Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Radiation therapy is an important modality in the management of patients with intrathoracic malignant neoplasms and primary and secondary chest wall neoplasms. Radiation therapy for these tumors usually results in radiation of the adjacent lung, and radiation-induced lung injury has been reported in association with treatment of lung, breast, esophageal, and thymic neoplasms and in lymphoma and pleural mesothelioma. Adverse effects of radiation therapy on the lung parenchyma—in particular, acute pneumonitis and chronic fibrosis—include ground-glass opacities or consolidation in the acute phase and traction bronchiectasis, volume loss, architectural distortion, and consolidation in the late phase.
Improvements in radiation techniques and delivery, including three-dimensional (3D) conformal radiotherapy (3D-CRT), intensity-modulated radiotherapy (IMRT), stereotactic body radiotherapy (SBRT), proton therapy, and altered fractionation schedules, have improved locoregional control and survival in patients with non–small cell lung cancer (NSCLC). Of importance, the imaging manifestations of radiation-induced lung disease associated with these newer therapeutic approaches often differ from those seen with conventional radiation therapy.
Knowledge of the expanded spectrum of manifestations of radiation-induced lung disease is important in the management of patients after radiation therapy and is useful in the diagnosis of local recurrence of malignant disease and in differentiating radiation-induced lung disease from infection. In this chapter we review the etiology and clinical and imaging manifestations of radiation-induced lung disease and emphasize unexpected or atypical manifestations that can occur when newer radiation techniques are used.
Radiation-induced lung disease rarely occurs with fractionated total doses less than 20 Gy and is commonly present in patients who receive doses greater than 60 Gy. A pooled analysis of 24 studies of 1911 patients with both small cell and NSCLC who received chemoradiation showed that the incidence of clinically significant radiation-induced pneumonitis (grade 2–3 according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer criteria) was 6% for total doses less than 45 Gy and 12% for doses greater than 55 Gy. Although the total dose of radiation delivered is important in the development of radiation-induced lung disease, other radiation technique factors that affect lung injury are the fractions into which the total dose is divided, the dose rate, and the volume of lung irradiated. The injury sustained by the lungs tends to decrease as the number of fractions in which the radiation is delivered increases.
Improved techniques of radiation therapy and dose escalation can modify the development and extent of lung injury. For instance, because the new fractionation strategies are often associated with an increase in radiation dose, there is a tendency to increase acute toxicity. However, by decreasing the treated volume, aggressive hyperfractionated schedules can limit acute radiation-induced injury of adjacent lung.
Various patient factors can increase the degree of injury sustained by the lung after irradiation of a thoracic neoplasm; these include age, low performance status, cigarette smoking, preexisting lung disease, and prior radiation therapy. The effect of neoadjuvant or concurrent chemotherapy on radiation-induced lung injury is not clear; some studies report an increase, whereas others have reported no significant association with pneumonitis. In addition, although steroids ameliorate radiation pneumonitis, an abrupt termination of administration can unmask latent radiation injury to the lung. Conversely, cytoprotectants such as amifostine, an organic thiophosphate, have shown promise in reducing the incidence and severity of injury associated with radiation therapy, although they have not been established as standard of care in preventing pulmonary toxicity.
Because of the complexity of factors that determine whether a patient with a thoracic malignant neoplasm will receive radiation therapy, including histology, stage, symptoms, and performance status, together with the heterogeneity of treatment options and numerous factors that can influence the degree of injury sustained by the lung after radiation therapy, the true prevalence of radiation-induced lung disease is difficult to ascertain for patients with lymphoma; pleural mesothelioma; and lung, esophageal, breast, and thymic cancers. However, radiation-induced lung injury is common in these patients, and subclinical changes in pulmonary function test results occur in an even larger percentage of patients.
Of the multiple thoracic malignancies, patients with lung cancer often receive the highest doses of radiation to the largest volume of lung and are accordingly more likely to develop radiation-induced lung disease. On the basis of a reported incidence of 10% to 20%, moderate to severe radiation-induced pneumonitis will probably occur in approximately 15,000 to 25,000 patients with lung cancer in 2016. This incidence is likely underestimated because the nonspecific symptoms of radiation-induced lung disease can be misattributed to preexisting respiratory or cardiovascular disease.
The goal of radiation therapy planning is to deliver a high dose of radiation to the tumor while sparing the surrounding normal tissue. To enable systematic tumor volume delineation, the International Commission on Radiation Units and Measurements has specified the use of concentric target volume delineations before radiation therapy. The gross tumor volume (GTV) is obtained outlining visible malignancy, including any involved nodes, on imaging studies. Next, a margin around the GTV is added for potential microscopic subclinical disease and is called the clinical target volume. An additional margin is added to the treatment plan to account for possible tumor movement during treatment because of respiratory motion and is called the internal target volume. The final volume treatment plan is the planning target volume and accounts for anticipated differences in patient positioning during the delivery of treatment.
