Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The transition from the kyphotic, rigid thoracic spine to the mobile, lordotic lumbar spine creates a biomechanically favorable location for burst fractures. Thoracolumbar burst fractures are a common injury, and there exists a broad range of classifications and treatment algorithms to aid surgeons in clinical decision making. The treatment goal of burst fractures is to optimize bony healing, maximize preservation of neurologic function, and allow early mobilization. Nonoperative management includes bracing, early mobilization, and close follow-up. Although the majority of thoracolumbar burst fractures are amenable to nonoperative treatment, there exists continued controversy regarding the transition point from conservative to operative care. Severe canal compromise with neurologic injury may benefit from an anterior approach, decompression, and fusion. The correct treatment choice should be tailored to the individual's clinical and radiographic presentation. Outcomes between operative and nonoperative care continue to be equivocal for those patients without neurologic injury. The anterior approach appears to maintain better sagittal correction; however, there has not been any clinical difference in functional outcomes compared with posterior-only and combined approaches.
Flexion-distraction injuries and fracture-dislocations mainly result from high-velocity injuries, either from high-velocity/high-energy trauma in motor vehicle accidents (MVAs) or from falls. The prevalence of these fractures is on the rise due to an increase in the occurrence of these accidents. Due to the mechanism of injury that is involved in the causation of flexion-distraction fractures or fracture-dislocations, these fractures have the potential for instability and neurologic deficits. This chapter discusses the various patterns of flexion-distraction and fracture-dislocation types of thoracolumbar fractures. A thorough clinical evaluation, along with critical assessment of the images, is necessary in the decision-making process. Patients with incomplete or complete neurologic deficits should be managed urgently. Conservative treatment is the treatment of choice for stable injuries with no neurologic deficits. Operative treatment is necessary for unstable fractures, fracture-dislocations, distraction injuries, and neurologic deficits. Operative treatments vary depending on the type of injury.
Low lumbar fractures are less common than those found in the lumbosacral junction. This is a result of lumbar lordosis significantly reducing the amount of kyphotic angle, resulting in fracture patterns that are somewhat different from those at the thoracolumbar junction. Traditionally, these fractures have been divided into compression fractures, burst fractures, distraction injuries, fracture-dislocations, and traumatic spondylolisthesis, which are frequently present at either L4 or L5. Current fracture classifications (e.g., Thoracolumbar Injury Classification System [TLICS]) have some usefulness when applied to the lumbar spine in directing the potential treatment of fractures in this region but tend to be less accurate in predicting outcomes because of the unique anatomy in this region, requiring treatment decision making based on sound medical judgment. Because of the differences in biomechanics, most lumbar fractures are either compression fractures or burst fractures without neurologic injury. These injuries are frequently associated with polytrauma, and patients need to be adequately assessed for other soft tissue and bony injuries. The development of a sound treatment plan for all the associated injuries follows acute cardiovascular stabilization. The vast majority of these lumbar fractures may be treated conservatively with brace treatment and palliative care, whereas fracture-dislocations, traumatic spondylolisthesis, and high-grade burst fractures with severe canal compromise and neurologic injury will require surgical reconstruction. When surgical intervention is required, protocols call for immediate reduction and long-term stabilization of these injuries to protect the patient from secondary neurologic injuries and potential complications related to treatment with prolonged bed rest. Both nonoperative and surgical interventions are extremely effective in treating both stable and complex injuries to preserve neurologic function, provide excellent long-term outcomes, and provide long-term stability of the spine.
Advances in the management of thoracolumbar trauma include percutaneous and fusionless spinal instrumentation, mini-open lateral and thoracoscopically assisted anterior decompression, and the concept of damage-control spine surgery. These advances have decreased patient morbidity and decreased the complication rate in comparison to conventional open procedures. Meticulous planning and proper choice of surgical indications are essential for success and avoidance of complications. Further research is needed to establish the role of these techniques.
Thoracic and upper lumbar spine injuries are the most common types of spinal injuries. The transition between the less mobile thoracic spine to the dynamic lumbar spine predisposes the thoracolumbar junction (T10–L2) to significant biomechanical stress compared with other regions of the spinal column. Approximately 160,000 people sustain a spinal column injury in the United States every year, with 15% to 20% of these fractures affecting the thoracolumbar junction. The prevalence of these injuries is on the rise due to the ever-increasing incidence of high-velocity/high-energy trauma. Because a majority of these fractures result from high-energy trauma, either from MVAs or falls, the potential for fracture instability and neurologic injury is significant. The mechanism of injury plays a major role in various fracture patterns, potential instability, and neurologic deficits. Caring for patients who have undergone such traumatic spinal fractures is challenging because a majority of these patients also have concomitant polytrauma with injury to other organ systems. Commonly associated injuries that occur with fracture-dislocations include closed head injuries, pulmonary contusions, splenic and hepatic lacerations, bowel injuries, vascular injuries, long bone fractures, and neurologic injuries.
Understanding the mechanism of injury is of utmost importance in expediting the diagnosis and appreciating the severity of the trauma. Patients need immediate stabilization in the field to prevent further worsening of the fracture, which could potentially lead to neurologic deficits. Flexion-distraction (seat-belt–type injuries) or extension-distraction injuries in the arthritic spine in elderly patients are unstable injuries, and timely identification of the pattern could prevent neurologic deficits in these patients. Understanding the mechanism of injury also aids in planning for definitive treatment.
An essential determinate of the treatment of thoracolumbar fractures is fracture classification.
Modern systems are based on cross-sectional imaging and assess the degree of instability, fracture morphology, and mechanism of injury.
A careful review of the history, physical examination, and imaging is needed to properly classify thoracolumbar injuries.
The Thoracolumbar Injury Classification System (TLICS) is a point system that assesses fracture morphology, neurologic function, and mechanism of injury.
The modified Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification system uses three fracture mechanisms: compression, distraction, and shear. Each is subdivided into common fracture morphology.
The thoracolumbar region consists of the upper, less mobile thoracic spine and the lower, dynamic lumbar spine. The cephalad 10 thoracic vertebrae are connected to the sternum anteriorly with the ribs, making this segment the most rigid part of the spinal column. The lumbar spine, on the contrary, affords flexibility due to the characteristic shape and orientation of the facet joints. The thoracolumbar junctional region (T10–L2) connects the less mobile thoracic region to the dynamic lumbar spine, which predisposes this region to traumatic fractures.
The spinal cord ends at approximately the L1–L2 level, and the roots of the cauda equina fill the thecal sac distal to this level. The lower end of the spinal cord, the conus medullaris, contains the upper neuron cells of the sacral nerve roots. A neurologic injury at the thoracolumbar region can present with different clinical scenarios. Spinal injuries above the level of L1 can lead to spinal cord injury and present with a typical upper-motor-neuron type of paralysis. Injuries below the level of L1–L2 result in injury to the nerve roots of the cauda equina, which results in a flaccid lower-motor-neuron type of paralysis. Injury to the conus medullaris usually results from an L1 burst fracture and presents with a unique clinical presentation. The injury in the conus medullaris results in an upper-motor-neuron paralysis of the bowel and bladder, whereas the lower motor nerve roots of the cauda equina escape the injury, preserving normal motor function in the lower extremities. Due to this wide spectrum of neurologic injuries in this region, a meticulous neurologic examination is of utmost importance.
Understanding the mechanism of injury is important in studying the fracture morphology and determining the potential for instability and/or neurologic injury. Forces of flexion, extension, distraction, rotation, or lateral shear, when strong enough, can result in an alteration of the structural integrity of the spine, leading to fractures, fracture-dislocations, or translations of the spinal column. The transition from a relatively rigid thoracic spine to a dynamic lumbar spine predisposes the thoracolumbar junctional segment to significant disruptive forces during high-velocity trauma. Concentration of these traumatic forces at the thoracolumbar junction results in fractures and fracture-dislocations, which have significant potential for instability and neurologic injury. As a result of these biomechanical effects, almost 50% of all vertebral body fractures and 40% of all spinal cord injuries are concentrated at the thoracolumbar junction. White and Panjabi reported that in a cadaveric evaluation of the spine, the flexion and extension ranges of motion in the lower thoracic spine increase from 5 to 12 degrees from T6–T7 to T12–L1 compared with the lumbar spine, which averages 15 degrees. They also demonstrated that the axial rotation and lateral side-bending of the thoracic spine is greater than that of the lumbar spine because of the coronal orientation of the facets compared with the more sagittal lumbar facet joints. The mechanisms that can create an unstable thoracolumbar fracture by exceeding the age-determined inherent range of motion and stability of the thoracolumbar spine include flexion, flexion-compression, flexion-rotation, flexion-distraction, extension, lateral shear or slice, and compression.
Spinal trauma patients, particularly those with high-energy–induced fracture-dislocations of the spine, often present with acute polytrauma and therefore require a thorough multispecialty assessment. Because these patients often present in shock, either hemorrhagic or neurogenic, they require extensive efforts to resuscitate and stabilize them, including intubation, intravenous (IV) fluids or blood products, chest tube placement, intra-abdominal assessment (for liver, spleen, pancreas, and bowel injury), and long bone splinting or external fixation. The trauma evaluation is critically important because more than half of patients with flexion-distraction injuries of the thoracolumbar spine have intra-abdominal injuries, including hollow viscus perforations. This number may be even higher in the pediatric population. An acute abdomen secondary to undetected intra-abdominal injury is associated with significant morbidity and mortality. It is recommended that a general assessment of the patient's overall mental functioning (Glasgow Coma Scale), polytrauma status (Injury Severity Score), neurologic status (American Spine Injury Association [ASIA] ), International Standards for Neurological Classification of Spinal Cord Injury, and comorbidities (Charlson Comorbidity Index ) be conducted. Although plain radiographs of the chest, abdomen, pelvis, spine, and long bones remain useful, most trauma centers now use more advanced imaging, such as computed tomography (CT) scanning and magnetic resonance imaging (MRI) of targeted areas. This is particularly true for obtunded patients, where a rapid CT scan of the chest, abdomen, pelvis, and total spine is required to rule out any unknown injuries.
