Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In 1924, Moschowitz reported a case of a 16-year-old girl who died of a previously undescribed illness characterized by microangiopathic hemolytic anemia (MAHA), petechiae, hemiparesis, and fever. Postmortem examination revealed numerous hyaline thrombi, most prevalent in the terminal arterioles and capillaries of the heart and kidneys. In 1936, four similar cases were reported by Baehr and colleagues, who proposed that the hyaline thrombi were secondary to agglutinated platelets. In 1947, Singer suggested that the term thrombotic thrombocytopenic purpura (TTP) be used to describe this disorder. In 1955, Gasser used the term hemolytic uremic syndrome (HUS) to describe a related syndrome consisting of Coombs-negative hemolytic anemia, thrombocytopenia, and renal failure. The physiology of these two disorders remained a mystery until 1982 when “unusually large” multimers of von Willebrand factor (VWF) were discovered in the plasma of patients with relapsing TTP. The clinical features of Shiga toxin-associated HUS (ST-HUS) were described in five young children who presented with renal failure following a diarrheal illness in 1962. An association between HUS and Shigella infections was recognized in 1975 and E. coli that produce Shiga toxin (Stx) were identified in 1983. These disorders are now collected under the rubric of thrombotic microangiopathies (TMA) , based on their shared features of MAHA, thrombocytopenia, and microvascular thrombotic lesions with resultant organ dysfunction.
MAHA and thrombocytopenia are cardinal signs/features of all TMA syndromes, leading to overlapping clinical and pathologic features. However, a multitude of different pathogenic pathways lead to vascular endothelial injury in these conditions ( Fig. 132.1 ). Most cases of TTP appear to be due to deficiency of a disintegrin-like and metalloprotease with thrombospondin type1 motif, family member 13 (ADAMTS13) either through an autoantibody or a congenital deficiency. This deficiency results in failure to control the interaction of VWF with platelets and subsequent organ dysfunction is a consequence of platelet-rich thrombi formation in the microcirculation (and occasionally in large vessels). The TMA syndrome with MAHA, thrombocytopenia, and renal failure not associated with diarrhea or severe ADAMTS13 deficiency had been called “atypical” hemolytic uremic syndrome (aHUS). This is mainly attributable to a defect in the regulation of the complement mechanism and unregulated deposition of complement factor C3b on cellular surfaces and these are currently designated as complement-mediated HUS (CM-HUS). A rare autosomal recessively inherited form of TMA occurs in patients with defects in the gene encoding diacylglycerol kinase epsilon (DGKε), which results in a shift of endothelial cells to a prothrombotic phenotype. Similarly, mutations in the methylmalonic aciduria and homocystinuria type C (MMACHC) gene results in methylmalonic aciduria and homocystinuria progressing to TMA with renal failure. Finally, in hemolytic uremic syndrome due to Stx-producing E. coli (STEC-HUS), endothelial injury is caused by Stx and inflammatory cytokines, perhaps inciting disease with increased frequency in individuals with specific genetic predispositions. STEC-HUS is the main mechanism underlying infection-associated HUS (IA-HUS), but several other infections may lead to TMA through other mechanisms. With improved understanding of the pathogenesis of TMA and the availability of targeted therapies, distinction among the various TMA syndromes is important.
The differential diagnosis of MAHA and thrombocytopenia is extensive ( Fig. 132.2 ). Vascular damage secondary to severe sepsis, autoimmune disorders (i.e., systemic lupus erythematosus, scleroderma, antiphospholipid syndrome), septic or tumor emboli, immune complex-mediated vasculitis (e.g., infective endocarditis), malignant hypertension, complications of pregnancy (severe pre-eclampsia, eclampsia, HELLP syndrome), cryoglobulinemia, infection with rickettsia, or, more rarely, hemorrhage-inducing viral organisms, may all lead to thrombotic microangiopathy. Occasionally, patients with disseminated intravascular coagulation secondary to malignancy or sepsis present with microangiopathy of sufficient severity to be confused with primary TMA (see Chapter 137 ). In the setting of renal transplantation, a biopsy may be required to distinguish TMA from allograft rejection or recurrence of a preexisting renal vascular disorder. Occasional patients present with acute pancreatitis, acute respiratory distress syndrome, memory and personality changes, or other poorly defined neurologic symptoms, which have a broad differential diagnosis.
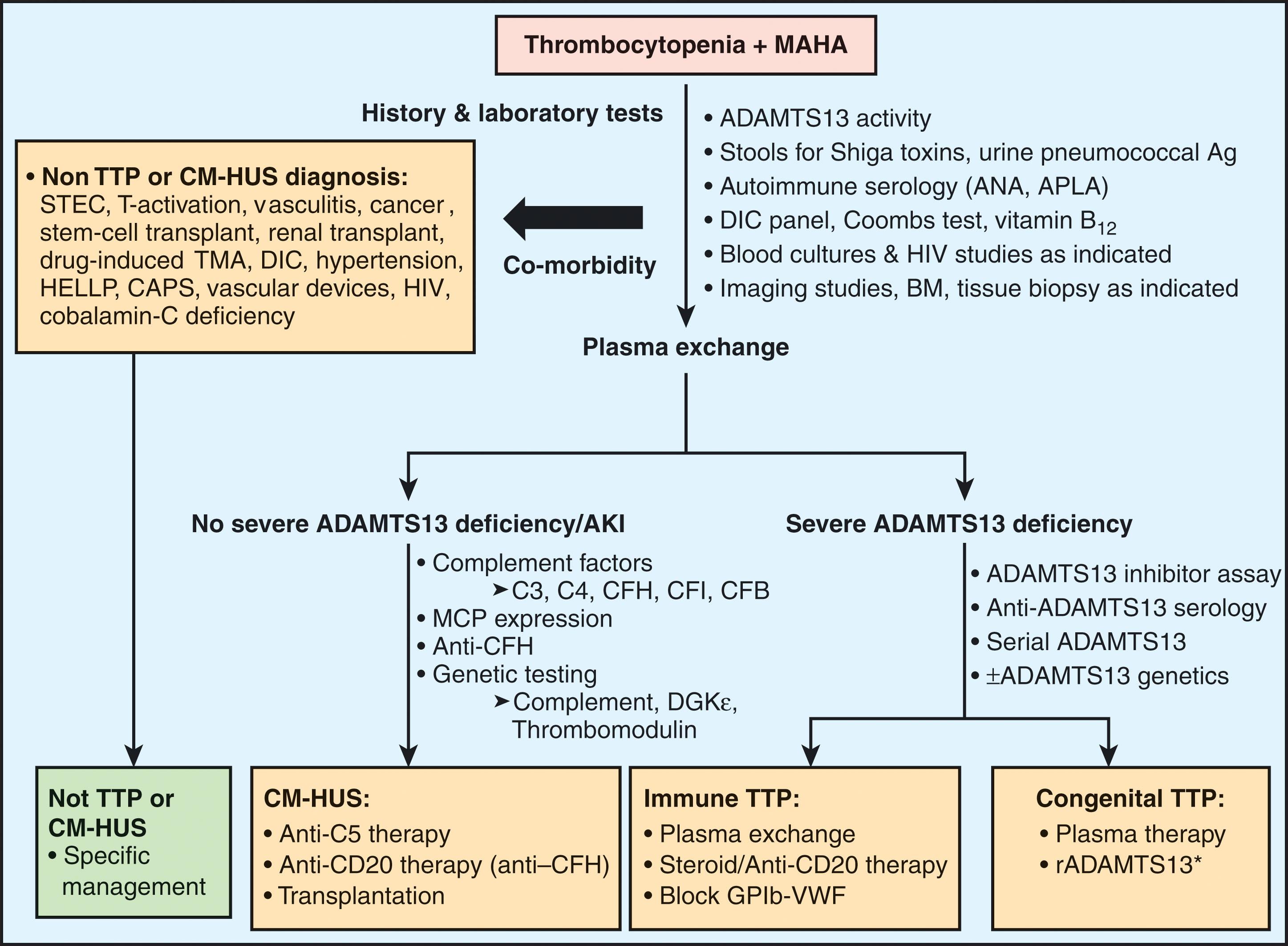
There are clues that have suggested that STEC-HUS examination should include the patient’s age at the time of presentation, ingestion of undercooked ground beef or other food that might have been contaminated by cattle, contact with farm animals, or concurrent clinical presentation by another family member. History of asynchronous presentation in a sibling suggests inherited TTP or CM-HUS. Historic approaches to distinguishing between TTP from CM-HUS relied on the age at presentation and the types of symptoms. However, as demonstrated by trials showing the utility of eculizumab, CM-HUS is not uncommonly diagnosed in adults. Similarly, there is significant end-organ dysfunction overlap between these two disorders. While the kidney remains a major target organ in CM-HUS, renal injury of sufficient severity to require dialysis may occur in up to 10% of patients with TTP. Neurologic symptoms are reported in 25% to 79% of patients diagnosed with TTP but may also be seen in 10% to 30% of patients with CM-HUS.
