Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Alexander performed the first cervical sympathectomy in 1889. A little more than a quarter of a century later, thoracic sympathectomy (TS) for hyperhidrosis became popular. Kotzareff was credited with the first TS for hyperhidrosis, and it was found that surgery contributes to anhidrosis from the nipple line upward. The role of TS has since expanded to include spastic paralysis to decreased muscle tone, and vasospastic conditions such as Raynaud’s disease and thromboangiitis obliterans (TAO).
Several open approaches for TS have been described, including supraclavicular (cervical), anterior transthoracic, posterodorsal, and dorsal midline transthoracic. These approaches have proved to be a great challenge for the surgeon to justify potential benefits from a significantly morbid procedure. That has led to a decrease in the number of open TS procedures being performed.
Endoscopic/thoracoscopic sympathectomy was pioneered by Goren Claes and Christer Drott in Sweden in late 1980. This minimally invasive technique—video-assisted thoracoscopic sympathectomy (VATS)—has revolutionized the indications and applicability of TS. It can be done as an outpatient procedure with a very low complication rate and acceptable cosmetic results.
Thoracic sympathetic ganglia are the most laterally placed structures in the posterior mediastinum, and each sympathetic trunk alongside the thoracic vertebrae enters the thorax by passing anterior to the neck of the corresponding rib and exits the chest by passing behind the medial arcuate ligament of the diaphragm. In the upper part of the thorax it lies on the necks of the ribs, and in its lower course, it lies on the vertebral bodies as the vertebrae become broader. The thoracic sympathetic trunk usually contains 11 or 12 ganglia. The first thoracic and inferior cervical ganglia might fuse together in front of the neck of the first rib to form the stellate ganglion ( Figure 1 ).
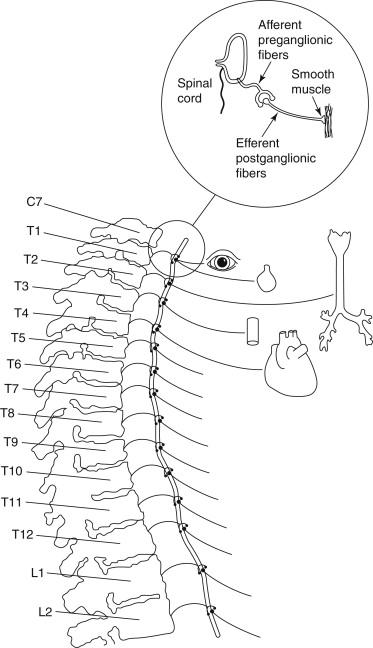
Each thoracic sympathetic ganglion is connected with the corresponding spinal nerve by white and gray ramus communications. The white ramus brings preganglionic fibers from the spinal nerve to the sympathetic ganglion, and the grey ramus carries postganglionic fibers from the ganglion to the spinal nerve (see Figure 1 ).
The preganglionic fibers from the T1 segment go mostly to the head and neck region; however, in about 10% of the population, T1 fibers supply the upper extremity as well. The 2nd, 3rd, and 4th ganglia give branches to the cardiac and pulmonary plexuses. The upper five ganglia give fine branches to the aorta to form an aortic plexus (see Figure 1 ).
The three lower branches of the thoracic sympathetic chain are the greater, lesser, and least splanchnic nerves. These sympathetic fibers pass to the abdominal cavity as follows: The greater splanchnic nerve arises from ganglia 5 to 9 and passes through the crus of the diaphragm to end in the celiac plexus; the lesser splanchnic nerve arises from the 9th and 10th thoracic ganglia and crosses the crus of the diaphragm and ends, joining the celiac plexus as well; and the least splanchnic nerve may arise from 11th and 12th ganglia and end in the rectal plexus. For clinical application, it is worthwhile to note that if spinal anesthesia is given high up, it can produce a temporary drop in the blood pressure by abolishing the effect of sympathetic vasoconstrictor fibers that arise from T5 downward and pass to the abdominal viscera by way of the splanchnic nerves.
Destruction of the white ramus of the 1st thoracic nerve produces Horner’s syndrome, because this nerve carries fibers to the dilator pupillae muscle, Muller’s muscle of the upper lid, and vasoconstrictor and secretory fibers to the sweat glands. This syndrome involves four clinical features: miosis (constricted pupil), ptosis (drooping upper lid), anhidrosis (loss of perspiration on the affected side), and enophthalmos (sunken eye). Therefore, sympathectomy for vascular diseases of the upper limb should be done below the level of the 1st ganglion. Most clinicians recommend excision of the 2nd and 3rd thoracic ganglia with their rami and their intervening part of the trunk to avoid Horner’s syndrome.
