Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Molecularly targeted anticancer agents (MTAs) are designed to target specific molecular features that are either uniquely or differentially expressed in cancer cells compared with normal host cells.
Molecular targets include the following: products of activating mutations and translocations, growth factors and receptors, aberrant signal transduction and apoptotic pathways, factors that control tumor angiogenesis and microenvironment, dysregulated proteins, DNA repair machinery, and aberrant epigenetic mechanisms.
Successful development of an MTA depends largely on the importance of the target in controlling tumor cell proliferation and survival and effective modulation of the target in the tumor at clinically achievable concentrations.
The development of MTAs requires innovative strategies that differ from those traditionally applied to conventional chemotherapy. An important objective in phase I trials of MTAs should be to determine a phase II dose based on optimal target modulation (i.e., a biologically effective dose) rather than solely on a toxicity-driven endpoint, such as the maximum tolerated dose. In addition, objective tumor response may not be an adequate end point for efficacy evaluations of some MTAs that have a primarily cytostatic effect. Alternate end points, such as progression-free survival, may be more appropriate.
Functional and molecular imaging will play an increasingly important role in the development of MTAs.
Patient selection is critical for trials with MTAs.
Improved knowledge of cancer biology and synthetic chemistry along with advances in biotechnology have generated extraordinary opportunities for the development of molecularly targeted cancer therapeutics. Molecularly targeted anticancer agents (MTAs) are defined here as agents that selectively target specific molecular features of cancer cells such as aberrations in genes, proteins, or pathways that regulate tumor growth, progression, and survival. By identifying ways that cancer cells differ from normal healthy cells at the molecular level, scientists can exploit these differences to develop drugs that selectively target cancer cells while sparing normal cells. Consequently, an increasing number of MTAs are being developed with the goal of producing more effective and less toxic anticancer therapeutics. Furthermore, progress in understanding cancer biology and in the development of MTAs can shape cancer therapeutics into a more individualized form of cancer medicine. This chapter reviews the principles of molecularly targeted therapy, including strategies for preclinical and clinical development.
The increasing number and assortment of molecular targets can be broadly categorized according to genetic or functional properties, including products of activating gene mutations and translocations; growth factors and receptors; aberrant signal transduction and apoptotic pathways; factors that control tumor angiogenesis and microenvironment; dysregulated proteins; DNA repair machinery; and aberrant epigenetic mechanisms ( Table 26.1 ).
| FDA-Approved Agent | Target | Disease Indication(s) |
|---|---|---|
| Alemtuzumab | CD52 | B-cell CLL |
| Atezolizumab | PD-1 |
|
| Bevacizumab | VEGF |
|
| Blinatumomab | Bispecific CD19-directed CD13 T-cell engager |
Ph− relapsed or refractory B-cell precursor ALL a |
| Cetuximab | EGFR |
|
| Dinutuximab | Glycolipid GD2-binding monoclonal antibody | Pediatric patients with high-risk neuroblastoma who have achieved a PR to first-line multiagent, multimodality therapy |
| Ibritumomab tiuxetan | CD20 | Relapsed or refractory, low-grade follicular B-cell NHL |
| Ipilimumab | CTLA4 |
|
| Obinutuzumab | CD20 |
|
| Olaratumab | PDGFRα | Soft tissue sarcoma a |
| Nivolumab | PD-1 |
|
| Ofatumumab | CD20 | CLL |
| Panitumumab | EGFR | KRAS wt+ metastatic CRC |
| Pembrolizumab | PD-1 |
|
| Pertuzumab | HER2 |
|
| Ramucirumab | VEGFR2 |
|
| Rituximab | CD20 |
|
| Siltuximab | IL-6 | Patients with multicentric Castleman disease who are HIV and HHV-8 negative |
| Ziv-aflibercept | VEGF, PlGF | Metastatic colorectal cancer resistant to or progressed following an oxaliplatin-containing regimen |
| Erwinia l -asparaginase | Asparaginase | ALL with hypersensitivity to Escherichia coli– derived asparaginase |
| Ado-trastuzumab emtansine | HER2+ | HER2+ metastatic breast cancer |
| Brentuximab | CD30 |
|
| Afatinib | EGFR |
|
| Alectinib | ALK | ALK+ NSCLC a |
| Axitinib | VEGFR | Advanced RCC after failure of systemic therapy |
| Bosutinib | BCR-ABL, Src | Chronic, accelerated, or blast phase Ph+ CML |
| Cabozantinib | VEGFR2, c-MET | Patients with advanced RCC who have received prior antiangiogenic therapy |
| Ceritinib | ALK | Patients with ALK+ metastatic NSCLC that progressed or who are intolerant of crizotinib a |
