Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Thyroid disease can present with overt symptoms, insidiously, or with isolated thyromegaly. Thyroid disease in children can encompass isolated biochemical abnormalities that have little or no physiological consequence, or with overt clinical symptoms. Clinically, hypothyroidism occurs more commonly than hyperthyroidism. Thyroid nodules and masses occur much less commonly than functional disorders but can portend the presence of thyroid cancer. Box 13.1 provides a classification of thyroid disorders in children. This chapter focuses on the most common conditions that affect the thyroid gland of children and adolescents.
Hashimoto thyroiditis, juvenile acquired hypothyroidism
Stimulating antibody, Graves disease
Blocking antibody, hypothyroidism
Suppurative thyroiditis
Subacute thyroiditis
Complete TBG deficiency
Partial TBG deficiency
TBG excess
Transthyretin variants
Loss-of-function hypothyroidism
Gain-of-function hyperthyroidism
Thyroid hormone beta receptor (TRβj) mutations
Peripheral tissue resistance syndrome
Pituitary resistance syndrome
Thyroid hormone membrane transport defects
Goiter
Mental impairment
Cretinism
Nonthyroidal illness
Thyroid neoplasia
Adenoma
Nonfunctional
Functional
Papillary-follicular carcinoma
Medullary carcinoma
MEN2A, 2B, Ret mutations
Sporadic
Undifferentiated
Metastatic
Few hormones exert as profound and essential role in human physiology as thyroid hormones. The major hormones released by the thyroid gland include tetraiodothyronine, or thyroxine (T4), and triiodothyronine (T3). The production of these hormones involves several discrete biochemical steps that are shown in Fig. 13.1 . Of these hormones, T3 plays the pivotal role in affecting physiology, being the molecule that principally binds to the thyroid hormone receptor (TRs). The thyroid hormone nuclear receptor belongs to the steroid hormone–retinoic acid receptor superfamily and is a regulator of deoxyribonucleic acid (DNA) transcription.
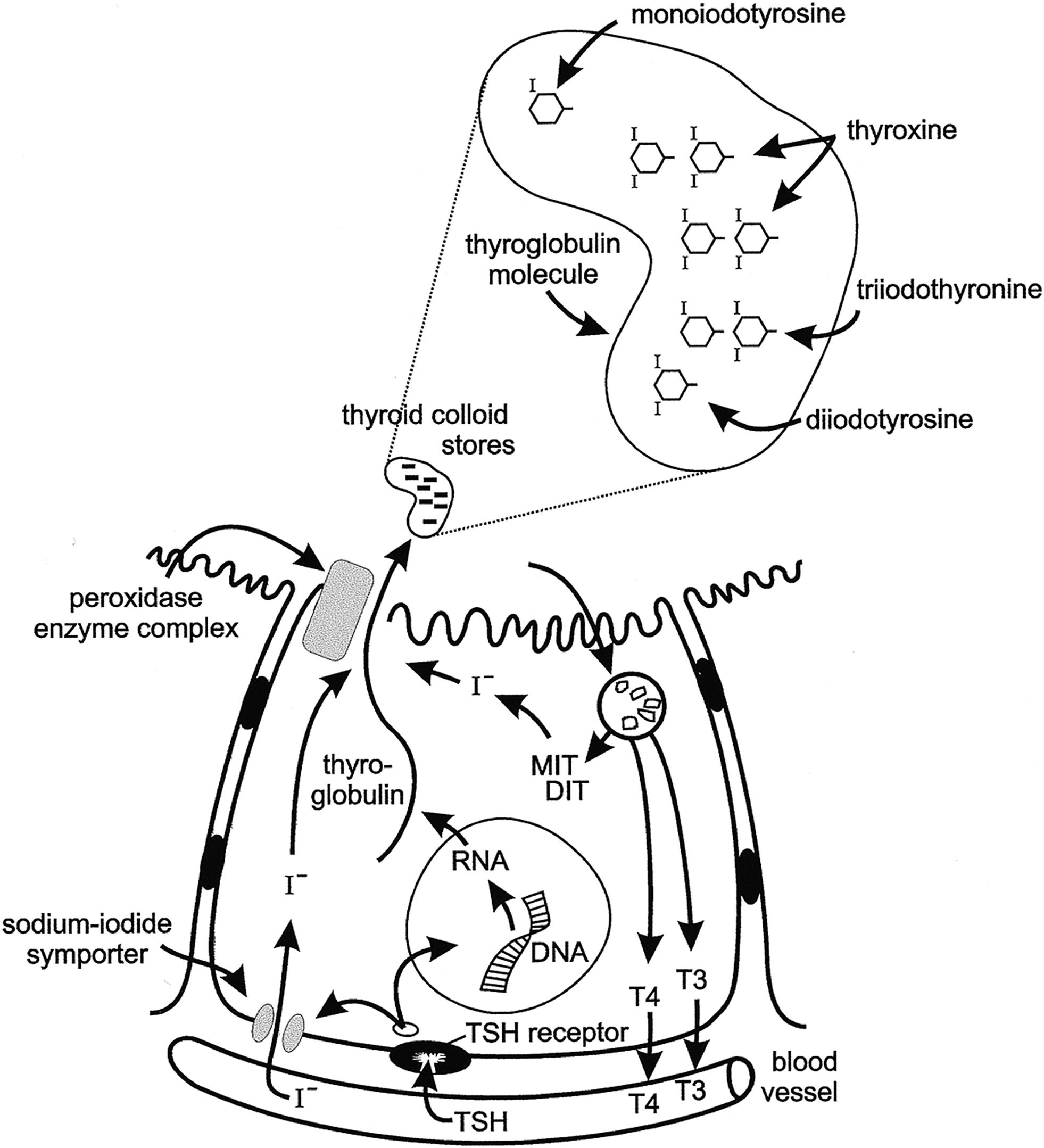
Two genes encode the TR; one on chromosome 17 designated alpha ( TRa ) and one on chromosome 3 designated beta ( TRb ). The TRs can exist as monomers or homodimers, and they can dimerize with other members of the family of nuclear receptors. After T3 binding to the TR, gene transcription is regulated in many tissues.