Choosing the appropriate therapy depends on an accurate diagnosis. Once MAHA and thrombocytopenia have been confirmed by examination of the peripheral smear, other systemic disorders (e.g., sepsis, progression of already established disease or complications of drug therapies) must be excluded. (See box on Management of Complement-Mediated Hemolytic Uremic Syndrome .)
A high index of suspicion is necessary to make a diagnosis of complement-mediated hemolytic uremic syndrome (CM-HUS)
CM-HUS is a systemic disorder
Advanced renal failure, hypertension and increased vascular permeability suggest a diagnosis of CM-HUS rather than thrombotic thrombocytopenic purpura (TTP).
Neurologic and gastrointestinal symptoms are common.
CM-HUS is a diagnosis of exclusion:
TTP should be excluded by ADAMTS13 assay
IA-HUS should be excluded with stool culture and/or tests for Shiga toxin.
Other causes of microangiopathic hemolytic anemia should be excluded with history, coagulation studies, infectious disease studies, serologies, etc.
Plasma studies of C3, C4, and complement proteins (CFH, CHI, CFB) and autoantibody to CFH may be normal, as may genetic studies (CFH, CFI, CFB, C3, MCP, THBD, and DGKε). Initiation of therapy should not be delayed while awaiting these study results.
Treatment of suspected acute CM-HUS:
Plasma exchange is recommended until a diagnosis of TTP is excluded.
Anti-complement therapy (such as eculizumab or ravulizumab) is currently the therapy of choice for CM-HUS.
Vaccination to prevent meningococcal infection is required; antibiotic coverage should be initiated pending immunization effect and chronic antibiotic prophylaxis should be considered.
Early treatment reduced the risk of long-term renal impairment.
Thrombocytopenia and hemolysis tend to respond early, but renal recovery may be delayed.
Subsequent follow-up:
Long-term anticomplement therapy is currently recommended. If anti-complement therapy is discontinued, close follow-up to detect early relapse with re-initiation of therapy (and potential prophylaxis during high-risk intervals) is suggested.
The probability of TTP can be calculated using scoring systems (discussed below) in addition to ADAMTS13 activity testing. In the setting of rapidly progressing renal failure, complement-mediated TMA should be considered because early use of eculizumab has been shown to prevent or reverse kidney injury. STEC infection should be ruled out as soon as possible if infection-ssociated HUS is considered, utilizing appropriate microbiologic studies (stool culture, immunoassay for Stx or fecal PCR). Children and young adults should be tested for a cobalamin C defect because defects in cobalamin metabolism may produce a similar hematologic picture (including thrombocytopenia, presence of bizarre microcytic red cells on the peripheral blood film, and elevations of lactate dehydrogenase [LDH] and bilirubin).
Acquired TTP or immune mediated (iTTP) accounts for 95% of all TTP cases and occurs with an estimated annual incidence of two to six cases per million (variation based on registry), but the incidence appears to be increasing, perhaps because of increased awareness. iTTP has a higher incidence in adults than in children and has a female/male ratio of 2–3:1 and a peak incidence in the fourth decade. Additional risk factors include blood group O, obesity, and African ancestry, which is also associated with an increased risk of relapse. Acquired TTP can occur in association with predisposing conditions such as autoimmune diseases, malignancy, infections, drugs, and pregnancy. TTP has been reported in approximately 0.3% of HIV-infected patients, usually in those with advanced disease.
Pregnancy is a common precipitating factor for both immune TTP (12% to 31% of individuals) and inherited severe ADAMTS13 deficiency. Clinical symptomatic TTP most frequently develops in the second or third trimester, with the decrease in ADAMTS13 levels and increase in VWF and factor VIII that occur in normal pregnancy perhaps acting as precipitants.
Congenital TTP (cTTP), also known as Upshaw-Schülman syndrome, is less common than immune TTP. In general, most estimates of cTTP range from 0.5 to 2 cases per million. This incidence varies based on the region.
TTP occurs when there is an inherited or acquired deficiency of ADAMTS13 (generally defined as less than 10%), leading to increased levels of “unusually large” VWF multimers that induce platelet aggregation in the microvasculature ( Fig. 132.3 ).
