Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Because of their tumor selectivity, monoclonal antibodies offer exceptional opportunities for targeted therapy.
As naked antibodies they can kill tumors by receptor blockade and by actively inducing apoptosis or by reenergizing endogenous antitumor T-cell immunity.
Tumor cytotoxicity is mediated through white cells by activation of antibody-dependent cell-mediated cytotoxicity, and in the presence of serum by complement-mediated cytotoxicity.
Bispecific or multifunctional constructs can greatly enhance antitumor effect of antibodies.
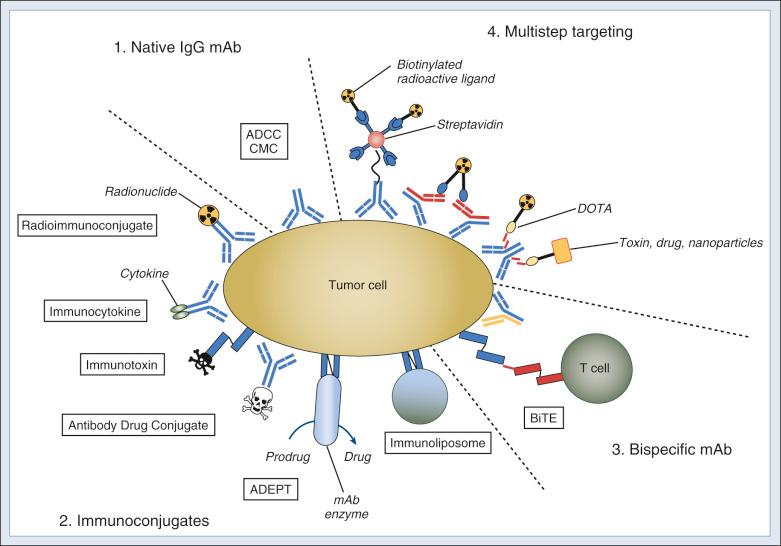
As immunoconjugates, antibodies can deliver effector molecules in the form of antibody drug conjugates, radioimmunoconjugates, immunocytokines, immunotoxins, immunoenzymes, immunoliposomes, and cellular immunoconjugates.
In general, antibodies do not have overlapping toxicity profiles with chemotherapy and radiation therapies, and dose-limiting toxicities of immunoconjugates vary depending on the cytotoxic moiety (e.g., myelosuppression in radioimmunoconjugates) being used.
Antibodies are likely to be most beneficial at the time of minimal residual disease, especially when used in conjunction with standard therapy.
The antibodies listed in Table 30.1 have been licensed in the United States or in the European Union over the last 20 years. In the coming decade, other antibodies currently under review or in various phases of clinical trial research may be added to the list. The prospect for further innovation in this established cancer treatment modality is highly favorable.
The clinical development of antibody therapy was accelerated by the introduction of the hybridoma technique in 1975 and the emergence of recombinant technology ( Table 30.1 ). Through these innovations, individual plasma cells can be immortalized, and cloning of heavy and light chain repertoires from animals and humans is now routinely done. In the last four decades, monoclonal antibodies (mAbs) have evolved from research tools to inclusion in a rapidly increasing list of licensed pharmaceuticals. Mouse-derived mAbs (*momab) have been chimerized (named as *ximab ) and humanized (*zumab), and use of mAbs derived from humans (*umab) is now routine. mAbs have generated excitement on many fronts and will likely play a pivotal role in the history of cancer medicine. Immunoediting using immune checkpoint inhibitors (ICIs) has recently opened new venues in treatment of advanced cancer. The clinical usefulness of mAbs for in vitro diagnosis and ex vivo manipulation of blood or stem cells is well recognized. Their role in the treatment and prophylaxis of graft-versus-host disease is detailed elsewhere (see Chapter 28 ). The use of B-cell idiotype and antiidiotypic antibodies as tumor vaccines is described in Chapter 103 . This chapter focuses on the application of naked cancer therapeutic mAbs and their conjugates in cancer therapy.