T4 is the predominant hormone released from thyroid follicular cells. After release, it circulates in protein-bound and free states at a ratio of about 1000 to 1. Thyroid hormone-binding proteins in the blood include thyroxine-binding globulin (TBG), prealbumin or transthyretin, and albumin. TBG is the predominant carrier protein for T4; TBG and albumin also carry T3. In the euthyroid study, the circulating concentration of free T4 (FT4) and free T3 are about 0.03% and 0.30%, respectively, of total hormone concentrations.
It is important to recognize that circulating levels of thyroid hormones and carrier proteins change with age ( Tables 13.1 and 13.2 ). Absolute mean free T4 and free T3 concentrations are about 10 and 4 pg/mL, respectively, and differ according to age. In adolescents and adults, the plasma concentrations of the several binding proteins are 1 to 3 mg/dL for TBG, 20 to 30 mg/dL for thyroxine-binding prealbumin, and 2 to 5 g/dL for albumin. TBG concentrations are greater in children than in adults, and they decline to adult levels during adolescence. Because the thyroid hormone-binding proteins are produced in the liver, they are acute phase reactants, with concentrations increasing during acute illness, they also increase in response to estrogen exposure.
| Age | TSH a (μU/mL) | T4 b (μg/dL) | TBG b (mg/dL) | Tg b (ng/mL) |
|---|---|---|---|---|
| Cord blood | 1–20 | 6.6–15 | 0.8–5.2 | 15–101 |
| 1–7 days | 1–39 | 11–22 | 0.8–5.2 | 1–110 |
| 1–4 weeks | 0.5–6.5 | 8.2–17 | 0.6–5 | 11–92 |
| 1–12 months | 0.5–6.5 | 5.9–16 | 1.6–3.6 | 12–113 |
| 1–5 years | 0.6–8 | 7.3–15 | 1.4–2.8 | 5–72 |
| 6–10 years | 0.6–8 | 6.4–13 | 1.4–2.8 | 3–40 |
| 11–15 years | 0.6–8 | 5.5–12 | 1.4–2.8 | 3–40 |
| 16–20 years | 0.5–6 | 4.2–12 | 1.4–2.8 | 2– 36 |
| 21–50 years | 0.5–6 | 4.3 –12 | 1.2–2.6 | 2–35 |
| T3 a (ng/dL) |
rT3 a (ng/dL) |
Free T4 b (ng/dL) |
Free T3 b (pg/mL) |
|
|---|---|---|---|---|
| Cord blood | 14–86 | 100–501 | 1.2–2.2 | — |