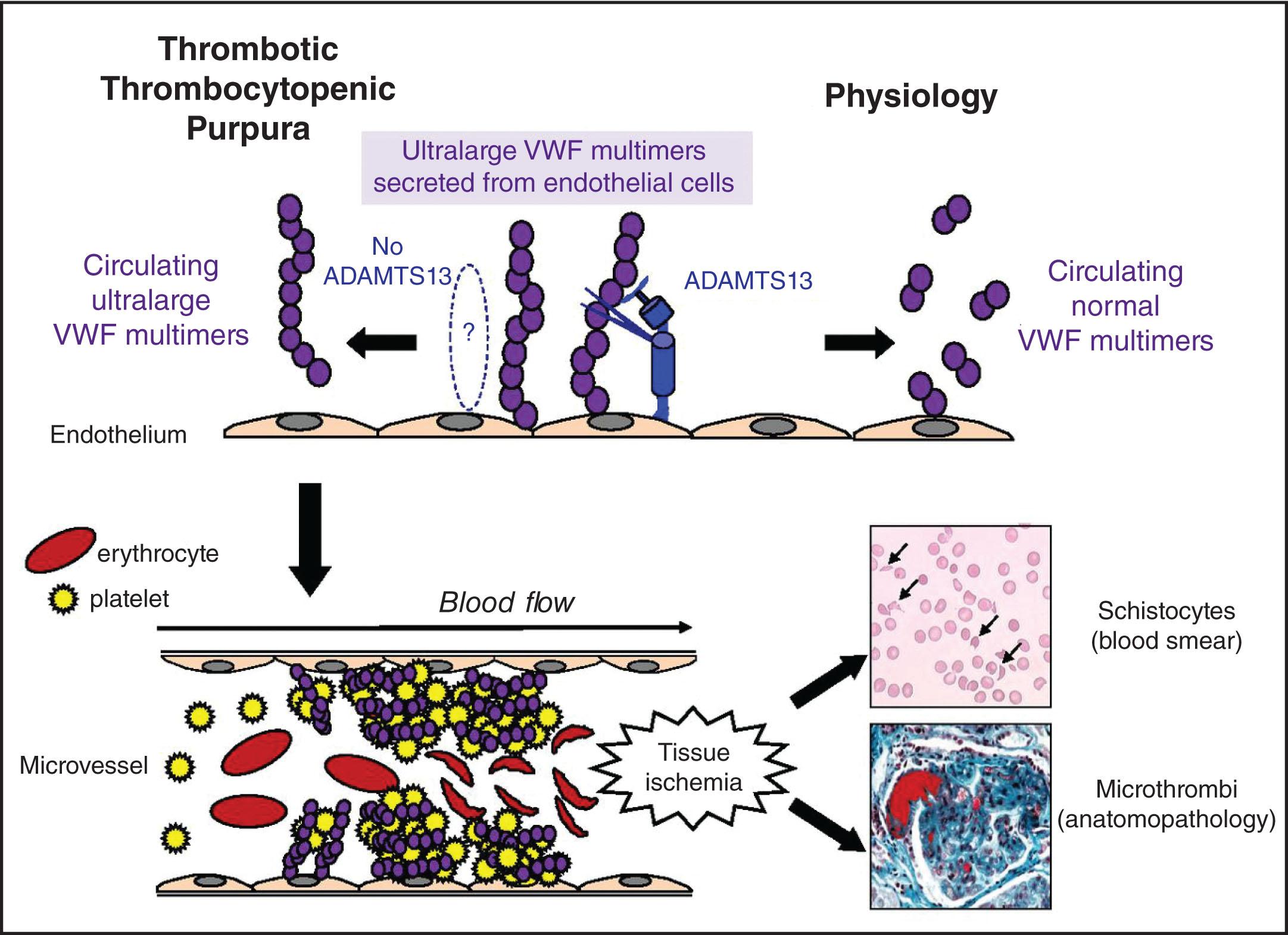
TTP was first linked to VWF by Moake et al., who identified unusually large VWF multimers between disease flares in the plasma of patients with chronic relapsing TTP. He proposed that such patients lacked a plasma enzyme that cleaves these unusually large multimers and that accumulation of abnormally large multimers caused uncontrolled intravascular platelet aggregation, thrombosis, and tissue infarction. Subsequently, two separate investigators identified the plasma metalloprotease that degrades VWF multimers by cleaving the Tyr 1605 -Met 1606 bond in the VWF A2 domain (see Chapter 133 ). This metalloprotease is lacking in patients with TTP, including a subset with a presumably inherited form of TTP, and mixing studies demonstrated the presence of an immunoglobulin inhibitor in many of the non-inherited cases. It was also demonstrated that patients with clinically suspected HUS were not deficient in the metalloprotease, supplying the first clear evidence that the pathogenesis of TTP differs from that of HUS.
The breakthrough in the identification of the VWF cleaving protease happened in 2001 when genome-wide linkage analysis revealed that Upshaw-Schülman syndrome is caused by mutations in the ADAMTS13 gene on chromosome 9q34 (discussed within the inherited TTP section). Almost simultaneously, three other groups working with the VWF-degrading protease (isolated from plasma or from commercial factor VIII/VWF concentrate) identified it as a member of the ADAMTS (A Disintegrin-like and Metalloprotease [reprolysin type] with ThromboSpondin type 1 motif) family of metalloproteases.; the designation 13 indicates that it was the 13th member of the ADAMTS family to be described. ADAMTS13 consists of multiple domains (Metalloprotease, Disintegrin-like domain, Thrombospondin type 1 repeat, Cysteine rich domain and Spacer domain, seven thrombospondin type 1 repeats, and two CUB [Complement components C1r and C1s, sea urchin protein Uegf, and Bone morphogenic protein-1] domains). The protein circulates in a folded conformation with the CUB domains interacting with the spacer domain. This folded structure suggests an autoinhibitory role in which the CUB domains shield exosites on ADAMTS13 to prevent unregulated enzyme activity and VWF proteolysis.
Endothelial cells secrete large VWF multimers that adhere to exposed collagen through their A3 domains. When VWF is tethered to collagen under high shear, these multimers elongate and reveal the platelet binding site for Gp1bα, which is in the A1 domain (see Chapter 123 ). This process facilitates platelet recruitment to the site of injury. While ADAMTS13 is generally maintained in a closed conformation with its CUB domains interacting and shielding the spacer domain, shear forces can open the conformation. Reciprocally induced conformational change in VWF and ADAMTS13 results in exposure of exosites on the enzyme and docking sites on VWF, culminating in regulated ADAMTS13-mediated cleavage of VWF in its A2 domain. Such cleavage reduces the length of VWF multimers and curtails its capacity to form thrombi particularly in areas of high shear (e.g., small arterioles and capillaries).
The factors that trigger sporadic episodes of TTP by causing endothelial damage or activation remain undefined. Some patients with congenital deficiencies of ADAMTS13 may not develop TTP until adulthood, or not at all despite chronic ADAMTS13 deficiency. The latter observations suggest that ADAMTS13 deficiency should be considered an important predisposing factor, but not the sole cause of this syndrome. This concept is supported by studies of ADAMTS13-deficient mice, in which a TTP-like syndrome develops spontaneously in some genetic backgrounds (high VWF levels) but requires a triggering factor, such as Stx, in other strains.
Antibodies (abs) against ADAMTS13 work by inhibiting the proteolytic activity of ADAMTS13 on VWF or by increasing the clearance of ADAMTS13 from plasma by forming immune complexes. Most inhibitory immunoglobulins are directed against the spacer domain of ADAMTS13, but antibodies directed against carboxyl-terminal CUB1-2 and TSP2-8 domains also have been described. Confounding this finding were reports from serologic studies indicating that anti-ADAMTS13 IgG may be present in 5% of healthy individuals, 20% of patients with a TMA other than TTP, 13% of patients with SLE, and 5% of patients with antiphospholipid syndrome. From a mechanistic standpoint, administration of the antibody alone resulted in a clinical syndrome resembling iTTP in baboon models. Work from Roose et al., also suggests that the conformational state of ADAMTS13 is important in the pathogenesis of the disease. Normally, ADAMTS13 is folded on itself in a “closed” and inactive conformation. However, ADAMTS13 opens when it docks with VWF. ADAMTS13 assumes an open conformation in the acute phase of the disease. This is important because, in the closed conformation, the spacer sites of ADAMTS13 are not accessible to autoantibodies. The factors that trigger the open conformation have yet to be discovered; however, one could hypothesize that there is a dynamic process, and that the presence of antibody stabilizes ADAMTS13 in the open conformation. From a clinical perspective, both high antibody levels and lower antigen levels are associated with mortality rates of 17% and 18%, respectively, while the combination appears to have a synergistic effect with a mortality rate of 27%.