| HISTORICAL MILESTONES | |||||
|
|||||
| International Nonproprietary Name | Brand Name | Target; Format | Indications | First EU Approval Year | First US Approval Year |
| Rituximab | MabThera, Rituxan | CD20; chimeric IgG1 | NHL | 1998 | 1997 |
| Trastuzumab | Herceptin | HER2; humanized IgG1 | Breast CA | 2000 | 1998 |
| Gemtuzumab ozogamicin | Mylotarg | CD33; humanized IgG4; ADC | AML | Under review | 2000 a |
| Alemtuzumab | MabCampath, Campath-1H; Lemtrada |
CD52; humanized IgG1 | CML a ; multiple sclerosis | 2001 a ; 2013 |
2001 a ; 2014 |
| Ibritumomab tiuxetan | Zevalin | CD20; murine IgG1 | NHL | 2004 | 2002 |
| Tositumomab and iodine-131 | Bexxar | CD20; murine IgG2a | NHL | NA | 2003 a |
| Cetuximab | Erbitux | EGFR; chimeric IgG1 | CRC, head and neck CA | 2004 | 2004 |
| Bevacizumab | Avastin | VEGF; Humanized IgG1 | CRC, cervical, ovarian, fallopian tube and primary peritoneal CA | 2005 | 2004 |
| Panitumumab | Vectibix | EGFR; Human IgG2 | CRC | 2007 | 2006 |
| Catumaxomab | Removab | EpCAM/CD3; rat-mouse bispecific | Malignant ascites | 2009 | NA |
| Ofatumumab | Arzerra | CD20; human IgG1 | CLL | 2010 | 2009 |
| Ipilimumab | Yervoy | CTLA4; human IgG1 | Metastatic melanoma | 2011 | 2011 |
| Brentuximab vedotin | Adcetris | CD30; chimeric IgG1; ADC | Hodgkin lymphoma, NHL | 2012 | 2011 |
| Pertuzumab | Perjeta | HER2; humanized IgG1 | Breast CA | 2013 | 2012 |
| Ado-trastuzumab emtansine | Kadcyla | HER2; humanized IgG1; ADC | Breast CA | 2013 | 2013 |
| Denosumab | Xgeva | RANKL; human IgG2 | Giant cell tumor of bone | 2014 | 2013 |
| Obinutuzumab | Gazyva | CD20; humanized IgG1; glycoengineered | CLL, FL | 2014 | 2013 |
| Ramucirumab | Cyramza | VEGFR2; human IgG1 | Gastric CA | 2014 | 2014 |
| Pembrolizumab | Keytruda | PD1; humanized IgG4 | Melanoma, NSCLC, squamous head and neck CA, MSI-H/dMMR solid tumors, urothelial CA, Hodgkin lymphoma, gastric or gastroesophageal junction CA | 2015 | 2014 |
| Blinatumomab | Blincyto | CD19, CD3; murine bispecific tandem scFv | B-cell precursor ALL | 2015 | 2014 |
| Nivolumab | Opdivo | PD1; human IgG4 | Melanoma, NSCLC, renal cell CA, Hodgkin lymphoma, squamous head and neck CA, urothelial CA, MSI-H/dMMR CRC, hepatocellular carcinoma | 2015 | 2014 |
| Dinutuximab | Unituxin | GD2; chimeric IgG1 | Neuroblastoma | 2015 | 2015 |
| Ramucirumab | Cyramza | VEGFR2; human IgG1 | NSCLC, gastric or gastroesophageal junction CA, CRC | 2015 | 2015 |
| Necitumumab | Portrazza | EGFR; human IgG1 | NSCLC | 2015 | 2015 |
| Elotuzumab | Empliciti | CD319 (SLAMF7); humanized IgG1 | Multiple myeloma | 2016 | 2015 |
| Daratumumab | Darzalex | CD38; human IgG1 | Multiple myeloma | 2016 | 2015 |
| Olaratumab | Lartruvo | PDGFRα; human IgG1 | Soft tissue sarcoma | 2016 | 2016 |
| Atezolizumab | Tecentriq | PD-L1; humanized IgG1 | Urothelial CA, NSCLC | Under review | 2016 |
| Avelumab | Bavencio | PD-L1; human IgG1 | Merkel cell carcinoma, urothelial CA | 2017 | 2017 |
| Durvalumab | Imfinzi | PD-L1; human IgG1 | Urothelial CA | Under review | 2017 |
| Inotuzumab ozogamicin | Besponsa | CD22; humanized IgG4; ADC | B-cell precursor ALL | 2017 | 2017 |
a Withdrawn or marketing discontinued for the first approved indication.
mAbs can mediate highly effective tumoricidal functions both in vitro and in vivo ( Fig. 30.1 ). These include signaling through receptor binding, antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-mediated cytotoxicity (CMC).
