing the inability of electron-dense proteins, such as hemoglobin and colloidal lanthanum, to pass through the TJs, these analyses led to the popular view of the TJ as an absolute barrier to paracellular flux. Although commonly thought of as an impermeant seal, it might be more appropriate to compare TJs to sieves. However, the characteristics of the selectively permeable TJ barrier vary widely among different tissues, within different cell types of a single tissue, and in response to physiological and pathophysiological stimuli ( Table 25.1 ). Thus, while the paracellular barrier is most often assessed by electrical conductance, TER, or transepithelial flux of small fluid phase markers, such as mannitol or polyethylene glycol, measurement of only one or two of these parameters provides an incomplete picture of overall barrier function.
Epithelia are classified as “tight” or “leaky” based on their overall electrical resistance. The small intestine, colon, and renal proximal tubule are typical examples of leaky epithelia, while gastric fundus, renal collecting duct, and urinary bladder are “tight” epithelia. In either case, paracellular ionic conductance can be measured by mounting tissue with the mucosal and serosal surfaces facing electrically isolated fluid-filled chambers with current and voltage electrodes on both sides. This is the standard Ussing chamber configuration. An equivalent circuit diagram of the epithelium can be developed as one that includes the transcellular pathway, represented by apical and basolateral membrane resistances series, in parallel with resistance of the paracellular pathway ( Fig. 25.1 ). Because membrane resistances are generally very high, conductance by the transcellular pathway can generally be discounted and the overall resistance interpreted as representing the paracellular pathway. However, this is not the case when transcellular and paracellular resistances are similar. This may occur when plasma membrane conductance is enhanced, as can occur when large numbers of ion channels, such as CFTR, are opened, or, alternatively, when paracellular resistance is very high, as in urinary bladder. Nevertheless, in most cases, the effects on overall transepithelial resistance reflect changes in paracellular resistance. Although the TJ and lateral intercellular space are arranged in series such that both contribute to paracellular resistance, the contribution of the lateral intercellular space is small and usually ignored. Since membrane resistances are generally high, it is the variation in TJ resistance that determined whether an epithelium is leaky or tight.
Electrophysiologic studies prior to about 1960 primarily used tight epithelia, such as frog skin and urinary bladder, where the paracellular resistance is very high (more than 1500 Ω cm 2 ). This reinforced the misconception that the TJ is an impermeant barrier. Such studies also provided evidence that barrier function requires viable tissue, as the progressive tissue injury following devascularization correlated with decreases in overall electrical resistance. Because leaky epithelia, such as gallbladder and small intestine, always demonstrated low resistance, it was assumed that this was due to tissue fragility and damage during mounting. Despite this interpretation, the high-conductance pathway across leaky epithelia displayed charge and size selectivity, which would not be expected if the “shunt” pathway was simply a result of tissue damage. The controversy was settled when the shunt pathway was localized to the paracellular space using conductance-scanning methods. By passing a microelectrode over the gallbladder epithelial surface a high conductance shunt could be demonstrated at the intercellular junctions. Use of smaller electron microscopic tracers, such as ionic lanthanum, also allowed ultrastructural visualization of permeation through the TJ. These findings led to a paradigm shift in which the idea of a selectively permeable TJ was accepted. Work over the past 40 years has characterized these permeability properties in detail and resulted in our current understanding of TJs as barriers that contain several classes of transport channels that can be distinguished in both functional and molecular terms.
Experimentally, ion selectivity is often measured as a ratio of cation to anion permeability. As sodium and chloride are the most common cation and anion, respectively, in physiological solutions, ion selectivity is most commonly reported as (PNa + /PCl − ) ( Table 25.1 ). However, care must be taken in interpreting PNa + /PCl − values, as the ratio in solution is 0.66 due to the smaller size of hydrated sodium, relative to chloride, ions. Thus, PNa + /PCl − values above 0.66 should be considered cation selective. This is true of most epithelia, although the actual ratio can vary 30-fold between anatomical sites. While this is of great physiological significance, it should be recalled that, relative to transmembrane ion channels, this level of discrimination between anions and cations is very low. Further, such transmembrane ion channels easily distinguish ions with similar size and charge densities, such as Na + and K + , while the ability of TJs to separate these is very poor. However, monovalent and divalent cations are treated differently by some TJs, and, as discussed below, we now know that this and overall ion selectivity reflect the composition of TJ in terms of claudin family members.
Charge selectivity is a characteristic feature of paracellular barriers and is essential for creating transepithelial gradients that direct passive paracellular transport. For example, many active transcellular absorptive processes within the intestine take advantage of the electrochemical gradient created by the Na + /K + ATPase. However, while the Na + /K + ATPase pumps Na + to the basolateral space, Na + -nutrient cotransport occurs at the luminal, or apical, plasma membrane. This requires that free luminal Na + be sufficient to drive these processes. Thus, if there was no effective means of serosal to mucosal Na + flux, Na + poor diets would result in malabsorption and osmotic diarrhea. Given that there are no defined mechanisms for transcellular Na + secretion (into the lumen), it stands to reason that this transport must occur by the cation-selective paracellular route that favors Na + over Cl − , which is the other most abundant ion in physiological settings. Defects in paracellular Na + transport may, in part, explain the abnormalities that occur when claudin-15, which enhances paracellular Na + flux, is knocked out in mice. Although inherited claudin mutations have not been associated with GI disease in humans, mutations that disrupt trafficking or expression of claudins 16 and 19 in the thick ascending limb of the renal tubule result in failure of paracellular Ca 2 + and Mg 2 + absorption and cause the autosomal recessive disease familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Nevertheless, alterations in expression and trafficking of claudin proteins are present in intestinal diseases and can be induced by specific inflammatory mediators. Whether these changes contribute to disease pathogenesis or, alternatively, represent an adaptive response, is an area in need of further study. In this respect, a recent study demonstrated that claudin-2-mediated enhanced water efflux offered relief from mucosal pathogen colonization, prolonged pathogen shedding, excessive cytokine responses, and major tissue injury in experimental Citrobacter rodentium colitis model.