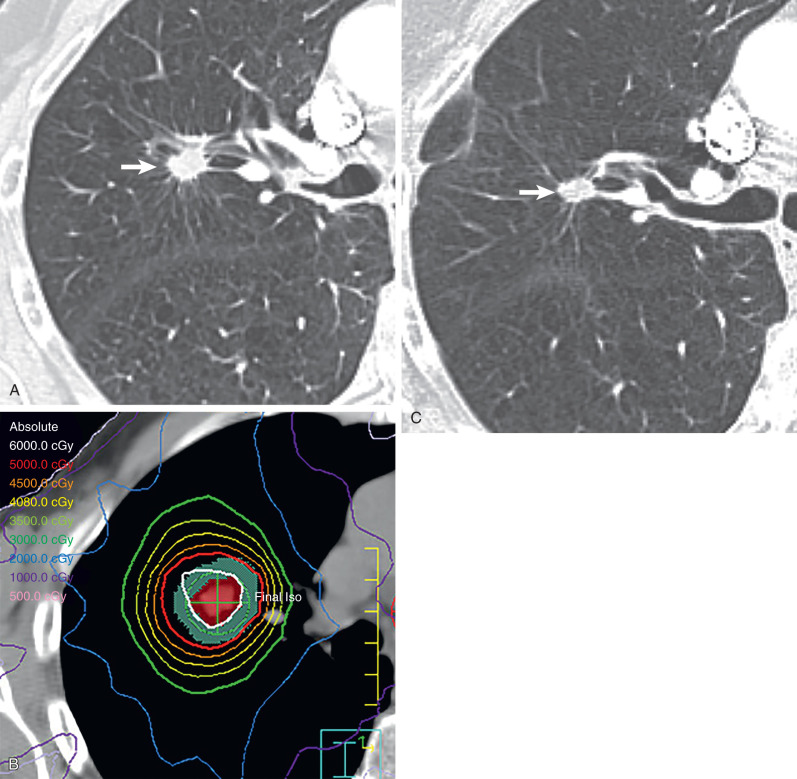
Sophisticated radiation therapy techniques, such as 3D-CRT, IMRT, and SBRT, allow the delivery of high therapeutic dose to the tumor, decreased radiation dose to adjacent tissue, and improved local tumor control rate. In contrast to conventional radiation therapy treatment planning, 3D-CRT uses a 3D image reconstructed from computed tomography (CT) scanning data to determine the target volumes to be treated. A computer planning system is used to design beam arrangements with a variety of orientations that deliver maximal radiation dose to the tumor while limiting exposure to normal structures. In IMRT the combination of powerful computer planning algorithms with multileaf collimators results in a high-precision dose delivered to the tumor with a steep dose fall-off outside the target, which decreases the volume of normal tissue that is radiated. Murshed and colleagues, in a study of 41 patients with advanced-stage NSCLC (stage III–IV), demonstrated a 10% improved target conformity of IMRT when compared with 3D-CRT. SBRT has steep dose gradients, allowing delivery of a highly precise dose conformed to the shape of the tumor. SBRT uses only a few fractions of radiation while delivering a significantly high biologic effective dose (>100 Gy). Because the target volume is small, SBRT is being used more frequently with curative intent in patients with peripheral early-stage NSCLC ( Fig. 66.1 ). In these patients SBRT is used rather than conventional radiation therapy or surgical resection as it achieves local control rates of 80% to 95% and improves overall survival compared with conventional radiation therapy.
Radiation therapy has improved considerably during the past few decades by refinements of existing techniques and introduction of new technology, including positron emission tomography (PET)-CT using the radiopharmaceutical 18 F-deoxyglucose, a d -glucose analog labeled with fluorine-18 ( 18 F-FDG–PET-CT), four-dimensional (4D) treatment planning, and proton therapy.
The increasing use of advanced CRT techniques such as IMRT and SBRT in thoracic radiation oncology makes precise target delineation important as these techniques have a higher likelihood of geographic misses. Use of FDG–PET-CT and 4D treatment planning improves the precision in radiation therapy by increasing the accuracy of tumor delineation and minimizing damage to surrounding structures.
FDG–PET-CT improves delineation of the extent of the tumor compared with CT alone when there is associated postobstructive atelectasis. The use of FDG–PET-CT results in less variation in lung cancer contouring compared with CT alone and reduces the risk of geographic misses with SBRT. In addition, because tumor volume delineation by FDG–PET-CT is typically smaller than that defined by CT, this can potentially facilitate dose escalation without increasing side effects. Also, the detection of unsuspected nodal metastases by FDG–PET-CT improves the target accuracy for nodal radiation and decreases isolated nodal failures. In addition to assisting with target volume delineation, PET-CT imaging may have an important role in adaptive planning. Adaptive planning is the modification of a radiation treatment plan during the course of therapy to account for changes in the tumor and/or adjacent tissue. The use of FDG–PET-CT has been reported to allow a smaller target volume to be radiated during radiotherapy delivery and to facilitate dose escalation.