The Fc region of immunoglobulin G (IgG) mAbs interacts with both activating and inhibitory Fc receptors (FcγRs). In humans, there are four activating FcγRs: FcγRI (CD64) is a high-affinity FcγR, whereas FcγRIIA (CD32A), FcγRIIIA (CD16A), and FcγRIIIB (CD16B) are low-affinity FcγRs carried on the alpha (α) chain. FcγRIIB (CD32B) is the only known inhibitory FcγR. All FcγRs are transmembrane glycoproteins except for FcγRIIIB, which is anchored on neutrophils by glycosylphosphatidylinositol (GPI). FcγRI also has an extracellular portion composed of three Ig-like domains, whereas FcγRII or FcγRIII has only two domains. This allows FcγRI activation by a single IgG molecule (or monomer), whereas the latter two Fcγ receptors must bind multiple IgG molecules within an immune complex to be activated. FcγRIIA carries the immunoreceptor tyrosine-based activation motif (ITAM) in its intracellular tail for activation. Other FcγRs do not have an ITAM in the alpha chain but interact with an adaptor protein called the accessory gamma (γ) chain (Fcγ subunit), which carries a cytoplasmic ITAM. ITAM becomes tyrosine phosphorylated by members of the Src-kinase family with subsequent recruitment of SH2-containing kinases. These events lead to the activation of phosphatidylinositol 3-kinase (PI3-K) and phospholipase-Cγ (PLCγ), followed by protein kinase C (PKC) activation and sustained calcium elevation. These biochemical cascades trigger phagocytosis, degranulation, cytokine release, and ADCC. In sharp contrast to activating FcγRs, FcγRIIB is a single-chain receptor that carries the immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain. Engagement of this inhibitory receptor activates phosphatase SHP-1 to remove phosphate groups from tyrosine residues, leading to downregulation of both biochemical and cellular functions. The ratio of activating to inhibitory FcγRs on immune cells, such as antigen-presenting cells (APCs), can influence the antitumor properties of mAbs.
Inflammatory mediators (interferon-γ or C5a) increase activating FcγRs and downregulate inhibitory FcγRIIB, whereas interleukin (IL)-4, IL-10, and transforming growth factor–β (TGF-β) upregulate FcγRIIB, thereby raising the thresholds for cell activation. Removing the inhibitory signals by FcγRIIB-blocking antibodies has shown efficacy in preclinical models. This may be relevant for cross-presentation of antigens that is acquired endocytically through Fc receptors on dendritic cells (DCs) during the induction of tumor-specific T-cell responses. In addition to these FcγRs, a unique class of Fc receptors called FcRn (neonatal) is found on endothelial cells and regulates antibody catabolism. FcRn is similar in structure to major histocompatibility complex (MHC) class I antigen and also associates with β2-microglobulin. It binds IgG at acidic pH of 6.0 to 6.5 but not at neutral or higher pH. When serum IgG is internalized by endothelial cells through pinocytosis, it becomes FcRn bound in the acidic endosomes and escapes lysosomal degradation by recycling to the cell surface for release back into blood, where the pH is neutral. This regulation of serum half-life by FcRn binding can be exploited in antibody engineering. Although most therapeutic antibodies have been primarily IgGs, both IgA1 and IgA2 can also mediate efficient ADCC by binding to FcαRI (CD89) on human neutrophils and monocytes or macrophages.
Depending on the affinity of the mAbs for the individual FcγRs, both natural killer (NK) cells (carrying FcγRII and FcγRIII) and neutrophils (bearing all three FcγRs) can mediate efficient ADCC. Because of its high affinity, FcγRI is generally occupied by monomeric IgG in human plasma. Human IgG subclasses (IgG1, IgG2, IgG3, and IgG4) have differential affinities for FcγRII and FcγRIII. Chimeric or humanized IgG1 antibodies can exploit FcγRIII for lymphocyte ADCC while using FcγRII for myeloid ADCC. Among the four IgG subclasses, IgG2 has the lowest affinity for the inhibitory receptor FcγRIIB. Mouse IgG3 (e.g., 3F8 specific for GD2) can engage both FcγRII and FcγRIII in ADCC, despite the low affinity of the monomer for human FcγRs. Polymorphic FcR alleles (FCGR2A and FCGR3A ) with higher affinity for human IgG1 have been reported to mediate more effective ADCC in vitro, and in some studies were associated with superior clinical responses. In addition to FcγRs, adhesion molecules are critical for mAb-mediated ADCC. These molecules include CR3 (CD11b/Cd18) and CD66b for neutrophil-mediated ADCC and LFA-1 (CD11a/CD18) for NK-mediated ADCC. Because cytokines can increase the expression of adhesion molecules, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ, IL-2, and IL-15 have been used to activate either myeloid- or NK-mediated effector models. Furthermore, because these cytokines expand the effector cell pools, they could enhance the effector-to-target ratio, a critical determinant of both in vitro and preclinical models of antibody-based therapy. Optimal combinations of mAbs and cytokines in the appropriate clinical setting are being explored.