The physical examination in the conscious patient should include the following: evaluation of the entire spine from the occiput to the sacrum for any points of tenderness, painful gaps, or step-offs between the spinous processes that may be indicative of a ligamentous injury; subcutaneous bruises or contusions; subcutaneous air; and the general alignment of the spine. In flexion-distraction injuries, soft tissue injuries can be detected by noting hematoma, significant paraspinous tenderness, and palpation of gaps between spinous processes. In fracture-dislocations, a gibbous deformity and step-off between spinous processes are palpable.
The neurologic examination includes a complete assessment of motor strength, sensory examination, proprioception, and rectal tone. If the patient is in spinal shock, the anal wink and bulbocavernosus reflex are less relevant for the prognosis of the injury because most of these patients should have had emergent reduction and stabilization of the spine within 72 hours of the injury. ASIA standards for the neurologic examination are used to guide physicians through a complete neurologic examination.
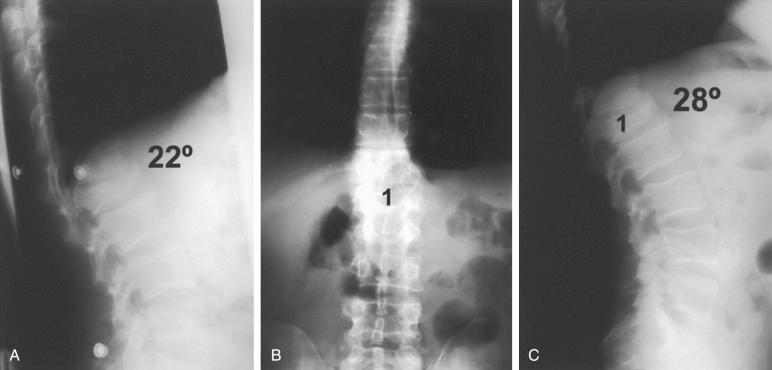
Imaging investigations are mainstays in diagnosis, decision making based on classification, and treatment of thoracolumbar fractures. Patients are managed with spine precautions until the imaging investigations and spine clearance are completed. In the past, anteroposterior (AP) and lateral radiographs were used for clearance of the TL spine. However, the use of radiographic images for the diagnosis of TL injuries had several limitations. These radiographs were usually completed in an area away from the emergency room (ER). Obtaining optimal visualization of the fracture site can be challenging in the scenario of trauma. The presence of multiorgan trauma also poses potential difficulties in obtaining necessary radiographs. Plain radiographs only have a specificity of 58% and a positive predictive value of 64%. Due to these limitations, helical CT scans of the chest, abdomen, and pelvis obtained for the diagnosis of visceral injuries have become the investigative tool of choice for the diagnosis of spinal injuries. CT scans have a sensitivity for thoracic and lumbar spine fractures of 97% and 99%, respectively.
CT scanning not only offers higher sensitivity and specificity, but also the time required for obtaining the CT scans has been found to be significantly less than that for the appropriate plain radiographs. The use of higher-quality CT of the chest, abdomen, and pelvis in the polytrauma setting is recommended. Dedicated CT scans are required for better definition if surgery is planned. Critical assessment of the sagittal and coronal CT imaging sequences is necessary to diagnose the fracture pattern and mechanism of injury. Axial sequences are used to diagnose coronal or sagittal plane injuries to the vertebral bodies, assess the severity of canal encroachment, and determine the presence of laminar fractures.
MRI can be used in a stabilized patient when there is a neurologic compromise. Other indications for MRI are the presence of unexplained neurologic deficits or neurologic deterioration and to determine the status of the posterior ligamentous complex. Further, MRI can aid in determining the acuity of osteoporotic-related fractures. The modern classification systems discussed herein are based on CT and, when indicated, MRI.
Injury classification systems allow for clear communication among healthcare providers and assist in decision making. Another advantage is more standardized care, which is lacking in the management of thoracolumbar trauma. Currently, there is significant international variation in the management of thoracolumbar trauma, likely related to the absence of a globally accepted classification system. Early attempts at the creation of a classification system focused on injury pattern and mechanism. More recent attempts at a clinically relevant classification system have included other aspects of the patients’ injuries, such as their neurologic status. The purpose of this chapter is to review the evolution of the thoracolumbar classification systems and provide a summary of the most clinically relevant systems being used today.
In 1938, Watson-Jones described a thoracolumbar classification system based on three distinct fracture patterns: the wedge fracture, the comminuted fracture, and the fracture-dislocation. Others identified unique fracture types that have become eponyms of the authors and have persisted to this day. One example is the Chance fracture, a flexion-distraction injury characterized by a wedge compression of the vertebral body and tension failure of the posterior elements, which was described by Chance in 1948. In 1970, Holdsworth first used the term burst fracture to describe a vertebral body compression fracture with involvement of the vertebral wall that he identified in a review of 1000 spinal injuries.
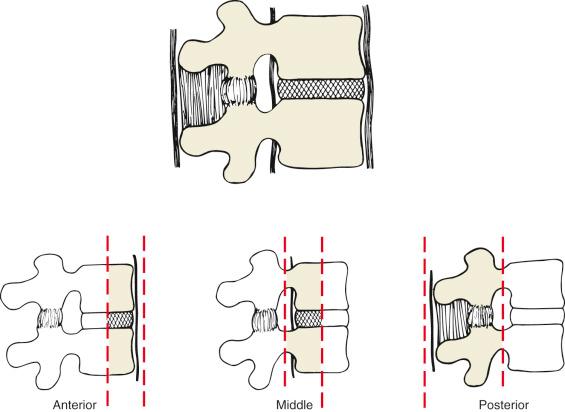
In 1968, Kelly and Whitesides first proposed the column theory to describe spinal stability. They described the spine as consisting of two columns, an anterior and posterior column. The anterior column includes the vertebral body and intervertebral disc and both longitudinal ligaments. The posterior column represents the entire neural arch and ligamentous attachments. They hypothesized that an injury to either one of the columns would be stable, but an injury to both columns would result in an unstable injury. This classification was modified by Denis, who divided the spine into three columns: the anterior, middle, and posterior columns ( Table 33.1 ). In this classification, the anterior column is composed of the anterior longitudinal ligament and the anterior two-thirds of the vertebral body and disc. The middle column represents the posterior third of the vertebral body/disc and the posterior longitudinal ligament. Lastly, the posterior column is described as including all structures from the pedicles on posteriorly ( Fig. 33.1 ). From this three-column theory, there are four major injury types (compression fractures, burst fractures, seat-belt injuries, and fracture-dislocations). The major injury types are further subdivided into 16 distinct subtypes. In the Denis classification, it is postulated that the middle column is the critical element of spinal stability. Therefore injury to either the anterior or posterior column in isolation would result in a stable injury, whereas injury of either of these columns and the middle column would create an unstable injury. This system has shown moderate interrater reliability with regard to injury type; however, it has shown poor reliability with regard to injury subtype. Although attracting some criticism because it is not comprehensive, this classification still has gained widespread acceptance. Further, it is often cited in providing justification for the operative treatment of burst fractures even in neurologically intact patients. Subsequent classification systems, described later in this chapter, have challenged this management principle.
| Compression (May Be Anterior or Lateral) | |
| Type A | Coronal split of the anterior column |
| Type B | Fracture of the superior endplate of the anterior column |
| Type C | Fracture of the inferior endplate of the anterior column |
| Type D | Anterior cortex fracture with intact endplates |
| Burst | |
| Type A | Fracture involving both endplates and the posterior wall |
| Type B | Fracture involving the superior endplate and the posterior wall |
| Type C | Fracture involving the inferior endplate and the posterior wall |
| Type D | Burst fracture associated with significant rotation |
| Type E | Lateral burst fracture that involves both endplates and the posterior wall but only involves the left or right side |
| Seat-Belt Type | |
| Type A | Single-level osseous injury |
| Type B | Single-level ligamentous injury |
| Type C | Two-level injury with osseous involvement of the middle column |
| Type D | Two-level injury with ligamentous involvement of the middle column |
| Fracture-Dislocations | |
| Type A | Flexion with rotation |
| Type B | Shear injury |
| Type C | Flexion-distraction injury |
McAfee et al. reviewed 100 thoracolumbar fractures utilizing the recently developed CT scan. This classification scheme combines the fracture morphology with the injury mechanism and groups the injuries into six main types: wedge compression, stable burst, unstable burst, flexion-distraction, Chance fractures, and translational injuries. This classification system does not challenge the need for surgical intervention being based on the integrity of the middle column; rather, this classification attempts to help determine the type of surgery needed. If the middle column injury is secondary to either a compressive force, such as a burst fracture, or a distractive force, such as a flexion-distraction injury, the authors recommended a posterior-based approach with distraction or compression instrumentation. However, if the injury is a translational injury, posterior distraction is not recommended, and instead, segmental instrumentation of each level is recommended.
Ferguson and Allen published a more comprehensive mechanistic classification, built on Denis's three-column theory. In the Ferguson and Allen system, there are seven main injury types with an additional five subtypes. These main types include vertical compression, compression-flexion, distraction-flexion, lateral flexion, translation, torsional flexion, and distractive extension ( Box 33.1 ). Although the classification nomenclature has become widely accepted, the validity of the classification and its usefulness for guiding treatment are unclear; it has never been independently validated. This is likely due to its reliance on an inferred injury mechanism as opposed to objective and verifiable imaging characteristics.