The use of 4D imaging in radiation treatment planning introduces the concept of time to the 3D conformal treatment planning and compensates for tumor motion, patient movement, and movement occurring either during a single fraction (intrafraction) or between multiple fractions (interfraction) of radiation. Intrafractional movement can cause significant target deformation resulting from respiratory motion, and interfractional variation can interfere in tumor delineation as tumor and anatomy may change significantly between each treatment session. 4D treatment planning allows an assessment of tumor motion across an entire respiratory cycle, and radiation therapy is delivered accordingly. 4D-CT is currently being used in conjunction with 3D-CRT and IMRT to achieve high-precision radiation dose delivery to the tumor. In addition, in the treatment of lung cancer, using SBRT with 4D-CT imaging allows a significant reduction of target volumes and decreased dose to surrounding tissue when compared with conventional 3D-CT. 4D-CT–based planning has also optimized radiation delivery in patients with lung cancer receiving proton therapy. In patients with target volumes that have a large amount of motion (typically >1 cm), two approaches can be used to further reduce the amount of lung treated. The first, respiratory gating, allows delivery of radiation only during certain phases of the respiratory cycle while the patient breathes normally. The second, deep inspiratory breath hold , has the patient “hold his or her breath” after deep inspiration. Because radiation is delivered when the tumor is in a relatively “fixed” position with either of these approaches, the full extent of the tumor motion does not need to be delineated.
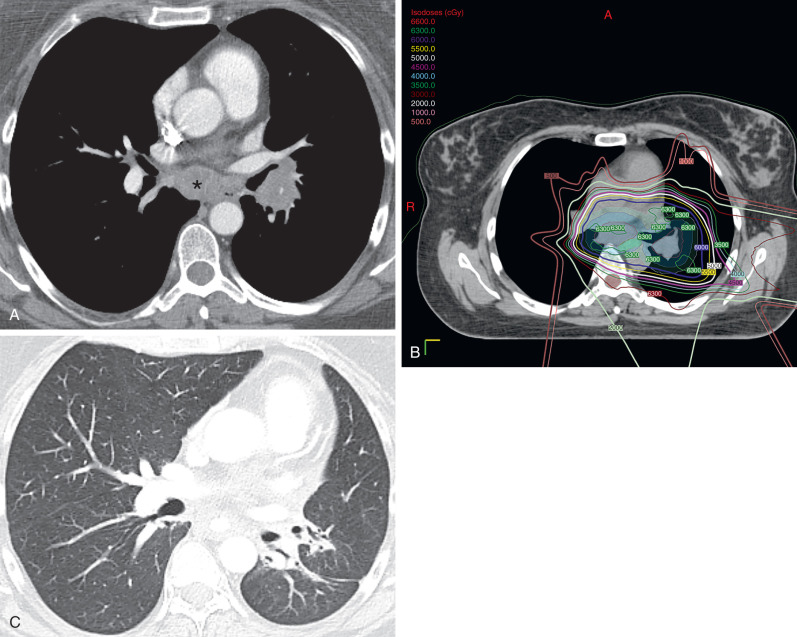
Proton therapy can allow high-dose radiation delivery to the tumor with minimal radiation exposure to the adjacent organs. The advantage of proton therapy relies on the physical characteristics of the proton particles, which deposit nearly all the therapeutic dose at a particular depth (Bragg peak), and this typically results in a negligible exit dose beyond the target. However, in aerated lung, proton beams travel beyond the distal edge of the tumor and can cause damage to adjacent lung tissue. A study assessing dosimetric values for stage I and III NSCLC treated with proton therapy, compared with 3D-CRT and IMRT, has shown that all three techniques provide adequate tumor coverage, but proton therapy delivers lower doses to the adjacent lung, esophagus, and spinal cord than the two other techniques. Sejpal and colleagues compared photon and proton-based RT and showed that proton therapy resulted in lower rates of esophagitis and severe pneumonitis than 3D-CRT and IMRT. However, the use of proton therapy in patients with lung cancer is not clearly defined. Currently, proton therapy has been indicated for centrally located tumors adjacent to the heart, spinal cord, and esophagus ( Fig. 66.2 ) as well as tumors close to the brachial plexus. In addition, a recent phase II randomized study from The University of Texas M.D. Anderson Cancer Center highlighted the need for adequate patient selection for this approach. Patients with locally advanced NSCLC were randomized to IMRT or passive scattering proton therapy (proton therapy using 3D-conformal techniques). The coprimary end points were the incidence of radiation pneumonitis and locoregional control, and in this unselected patient population, there was no difference between the two modalities. Further analyses are underway comparing IMRT with intensity-modulated proton therapy (proton therapy incorporating features of inverse planning and with greater conformality than the passive scattering approach), which use selective dose escalation based on normal tissue doses.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here