| 4–7 days | 36–316 | 34–258 | 2.2–5.3 | 1.3–6.1 |
| 1–4 weeks | 105–345 | 26–290 | 0.9–2.3 | 2.2–8 |
| 1–12 months | 105–245 | 11–129 | 0.8–2.1 | 2.5–7 |
| 1–5 years | 105–269 | 15–71 | 0.8–2 | 2.8–5.2 |
| 6–10 years | 94–241 | 17–79 | 0.8–2 | 2.8–5.2 |
| 11–15 years | 83–213 | 19–88 | 0.8–2 | 2.9–5.6 |
| 16–20 years | 80–210 | 25–80 | 0.8–2 | 2.4–5 |
| 21–50 years | 70–204 | 30–80 | 0.9–2.5 | 2.4– 4.4 |
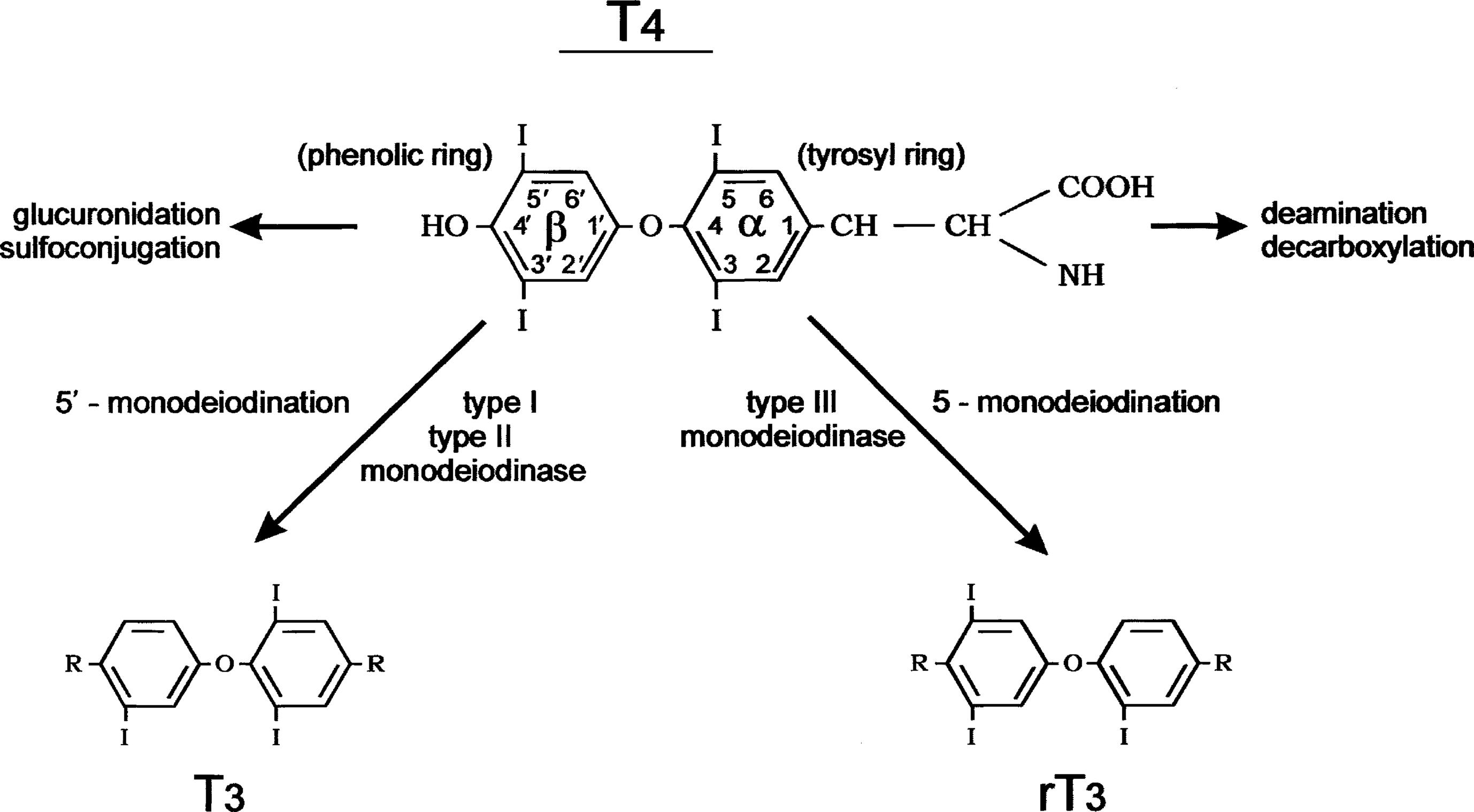
Conversion of T4 to T3 involves the deiodination of T4 ( Fig. 13.2 ). Monodeiodination of the beta or outer ring by monodeiodinase (MD) type II produces T3. Monodeiodination of the alpha or inner ring produces reverse T3 (rT3), which is inactive metabolically. Under normal circumstances, T3 and rT3 are produced at similar rates. About 70% to 90% of circulating T3 is derived from peripheral conversion of T4, and 10% to 30% of circulating T3 is from the thyroid gland. Reflecting the age-related changes in the hormones that regulate T4 stability, the clearance of T4 generally decreases from infancy to adulthood ( Table 13.3 ).
| Thyroxine Kinetic Parameter | Children (3–9 years) | Adolescents (10–16 years) | Adults (23–26 years) |
|---|---|---|---|
| Half-life (d) | 5 (0.13) |
6 (0.35) |
6.7 (0.30) |
| Fractional clearance b | 0.14 (0.005) |
0.12 (0.008) |
0.11 (0.004) |
| Distribution volume (L/kg) | 0.16 | 0.16 | 0.12 |
| (0.008) | (0.014) | (0.005) | |
| Thyroxine turnover (μg/kg/day) | 1.9 (0.09) |
1.5 (0.07) |
1.1 (0.06) |
The production of T4 and T3 within the thyroid gland is regulated by the thyroid-stimulating hormone ([TSH]; also called thyrotropin ), which is released from the anterior pituitary gland ( Fig. 13.3 ). TSH receptors are present on thyroid follicular cells and are G protein–coupled receptors with a large extracellular amino terminus. Mutations of the TSH receptor can result in constitutive activation of the receptor with severe hyperthyroidism, whereas inactivating mutations result in TSH unresponsiveness and hence hypothyroidism.