All sympathetic preganglionic fibers exit the spinal cord in the anterior nerve roots of the thoracic and first two lumbar nerves. These fibers (white ramus) either synapse in the thoracic and lumbar ganglia or run up or down through the sympathetic chain to synapse with other ganglia (see Figure 1 ). Some of these fibers might pass through ganglia to synapse in a more peripheral ganglion. The term white ramus is used because all of the preganglionic fibers are myelinated. The fibers leaving the ganglia are called efferent postganglionic cells; these are unmyelinated and gray.
These fibers are distributed via branches of the spinal cord to the target blood vessel (adrenergic fibers), sweat glands (cholinergic fibers), and arrector pili muscles (adrenergic fibers). These fibers are vasomotor, sudomotor, and pilomotor. These blood vessels have no other antagonistic fibers; therefore, vasoconstriction is secondary to stimulation of sympathetic fibers, and absence of sympathetic stimulation contributes to vasodilation. Every spinal nerve except the cervical and lower lumbar receives a gray ramus; therefore the thoracic and first two lumbar spinal nerves have a preganglionic white ramus, ganglia, and gray ramus, which constitutes the somatic branches of the sympathetic ganglia. Distribution of the autonomic nervous system is more prominent in the arteriolar bed. Therefore, skin hyperemia can result from stimulation of the autonomic nervous system.
The highest concentration of sweat glands is on the palms and the soles. Cholinergic stimulation of the sympathetic nervous system will activate postganglionic gray matter and nonmyelinated C fibers, consequently producing sweating from sudomotor effect. This understanding supports the concept of sympathetic block that has been advocated by some before any permanent sympathectomy is done. Also, for complete sympathectomy, excision of the ganglia as well as postganglionic fibers is crucial.
Sweating is not based completely on the above mechanism. There are other mechanisms: Emotional sweating is cortically mediated, thermal sweating is mediated via the hypothalamus, gustatory sweating is controlled via medullary nuclei, and spinal sweating is controlled by spinal cord.
The following are the most important indications in this order: hyperhidrosis, complex regional pain syndrome (CRPS), vasoocclusive disorders and vasospastic disorders (Raynaud’s syndrome), and refractory long QT syndrome.
There are two common types of hyperhidrosis: idiopathic (primary) hyperhidrosis, which is the focus of this chapter, or secondary hyperhidrosis, which is related to several medical conditions such as thyrotoxicosis and some immune and rheumatologic disorders. Idiopathic hyperhidrosis is an increase in the sudomotor activity and is defined as an excessive secretion of the sweat glands beyond what is required for thermoregulation and usually interferes with daily activity.
Hyperhidrosis is usually secondary to central stimulation of sympathetic fibers, with subsequent intense sweating, mainly in the axillae and palms bilaterally. It affects up to 3% of the population, with an equal distribution between males and females. Genetic predilection has been reported in up to 50% of patients with hyperhidrosis. Female patients are more likely to seek medical attention. Although the onset usually occurs in childhood, presentation is often delayed until adolescence and is associated with significant psychosocial, professional, and functional impairment. Conservative treatment, including topical aluminum chloride, oral anticholinergics, or psychotherapy, has been attempted, but the results have been disappointing. Botulinum toxin has been recommended by some, but its exact role and effects have yet to be determined.
Cervical thoracic (open) sympathectomy has traditionally been used to treat idiopathic hyperhidrosis, but development of VATS as a minimally invasive procedure has revolutionized the treatment of this entity since 1980. Advances in the surgical techniques have encouraged some to recommend thoracoscopic sympathectomy as the first line of treatment for idiopathic hyperhidrosis. Most clinicians reserve this procedure for patients with a body mass index (BMI) less than 30, as demonstrated by the Hospital das Clínicas, Medical College, University of São Paulo (HC-FMUSP) study, which demonstrated more compensatory hyperhidrosis in the postoperative period. The same study also showed the adequacy of resecting the two to four thoracic sympathetic chains. Others have limited sympathectomy to the 4th ganglion to decrease the incidence and severity of compensatory hyperhidrosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here