| Crizotinib | ALK |
|
| Dabrafenib | RAF | Unresectable or metastatic melanoma with BRAF V600E mutation |
| Dasatinib | BCR-ABL, Src |
|
| Erlotinib | EGFR |
|
| Everolimus | mTOR |
|
| Ibrutinib | BTK |
|
| Idelalisib | PI3K |
|
| Imatinib | BCR-ABL, KIT, PDGFRβ |
|
| Lapatinib | HER-2, EGFR |
|
| Lenvatinib | VEGFR1, VEGFR2, VEGFR3 FGFR PDGFRα |
|
| Nilotinib | BCR-ABL | Ph+ CML progressed on imatinib |
| Osimertinib | EGFR T790M | NSCLC with EGFR T790M mutation progressed on EGFR TKI a |
| Palbociclib | CDK 4/6 inhibitor | Hormone receptor–positive, HER2− advanced or metastatic breast cancer a |
| Pazopanib | VEGFR, PDGFR, FGFR, c-KIT |
|
| Ponatinib | BCR-ABL |
|
| Regorafenib | VEGFR2, TIE2 |
|
| Ruxolitinib | JAK |
|
| Sorafenib | VEGFR, PDGFR, RAF |
|
| Sunitinib | PDGFR, VEGFR, c-KIT, RET, CD114, CD135 |
|
| Trametinib | MEK | Unresectable or metastatic melanoma with BRAF V600E or V600K mutation |
| Vandetanib | VEGFR, EGFR, RET | Medullary thyroid cancer |
| Vemurafenib | BRAF | Unresectable or metastatic melanoma with BRAF V600E mutation |
| Abiraterone acetate | CYP17 inhibitor | Metastatic castrate-resistant prostate cancer |
| Azacitidine | DNMT | Myelodysplastic syndrome |
| Belinostat | HDAC | Relapsed refractory peripheral T-cell lymphoma a |
| Bortezomib | Proteosome inhibitor | MM, mantle cell lymphoma |
| Carfilzomib | Proteosome inhibitor | MM |
| Decitabine | DNMT | Myelodysplastic syndrome |
| Enzalutamide | AR | Metastatic castrate-resistant prostate cancer |
| Omacetaxine Mepesuccinate | A-site cleft of ribosomes | Chronic or accelerated phase CML with resistance to two or more TKIs a |
| Olaparib | PARP | BRCA mutated ovarian cancer after three or more prior lines of chemotherapy |
| Panobinostat | HDAC | MM after two prior regimens, including bortezomib and an immunomodulatory agent |
| Romidepsin | HDAC |
|
| Temsirolimus | mTOR | RCC |
| Vismodegib | Hedgehog | Basal cell carcinoma |
| Vorinostat | HDAC | Cutaneous T-cell lymphoma |
| Radium-223 dichloride | Alpha particle–emitting radioactive agent | Hormone-refractory prostate cancer with symptomatic bone metastases and no known visceral metastatic disease |
The most promising molecular targets are those solely responsible for sustaining tumor growth and survival without playing a significant role in normal cells—providing the therapeutic window that allows highly efficacious doses to be administered safely. One of the best examples of a critical target is BCR-ABL in patients with chronic myelogenous leukemia (CML). BCR-ABL is a fusion protein formed by the reciprocal translocation of chromosomes 9 and 22. Knowledge that this dysregulated tyrosine kinase played a causal role in the pathogenesis of essentially all cases of CML spurred preclinical studies, which led to the development of a potent and selective ABL tyrosine kinase inhibitor (TKI), imatinib mesylate (previously known as STI571). Subsequent clinical trials established imatinib mesylate as the first highly effective molecularly targeted therapy for CML and a prototype for the development of others in the class. Imatinib mesylate is also a potent inhibitor of other tyrosine kinases including platelet-derived growth factor receptor (PDGFR) and KIT, and is highly effective in the treatment of gastrointestinal stromal tumors (GISTs) bearing activating c-KIT mutations and in some GISTs bearing activating PDGFR mutations.
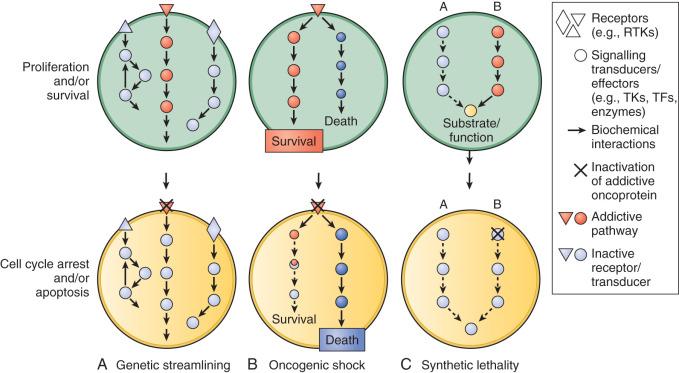
Unfortunately, most human tumors are genetically complex and do not have a single critical target, and the relative importance of a target in different tumors may vary. The existence of multiple abnormalities in one or more molecular pathways driving growth and survival contributes to resistance and provides a rationale for treatment strategies combining two or more targeted agents. However, cancer cells may become “addicted” or physiologically dependent on the sustained activity of specific oncogenes for maintenance of a malignant phenotype and for survival. This dependence mechanism, termed oncogene addiction ( Fig. 26.1 ), is associated with differential attenuation rates of prosurvival and proapoptotic signals stemming from the oncoprotein, with predominant apoptotic signals resulting in cell killing. The latter process, termed oncogenic shock, could explain the remarkably rapid clinical responses to TKIs in some patients with solid tumors, including those typically having complex molecular abnormalities. Other possible factors controlling sensitivity or resistance to molecularly targeted therapy include increased expression of the target due to gene amplification or transcription, emergence of resistant target gene mutations, and overexpression of multidrug transporter membrane proteins.