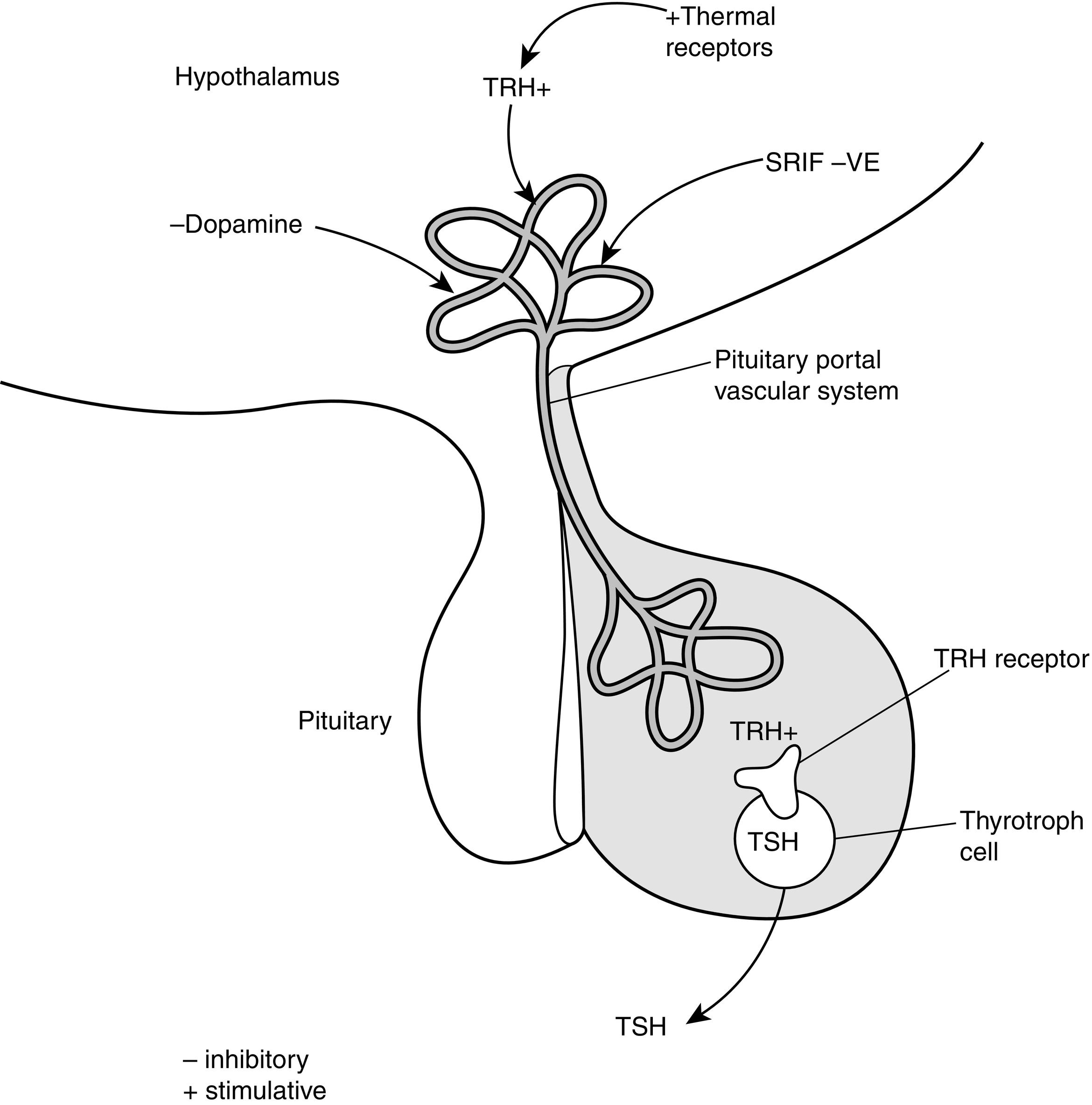
TSH receptor activation stimulates adenylate cyclase accumulation within follicular cells, which in turn causes accumulation of cyclic adenosine monophosphate (cAMP). Increased cellular concentrations of cAMP promote iodide trapping, iodotyrosine synthesis, thyroglobulin (TG) synthesis, and hormone release.