Many theories have been proposed to explain the onset of iTTP. The most prominent of these is the link between infections and iTTP. Proposed mechanisms include a two-hit model, cross reaction with CD4 + T cells targeting microbiome derived peptides, variants in TLR-9 being more prone to producing Th1 cytokines and activation of the alternate complement pathway through interaction with unusually large VWF multimers. Since the HLA system has been implicated in the development of autoimmune disease, research efforts have been directed to evaluation of the HLA system as a factor in the development of iTTP. The first association was reported in the 1990s with the antigens DR52 and DR53 from the HLA-DRB3 and HLA-DRB4 genes, respectively. In 2010, two European studies demonstrated that HLA class 2 loci DRB1*11, DRB1*15, DQB1*03, and DQB1*06 were predisposing haplotypes for iTTP. Interestingly, HLA-DRB1*04 and its associated HLA-DRB4-encoded serotype HLA-DR53 were shown to be protective against the development of iTTP. A Japanese group recently reported an association between HLA alleles DRB1*08:03, DRB3/4/5*blank, DQA1*01:03, and DQB1*06:01 and iTTP. The increased incidence of iTTP in persons of African origin may reflect the low prevalence of the protective allele DRB1*04 in this ethnic group.
The classic pentad of signs and symptoms of TTP syndrome include MAHA, thrombocytopenia, neurologic impairment, fever, and renal dysfunction. The complete pentad was most often described before the routine use of therapeutic plasma exchange. Earlier recognition of this syndrome and the established efficacy of plasma exchange, however, have led to the appreciation that MAHA and thrombocytopenia, in the absence of an obvious precipitating condition, are sufficient to make a presumptive diagnosis of TTP. In fact, at diagnosis less than 10% of patients present with the full pentad.
Approximately 10% to 40% of patients with TTP recall an upper respiratory tract infection or flu-like syndrome in the weeks preceding the diagnosis. Patients may present with malaise, fatigue, fever, or other nonspecific symptoms days to weeks in duration and are unresponsive to antibiotics or symptomatic management. The frequency of end-organ involvement is variable with the renal system, GI system, central nervous system, and cardiac system most often affected.
The central nervous system is the most often affected visceral organ at presentation with symptoms ranging from headache or transient confusion to severe symptoms such as seizures, TIA or stroke. Most patients have normal findings on imaging. Gastrointestinal symptoms occur in 35% to 40% of patients and include nausea, vomiting, abdominal pain or diarrhea. Acute renal failure requiring dialysis occurs in up to 5% to 15% of patients and severe renal dysfunction is more common in HUS. An elevated troponin may be identified in up to 70% of patients with TTP and is associated with worse prognosis.
Scoring systems incorporating clinical and laboratory parameters have been developed to enable prompt identification of patients with thrombotic microangiopathy who may benefit from plasma exchange ( Table 132.1 ). These include the French score, the Bentley score and the PLASMIC score. The French score, which was derived from a cohort of 214 patients, has three components, (thrombocytopenia, creatinine level, and ANA) and has a negative predictive value of 93% with a score of zero. The PLASMIC score contains seven components, and stratifies patients into low-, intermediate-, and high-risk categories. It was developed to predict the likelihood of ADAMTS13 activity less than 10%. A high PLASMIC score (six to seven) is predictive of ADAMTS13 activity ≤10 with a sensitivity of 94%. Similarly, a low PLASMIC score predicts the absence of severe ADAMTS13 deficiency 98% of the time. A high probability score with either the French or the PLASMIC scoring system supports immediate initiation of plasma exchange.
| Parameter | French Score | PLASMIC Score |
|---|---|---|
| Platelet count | <30,000/μL: +1 | <30,000/μL: +1 |
| Serum creatinine level | <2.26 mg/dL: +1 | <2.0 mg/dL: +1 |
Markers of hemolysis
|
a | +1 |
| No active cancer in previous year | a | +1 |
| No history of solid organ or stem cell transplant | a | +1 |
| International normalized ratio (INR) < 1.5 | a | +1 |
| Mean corpuscular volume (MCV) < 90 fL | — | +1 |
| Likelihood of severe ADAMTS13 deficiency (< 10%) | Score = 0: 2%Score = 1: 70%Score = 2: 94% | Score = 0–4: 0%–4%Score = 5: 9%–24%Score = 6–7: 62%–82% |
a French score assumes microvascular hemolysis (schistocytes present) and absence of clinical evidence for associated cancer, transplantation or disseminated intravascular coagulation, and did not incorporate mean red cell volume.
Older adults (greater than 60 years) may have a distinct presentation compared with young adults. Atypical neurological symptoms such as confusion, headache, delirium, behavioral changes, TIAs, and stroke are commonly seen. Older patients present with greater renal impairment and less severe thrombocytopenia and anemia compared with younger patients. Consequently, the sensitivity of the PLASMIC score may be lower in older individuals, which can lead to a delay in diagnosis. Critically, the scoring systems developed for use in TTP were validated with populations containing an over representation of younger patients. In a study by the French group for TMA, only 61% of older patients with severe ADAMTS13 deficiency were as high risk using the French score.