Vertical compression
Burst fracture with diffuse retropulsion
Burst fracture with retropulsion at the superior and inferior endplates
Compression flexion
Anterior wedge
Anterior wedge with associated posterior tension-band disruption
Burst fracture with associated posterior tension-band disruption
Distraction flexion
Lateral flexion
Translation
Torsional flexion
Distractive extension
In 1990, White and Panjabi proposed an important new concept of spinal stability that has altered the subsequent interpretation of spinal injury. They defined stability as the ability of the supporting elements of the spine to resist physiologic loads so as to prevent neurologic injury, deformity, or pain. This was an important step in refuting the notion that all fractures that involve the middle column are unstable. However, in spite of the work by White and Panjabi, the next major classification system proposed by Magerl and colleagues failed to challenge the assertion that all fractures involving the middle column are unstable. The Magerl system is a hierarchical system that first divides the injury into one of three major types based on mechanism and then subdivides each injury based on morphology. The major types, identified as type A, B, and C, refer to compression, distraction, or rotational injuries, respectively. However, although this classification increased the granularity of differing fracture morphologies, with 53 unique injuries described, the complexity has resulted in only poor to fair interobserver reliability. Furthermore, because of its reliance in the middle column to determine stability, it does not alter the treatment algorithm of thoracolumbar injuries. This, along with its failure to appreciate the importance of the neurologic status of the patient or the integrity of the posterior ligamentous complex, has limited the clinical utility of the Magerl classification.
The Thoracolumbar Injury Classification System (TLICS) was developed by Vaccaro and colleagues in 2005 with the goal of serving as a compressive classification system that would guide clinical decision making. Classification of an injury with this system is first evaluated by three parameters: morphology, integrity of the posterior ligamentous complex (PLC), and neurologic status of the patient. The morphology is described as one of three major categories: compression injuries, translational/rotational injuries, and distraction injuries. The PLC is qualified as either intact, disrupted, or indeterminate. Lastly, the patient's neurologic status is described as intact, nerve root deficit, complete spinal cord injury, or incomplete spinal cord injury/cauda equina syndrome. An associated injury severity score can be applied to the TLICS to assign a score to each injury. That score (between 1 and 7) can be used to help guide clinical decision making in that a score of less than 4 would suggest nonoperative management, a score greater than 4 suggests operative treatment, and a score of 4 is an equivocal recommendation that makes no specific recommendation on treatment ( Table 33.2 ).
| Variable | Points |
|---|---|
| Injury Morphology | |
| Compression | 1 |
| Burst | +1 |
| Translation/rotation | 3 |
| Distraction | 4 |
| Neurologic Status | |
| Intact | 0 |
| Nerve injury | 2 |
| Cord, conus medullaris | |
| Incomplete | 3 |
| Complete | 2 |
| Cauda equina | 3 |
| PLC Integrity | |
| Intact | 0 |
| Indeterminate a | 2 |
| Injured | 3 |
a Indeterminate status is attributed to patients without evident disruption on computed tomography (CT) scan reconstructions (without clear dislocation) but with suggested ligamentous injury on the short tau inversion recovery (STIR) or T2-weighted magnetic resonance imaging (MRI) sequence.
The TLICS is distinguished from its predecessors by several unique characteristics. First, with regard to its classification, it is the first system to include either the integrity of the PLC or the patient's neurologic status in its injury categorization. Second, implicit in its injury severity score and treatment recommendation is the idea that not all injuries to the middle column are unstable. Lastly, the TLICS represents the first thoracolumbar injury classification system that has been externally validated. The TLICS utilizes modern imaging such as multidetector CT and MRI, which historical systems did not have available. Furthermore, the TLICS has been validated in the pediatric population.
Most likely the most difficult fracture type to determine treatment for is the burst fracture. Despite the usefulness of the TLICS, controversy persists regarding the best treatment for burst fractures in neurologically intact patients. In this scenario, if the PLC is intact, then nonoperative treatment is recommended (injury score of 2). If the PLC is disrupted, then operative management is recommended (injury score of 5). However, if the integrity of the PLC is unknown, then no formal recommendation is given (injury score of 4). However, using the PLC as a key determinant of spinal stability is challenging because, despite the high-quality imaging that is available to most surgeons today, multiple studies have shown that there is poor reliability in assessing the integrity of the PLC. Schroeder et al. found poor reliability (κ = 0.11) when more than 500 spine surgeons from around the world evaluated 10 compression-type injuries for the integrity of the PLC. Similarly, Harrop et al. found only marginally better reliability (κ = 0.34) when 48 highly trained academic spine surgeons reviewed 56 injuries to the thoracolumbar spine.
Another critical flaw in the TLICS system is that there are dramatic differences across the globe in the treatment for a neurologically intact patient with a burst fracture. So although the TLICS makes a firm recommendation against operative fixation for neurologically intact patients with a burst fracture, surgeons in some parts of the world still consider the middle column the cornerstone to spinal stability and therefore recommend surgical intervention for all of these fractures. This has caused these regions of the world to reject the TLICS classification.
In an effort to create a globally accepted thoracolumbar classification system, Vaccaro and colleagues combined the Magerl classification and TLICS to create a new system in 2013: the AOSpine Thoracolumbar Spine Injury Classification System. The Magerl classification was modified to create three main patterns with a total of nine subtypes. The three main patterns are as follows: type A—compression injuries, type B—tension band injuries, and type C—translation injuries. Type A injuries are from compression forces and are subclassified into five subtypes: A0—a trivial injury that does not affect the stability of the spine, such as spinous or transverse process fracture; A1—a fracture of one endplate without posterior wall involvement; A2—a fracture involving the cephalad and caudal endplates without posterior wall involvement; A3—a fracture of one endplate with posterior wall involvement (incomplete burst); A4—a fracture involving the cephalad and caudal endplates with posterior wall involvement (complete burst). Type B injuries are distractive injuries with three subtypes: B1—an osseous injury to the tension band injury (a bony Chance fracture), B2—an injury that disrupts the posterior ligamentous tension band, and B3—a distractive injury of the anterior tension band. Type C includes injuries that result in intervertebral translation and may be along any major axis, including anteroposterior, axial, and vertical.
Kepler and colleagues conducted a reliability analysis with data from 100 surgeons around the world, and for the three main types, they found substantial (κ = 0.74) interobserver reliability and excellent (κ = 0.81) intraobserver reliability. When the subtypes were evaluated, the results were not as impressive, but they did find moderate interobserver (κ = 0.56) and intraobserver (κ = 0.43 to 0.57) reliability of the fracture subtypes. Furthermore, these results have since been validated by multiple unaffiliated authors, with Urrutia et al. reporting substantial interobserver reliability of the main types (κ = 0.62) and moderate reliability of the subtypes (κ = 0.55); similarly, Azimi and colleagues found excellent interobserver reliability of the three main types of injuries (κ = 0.83 to 0.89).
Schroeder and colleagues verified the hierarchical nature of the classification, and Kepler and colleagues used these results to develop the Thoracolumbar AOSpine Injury Score (TL AOSIS). The TL AOSIS is similar to the TLICS injury severity score such that it assigns a value to each injury pattern that can be used to guide clinical decision making ( Table 33.3 ).
| Fracture Morphology | |
| A | Compression |
| A0 | No injury/process fracture |
| A1 | Wedge/impaction |
| A2 | Split/pincer |
| A3 | Incomplete burst |
| A4 | Complete burst |
| B | Tension-Band Injuries |
| B1 | Posterior transosseous disruption |
| B2 | Posterior ligamentous disruption |
| B3 | Anterior ligamentous disruption |
| C | Translation/Displacement |
| Neurologic Status | |
| N0 | Intact |
| N1 | Transient, resolved |
| N2 | Radiculopathy |
| N3 | Incomplete spinal cord or cauda equina injury |
| N4 | Complete spinal cord injury (ASIA grade A) |
| Clinical Modifiers | |
| M1 | Indeterminate tension band |
| M2 | Patient-specific comorbidities (AS, DISH, osteopenia, etc.) |
Lastly, Vaccaro et al. reported on a worldwide survey of over 500 surgeons to determine the treatment algorithm for the AOSpine Classification. The algorithm itself was developed with input from over 500 surgeons from around the world, with the hope that with this worldwide input, the global community would implement the treatment algorithm. A vignette of all controversial thoracolumbar injury patterns was described to the participating surgeons, and they were asked to recommend surgical or nonsurgical care. A recommendation of nonoperative care was given to injuries in which less than 30% of the participating surgeons suggested initial surgical management. Similarly, a recommendation of surgical management was established when greater than 70% of surgeons recommended operative intervention for the injury. Using these results and the TL AOSIS, a surgical algorithm that is simple and easy to use was proposed. Patients with a total TL AOSIS of less than 4 should undergo a trial of nonoperative treatment, and early operative intervention is appropriate for patients with a TL AOSIS of more than 5. Treatment of patients with a TL AOSIS of 4 or 5 should be individualized based on surgeon and patient variables because operative or nonoperative care may be appropriate ( Table 33.4 ).
| Subgroup | Description | TL AOSIS |
|---|---|---|
| Type A—Compression Fracture | ||
| A0 | An injury that has no possibility of affecting the structural integrity of the spine (i.e., fracture spinous or transverse process fracture) | 0 |
| A1 | A fracture through a single endplate that does not extend into the posterior wall | 1 |
| A2 | A fracture through both endplates that does not extend into the posterior wall | 2 |
| A3 | A fracture through a single endplate that does extend into the posterior wall (incomplete burst) | 3 |
| A4 | A fracture through both endplates that does extend into the posterior wall (complete burst) | 5 |
| Type B—Tension-Band Injuries | ||
| B1 | A completely osseous tension-band injury (i.e., a bony Chance fracture) | 5 |
| B2 | An injury that disrupts the posterior tension band | 6 |
| B3 | An injury that disrupts the anterior tension band | 7 |
| Type C—Translational Injuries | ||
| C | Any injury that results in translation of the vertebral body | 8 |
The evolution of thoracolumbar classification systems has led to the creation of increasingly comprehensive systems, each with the goal of providing clear and accurate injury description, providing assistance in decision making, and achieving global acceptance. Presently, several systems are in use and demonstrate regional variability. The most recent system, the AOSpine Thoracolumbar Spine Injury Classification System, was created with global input in the hope of unifying our nomenclature and improving our ability to deliver spinal care around the world.