TSH release is regulated by the hypothalamic hormone thyrotropin-releasing hormone (TRH) (see Fig. 13.3 ). This peptide hormone is produced in medial neurons of the paraventricular nucleus of the hypothalamus and is released into the portal circulation of the pituitary gland. Several different neurotransmitters have been observed to influence TRH release.
In addition to normal regulation of TSH receptor activity by TSH, thyroid function can be adversely affected by antibodies that can either stimulate or block TSH action. TSH receptor–stimulating immunoglobulin antibodies (TSIs), or thyroid receptor antibodies (TRAbs), are present in the circulation of individuals with Graves disease (GD) and activate TSH receptors. Conversely, TSH receptor–blocking immunoglobulins (TBIs) antagonize TSH action and can lead to hypothyroidism.
Thyroid disease can present with overt symptoms, insidiously, or with isolated thyromegaly. Thus evaluation of the thyroid gland should be included in the routine examination of children. The thyroid gland can be visualized by having the patient look to the ceiling and swallow. As the thyroid moves, the margins of the gland are viewed to estimate size and symmetry. The thyroid should be palpated to assess size, consistency, and symmetry. This can be performed with the clinician standing behind the patient and palpating the neck with the fingertips. The texture of the thyroid can be assessed to determine if it is smooth or irregular and if nodules are present, which may feel firm or soft. If any asymmetry or abnormal thyroid fullness is noted, ultrasonographic evaluation is recommended because pathological thyroid nodules may feel like normal tissue.
To assess gland size, one may estimate the size of each thyroid lobe relative to that of a teaspoon (5 g) or a tablespoon (15 g). In general, until the end of puberty, gland size (in grams) approximates the patient's age in years times 0.5 to 0.7. Thus each thyroid lobe of a 10-year-old child is approximately one-half of a teaspoon for a total gland size of 5 to 7 g. For teens and adults, each lobe of the thyroid may reach one teaspoon in size for an approximate total gland size of about 10 g.
In newborns and young infants, the thyroid can be examined by placing the infant supine on the parent’s lap, with the head toward the parent’s knees. The head can then be gently lowered backward to expose the neck, which facilitates thyroid palpation. If the examiner can palpate each ring of the trachea from the sternal notch to above the larynx, then the absence of pretracheal thyroid tissue is suggested, which is observed. Such an absence occurs in cases of failure of thyroid formation or migration. Failure to detect pretracheal thyroid tissue in older children warrants visual examination of the base of the tongue for ectopic thyroid tissue.
When a sublingual thyroid gland is discovered late in childhood or in adolescence, the tissue should be palpated with a gloved finger, during regular office visits, because nodules and malignancies may develop in ectopic thyroid glands. In contrast, when an ectopic thyroid is detected in infancy and replacement therapy is started, the residual thyroid tissue becomes atrophic and does not present long-term problems.
Approximately 97% of the thyroid hormone released from the thyroid gland is T4. After its release, less than 1% of T4 remains free. The rest of the thyroid hormone circulates bound to the proteins thyroglobulin (TBG; 70%), prealbumin (transthyretin; 10%), and albumin (15% to 20%) . T3 is also released from the thyroid and is generated peripherally. Although T4 constitutes the bulk of circulating thyroid hormone, T3 has far greater affinity for nuclear TRs and exerts most of the potent cellular effects of thyroid hormone action.
Thyroid function can be assessed by measurement of total T4 and total T3 levels, along with indices that reflect thyroid hormone-binding proteins (T3 or T4 resin uptake). The levels of estimated free (unbound) T4 (FT4) are measured to assess thyroid hormone status, without the confounding influences of carrier proteins.
Several conditions are seen in which thyroid hormone levels are abnormal, yet the individual is euthyroid. Because of their confusing nature, these conditions may result in the patient being erroneously diagnosed or treated for hypothyroidism or hyperthyroidism.
When FT4 values are normal, yet total T4 values are high, familial dysalbuminemic hyperthyroxinemia needs to be considered. This autosomal dominant disorder is most commonly seen in Hispanic individuals and can be diagnosed by thyroid hormone-binding protein electrophoresis. If FT4 values are normal but total T4 values are low, the possibility of TBG deficiency must be entertained. TBG deficiency is an X-linked disorder that may be associated with color blindness. In these and other conditions affecting thyroid hormone binding, treatment is not needed and the patient should be educated about the condition to avoid treatment by unsuspecting practitioners.
T4 is much more abundant in the circulation and T3 is the more metabolically active thyroid hormone. T3 is produced peripherally from T4 and is also secreted by the thyroid. A metabolically inactive form of T3', reverse T3', is also produced, and its level is elevated in conditions, such as euthyroid sick syndrome, or nonthyroidal illness syndrome (see Fig. 13.2 ).