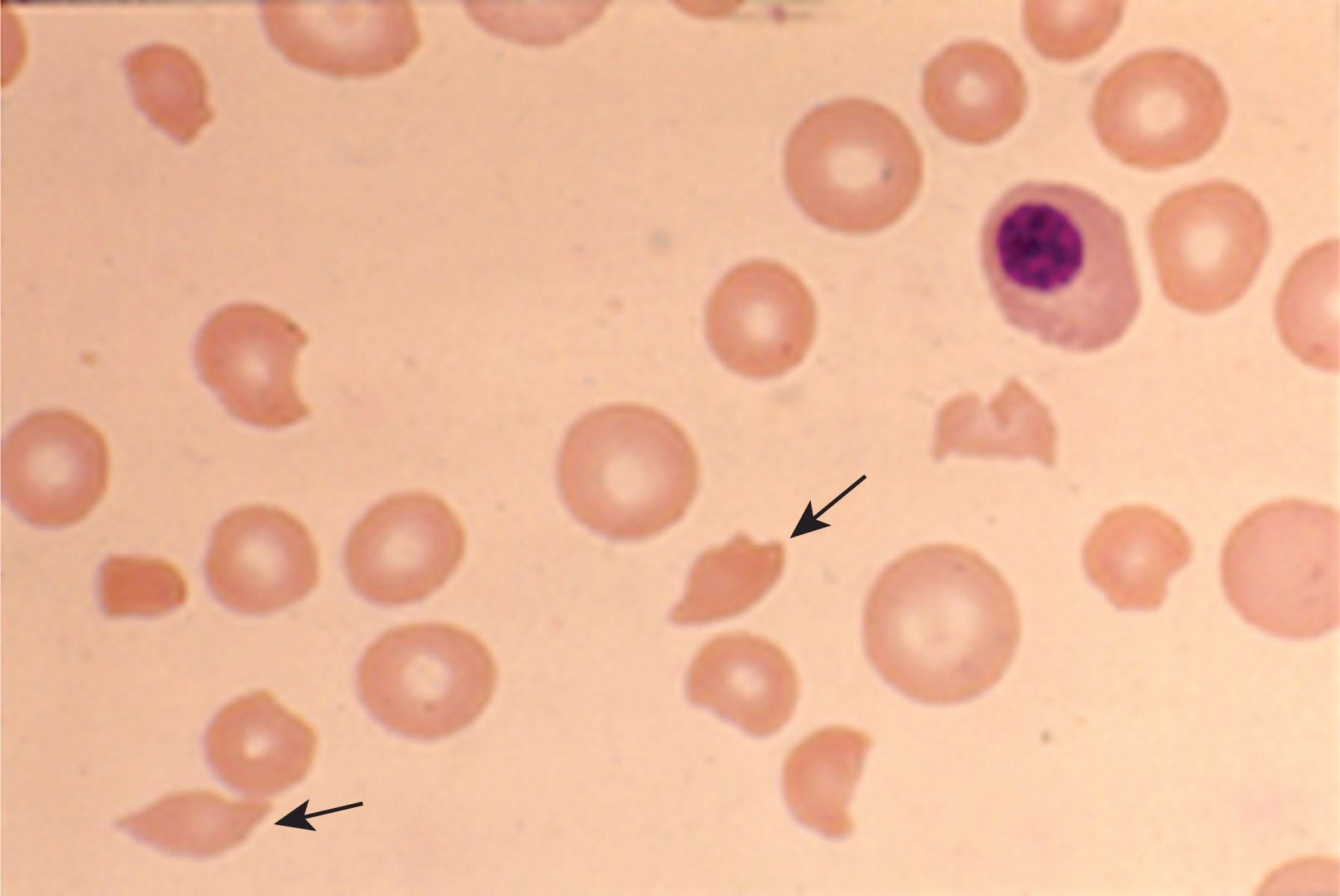
The presence of schistocytes on the peripheral blood smear is a characteristic laboratory finding of TTP ( Fig. 132.4 ). In most TMAs, schistocytes account for more than 1% of all erythrocytes. A finding of two or more schistocytes per high-power field is suggestive of MAHA. However, there is no threshold number below which the possibility of TTP can be excluded. More subtle signs of RBC fragmentation may be seen early in the course of the disease. Thrombocytopenia in TTP reflects deposition of platelets in microthrombi. In a series of 70 patients with TTP, the mean platelet count on presentation was 10,000.
ADAMTS13 activity testing is critical for the diagnosis of TTP. However, therapy should not be delayed while waiting for results of this test if the clinical suspicion for TTP is high. An activity level of less than 10% confirms the diagnosis of TTP in patients with MAHA and thrombocytopenia without any other obvious cause. ADAMTS13 activity less than 10% has been noted in rare cases of sepsis and systemic cancer and thus cannot be used exclusively as a diagnostic test; the clinical context must also be considered.
ADAMTS13 activity assays involve incubation of plasma with substrate full-length VWF multimers or truncated peptides containing the ADAMTS13 cleavage site. The substrate undergoes proteolysis by ADAMTS13 in the test plasma and enzyme activity is measured by detecting the cleavage product or by measuring the residual VWF. The first generation of assays used VWF as the substrate with urea or guanidine hydrochloride to expose the cleavage site in the VWF A2 domain. Current methods for ADAMTS13 level measurement utilize VWF peptides that undergo rapid cleavage when exposed to ADAMTS13, with endpoints based upon detection of fluorescence due to separation of emitter and quencher adducts that become separated through the cleavage process (fluorescence resonance energy transfer [FRET]–based assays utilize substrates such as VWF-VWF73 peptide), immunologic detection of the cleaved end of a solid-phase adherent residual peptide or a surface-enhanced laser desorption/ionization time-of-flight mass spectrometry. The antigen assays quantify the ADAMTS13 protein levels in plasma which includes both free active enzyme as well as inactive ADAMTS13 in complex with autoantibody.
The measurement of ADAMTS13 inhibitor levels using a functional inhibitor assay and/or autoantibody assay should also be performed when possible because the results are useful for predicting the clinical course and for distinguishing between iTTP and cTTP (see discussion in pathophysiology). Functional inhibitor assay is performed by mixing patient plasma with a source of ADAMTS13 (such as normal pooled plasma) and measuring the residual ADAMTS13 content. Inhibitor units are reported in a fashion analogous to how Bethesda units are quantified in the setting of hemophilia; one inhibitor unit is defined as the amount of inhibitor that will neutralize 50% of the ADAMTS13 activity when mixed into an equal volume of normal pooled plasma. Neutralizing antibodies interfere with ADAMTS13 proteolytic activity whereas non-neutralizing antibodies promote ADAMTS13 clearance from plasma. Several factors can affect the detected ADAMTS13 activity and inhibitor. Samples for testing cannot be collected in EDTA because it chelates the metal ions required for ADAMTS13 activity. Other interfering substances include hyperbilirubinemia and severe lipemia, which interfere with the FRET-based assay. Antibody assays quantify anti-ADAMTS13 antibody levels (usually IgG but may also be IgM and IgA) using ELISA, western blotting, or immunoprecipitation. ADAMTS13 inhibitor assays identify and quantify anti-ADAMTS13 antibodies that functionally inhibit ADAMTS13 in vitro. If severe ADAMTS13 deficiency persists and inhibitor or antibody assays are negative, a diagnosis of cTTP should be considered and ADAMTS13 sequencing should be performed.
TTP was initially considered an acquired disorder, with the term TTP suggested in 1947 (after the discovery of systemic microvascular thrombosis) to distinguish this disorder from immune thrombocytopenic purpura (ITP). The hereditary occurrence of TTP was first described in 1975 by Wallace et al., who noted an autosomal recessive inheritance pattern. A series of reports, including one by Upshaw, reporting improvement in clinical outcomes with plasma infusion resulted in the eponymous name. Subsequently, genome-wide linkage analysis revealed that Upshaw-Schülman syndrome is caused by mutations in the ADAMTS13 gene on chromosome 9q34.
ADAMTS13 is mainly synthesized by hepatic stellate cells but also by vascular endothelial cells and renal glomerular podocytes. Small amounts of functional ADAMTS13 are present in platelets. More than 200 mutations that can cause inherited TTP have been reported, and they involve almost all structural domains of ADAMTS13. Most of these mutations are single amino acid missense substitutions and most patients are carriers of compound heterozygous mutations. Mutations affecting the highly conserved N-terminal domains of ADAMTS13 are associated with lower residual enzyme activity, more severe clinical phenotype, and probably presentation of disease earlier in life. Expression studies have shown that missense mutations usually prevent the secretion of ADAMTS13, although some also impair catalytic activity. Specific mutations have been noted in population clusters with studies in Norway identifying the mutation p.R1060W and the insertion c.4143_4144dupA in approximately 0.3% to 1% of the population. Similarly, other clusters have been noted in regions around the Baltic Sea, central Europe, Japan, and in Scandinavia. Because some of these mutations are associated with residual ADAMTS13 activity, the disease is often identified during pregnancy with patients sometimes presenting with severe pre-eclampsia. However, residual activity is not the only determinant of age at first disease manifestation.