Consider each injury as potentially unstable until the fracture is classified and stability is determined in a systematic manner.
The neurologic examination should be performed and recorded per American Spinal Injury Association standards.
CT is the most sensitive examination to identify bony injuries.
If the status of the PLC is questioned, then MRI with fat suppression is indicated.
Substantial supra-physiologic axial load to the anterior and middle columns of the thoracolumbar spine can result in a burst fracture. These injuries are defined when there is a vertebral body fracture with extension into the posterior cortex of the body. The vast majority have some component of fracture comminution with retropulsion of the posterior vertebral body wall into the spinal canal. The fracture may involve one or both vertebral endplates. Posteriorly, the pedicles are widened, with some element of facet joint disruption. Due to tensile forces, there may be various degrees of PLC disruption.
Burst fractures account for approximately 50% of the thoracolumbar fractures that cause neurologic injury. The fractures commonly occur at the thoracolumbar junction due to relatively abrupt transition from a fixed, kyphotic thoracic spine to the mobile, lordotic lumbar spine caudally. Burst fractures make up 10% to 20% of all fractures in this transitional zone. The goals of treatment for thoracolumbar burst fractures are to (1) maintain and restore spinal stability, (2) preserve neurologic function, (3) minimize loss of spinal mobility, (4) achieve a healed and stable spinal column, and (5) prevent long-term disability. To maximize the chance of neurologic recovery, decompression of the neural elements should occur when indicated. Additionally, in the setting of polytrauma injuries, treatment should aid in the early mobilization of these patients.
Biomechanically favorable conditions cause thoracolumbar burst fractures to be one of the most frequent fractures encountered by spine surgeons due to the transition from the rigid, kyphotic thoracic spine to the mobile, lordotic lumbar spine.
Multiple fracture classification systems exist to aid surgeons in operative decision making. Currently, the TLICS may be the best system available for therapeutic decision making; however, the TL AOSIS is an emerging schema and may provide additional assistance regarding when to choose surgical intervention. Future prospective studies are needed to decide whether the TL AOSIS schema leads to better agreement among surgeons.
Disruption of the PLC as determined by advanced imaging, upright radiographs demonstrating instability, and/or neurologic injury is an indication for surgical treatment of thoracolumbar burst fractures.
In cases with incomplete neurologic injury, there is no difference in neurologic or functional outcome between anterior, posterior, or combined anterior/posterior approaches.
Correction of kyphosis appears to be maintained with a combined anterior/posterior approach.
Neurologic decline is rare in thoracolumbar burst fractures.
The thoracolumbar junction, generally considered to include T10 to L2, is the transition point from the rigid, thoracic spine to the mobile, lumbar spine. In addition to the bony elements, the PLC provides essential tension-band stability. The PLC includes the interspinous ligaments, supraspinous ligament, facet capsules, ligamentum flavum, and thoracolumbar fascia. In severe injuries, if the entire complex is disrupted, it can cause significant instability. Assessment of the PLC has been a key feature of modern methods of classification schemes of thoracolumbar fractures. Additionally, an intact PLC can aid in the indirect decompression of burst fractures through ligamentotaxis. This is important surgically because if the PLC has been disrupted, full decompression may not be achieved, and a laminectomy may be needed.
The spinal cord in this region transitions from the conus medullaris (upper motor neurons) to the cauda equina (lower motor neurons). The conus medullaris begins at T11 and ends around the L1–L2 space. Patients presenting with burst fractures occurring at this transition point, such as T12, may have a mixed neurologic examination consisting of upper motor-neuron signs with lower motor weakness and radiculopathy. After transitioning to the cauda equina, there is more space available in the spinal canal. This allows for significant canal compromise before neurologic changes are seen clinically.
Various classification schemes have been devised to aid surgeons in assessing the stability of the burst fracture and the need to perform operative stabilization. Beginning in 1943, Watson-Jones first described the wedged, comminuted fracture and emphasized the integrity of the PLC. Nicoll's classification of thoracolumbar spine trauma further defined stability based on the integrity of four structures: the vertebral body, the disc, the intervertebral joint, and the interspinous ligaments. In the 1970s, wedge compression fractures were distinguished from “compression burst fractures”; however, the stability of these fractures was still in question.
In the 1980s, Denis categorized thoracolumbar injuries into four categories, including the thoracolumbar burst fracture. He divided the spine into three columns. Denis defined instability when two of three columns were disrupted. Using this algorithm, all burst fractures are considered unstable, and neurologic instability is defined as spinal trauma–induced neurologic deficit.
The question of which burst fractures are stable and which require surgical intervention continued to afflict authors. Based on 100 CT examinations of the thoracolumbar spine, McAfee and colleagues classified these injuries into six groups, delineating stable and unstable fractures. In this system, the PLC is a key component in fracture stability. Characteristics of the fracture, such as the degree of comminution, fracture fragment displacement, and deformity, were grouped and graded by McCormack and colleagues. These characteristics are given scores from 0 to 3 and summed together. Higher scores indicate greater degrees of instability and likelihood for later kyphotic angulation despite treatment.
The Spine Trauma Study Group developed the TLICS. According to this classification system, burst fractures are categorized as moderate injuries. Specific injury characteristics are qualified and then quantified based on a preset scoring system. These characteristics include injury morphology, neurologic status, and the integrity of the PLC ( Table 33.5 ). Compression fractures are assigned 1 point, burst fractures receive 2 points, rotational and translational injuries 3 points, and distraction injuries 4 points. Neurologically intact patients receive 0 points; an isolated radiculopathy 2 points; complete injuries 2 points; and incomplete spinal cord injuries, conus medullaris, and cauda equina syndrome are assigned 3 points. If the PLC is intact, it receives 0 points. Suspected PLC injuries, such as high-intensity signal observed on short tau inversion recovery (STIR) MRI, without overt tear are assigned 2 points, and obvious tear of the PLC defined by MRI or interspinous widening on plain radiographs or CT receives 3 points. According to the TLICS algorithm, scores less than 4 points can be treated with nonoperative means, whereas scores greater than 4 points are indicated for surgical management. Injuries assigned a score of 4 can be treated conservatively or with operative management depending on the overall clinical picture of the patient. Burst fractures with an intact PLC in neurologically intact individuals have a TLICS score of 2, whereas those with deficits or PLC disruptions are greater than 5 points.
| Injury Morphology | Qualifiers | Score |
|---|---|---|
| Compression | 1 | |
| Burst | 1 | |
| Rotation/translation | 3 | |
| Distraction | 4 | |
| Posterior Ligamentous Complex | ||
| None | 0 | |
| Suspected/indeterminate | 2 | |
| Disrupted | 3 | |
| Neurologic Function | ||
| Intact | 0 | |
| Nerve root injury | 1 | |
| Cord, conus medullaris injury | Complete | 2 |
| Incomplete | 3 | |
| Cauda equina syndrome | 3 | |
| Total a | ||
a Nonoperative management is recommended for a total score of ≤3. A score of 4 can be treated with either surgical or nonoperative management as indicated. A score of ≥5 indicates surgical intervention.
The AOSpine Classification Group created a new scheme using elements of the Magerl and TLICS to better describe pathomorphology and estimate injury severity using all the information available to the clinician. The classification system describes the mode of failure based primarily on features identified on CT scans: type A fractures include compression injuries with intact tension band, type B includes injuries with failure of the posterior or anterior tension band through distraction with maintenance of alignment of the spinal axis, and type C includes failures of all elements leading to dislocation, translation, or displacement in any plane. Type A and type B injuries are further subdivided into five and three types, respectively. Neurologic status is classified with the N modifier, with 0 to 4 increasing from no injury to complete spinal cord injury, respectfully. The NX modifier is used for a neurologic examination that is unobtainable. Two additional modifiers were added. M1 is assigned to compression-type fractures where the status of the PLC is unknown. M2 is assigned to patients in whom patient-specific morbidities affect the treatment, such as polytrauma or ankylosing spondylitis (AS).
Burst fractures are types A3 and A4. The lack of involvement of posterior structures clearly differentiates them from other injury patterns. Posterior tension-band disruptions or Chance fractures are considered B1 and B2 fractures. A recent multicenter study comparing TLICS and the new AOSpine Classification showed better reliability for identifying fracture morphology with the new scheme.
The posterior spine is examined to note areas of abrasion/contusion and hematoma. The posterior midline spine can be palpated to assess for step-offs, gaps between spinous processes, or hematoma formation. Posterior midline edema or significant pain with palpation may increase the clinician's suspicion for PLC injury.
The neurologic examination, including perineal examination, is performed as outlined later in this chapter. Patients with thoracolumbar burst fractures may present with upper- or lower-motor-neuron signs due to the transition from cord to nerve roots depending on the level of injury. The severity of neurologic injury may be related to the initial displacement and amount of canal encroachment of the retropulsed fragments at the time of injury. Careful evaluation of perineal sensation and sphincter tone should be performed to rule out conus medullaris or cauda equina syndrome.
Plain radiographs may have been obtained in some cases in patients with a low-energy mechanism but are less sensitive than CT. Characteristic radiographic findings of burst fractures include loss of posterior vertebral body height, narrowing of the spinal canal on lateral views, and increased interpedicular width on AP views.
The modern practice for trauma patients widely uses CT, which can easily be formatted to assess the thoracolumbar spine. On the sagittal CT scan, findings include a decrease in vertebral body height; a lack of smooth, defined cortices; retropulsion of the posterior cortex into the canal; and an increase in local kyphosis. Clinicians should have a high level of suspicion for instability if there is evidence of interspinous widening. If there is an indeterminate PLC injury or there is suspicion of worsening neurologic compromise, MRI should be obtained. Other indications for MRI include unexplained neurologic deficits, progressive neurologic deterioration, the timing of the acuity of fracture, and preoperative planning in some cases.