It is important to consider that estimated FT4 values may not be accurate in infants because of the fact that circulating levels of TBG are elevated, confounding biochemical assessment in this age group. FT4 levels determined by equilibrium dialysis assay, though, are accurate.
Ultrasensitive thyrotropin or TSH assays have been developed, and assessment of TSH has greatly improved the evaluation of thyroid status. TSH levels help to distinguish many thyroid disorders that present with either low or high T4 levels in most cases. TSH values within the normal range for the assay are indicative of a euthyroid state if the hypothalamic pituitary axis is intact. Elevations of TSH generally indicate primary thyroid dysfunction; suppressed TSH values indicate hyperthyroidism. When both FT4 and TSH levels are elevated, TSH-producing pituitary adenomas and thyroid hormone resistance need to be considered.
Hyperthyroidism is distinguished from subclinical hyperthyroidism, a condition in which levels of T4, FT4, and T3 are normal, but TSH levels are suppressed in the absence of thyroid overt disease. The causes of subclinical hyperthyroidism are similar to those of overt hyperthyroidism. Thus it is important to reevaluate individuals with isolated suppression of TSH levels every 3 to 6 months, until the clinical situation declares itself.
Critical in the interpretation of TSH levels in children is recognition that the normative range differs from that of adults (see Box 13.1 ), which is defined by an upper limit values of about 4 μU/mL or less. In comprehensive studies of this issue, the upper limit of TSH values in healthy children and adolescents without thyroid disease is about 7 μU/mL. The application of adult reference range to children thus results in the erroneous diagnosis of subclinical hypothyroidism and results in the unnecessary referral of children for subspecialty care by primary care providers.
Disorders of the thyroid lead to hypothyroidism much more commonly than to hyperthyroidism. Hypothyroidism may be present at birth, acquired during childhood or adolescence, present with or without symptoms, or present gradually or acutely.
It is commonly believed by the public and many practitioners that hypothyroidism is associated with and is a cause of obesity, yet there is little support for the notion that the hypothyroid state contributes to obesity. It is also important to note that TSH levels are slightly higher in obese individuals than nonobese individuals. With weight loss, TSH levels normalize in these children. Thus slight elevations in TSH in obese individuals are physiological and do not warrant therapy.
Hypothyroidism may be elusive, with symptoms elicited only in retrospect. In the extreme, hypothyroidism can be associated with cold intolerance, bradycardia, carotenemia, coarse and brittle hair, dry skin, pallor, and myxedema. These symptoms may not be distressing, which allows prolonged hypothyroidism to escape detection.
The most common causes of hypothyroidism in children are autoimmune processes resulting in Hashimoto thyroiditis. Autoimmune thyroiditis also leads to juvenile acquired hypothyroidism that can present with growth failure when chronically present. Hypothyroidism in children can be caused by iodine exposure or hypothalamic pituitary dysfunction. Other causes of hypothyroidism include exogenous goitrogens, cystinosis, acute thyroiditis, and thyroid irradiation during cancer treatment. Hypothyroidism in the newborn is a serious health concern and is detected by newborn screening programs.
Autoimmune thyroiditis with thyroid enlargement is one of the most common presentations of childhood thyroid disease. It is associated with antibodies against thyroglobulin and thyroperoxidase and is characterized by lymphocytic infiltration of the thyroid gland, which results in thyromegaly. Depending on the nature of the antithyroid antibodies, Hashimoto disease may be associated with a euthyroid state, hypothyroidism, or transient hyperthyroidism.
Hashimoto thyroiditis may rarely occur in very young infants, and typically presents in adolescents, affecting females more than males. The thyroid gland is usually diffusely enlarged and may have an irregular, cobblestone feel. Asymmetric thyroid enlargement, mimicking a thyroid nodule, may be noted. The presence of antithyroid antibodies and the absence of nodules on ultrasonography can distinguish inflammation from other pathologic processes.
Importantly, the presence of antithyroid antibodies does not portend the development of complete or partial thyroid failure that will warrant therapy. In the healthy adult population, up to 5% of individuals had circulating antithyroid antibodies that are present. Less than 10% of these individuals will develop hypothyroidism, with those having elevated antithyroperoxidase (anti-TPO) antibodies being much more at risk than those having anti-TG antibodies.
In children, the incidence of antithyroid antibodies at a population level is not generally known. Of those children with antithyroid antibodies, about 20% are reported to develop hypothyroidism needing therapy. These children often have very high antithyroid antibody titers. Thus if a child is found to have low levels of antithyroid antibodies, it is reasonable to assess thyroid indices every 6 to 12 months and initiate therapy when the TSH rises above the upper limit of normal for children. However, if high titers are present at presentation, it is reasonable to initiate therapy at that point.