Patients with cTTP are at risk for manifestation at any time in life. In general, however, the risk is highest at birth and with pregnancy. A 2011 study describing the natural history of cTTP in Japan identified 43 cases in patients ranging in age from childhood to 79 years. Of these cases, 42% were diagnosed during childhood, 36% between the ages of 15 and 45 (all female, most commonly presenting during pregnancy), and 12% beyond 45 years of age. Likewise, a registry from the United Kingdom also reported a large variation in age at symptom onset ranging from childhood to adulthood.
Newborns with TTP may present with hyperbilirubinemia, anemia, and severe thrombocytopenia. The pathophysiology of the presentation is due to an abrupt increase in systemic vascular resistance after circulation to the placenta stops in the setting of a high hematocrit and ultra large VWF multimers. Plasma infusion is the treatment of choice in this setting. Children who survive often remain asymptomatic for years.
In the Japanese cohort of 43 patients, 9 of 20 women were diagnosed with TTP during pregnancy. Antecedent pregnancies were associated with severe complications. cTTP should be considered in women presenting with pre-eclampsia at less than 25 weeks of gestation. Patients with cTTP have a high risk of stroke and TIA. In a cohort of 73 patients in the United Kingdom, 19% of patients had a stroke and another 8% a TIA. The reported median age at presentation was 19 years; however, cases have been reported in patients over 60 years of age. Strokes can occur in the absence of hemolysis or thrombocytopenia. It has been estimated that 25% of strokes may be embolic with an undetermined source (see Chapter 144 ). Interestingly, rates of myocardial infarction are much lower compared with that of stroke. Acute kidney injury and chronic kidney disease have also been reported in patients with cTTP. As men do not experience the stress of pregnancy, recurrent stroke in males of young age should raise the possibility of hereditary TTP.
The absence of a functional ADAMTS13 inhibitor or antibody is characteristic of cTTP. False positive results with FRET-based functional assays can occur with severe hemolysis and a high hemoglobin concentration. The diagnosis of cTTP is further suspected if ADAMTS13 deficiency persists when the patient is in clinical remission and is confirmed by genetic analysis. As noted earlier, CNS symptoms may occur without thrombocytopenia or evidence of hemolysis; patients may also present with AKI. Fortunately, from a therapeutic perspective, plasma therapy results in rapid clinical recovery. In pregnant women with cTTP, monitoring of blood counts and prophylactic administration of plasma decrease the incidence of pregnancy complications and fetal loss, fostering a safer antenatal course.
The half-life of ADAMTS13 activity in patients receiving prophylactic plasma infusions is 2.5 to 3.5 days. Treatment intervals between plasma infusions have been empirically determined and depend on clinical symptoms and maintaining a normal platelet count. As a result, there is a wide variation in the recommended treatment intervals between infusions. Plasma derived factor VIII/VWF concentrates containing ADAMTS13 (Koate DVI and BPL 8Y) have been used for the treatment and prophylaxis of hereditary TTP. As a cautionary note, inhibitory alloantibodies can develop in patients receiving plasma therapy. In a phase 1 study, recombinant, fully glycosylated ADAMTS13 expressed in Chinese hamster ovarian cells (BAX 930; SHP 655; rADAMTS-13) was safe and well tolerated. This product is under investigation in a multicenter study with results expected in 2023.
Multiple questions remain in the field of cTTP. From an epidemiological perspective, additional studies with case finding strategies need to be performed. In addition, strategies to optimize replacement therapy protocols using plasma derived or recombinant ADAMTS13 are required to avoid complications of pregnancy, preserve renal function and prevent large vessel occlusive disease (stroke and myocardial infarction).
The goal of treatment in TTP is to prevent microvascular obstruction and replenish ADAMTS13 levels. Depending on the underlying etiology of ADAMTS13 deficiency—inherited or acquired (autoimmune)–replacement or replacement with immunosuppression +/− interruption of thrombus formation forms the thrust of therapy. The clinical presentation of TTP can be dramatic. When hemolysis and thrombocytopenia raise the specter of TTP, the PLASMIC score is useful to make a presumptive diagnosis and guide therapy. The reason for haste is the high mortality rate, which in the absence of therapy can exceed 90%, and the fact that the clinical situation can rapidly deteriorate.
The initial approach to TTP through the mid-70s focused on splenectomy, antiplatelet agents, high doses of glucocorticoids and whole blood exchange transfusions. The proposed rationale for exchange transfusion was to remove “a toxic substance.” Byrnes and Khurana established that plasma provides the critical factor for the treatment of TTP, ushering in a naive era in its treatment. (See box on Management of Acute Thrombotic Thrombocytopenic Purpura .)
A high index of suspicion is necessary to make a diagnosis of thrombotic thrombocytopenic purpura (TTP)
Evaluate pretest probability of TTP based upon clinical assessment and risk assessment tool (PLASMIC or French Score)
Send ADAMTS13 assays (activity and either inhibitor or IgG antibody) before initiating therapy if such testing is available. In an appropriate clinical setting, an ADAMTS13 activity result <10% confirms a diagnosis of TTP
If pre-test probability is high, begin therapy for TTP. If pretest probability is intermediate or low, initiation of treatment of TTP is based upon clinical judgement while awaiting ADAMTS13 studies.
Initial treatment of an acute episode of TTP:
Start plasma-based therapy. Plasma exchange (1.0–1.5 plasma volume) is recommended. Plasma infusion may be considered until exchange can be initiated, or potentially in a patient with known congenital TTP (cTTP)
In a patient with suspected or confirmed iTTP, begin corticosteroid therapy.
Anti-VWF therapy with caplacizumab is guided by ADAMTS13 activity assay results. Consider anti-VWF therapy with caplacizumab before ADAMTS13 results return if results are pending and patient has a high pretest probability, or after ADAMTS13 activity result returns at <10% for patients with low or intermediate pretest probability (use clinical judgement for activity result 10%–20%).
Consider early immunotherapy (e.g., rituximab) if iTTP is confirmed with ADAMTS13 inhibitor or anti-ADAMTS13 IgG assay, or if such diagnosis is highly suspected.
Platelet transfusion should be reserved for life-threatening bleeding. Packed red cell transfusion can be safely transfused in TTP.
Subsequent treatment of an acute episode of TTP:
Continue plasma-based therapy until patient achieve clinical remission based on clinical symptoms, lactate dehydrogenase, and platelet count.
In treatment responders:
Continue anti-VWF therapy with caplacizumab for 30-day course or based upon ADAMTS13 activity results.
In patients with iTTP, complete immunotherapy treatment course.
In treatment non-responders, consider investigation for confounding conditions (such as infection). Alternative/additional therapies should also be considered.