In addition to charge selectivity, the TJ discriminates between solutes on the basis of size. This property has been recognized for over 30 years, but, for the most part, detailed analysis has not been possible due to the limited number of probes available. This obstacle has been overcome using mixtures of polyethylene glycol oligomers with hydrodynamic radii ranging from ~ 3 to ~ 7 Å. The relationship between size and apparent permeability of these probes changed sharply at radii of ~ 4 Å, indicating a size-restricted population of paracellular pores. This is similar to the ~ 6 Å pores reported within small intestinal villous epithelium and may reflect the same structure. Notably, recent data have shown that claudin-2 expression, which enhances paracellular flux of monovalent cations, also increases flux of polyethylene glycol oligomers with radii less than 4 Å. Thus, it appears that a claudin-based class of paracellular channels defines overall charge selectivity as well as size selectivity of this route, which has been termed the pore pathway.
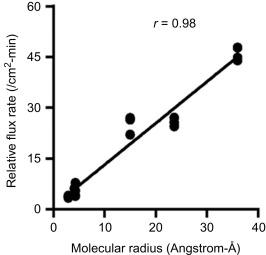
Despite the claudin-2-based cutoff at ~ 4 Å radii, larger polyethylene glycol oligomers are still able to traverse epithelial monolayers, albeit at much lower rates than smaller solutes. The magnitude of this component of paracellular flux does not vary substantially with solute radius, suggesting that these molecules traverse a size nonrestricted route that is distinct from the claudin-based pore pathway. The route of large solute flux has been referred to as the leak pathway and may correlate with the 50–60 Å channels described within crypt epithelium. Enhanced leak pathway flux is the means by which IFN-γ and TNF-α increase paracellular permeability, and it has, therefore, been proposed that leak pathway regulation is primarily associated with pathologic processes. While this may be true, the converse is not, as some inflammatory mediators, for example, IL-13, which enhances claudin-2 expression, increases flux across the pore pathway without affecting the leak pathway. Based on recent studies, occludin-dependent regulation of leak pathway is now clearly recognized. Transient occludin depletion in mature Caco-2 cell monolayers was shown to cause a progressive size-dependent increase in paracellular flux rate (three- to fourfold increase for urea (molecular radius 2.9 Å), five- to sixfold increase for mannitol (molecular radius 4.1 Å), ∼ 25-fold increase for inulin (molecular radius 15 Å), ~ 25-fold increase for 10-kDa dextran (molecular radius 23 Å), and ~ 45-fold increase for 70-kDa dextran (molecular radius 36 Å) ( Fig. 25.4 ). These findings were supported by another study showing charge-nonselective reduction in macromolecular flux with stable knockdown of occludin in Caco-2BBe cells. In the same study, C-terminal occludin/ELL domain was shown to be critical for anchoring and exchange of occludin at the TJ. Although occludin knockdown has been shown to have differential effect on the expression of claudins, possible due to different approaches and heterogenicity of cell lines, the contribution of claudins to the noncharge selective, large-size leak pathway in the absence of occludin is unclear.
Myosin light chain kinase (MLCK), which causes contraction of peri-junctional actomyosin, is also a key regulator of TJ permeability. Close association of TJ with the cytoskeleton described by Madara was later shown to be the basis for cytoskeletal control of tight-junction permeability. Under physiological conditions, MLCK-induced increase in pore pathway permeability during Na + glucose cotransport is associated with condensation of peri-junction actomyosin. Under physiopathological conditions, such as TNF-α-mediated increase in TJ permeability, the enhancement in leak pathway permeability is associated with MLCK-dependent occludin endocytosis. While MLCK activation do not affect pore pathway directly, in vivo secondary immune activation in constitutively active MLCK expressing mice has been shown to increase claudin-2 expression and alter TJ Na + /Cl − selectivity in IL-13-dependent way. Also, proinflammatory cytokines are known to increase leak pathway permeability in MLCK transcription and activation-dependent way, as discussed in detail, later in this chapter.
Beginning in 1986 with identification of ZO-1 as the first TJ-associated protein, there has been continuous growth to almost 40 distinct proteins or protein families ( Table 25.2 ). Surprisingly, this number far exceeds the known components of other intercellular junctions, such as adherens, desmosomes, and gap junctions ( Fig. 25.2 ), and it is likely that many identified TJ proteins serve regulatory, rather than structural functions. This is certainly the case of enzymes, such as atypical protein kinase C isoforms and MLCK, which localize to the TJ and have been shown to regulate epithelial polarity, junction assembly, and barrier function. These will be discussed later in the context of specific regulatory events. In contrast, it is now clear that claudin proteins define the charge, and size selectivity of the TJ barrier. The specific roles played by other TJ-associated proteins, such as ZO-1 and occludin are less certain, but recent data have shed light on their functions in TJ stabilization and barrier regulation. Through an interesting evolutionary convergence, many tight and adherens junction proteins also serve as receptors or coreceptors for pathogen entry. Finally, polarity complexes are concentrated at the TJ.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here