Untreated in some children, Hashimoto thyroiditis can result in progressive thyromegaly and hypothyroidism. Treatment with levothyroxine prevents hypothyroidism and the TSH elevations that stimulate gland enlargement. When T4 levels are modestly depressed (> 5 μ/dL) or normal, treatment can be initiated with 1 to 2 mcg/kg/day of levothyroxine. If profound hypothyroidism is present, pseudotumor cerebri may develop when children are treated with conventional doses. Thus treatment is often initiated with one-third to one-half of the usual dose of levothyroxine. After 2 to 4 weeks, the patient can be advanced to conventional doses. However, children with profound hypothyroidism can develop pseudo tumor cerebri, even if treatment is initiated with low doses of levothyroxine.
Interestingly, it has been recently reported that severe hypothyroidism in children with TSH elevations > 500 μU/mL, experience complete resolution of the hypothyroid state. Based on the experience of others, this happenstance is very uncommon.
Although it has been reported that there are some differences in the oral bioavailability of different levothyroxine preparations, from a practical vantage, these differences are small. Thus the routine use of less expensive generic compounds versus more expensive brand-name products is justified.
The timing of levothyroxine ingestion has been the subject of study. Taking the medication at bedtime is associated with higher T4 levels and lower TSH levels over the course of the day. This is believed to be related to better gastrointestinal absorption in the evening than day.
The suggestion has also been made that hypothyroidism in adolescents can be treated with a single dose given weekly. This approach is not recommended, however, because thyroid hormone levels are high, shortly after the dose is administered, and are low by the week’s end. Treatment of congenital hypothyroidism with weekly doses of levothyroxine also can result in mental retardation. It is also recognized that excessive soy intake can interfere with the absorption of levothyroxine.
Of note, other potential therapies that may theoretically alter the autoimmune process have been tested. No proven benefit has been observed in patients who have taken selenium.
Uncommonly, patients may present with Hashitoxicosis, in which immunologic destruction of thyroid tissue results in the release of preformed thyroid hormone, which leads to elevated T4 levels. In contrast to GD, hyperthyroidism is transient, eye findings are absent, radionuclide uptake is low, and elevated levels of thyroid-stimulating immunoglobulins are not present.
Hashimoto thyroiditis may be associated with other autoimmune diseases, including diabetes mellitus, adrenal insufficiency, vitiligo, and hypoparathyroidism. Autoimmune thyroiditis is also seen in patients with inflammatory bowel disease and juvenile arthritis. Annual surveillance of thyroid gland size and TSH levels should thus be considered for children with other autoimmune problems, and clinicians should be vigilant for signs of hyperthyroidism or hypothyroidism. Conversely, children with autoimmune thyroiditis should be observed for signs of diabetes mellitus and Addison disease.
The incidence of celiac disease in the setting of Hashimoto thyroiditis is about 1%. If patients manifest abdominal discomfort, weight loss, or gastrointestinal symptoms, Celiac disease screening should be performed, but it does not need to be done routinely in children with thyroid disease. We have found a 1% incidence of autoimmune liver disease in children with autoimmune thyroid disease. Because such liver disease can be occult, we annually assess circulating transaminase levels, and if levels are elevated, evaluation of possible liver disease is initiated.
Several groups of children are at risk for autoimmune thyroiditis. Because girls with Turner syndrome are predisposed to autoimmune thyroiditis, TSH levels should be assessed annually. Turner syndrome should also be considered in girls with hypothyroidism, especially if the child is prepubertal at presentations. Children with Down syndrome also warrant annual screening for hypothyroidism.
Subclinical hypothyroidism refers to a condition in which circulating T4 and T3 levels are normal, but TSH levels are elevated. As noted earlier, many children are erroneously diagnosed with this condition when TSH levels are found to be elevated relative to adult reference range values. However, if proper pediatric-based TSH levels are applied, the vast majority of children so diagnosed will not have hypothyroidism. Thus some experts have questioned if subclinical hypothyroidism is a real entity in children.
Studies of children with mild TSH elevations (5–10 μU/mL) reveal that only a small fraction will progress to TSH elevations over 10 μU/mL. Data also show that treatment of children with TSH levels 5 to 10 μU/mL does not exhibit somatic or other benefits when treated with levothyroxine. Thus treatment of children with TSH levels lower than 10 μU/mL is not needed. For children with TSH levels over 10 μU/mL, treatment with low doses of levothyroxine is indicated.
When autoimmune thyroiditis occurs during childhood, it is referred to as juvenile acquired hypothyroidism. In children, severe hypothyroidism can be well tolerated. Thus prolonged hypothyroidism may not be detected until growth failure occurs.
Because untreated infantile hypothyroidism is associated with mental retardation, the assumption is often made that juvenile hypothyroidism is associated with learning problems and poor academic performance. This notion is not correct, as children with juvenile hypothyroidism can be successful academically and do not manifest overt learning problems related to the hypothyroid state, nor cognitive impairment.