Subsequent follow-up:
For patients with iTTP, continued monitoring of ADAMTS13 activity is suggested as pre-emptive immunotherapy retreatment has been shown to decrease incidence of clinical disease relapse.
For patients with cTTP, ADAMTS13 replacement therapy (with plasma) prevents relapses and improves outcomes of pregnancy. Indications for chronic or episodic prophylactic replacement therapy remain to be defined, and clinical judgement is required.
With the use of plasma infusion and plasma exchange, the survival of patients with TTP has increased to over 80% to 90%. The overarching debate in the late 70 s and early 80 s was the effectiveness of plasma infusion versus plasma exchange. In a nationwide trial conducted in Canada over a 7-year period, investigators addressed this question by randomizing patients to treatment with aspirin and dipyridamole and either plasma exchange or plasma infusion with fresh frozen plasma (FFP). At 6 months, complete remissions were seen in 78% of patients treated with exchange versus 31% of those treated with plasma infusion. This study confirmed the superiority of plasma exchange over plasma infusion likely reflecting the fact that plasma exchange results in both the removal of the IgG inhibitor of ADAMTS13 and replacement of the deficient protein. In contrast, plasma infusion (or infusion of ADAMTS13 concentrate) suffices for patients with congenital ADAMTS13 deficiency. Large volumes of plasma are more easily administered by exchange, and unless a genetic deficiency of ADAMTS13 has been documented, plasma infusion should be reserved for situations in which exchange is not immediately available. The recovery of ADAMTS13 levels may lag behind other indicators of clinical response, and the utility of monitoring ADAMTS13 levels during acute treatment has not been established (see below concerning anti-VWF therapy with caplacizumab).
Plasma (either FFP or thawed plasma) is used for plasma exchange. There is no clear advantage for the use of cryo-poor plasma (CPP), which is depleted of VWF. These findings are consistent with a study showing similar concentrations and stability of ADAMTS13 during storage at 1°C to 5°C in CPP and FFP. Pilot studies suggest that solvent-detergent treated plasma (SDP), which contains ADAMTS13 at concentrations approximately 20% lower than those in FFP is as efficacious as FFP and is associated with fewer allergic/urticarial reactions. In the UK, SDP is used for all patients with TTP.
Treatment is generally initiated with the goal of exchanging 1 to 1.5 plasma volumes (40 to 60 mL/kg) daily, although the optimal regimen has not been determined. Some experts advocate increased volumes of exchange if the initial response is poor. Neurologic improvement occurs most rapidly, often within hours to days. The serum LDH level typically falls by 50% within 3 days in responders, and the platelet count begins to rise at a mean of 5 days, though normalization may take several weeks. Improvement in renal function and disappearance of schistocytes occur more slowly.
Daily plasma exchange should be continued until neurologic symptoms have resolved and both a normal serum LDH and platelet count have been achieved; many experts recommend an additional 2 to 3 days of plasma exchange thereafter. Approximately 85% to 90% of patients exhibit a clinical and laboratory response to plasma exchange within 3 weeks, most often within 10 days (mean: 16 days; range: 3 to 36 days). However, 20% to 40% of patients will experience an exacerbation of disease within 30 days after stopping plasma exchange, whereas approximately 30% will relapse at later dates, usually within the first year. Limited data are available to support the decision between abrupt discontinuation or “tapering” of plasma exchange after remission.
Complication rates associated with plasma exchange therapy have been reported to be as high as 30%, but recent reports suggest a lower rate. Most adverse events are related to central venous catheter insertion, infection, allergic reactions to plasma, and occasionally thrombosis.
Plasma exchange is effective for patients with or without demonstrated inhibitors, and clinical responses frequently occur despite persistence of both the inhibitor and severe ADAMTS13 deficiency, although patients with high titer inhibitors may respond more slowly and relapse more often. Both congenital and acquired ADAMTS13 deficiency is characterized by unpredictable periods of stability between relapses, probably reflecting exacerbation of the disease by co-morbid conditions that activate or damage the endothelium, thereby increasing the release of unusually large VWF multimers, and triggering microvascular thrombosis.
Immunosuppression with glucocorticoids and anti-CD20 monoclonal antibody (rituximab) are a cornerstone of therapy in patients with iTTP. Glucocorticoids aid recovery by reducing production of autoantibodies to ADAMTS13. Therapy is typically started with oral prednisone at a dose of 1 to 2 mg/kg/day; a higher dose of methylprednisolone (1000 mg daily) may be used for critical illness. Prednisone was compared to cyclosporine in a randomized control trial and was found to be more effective in increasing ADAMTS13 activity and suppressing antibodies (study stopped after an interim analysis).
Anti-CD20 therapy with rituximab has been shown to reduce the risk of exacerbations and relapse and to hasten the response to therapy. Consequently rituximab is often given before plasma exchange is completed. Rituximab should ideally be given after plasma exchange to minimize its removal by the procedure, but its use should not delay plasma exchange. Rituximab has been shown to effectively deplete CD20 + lymphocytes within 24 hours of infusion even with ongoing plasma exchange therapy.
The data supporting the utility of rituximab come mainly from observational studies. The STAR trial evaluated the benefit of adding rituximab to plasma exchange and glucocorticoids as initial treatment. Unfortunately, the trial was stopped because of poor accrual. Data from a phase 2 study suggested that rituximab was safe and effective when given within 3 days of an acute TTP admission. Compared with historical controls, inpatient stay was reduced by 7 days, and 10% of patients given rituximab relapsed compared with 57% in the historical control population. The dose of intravenous rituximab used for acquired TTP has generally followed the traditional schedule of 375 mg/m² once weekly for 4 consecutive weeks. A prospective multicenter single arm phase 2 trial using a fixed dose of 100 mg weekly for four doses showed response rates similar to those achieved with the higher dose. Another approach pioneered by the French Thrombotic Microangiopathy group has been to use an adaptive dose of rituximab in response to B cell depletion. Rituximab use in the acute phase of TTP prompts a high response rate (greater than 90% in the first month) and extends the time to relapse. The median time to relapse across studies conducted in the United Kingdom and France and using data from the Oklahoma TTP registry indicate a median time to relapse between 24 and 29 months.