It remains the standard to treat stable burst fractures without significant PLC disruption and absence of neurologic injury nonoperatively. Wood et al., in a multicenter, randomized clinical trial, studied 47 consecutive patients from 1992 to 1998 with stable, neurologically intact burst fractures, comparing nonoperative and operative treatments. Patients in the nonoperative group seemed to do better compared with the operative group based on radiographic criteria, Short Form 36 (SF-36), and Oswestry questionnaires at an average follow-up of 44 months. The operative group showed more frequent complications and greater costs. Surgical treatment was heterogeneous and up to the discretion of the treating surgeon. In a recent publication consisting of 16- to 22-year follow-up of the same cohort, these authors presented long-term follow-up results. Of the original randomized 47 patients, 78% were included, and again, patients treated nonoperatively showed better outcomes in regard to pain and function. A later randomized controlled trial (RCT) of 34 subjects by Siebenga et al. found that stable burst fractures without neurologic deficits treated with operative intervention had better functional outcomes and higher return to preinjury work than the nonoperative group. These authors recommended that treatment should take into consideration the amount of deformity and patient preference with respect to complication risks.
Traditionally, stable burst fractures without neurologic injury were treated with immobilization through bracing. An “off-the-shelf” thoracolumbar spinal orthosis (TLSO) or hyperextension brace, such as a Jewett brace, may be used. These can be used in the midthoracic (T5–T10) and upper thoracic (T1–T5) with the addition of a neck extension added to the TLSO.
Recently, authors have questioned the use of bracing in burst fractures. A prospective RCT from Canada found no difference in the outcomes or complications of patients treated with activity restriction and guidance versus those who received prefabricated and customary orthotics. Another study showed similar results, with no difference in progression of kyphosis, vertebral height loss, outcomes, or complications between treatment with or without external immobilization. Urquhart et al. compared the long-term follow-up (5 to 10 years) of 16 patients treated with a TLSO versus 20 patients without bracing. There was no significant difference between groups in function or pain relief. It is critical for surgeons to discriminate between patients who may benefit from a brace versus those who would be better served by early mobilization. It may depend on the severity of vertebral height loss and kyphotic deformity. In those cases where a custom brace or hyperextension brace is required, surgical fixation may be a reasonable alternative to reduce the discomfort and burden of external immobilization.
Despite numerous classification systems and RCTs, the surgical treatment of burst fractures with no or transient neurologic injury remains controversial. Recognizing this knowledge gap, the AOSpine group has created the Thoracolumbar AOSpine Injury Score (TL AOSIS). This system assigns points for three domains (morphology, neurologic injury, and PLC/polytrauma modifiers) to aid in the decision making for surgical and nonsurgical treatment ( Table 33.6 ). In this schema, an AOSpine injury score of 3 or less should be treated with a trial of conservative treatment. A score of greater than 5 points based on fracture and clinical characteristics should be considered for operative intervention. It is important to note that this system has not been validated clinically. Controversy remains regarding AOSpine A3 and A4 injuries without neurologic injury or posterior tension-band injuries. Currently, there is not a consensus on surgical treatment at this threshold. For example, the TL AOSIS scores A4N0M0 (both-endplates burst fracture without neurologic injury and intact PLC) at 6, and that indicates surgery. In a survey of 483 spine surgeons distributed throughout the world, there was not a consensus of surgical treatment for these fractures, with 60% of respondents choosing nonoperative treatment for these fracture types. The AOSpine group has sponsored an ongoing study that will prospectively collect outcome data in patients with A3 and A4 fractures that may adjust this scoring system in the future.
| Classification | Points |
|---|---|
| Type A—Compression Injuries | |
| A0 | 0 |
| A1 | 1 |
| A2 | 2 |
| A3 | 3 |
| A4 | 5 |
| Type B—Tension-Band Injuries | |
| B1 | 5 |
| B2 | 6 |
| B3 | 7 |
| Type C—Translational Injuries | |
| C | 8 |
| Neurologic Status | |
| N0 | 0 |
| N1 | 1 |
| N2 | 2 |
| N3 | 4 |
| N4 | 4 |
| NX | 3 |
| Patient-Specific Modifiers | |
| M1 | 1 |
| M2 | 0 |
a A score of 4 or less should be considered for nonoperative treatment, a score of 5 can be treated either with or without surgery, a score of 6 or greater should be considered for surgery.
Conversely, using the TLICS system, burst fractures assigned a score of 4 without neurologic injury (a morphology score of 2 and PLC integrity score of 2) may undergo a trial of nonoperative management. TLIC scores of 5 or greater are indicated for operative management. In a retrospective series evaluating the TLIC scoring system, all burst fractures graded a 4 had no appreciated harm if initially treated conservatively. When deciding between operative and nonoperative management, the PLC must be closely evaluated because an indeterminate value would not cross the operative threshold in the TLICS schema. If there is a neurologic injury with the burst fracture, operative treatment would provide stabilization to prevent further injury to the neural structures.
The majority of burst fractures are stable injuries and can be successfully treated with nonoperative care. Ideal patients for conservative care are those with burst fractures that are neurologically intact without PLC disruption. These patients should obtain upright radiographs to ensure physiologic loading does not induce deformity progression. A brace may or may not be used to aid in the comfort of the patient when mobilizing. If a brace is used, an “off-the-shelf” brace is recommended to reduce costs. After mobilization, pain and neurologic function should be assessed. Changes in either suggest fracture translation or distraction and would require surgical intervention. Patients should mobilize as quickly as possible to reduce the potential complications associated with bed rest—venous thromboembolism events, deconditioning, aspiration pneumonia, and decubitus ulcers. Physical therapy and occupational therapy are consulted during the hospital admission to improve body mechanics and aid in the patient's activities of daily living. Heavy lifting, bending, and twisting activities should be restricted in the first 3 months after injury. Appropriate orthosis management and activity restrictions are reinforced by the physical and occupational therapists before discharge. Patients are seen 2, 6, and 12 weeks after their injury, with repeat radiographs and clinical assessment. Patients should be warned to seek evaluation if there is a change in the neurologic examination or new bowel/bladder incontinence. At 4 months after injury and radiographic demonstration of fracture healing without pain, patients may progress back to regular preinjury activity or work.
Every patient considered for nonoperative care should undergo upright, standing if possible, radiographs to evaluate coronal and sagittal balance. Mehta et al. demonstrated that upright imaging altered nonoperative care for up to 25% of patients with thoracolumbar compression or burst fractures. When evaluating postmobilization radiographs, any new translation or distraction demonstrates instability, and surgical intervention should be sought. Diagnosis of a PLC injury on plain radiographs may be difficult. A recent study found that a 20-degree kyphotic Cobb angle measured at the fractured vertebra from the superior and inferior endplates and increased interspinous distance were associated with PLC injury. Conversely, a retrospective, case-controlled series did not show that local kyphosis of greater than 20 degrees or vertebral body height loss greater than 50% was predictive of PLC injury. Rather, translation of greater than 3.5 mm was associated with PLC disruption.
The treatment of osteopenic or osteoporotic thoracolumbar fractures presents unique challenges due to the poor bone quality and potentially rapid progression of deformity. The higher risk of kyphosis progression may lead to neurologic compromise, and these patients should be monitored closely. The patients should be mobilized as early as possible to reduce the risk of complications associated with recumbence. Patients should be referred to an osteoporosis medical clinic for medication management that may reduce pain in acute fractures.
Surgical treatment of thoracolumbar burst fractures is indicated in those patients with evidence of neurologic compromise or demonstration of instability. The TLICS and TL AOSIS can aid in surgical decision making. Additionally, there is some emerging evidence indicating surgical management of stable burst fractures without neurologic injury to promote early mobilization, avoidance of orthoses, and prevention of deformity. There are also some advantages of operative management for those who cannot tolerate brace wear due to polytrauma, skin lesions, and inability to wear orthosis due to body habitus. If operative intervention is chosen, the approach and technique should consider the fracture morphology, the need to decompress the neural elements, and the need to stabilize the injured tension-band construct. The goals of operative intervention are to (1) adequately stabilize the fracture, (2) correct kyphotic deformity, (3) reverse neurologic compression, and (4) allow for early mobilization.
Anterior, posterior, or combined anterior and posterior approaches may be used for burst fracture management. Fractures may be stabilized with or without fusion. Posterior instrumentation is generally short-segment fixation with or without the addition of screw fixation at the level of the fracture. Decompression can occur either indirectly by restoring the sagittal alignment or directly through corpectomy/laminectomy. In addition, minimally invasive surgery (MIS) is increasingly being utilized, which may change surgical indications in the future.
Advantages of an anterior approach include direct visual decompression to remove fracture comminution, torn disc, or disrupted ligaments within the canal. Anterior visualization may be particularly helpful in the subacute or chronic fracture setting, where callus may inhibit fragment mobilization. The ability to restore sagittal alignment and provide anterior column reconstruction potentially prevents the development of the kyphosis often seen with posterior treatment and is an important advantage of the anterior approach. Fewer levels may need to be fused through this approach. Anterior approaches in general are more invasive, are associated with greater blood loss and surgical time, and have less secure fixation than posterior approaches.
Posterior spinal instrumentation and fusion is an established and effective treatment for thoracolumbar burst fractures. The posterior approach is relatively safer compared with the anterior approach due to the reduced risk of pulmonary, visceral, and vascular injury. In cases with incompetent PLC, posterior instrumentation stabilizes the posterior elements. Disadvantages include potentially inadequate indirect reduction, mobilization of the dura if anterior compression is needed, failure of instrumentation, or inadequate stabilization in the setting of severe anterior vertebral body comminution or poor bone quality. In posterior instrumentation and fusion, the most frequent complications are implant failure and donor-site pain. Combined anterior and posterior approaches offer the advantages of both and may be necessary in the setting of severe comminution and neural injury.