Children with severe hypothyroidism may manifest cold intolerance, decreased frequency of bowel movements, and decreased physical activity. Bradycardia, facial puffiness, delayed reflexes, and carotenemia may be present. In comparison with Hashimoto thyroiditis, the thyroid gland is either small or only modestly enlarged. Antithyroid antibodies are usually present. These patients are generally not obese, and body mass index values are similar before and after treatment. The development of slipped capital femoral epiphyses may antedate the detection of hypothyroidism.
Some children with juvenile hypothyroidism may present with signs of puberty without pubic hair. Boys may present with testicular enlargement and girls may present with menarche, with or without breast development. With treatment of the hypothyroid state, these characteristics may regress. Available evidence suggests that the hypothyroid state leads to increased gonadotropin secretion, which triggers gonadal activity. In some children, puberty may develop within a year or two of treatment onset, which may limit catch-up growth.
Juvenile hypothyroidism may not be recognized until a sizable statural deficit is present, and the lost height is usually not completely recovered. Children with juvenile hypothyroidism who present with growth failure manifest very low T4 values that are often less than 2 mcg/dL, and profoundly elevated TSH levels that are higher than 250 μU/mL. Hypercholesterolemia and anemia may be present.
The magnitude of the height deficit is proportional to the duration of hypothyroidism, which can be estimated as the difference between the chronologic and bone ages. When the individual is treated with conventional doses of levothyroxine, accelerated skeletal maturation is observed, with the skeletal age advancing disproportionately faster than gains in height. Thus predicted heights fall, and generic growth potential is not achieved.
Because of the poor outcomes of patients with hypothyroidism, we have treated these patients with low doses of levothyroxine (0.25–0.5 mcg/kg/day; e.g., 50 mcg for a 10-year-old). Interestingly, we find that, with low-dose levothyroxine therapy, T4 values normalize (6–7 mcg/dL) within 2 months, and TSH levels normalize or remain only modestly elevated. When serial bone age determinations have been made, we have not observed the disproportionate advancement of skeletal age seen with conventional therapy. However, we do not know if this approach leads to more favorable height outcomes. Some have also suggested that treating these children with gonadotropin-releasing hormone analogs will lead to improved long-term growth. Yet, we have found that catch-up growth slows markedly in some hypothyroid children receiving gonadotropin-releasing hormone analog therapy and predicted adult heights fall and others have not observed added benefit. Because the loss in adult height is proportional to the duration of hypothyroidism, early detection of this disorder is the best intervention for preventing statural deficits.
Iodine is a trace clement that is essential for thyroid hormone formation (see Fig. 13.1 ). Recommended dietary iodine intake is approximately 8 pg/kg, or 100 to 150 pg/day, for adolescents and adults. Although modest iodine intake is essential for thyroid function, high-level iodine exposure results in an acute block in the release of preformed thyroid hormone and impaired thyroid hormone synthesis, a phenomenon referred to as the Wolff-Chaikoff effect . When iodine-induced hypothyroidism is suspected, it can be diagnosed by the detection of high iodine levels in urine samples.
In children, iodine can be absorbed through the skin, and iodine-induced hypothyroidism has been observed after cutaneous iodine or betadine use. We have also observed iodine-induced suppression of thyroid hormone production in children with central intravenous lines, when regular cleansing of the insertion site with iodine was included in central line care. Neonatal hypothyroidism has also been associated with maternal povidone iodine exposure at the time of delivery.
In preterm infants, iodine-induced hypothyroidism warrants special attention, because the suggestion has been made that cutaneous iodine exposure is a major cause of hypothyroidism in premature infants. Fortunately, studies show that iodine-induced hypothyroidism is uncommon in the United States.
Significant iodine exposure also occurs from amiodarone, an antiarrhythmic drug that contains 37% iodine. Hypothyroidism occurs in 10% of individuals treated with this compound. Amiodarone can also reach the fetus by transplacental passage and induce fetal hypothyroidism.
In addition to iodine excess, iodine deficiency also leads to hypothyroidism. Estimates are that more than 1 billion people worldwide are at risk for iodine deficiency. Clinically, iodine deficiency is associated with goiter, hypothyroidism, and endemic cretinism.
In the United States, geographic areas of iodine deficiency exist. With the prevalent use of iodized salt, however, the incidence of iodine deficiency has been markedly reduced, and hypothyroidism and goiter caused by iodine deficiency are rare. Of note, the iodine intake in the United States has declined over the past decade, an issue that may have future clinical implications. In Australia, reduction in iodine intake has been recently observed, with potential implications for pregnant and lactating women. The exclusive use of deiodized salt, which includes sea salt, is thus not recommended.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here