Microvascular thrombosis, the hallmark of TTP, occurs because of unregulated interaction of ultra-large VWF with platelets. Interruption of that process with anti-VWF therapy has the potential to halt microvascular obstructions and organ ischemia. Caplacizumab is a humanized, bivalent, variable domain only immunoglobulin fragment that targets the A1 domain of VWF and blocks its interaction with platelet glycoprotein lb-IX-V. In the randomized controlled phase 3 HERCULES trial, 145 patients with acquired TTP were randomly assigned to receive caplacizumab or placebo daily until 30 days after the last daily plasma exchange (plasma exchange was performed daily until at least 2 days after normalization of the platelet count). All patients received daily plasma exchange and glucocorticoids and could receive other immunosuppressive therapies (slightly less than half received rituximab). Caplacizumab was associated with faster normalization of the platelet count, fewer days of plasma exchange, fewer exacerbations in the 30 days after stopping plasma exchange (4% vs. 38%) and a shorter course of hospitalization (10 vs. 14 days). Caplacizumab was associated with more bleeding (65% vs. 48%) which resolved spontaneously. In addition, a composite outcome of TTP related death, recurrence of TTP or a major thromboembolic event occurred in 12% of patients in the caplacizumab group and in 49% of those in the placebo group. Normalization of markers of organ dysfunction occurred sooner with caplacizumab than with placebo. The findings of more rapid platelet count response, reduced organ damage and conservation of resources, prompted the recent ISTH guidelines to suggest that caplacizumab be initiated in all patients with a high probability of having TTP in whom confirmation via ADAMTS13 assay is anticipated. Based upon the initial phase 2 Titan and the phase 3 Hercules trial experience, it is recommended that caplacizumab be continued for 30 days after discontinuation of plasma exchange. Relapses were noted in patients who had severely suppressed ADAMTS13 activity below 10% at the end of the 30-day period after the last plasma exchange. As supported by the HERCULES trial data, some groups recommend monitoring ADAMTS13 activity level at that point and continuing caplacizumab therapy until ADAMTS13 levels recover to above 20% to prevent exacerbations of disease. Successful treatment of TTP with caplacizumab without concomitant plasma therapy has been reported in several individual cases and a recent case series. Situations prompting this approach have included patients with contraindications to plasma therapy (such as history of severe allergic reactions to plasma or inadequate venous access) and patient refusal of plasma therapy (including religious objection to blood products). Because plasma exchange is the standard of care for TTP and use of caplacizumab alone is off-label, the decision to omit plasma-based therapy must be shared between the patients and their physicians. Trials of caplacizumab alone are needed in patients with TTP.
Before the introduction of plasma therapy, splenectomy was the first-line treatment for TTP and induced remission in up to 50% of patients. Currently, splenectomy is reserved for patients who are refractory to all other modes of treatment. Antiplatelet agents have fallen out of favor and are now part of the historical construct of TTP treatment. Similarly, immunosuppressive agents like cyclophosphamide and vincristine are no longer used now that rituximab is available. Other adjunctive therapies that have fallen out of favor include azathioprine, mycophenolate mofetil, staphylococcal protein A immunoadsorption, and bortezomib. However, these may still be considered in refractory cases or in patients with iTTP whose ADAMTS13 levels fail to rise with rituximab.
Historically, platelet transfusions have not been recommended for patients with TTP because of their potential to provoke fatal thrombotic events. Data from the Oklahoma TTP-HUS registry challenged this dogma. In a cohort of 54 consecutive TTP patients (of whom 47 had ADAMTS13 activity less than 10%), 33 patients received platelet transfusions. There was no difference in the incidence of death or severe neurologic events. The authors of the study concluded that there was “uncertain evidence of harm” with platelet transfusion in TTP. Although platelet transfusion might be considered prior to vascular access device placement, catheters for apheresis can safely be inserted even in the face of severe thrombocytopenia (less than 10,000). Therefore, based on accumulating evidence, platelet transfusion in TTP patients should be reserved for those with life-threatening bleeding.
Once a patient with TTP is stabilized, subsequent treatment is dictated by close monitoring for disease relapse and by immunosuppression. Disease response to therapy can be assessed using the platelet count. A good response is a platelet count over 150,000/μL for at least 3 days or a stable plateau. In addition to the platelet count, weekly monitoring of the LDH and ADAMTS13 level is suggested but well-established protocols do not exist. Schistocytes may persist for some time after the thrombocytopenia has resolved. Ultimately, normalization of the platelet count and improvement in symptoms are the most reliable indicators of response to therapy, while an increase in ADAMTS13 levels indicates a biochemical response.
Plasma exchange can be stopped once the platelet count is over 150,000 for at least 2 days. Plasma exchange may be stopped abruptly or may be tapered off, but the tapering is not of proven benefit. In the first month after discharge from the hospital, patients with TTP should have frequent blood counts and monitoring for symptoms (e.g., starting three times weekly and gradually tapering to once a week by the end of the first month if there has been no relapse). A relapse is generally preceded by a drop in the platelet counts below 100,000/μL. More frequent monitoring of symptoms and blood counts should be considered if the platelet count falls below 150,000. If the platelet count falls below 100,000 and ADAMTS13 activity is low or symptoms suggestive of a relapse (e.g., neurologic symptoms) develop, plasma therapy and/or further treatment with immunosuppression or anti-VWF therapy is indicated. If there is no relapse in the first month, glucocorticoids are generally tapered over 2 to 3 weeks while the rituximab course is completed. ADAMTS13 levels should be measured periodically after stopping plasma exchange until levels normalize. Patients with intermediate ADAMTS13 levels (20%—the lower limit of normal) may merit more frequent monitoring. Patients are at an increased risk for relapse in the first year with illness or surgery serving as potential triggers. ADAMTS13 levels should be checked every 3 months for the first year with more frequent if the level falls below 40%.
The use of immunotherapy and anti-VWF therapy (caplacizumab and rituximab) reduces the risk of exacerbations and relapses. Rituximab has decreased the frequency of relapse to about 13% with most relapses occurring over a year after plasma exchange is discontinued. The use of preemptive rituximab is supported by the results of a study from the French TMA group ; 48 of 233 patients had an ADAMTS13 level less than 10% and 30 were treated with rituximab preemptively. Whereas the 18 patients not treated preemptively had a median relapse free survival of 9 years, patients given rituximab did not reach a median relapse-free survival. This report was followed by a second study demonstrating a decrease of relapse from 74% with a median follow-up of 7 years versus 15% with a follow-up of approximately 3 years.
Therapy for patients with a history of iTTP whose ADAMTS13 level fall over time remains uncertain. Some experts suggest a single dose of rituximab followed by re-evaluation of the ADAMTS13 level in a month and withholding further therapy if the level is greater than 40% and giving additional weekly doses if the level remain less than 40%. Other experts suggest “maintenance” doses of rituximab, including the use of lower doses. The UK TTP registry demonstrated equivalence among 3 dose levels (375 mg/m 2 once per week for 4 weeks, 200 mg once per week for 4 weeks, 500 mg once per week for 4 weeks) with no differences in time for the recovery of ADAMTS13 levels and need for retreatment. If the patient has had multiple relapses with no effect of maintenance rituximab on ADAMTS13 levels, other immunosuppressive agents or splenectomy could be considered.
Patients with acquired TTP that does not respond to initial treatment with plasma exchange and immunosuppression within 4 days or with ongoing disease activity, as evidenced by thrombocytopenia or neurological symptoms, are considered refractory. In these patients, therapy may be intensified using a higher dose of glucocorticoids (1 g IV of methylprednisolone daily for 3 days) and addition of caplacizumab and rituximab (if not given already). Alternate diagnoses that could contributing to the refractory state need to be considered. These include infection, occult malignancy, or medications. With refractory disease, cyclophosphamide, bortezomib, cyclosporine, mycophenolate may be considered.
Patients with iTTP are susceptible to subsequent relapses and must be educated about the need to seek immediate medical care should symptoms arise. They also are at higher risk for hypertension, major depressive disorders, autoimmune diseases (SLE), and stroke compared with age and sex matched controls.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here