Short-segment pedicle fixation is one of the most standard approaches to treat thoracolumbar burst fractures. These constructs consist of fixation at the levels directly cranial and caudal to the fractured vertebra. Due to modern instrumentation systems, levels can be preserved from fusion with shorter fixation segments. However, posterior-only instrumentation may fail due to the lack of anterior structural and mechanical stability at the fracture segment. The addition of a screw at the fractured vertebra can augment the short-segment posterior fixation and can protect the anterior column by increasing the stiffness of the construct. In most patients with burst fractures, the pedicles remain viable for screw fixation ( Fig. 33.2 ). Kanna et al. reviewed their series of 32 patients with severe, unstable thoracolumbar injuries with posterior fixation including the fractured vertebra over 2 years. There were no instances of implant failure in their series and minimal progression of kyphosis. Long-term follow-up outcomes remain to be seen.
The goal to reduce the morbidity of the surgical approach by reducing infection risk, blood loss, operative time, and iatrogenic injury leading to functional deficits has led to the emergence of MIS in the treatment of thoracolumbar burst fractures. Posterior percutaneous pedicle screw fixation, external spine fixators, kyphoplasty reduction and cement augmentation with or without pedicle screw constructs, and anterior endoscopic decompression and stabilizations have been developed as new techniques. These technologies may provide the ability for surgeons to reduce perioperative morbidities. Currently, there remains limited evidence to support routine use.
There remains controversy regarding whether to preserve motion after posterior instrumentation rather than fusing the segments. Fusionless fixation has demonstrated similar long-term outcomes to those of fused fixation. Advantages of nonfusion include decreased intraoperative blood loss and operative time, preservation of segmental motion, and avoidance of donor-site morbidities. Typically, the hardware is removed 9 to 12 months after the initial operation. Fusion provides a lower risk of implant failure and improvement in radiographic parameters. In an RCT of stable burst fractures with a 5- to 7-year follow-up, patients were treated with or without fusion. There were no clinical or radiologic differences, and the nonfusion group had less operative time and blood loss. In a prospective RCT comparing fusion and nonfusion care, there was no difference in functional status, neurologic status, or pain between the two groups. Lan et al. performed a meta-analysis of the available English literature comparing fusion to nonfusion in treating thoracolumbar burst fractures and demonstrated satisfactory clinical and radiographic results in the nonfusion groups. There was no difference in hardware failure between the two groups.
Neurologic injury severity is related to the volumetric amount and time of compression. A systematic review of the literature regarding the timing of neurologic decompression strongly recommended, with a moderate level of evidence, consideration of early surgical decompression (within 24 hours) for any patient with spinal cord injury. Additionally, due to the implementation of modern resuscitation and stabilization techniques, there appears to be a gradual improvement in the prognosis for neurologic recovery.
For fractures at the thoracolumbar junction between T10 and L3, an anterior retroperitoneal approach may be used for direct decompression and provision of structural stability. For fractures above T10, a right-sided transthoracic approach or video-assisted thoracic surgery (VATS) approach is needed. Although VATS can potentially reduce the risk of thoracic injury, it is technically demanding. Additional indications include the following: a large retropulsed fracture fragment that cannot be reasonably reduced via the posterior approach, inadequate reduction of kyphosis, severe vertebral body comminution requiring structural support, or inadequate decompression after a posterior approach.
The anterior approach can provide direct visualization of the fracture, provide significant structural support, and directly distract the fracture segment, improving the kyphosis. This approach will only address the ventral columns. If there is posterior-element injury, posterior stabilization may be required. Relative contraindications in the polytrauma patient include chest and abdominal injuries. Those patients with pulmonary comorbidities, such as chronic obstructive pulmonary disease, may not have enough reserve to undergo an anterior approach.
The patient is placed in the right lateral decubitus position for a left-sided approach in burst fractures at T12 or below ( Fig. 33.3A ). This avoids obstruction by the liver or having to mobilize the vena cava. More cranial thoracic fractures can be approached from the right or left side due to the ability to mobilize the aorta. If a thoracotomy is used for lower thoracic injuries, the anesthesiologist should intubate with a double-lumen tube to allow for deflation of the right lung to assist with the approach (see Fig. 33.3B ). The abdomen is allowed to fall forward, aiding the approach. Patients should be placed laterally, perpendicular to the ground, to maintain orientation of the vertebral body during the approach, corpectomy, and instrumentation. An axillary roll should be placed underneath the right axilla to prevent a brachial plexopathy. Elbows and knees are padded with gel rolls and pillows to avoid injuries caused by peripheral nerve compression. The left hip is flexed to decrease tension on the psoas muscle. The head and legs on the operating room table are lowered to elevate the fractured vertebral body and aid in the exposure. Additionally, a wrinkle-less, square bump made from bedsheets can be placed underneath the fracture level to assist in flexing the patient's surgical level. Before final instrumentation, the table should be leveled so as not to induce coronal deformity.
After correctly identifying the correct level by fluoroscopy, the skin incision is made along the rib attached to the vertebra one to two levels above the fractured level. An L1 fracture would have an incision over the 12th or 11th rib, which would extend obliquely antero-inferiorly toward the umbilicus. For T11 and some T12 fractures, the incision should be made at the rib just cranial to the involved level (10th rib and 11th rib, respectively). The length of the incision is dependent on the number, location, and severity of fractures as well as the body habitus of the patient and prior thoracic operations.
The chest muscles overlying the rib and abdominal muscles distal to the costal cartilage are incised using electrocautery. The latissimus dorsi can be retracted posteriorly. At this point, the rib is exposed with subperiosteal dissection and can be resected if necessary. Care is taken to protect the neurovascular bundle that lies just caudal to the rib. The resected rib can be later used for structural or crushed bone graft. The pleura parietalis is opened at the posterior aspect of the incision, and the lung is exposed. The diaphragm attaches to the chest wall further anteriorly. At the anterior part of the incision, the layers of the abdominal wall are split, and the retroperitoneal cavity is entered.
Access to T10–T12 should follow the principles of a left-sided thoracotomy. During this approach, the diaphragm may not require division. After the rib is resected, the lung is deflated and retracted anteriorly. A self-retaining retractor system may be used to gently retract the lung and vessels. The parietal pleura is incised over the injured level. A localized hematoma should aid in localization of the fractured level, but intraoperative fluoroscopic images should be obtained (see Fig. 33.3C ). The segmental vessels are identified and ligated in the mid-body. A subperiosteal dissection is performed from the anterior longitudinal ligament to the rib head.
Exposure of T12 or L1 requires sectioning of the diaphragm. After entering the retroperitoneal space, the peritoneum is swept away from the diaphragm. Innervation of the diaphragm comes from the phrenic nerve, which lies adjacent to the esophagus, so any diaphragmatic muscle cut will be left de-innervated. Therefore the diaphragm should be incised near the chest wall, ensuring to leave a 1- to 2-cm cuff of tissue. To aid in repair during closure, tag sutures should be placed every 3 to 4 cm. The diaphragm is retracted anteriorly. At this point, the vertebral body and discs will appear as a “valley” and “hills,” respectively, underneath the parietal pleura. Above L1, the parietal pleura is incised to the cranial vertebral body. The sympathetic chain can be bluntly retracted.
L1 or L2 is approached retroperitoneally where the peritoneum is retracted anteriorly and the psoas muscle is mobilized to retract it posteriorly. Dissection is started anteriorly at the disc space cranial and caudal to the fracture site. The segmental vessels are identified and ligated. Posterior dissection is carried out subperiosteally to the pedicles. Anterior dissection is carried toward the anterior longitudinal ligament. The sympathetic chain can be identified and reflected anteriorly.
Decompression should begin with discectomy (see Fig. 33.3D ). This allows the surgeon to visualize the cranial and caudal limit of the spinal canal. The rib head should be identified and removed with an osteotome or burr. In some instances, the pedicle may need to be removed to identify the exiting nerve root and location of the thecal sac. A triangle-shaped opening in the endplates above and below the disc is created to aid in the evacuation of compressive fragments with minimal spinal cord manipulation. Completion of the discectomy is performed by incising the annulus fibrosis and removing the disc material with Kerrison rongeurs and curettes to the posterior annulus and posterior longitudinal ligament.
The corpectomy is started at the anterior two-thirds of the body with the use of Leksell rongeurs and osteotomes. If possible, a rim of anterior body is left to protect the great vessels and to facilitate fusion (see Fig. 33.3E ). A high-speed burr is used to remove the remaining posterior vertebral body. Retropulsed fragments can be removed with small curettes or thin Kerrison rongeurs (see Fig. 33.3F–H ). Care should be taken to protect the dura, and epidural bleeding should be controlled.
Reconstruction can be performed using an autograft or allograft structural graft or a metallic or synthetic cage. A structural autograft can be a tricorticate iliac crest or the resected rib. A Morcellized resected vertebral body or resected rib can be used for a graft within a cage. The cartilage on the superior and inferior endplates should be removed, but it is essential not to remove the bony structure to prevent later subsidence of the graft or cage. Alignment should be evaluated with fluoroscopy and reduction maneuvers performed. This can be achieved with manipulation of the spine from distraction within the corpectomy with the use of a laminar spreader or expandable cage ( Fig. 33.4A and B ). Also, external corporeal pressure can also be applied to correct deformity. The instrumentation system can be used to restore lordosis.