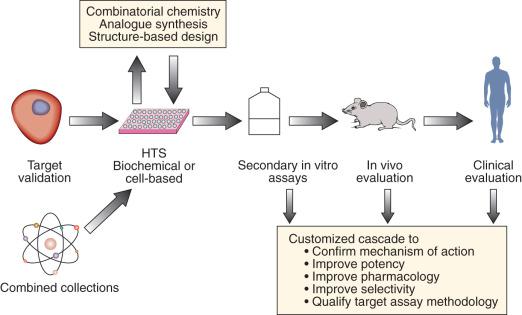
The discovery and development of molecularly targeted therapies require closely aligned laboratory and clinical research, integrating drug discovery, development, and clinical investigation. In such a cooperative setting, researchers can effectively take rational and iterative steps from target identification to clinical evaluation ( Box 26.1 and Fig. 26.2 ). A crucial early step in developing a molecularly targeted therapy is target validation, defined as experimental evaluation of the role of a given gene or protein. The target validation process (i.e., that affecting the target inhibits tumor growth, progression, or survival) involves a variety of preclinical approaches, including genetic, cell-based, and animal models. Validation and prioritization of molecular targets for therapeutic development depend on a variety of criteria, taking into consideration chemical, biologic, clinical, and practical factors ( Box 26.2 ). The next major step is finding or synthesizing compounds directed against that target.
Identify molecular target against which an agent will be developed
Validate molecular target—affecting the target inhibits tumor growth or survival
Screen for compounds that “hit” target—high-throughput screening
Optimize compounds—select or modify structure to increase activity and selectivity while maintaining favorable pharmacologic drug properties
Qualify assay methodologic methods for measuring drug levels and drug-target effect, in vitro and in animal models
Evaluate lead drug(s) in vivo for efficacy and safety
Prioritize and select drug candidate(s) for clinical testing
Conduct necessary preclinical animal toxicologic and pharmacokinetic studies to support an investigational new drug application for first-in-human trial
High frequency of genetic or epigenetic deregulation of the molecular target or pathway in human cancer indicates that the target or pathway is likely important in driving the disease.
Linkage of the deregulation to clinical outcome strengthens the case for causal involvement.
Evidence in a model system that the target pathway causes or contributes to the malignant phenotype demonstrates a direct causal role in malignancy.
Demonstration of reversal of the malignant phenotype provides greater confidence that modulation of the target by a drug will produce an anticancer effect.
Demonstration of “drugability” of the target—for example, enzymes are generally much more “drugable” than are large-domain protein-protein interactions.
Availability of a robust, efficient biologic test cascade to support the drug discovery program to allow evaluation of lead compounds and to select a development candidate for preclinical toxicologic testing and clinical trials.
Feasibility of establishing, validating, and running an affordable and robust high-throughput screen.
Potential for a drug design approach based on structural biology; such an approach, based on an x-ray crystallographic or nuclear magnetic resonance spectroscopy structure, can be highly complementary to a screening strategy.
Empiric approaches traditionally used to screen for cytotoxic agents are not optimal for MTAs. Rather, screening for MTAs should be target-based. The increasing number of potential targets and the availability of sophisticated high-throughput screening technologies have resulted in tremendous opportunities to screen an enormous set of diverse small-molecular compounds for promising therapeutics. Furthermore, the availability of genetically engineered mouse models provides the opportunity to better screen compounds in vivo. A more in-depth discussion of these important drug discovery tools and their implications for the molecularly targeted drug development process is beyond the scope of this chapter.
Optimal development of an MTA requires careful assessment of pharmacokinetic (PK) and pharmacodynamic (PD) effects in relevant nonclinical models before initiation of clinical trials. Preclinical in vivo pharmacologic and toxicologic testing is necessary to establish the starting dose and schedule for clinical trials, to evaluate effects on normal host tissue, and to help make predictions about serum and tissue levels required for target modulation, as well as effects on tumor growth. Currently, most in vivo efficacy studies involve human tumor cell murine xenograft models. However, xenograft models have limitations, including the requirement for an immunocompromised host, and they are not ideal for simulating the complex relationship between tumor and microenvironment. Moreover, xenograft models have not been very predictive of drug efficacy in patients with cancer; activity in a particular histologic type in a xenograft tumor model does not closely correlate with activity in the same human cancer histologic type. However, agents that have activity against a broad range of tumor types in xenografts have a better chance of clinical activity. Major variables to consider in designing xenograft studies include the molecular characteristics of the tumor, site of implantation, size of the tumor at commencement of drug treatment, and dose and schedule of administration. Clearly, future development of MTAs would benefit from more predictive in vivo models. Although limitations exist, prudent use of genetically engineered mouse models in conjunction with traditional xenograft models holds promise for accelerating targeted drug development.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here