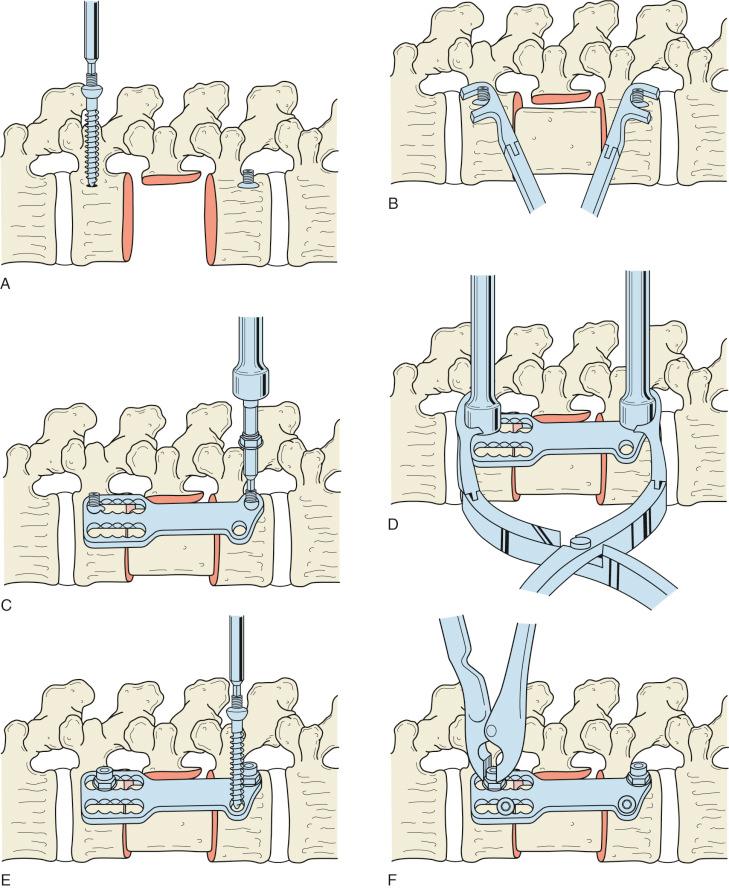
Internal fixation can augment the structural graft or cage (see Fig. 33.4C–F ). Transvertebral screws and rods or plates are placed posterio-laterally on the vertebral body to avoid irritation of the vessels and viscera. Screws are aimed laterally to the contralateral side. The screws are placed parallel to the endplates. Care must be taken to ensure that the screws do not violate the spinal canal. Screws should be precise in length to prevent iatrogenic visceral injury.
A layered closure should be carried out after meticulous hemostasis. The parietal pleura should be sutured over the instrumentation. A chest tube is introduced in the midaxillary line, and the diaphragm is repaired with interrupted sutures. The abdominal layers are approximated and closed in a layered fashion. If the rib was not resected, the layers can be approximated with nonresorbable sutures. Finally, subcutaneous tissues and skin are sutured in a layered fashion.
Patients should be mobilized as early as possible. Bracing is up to the surgeon's discretion and is often dependent on the patient's fixation, bone quality, and postoperative pain. Upright radiographs should be obtained as early as possible to ensure stable fixation. Chest radiographs are followed until there is resolution of the pneumothorax and stable, low output. At this point, the chest tube can be safely removed. The patient should be monitored for ileus.
Surgical complications from the anterior approach often occur due to suboptimal exposure. The appropriate rib must be chosen to ensure that the approach is direct to the fractured vertebra. If an approach surgeon is used, the spine surgeon should be present and assist in the exposure to the injured vertebra. Anterior placement of instrumentation could lead to vascular impingement or violation during instrumentation.
Complications specific to the anterior approach may arise due to the approach, during decompression, or from construct failure. The prevalence of complications in the anterior approach alone ranges from 11.5% to 31%. McDonnell et al. stratified complications into major (11%) or minor (24%) for 447 patients. The most common minor complication was genitourinary, and the most common major complication was pulmonary. In another large retrospective review of anterior fusions in the thoracolumbar spine, the anterior approach was associated with postoperative pneumonia, retrograde ejaculation, post-thoracotomy pain syndromes, and meralgia parasthetica.
Iatrogenic injury to the neural elements or iatrogenic durotomy is less likely in the anterior approach due to the direct visualization during decompression. However, if a durotomy is present, it should be closed in an expedited, normal fashion. In osteoporosis or other manifestations of poor bone quality, there may be settling of the graft or cage, leading to progression of kyphosis. In these cases, posterior fixation should be considered to augment the anterior construct.
The posterior approach to thoracolumbar fractures is discussed later in this chapter. Patient positioning, anesthesia considerations, and instrumentation are similar when treating burst fractures. Posterior decompression can be performed using laminectomy and direct manipulation of retropulsed fragments. This is indicated in the presence of neurologic deficits in the lower lumbar spine where anterior decompression is more difficult. The retropulsed bone fragments can be difficult to mobilize, and it is best to perform a discectomy to create space. The burst fragments can then be pushed forward using a down-angled curette. Removal of the pedicle can give greater exposure and ease of decompression. If needed, subtotal corpectomy can also be done through a transpedicular posterior approach. Dural tears should be anticipated, and primary closure, if possible, is the best treatment. When laminectomy is performed, then fusion is mandatory, and a fusionless technique should be avoided. Further laminectomy increases instability. In these instances, longer instrumentation constructs at least two levels above and below should be considered.
Posterior reduction maneuvers are dependent on the direction of fracture instability. The preponderance of burst fractures is a kyphosing event and necessitates a combination of lordosis and compression to correct the deformity. Inducing lordosis can be achieved through a cantilever rod-reduction maneuver or in situ rod-bending techniques. When reducing the fracture, the rod loses some lordosis, so it is recommended to over-contour the rod before reduction. The superior articular process may block reduction and can be removed to aid in reduction. The rod should be inserted into the proximal screws bilaterally, and sequential reduction should be performed with the use of a persuader. Compression between pedicle screws after rod reduction may induce additional sagittal correction if needed.
AOSpine type A3 or A4 bursts without neurologic injury and/or PLC injury are common fracture variants in the thoracolumbar spine. Throughout the world, there remains a wide range of “standard” treatment, which is highly region dependent. Treatment ranges from early mobilization without bracing to combined anterior/posterior surgical intervention. Surgical stabilization with or without decompression may result in earlier mobilization, decreased length of stay, and earlier return to work. These patients are at risk for surgical complications and revision surgery. Nonoperative management with symptomatic pain control and early mobilization in stable fractures would avoid the inherent risks of surgical intervention. A recent Cochrane review of RCTs of thoracolumbar burst fracture management was unable to conclude if there was a significant improvement in pain or functional outcomes between nonoperative and operative care.
Wood et al., in an RCT of 47 patients treated with either nonoperative management or surgery from 1992 to 1998, presented favorable outcomes for nonoperative treatment. At an average of 44 months, there were no clinical or radiographic differences. It should be noted that many of the surgical techniques used in this study would unlikely be used today. These authors again reported on the same cohort 16 to 22 years after treatment randomization. The surgical group had significantly worse functional outcome scores compared with the nonsurgical group. The average kyphosis between the two groups was not significantly different.
A multicenter, prospective RCT from the Netherlands of AO type A fractures without neurologic deficits demonstrated superior return to work in the operative group compared with the nonoperative group, 6.7 months versus 13.6 months, respectively. The surgical group underwent short-segment fixation with pedicle screws and fixed-angle dedicated fracture-reduction implants. Additionally, a multicenter study from German and Austrian trauma centers reported favorable outcomes and lower complications than previously reported for surgically treated patients. A meta-analysis in 2012 reviewed four trials consisting of 79 patients and compared the two treatment methods. Their findings showed improved kyphosis with the surgically treated but did not show improved function 4 years after injury. Additionally, there were increased complication rates with surgery.
In a prospective RCT, the authors compared nonoperative care with posterior instrumentation and showed that surgical intervention provided early pain control and kyphosis correction. Neurologic deteriorations did not occur in either group, and both showed progressive kyphosis over time. The surgical group had statistically significant improved pain scores at 1 and 3 months postoperatively but no difference at 6 months and beyond. In this study, a large percentage of patients who underwent surgical intervention were able to return to heavy labor compared with nonoperative patients.
Anterior versus posterior approaches have been shown to be equivocal for radiographic and patient-reported functional outcomes. A systematic review determined that there were no relative advantages to the posterior, anterior, or combined approach for neurologic recovery. These authors concluded that the combined anterior and posterior approach appeared to maintain kyphosis correction. A meta-analysis, which reviewed seven RCTs including 331 patients, compared the anterior and posterior approaches and found no significantly superior deformity correction, neurologic recovery, or return to work between the two groups. In this study, there were no differences in complication rate. However, the anterior approach appeared to have increased operative times, blood loss, and costs compared with posterior approaches.
Lin et al. compared the two techniques in patients with neurologic injury in a prospective, randomized study. Sixty-four patients underwent either an anterior-approach fusion or a posterior approach that consisted of a subtotal corpectomy, decompression, and reconstruction of the spine. At a minimum of 2 years, all patients in the study went on to achieve a successful fusion, and there was no significant difference in motor-examination improvement between the two groups, although the posterior-approach group experienced fewer pulmonary complications, decreased rates of blood loss, fewer complications, and a shorter operative time.
A prospective multicenter registry performed in Germany compared the anterior and posterior approach versus the posterior approach in 733 patients. These authors reported no difference in neurologic recovery between the two groups. Correction of deformity was greater in the combined group. The single posterior approach showed superior functional results, including shortened return to work and reduced pain.
Loss of sagittal correction after a posterior-only approach may be due to a failure to reconstruct and maintain the anterior column. If using posterior-only instrumentation, high cantilever bending loads are placed on the pedicle screws, and increased kyphosis may occur over time. Sasso et al. reviewed sagittal alignment in 53 patients who sustained unstable thoracolumbar burst fractures. Forty patients underwent an anterior approach with strut placement, and 13 patients underwent short-segment posterior-only instrumentation. Although both groups initially achieved statistically significant kyphosis correction, the posterior-approach group demonstrated increased kyphosis deformity.
Thoracolumbar burst fractures are common injuries at the junction between the rigid, kyphotic thoracic spine and mobile, lordotic, lumbar spine. Burst injuries are defined by a fracture line that extends into the posterior cortex with retropulsion of fracture fragments into the canal. New classification schemes based on fracture morphology may make treatment decisions easier for surgeons. Operative intervention is determined by the morphology of the fracture leading to instability and neurologic impairment due to compression or deformity. Restoring sagittal alignment and decompression of the neural elements may be approached anteriorly, posteriorly, or with a combination of approaches, with equivocal results. The anterior approach appears to maintain sagittal correction over time compared with the posterior-only approach due to the reconstruction of the anterior column. Posterior instrumentation, with or without fusion, restores the tension band in burst fractures with PLC injuries. The addition of fracture-level pedicle screw fixation may augment short-segment fixation in the setting of severe anterior vertebral body comminution.
Thoracolumbar burst fractures are commonly due to a high-energy mechanism, and the patient should be evaluated for additional injuries at initial presentation.
Most thoracolumbar burst fractures can be treated with nonoperative management.
If nonoperative management is selected, upright radiographs must be obtained to confirm the stability of the fracture. Increased translation or distraction confirms significant injury to the PLC, and operative care must be considered to prevent further deformity and neurologic injury.
Appropriate rib selection should be made to adequately expose the desired level and to minimize surgical complications.
In either operative or nonoperative management of thoracolumbar burst fractures, patients are mobilized immediately with or without an “off-the-shelf” TLSO.
Flexion-distraction and fracture-dislocation injuries are two common fracture types that can be seen as a continuum due to hyperflexion forces. They also may be combined with rotation or lateral shear so that the injuries may be asymmetrical between the left and right sides. Regardless, most of these injuries are unstable and will usually have a significant component of ligamentous injury, which, except in younger patients, has a reduced potential for healing. Because of translation or significant distraction, neurologic injuries are common. These types of injuries occur from high-energy trauma and may be associated with other injuries, especially thoracic and intra-abdominal injuries; thus critical evaluation of the patient is required before treatment of the spinal condition. This section reviews the proposed mechanism of injury of these fracture patterns, their treatment, and outcomes.
The thoracolumbar junction has an inherent predisposition to fractures compared with the rest of the spine.
The mechanism and force of the injury determine the extent of fractures and fracture-dislocations.
A multidetector CT scan is the imaging investigation of choice considering the time taken and the accuracy of imaging in diagnosing these fractures.
MRI is indicated when fracture instability is in question and in the presence of neurologic deficits.
The mechanism of injury/fracture morphology, integrity of the PLC, and neurologic status of the patient must be considered in the treatment decision-making process.
Stable burst fractures and compression fractures can be managed conservatively with or without bracing.
Unstable burst fractures, fracture-dislocations, and distraction injuries are treated surgically.
Flexion injuries result from forceful flexion of the vertebral column where the force vector goes through the anterior to the vertebral body or through the vertebral body. The flexion force is usually associated with some sort of axial loading. According to the AO Classification system discussed earlier, fracture types that result from flexion injuries are compression type (AO type A), including compression fractures and bursting fractures; distractive lesions (AO type B), such as Chance and flexion-distraction injuries; and translation injuries (AO type C), such as fracture-dislocations.
Flexion-distraction injuries are from hyperflexion involving one or two spinal segments. In these types of injuries, the distraction force is the predominant force over the compression component. In the more common flexion-distraction injury, the center of spinal rotation is within the vertebral body. The posterior elements, including the pedicle, posterior longitudinal ligament, and posterior disc annulus, fail in tension. The anterior vertebral body, which is ventral to the center of rotation, is compressed, usually resulting in a compression fracture. Alternatively, with greater force, a burst fracture can occur. Thus the flexion-distraction injury has a tensile failure of the posterior and middle columns and compressive failure of the anterior column. Flexion-distraction injuries typically result from a seat-belt injury. This injury pattern is prototypical of the mechanism and has facilitated the clarification of the actual mechanism of these injuries as being caused by the spine rotating around a fixed fulcrum anteriorly (e.g., a lap belt) and sustaining a hyperflexion rotational moment ( Fig. 33.5 ).
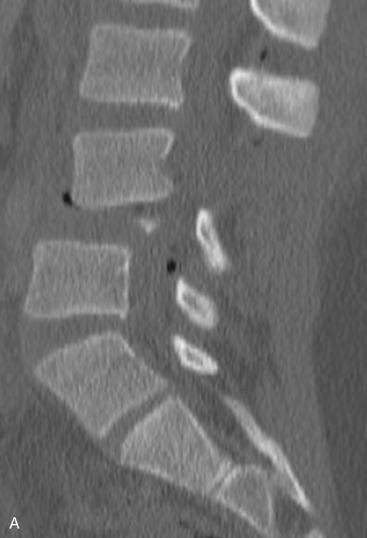
The Chance fracture is a unique pattern because it is a purely bony distractive injury. The posterior spinal processes, pedicles, and vertebral body fracture and displace as they fail under tension as the spine rotates around an anterior fulcrum, whereas the anterior longitudinal ligament often remains intact. The force of this injury may also result in numerous severe intra-abdominal soft tissue injuries. In other types of injuries, an anterior column injury may occur through the disc, and the vertebral body may remain intact, whereas a posterior column injury may manifest as facet dislocations or posterior ligamentous disruptions. These purely discoligamentous injuries may be associated with bony fractures. These types of injuries are known as Chance variants. The vertebrae may realign after the initial injury, and the PLC injury may be missed in these patients unless an MRI is obtained. CT scans aid in revealing facet fracture or subluxation ( Fig. 33.6 ).
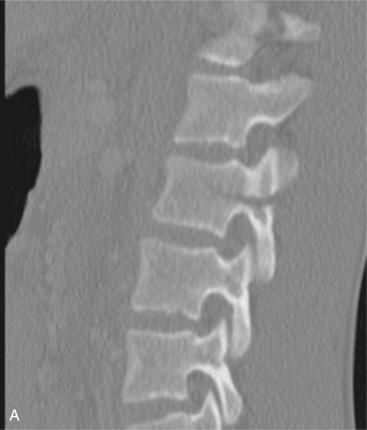
Due to the violent forces involved, flexion-distraction injuries are usually associated with severe intra-abdominal injuries, including trauma to the major blood vessels or hollow viscera. Owing to the potentially fatal nature of these injuries, a person with flexion-distraction injuries should be critically evaluated to rule out these injuries, and appropriate general surgical consults should be obtained.
Lateral shear injuries are rare and generally very unstable. They are often associated with other fracture patterns, including flexion-rotation and lateral compression, and thus create very complex injuries. These injuries often involve a combination of facet fractures, have oblique fractures through the vertebral body or a bursting component, can displace in any direction, and have a high association with concurrent complete neurologic injury. They are often associated with severe polytrauma, including to the chest and abdomen.
Flexion-rotation injuries occur after rotational forces are applied simultaneously with a flexion moment to the thoracolumbar spine, resulting in the complete disruption of the PLC and extension through the anterior disc and vertebral body. These injuries are often associated with facet fractures, are highly unstable, and can progress to a complete disassociation of the involved spinal segments. They carry a high likelihood of a complete neurologic injury. The hallmark of these injuries is lateral or rotational displacement and fracturing of the transverse processes.
Any of the aforementioned forces of flexion, extension, lateral shear, distraction, and/or rotation, when severe enough, may lead to translation of the cephalad vertebra over the caudal vertebra, with dislocation of one or both facets. Vertebral translations can occur in the sagittal or coronal planes. These injuries are considered highly unstable, and the potential for neurologic injury is elevated. These fractures can be diagnosed on plain radiographs based on the translation of the vertebra and facet dislocation/subluxation. Axial CT scan imaging can also show the presence of an “empty facet sign” in the presence of bifacetal dislocations. Fracture-dislocations in neurologically intact patients need to be investigated emergently with MRI to assess the discoligamentous complex, and immediate surgical treatment is warranted.
In addition to the nature and direction of the force applied to the spine during a violent traumatic event, the strength of the bone and elasticity of the soft tissue are critical in determining the severity of the injury. Additional modifiers to the type of resultant fracture include the age of the patient, the level of fracture, the degree of comminution, the presence of osteoporosis and osteomalacia, the development of avascular necrosis, and damage to supporting structures such as the ribs and sternum. Preexisting deformities, including spondylolisthesis, kyphosis, and scoliosis, can also impact the fracture pattern and stability ( Fig. 33.7 ).
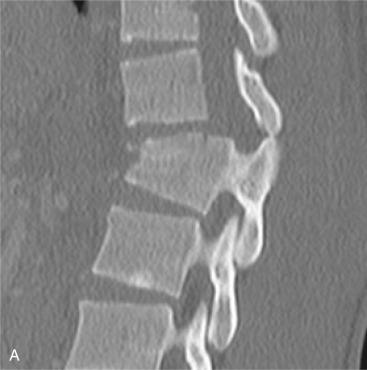
Clinical and radiologic evaluation of the patient allows surgeons to determine the nature of the thoracolumbar injury. Surgeons are able to assess the neurologic status, morphology of the fracture, mechanism of injury, potential for instability, and presence of other injuries. The mechanism of injury can differentiate between low-energy or fragility fractures versus high-velocity injuries caused by falls from a height or MVAs. It is equally important to consider the overall general condition of the patient with respect to other skeletal and visceral injuries and the feasibility of operative treatment if it is considered.
After the initial assessment, the decision making for nonoperative versus operative treatment depends mainly on the presence or absence of a neurologic injury and the integrity of the PLC. In flexion-distraction injuries, MRI may be useful when the status of the PLC is indeterminate. On fat-suppressed T2 MRI, an increased signal transverses through the supraspinous and intraspinous ligaments or disruption of facet joints, indicating disruption of the PLC. In the presence of a PLC injury or neurologic deficits, the preferred treatment of choice is surgical stabilization with or without decompression. In the absence of a neurologic injury and with an unequivocally intact PLC, the choice of treatment is mainly nonoperative.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here