IgG initiates the classic complement cascade by binding C1q to its CH2 domain. C1q is more avid for human IgG1 and IgG3 than IgG2, and has no affinity for IgG4. CMC potency of individual mAbs is also correlated with its slow off-rate. Although some tumor cell lines (e.g., lymphoma and neuroblastoma) are sensitive to CMC, many are resistant to complement because of anticomplement surface proteins such as decay-accelerating factor (DAF, CD55), homologous restriction factor (CD59), and membrane cofactor protein (CD46). The effect of complement activation extends beyond direct tumor lysis. Following complement activation, tumor-bound C3b is cleaved rapidly by plasma protease factor I to iC3b. Through the iC3b receptors, CR3 (Mac-1 or α M β 2 -integrin) and CR4 (CD11c/CD18, α X β 2 -integrin) on leukocytes, iC3b acts as opsonin. C3a and C5a, byproducts of complement activation, are also potent mediators of inflammation and are chemotactic for phagocytic leukocytes, drawing them to the tumor sites. C5a can also downregulate the inhibitory receptor FcγRIIB or induce secondary cytokines to increase vascular permeability for both mAbs and effector cells. Preclinical models of anti-CD20 immunotherapy showed that CMC could interfere with ADCC, and the adverse clinical outcome correlations with C1qA levels supported this observation.
When the antigen is a tumor cell surface receptor , its clustering by multivalent mAbs can induce apoptosis. Apoptosis increases with hyper-cross-linking (e.g., CD20 target on lymphoma cells). Both caspase-dependent and caspase-independent programmed cell death pathways appear to be involved. In AIDS-related lymphoma (ARL), anti-CD20 mAb diminishes p38MAPK signaling and Bcl-2 expression, whereas in non–AIDS-related lymphoma, signaling through CD20 inhibits AP-1 and nuclear factor–κB (NF-κB), leading to downregulation of Bcl-xL, thereby sensitizing lymphoma cells to chemotherapy. Direct receptor blockade by mAbs has also been reported for epidermal growth factor receptor 1 (EGFR1) and human epidermal growth factor receptor 2 (HER2; EGFR2), leading to upregulation of the BH3-only protein Bnip3L, thereby sensitizing tumor cells to chemotherapy. In addition, induction of cell death through death receptor family functions such as those of tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) receptors on tumor cells can be successfully applied for cancer therapy.
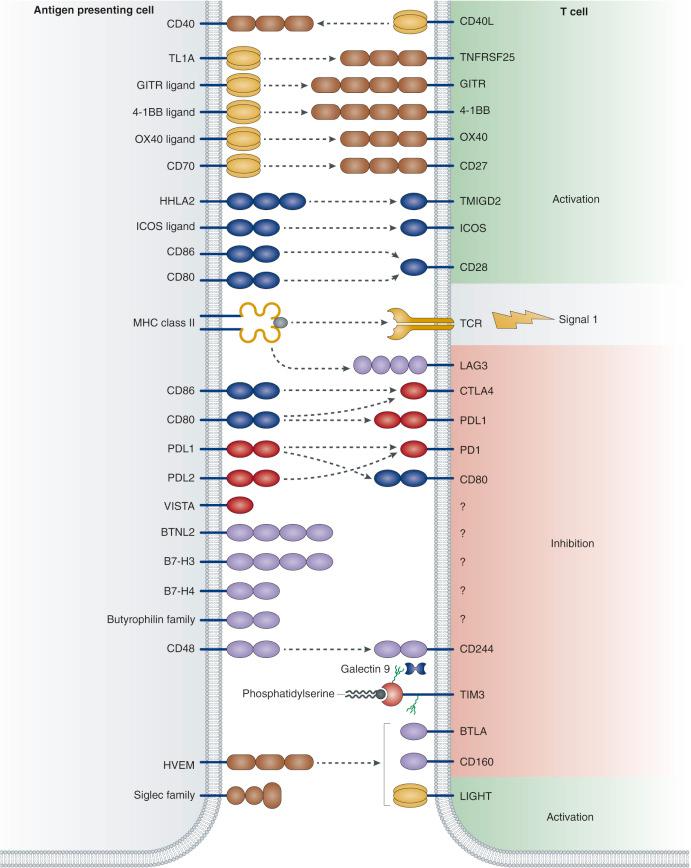
Activating signaling receptors on human cytotoxic lymphocyte and specifically NK cells can enhance ADCC functions. Although most relevant for CD8+ T cell–mediated tumor lysis (e.g., CD137 [4-1BB], CD134 [OX40], and glucocorticoid-induced tumor necrosis factor receptor–related protein [GITR]), agonistic antibodies to CD137 can also potentiate NK-ADCC in anti-CD20 and trastuzumab therapy. Conversely, antagonistic antibodies to remove immune checkpoint blockade allow T cells to perform more effective surveillance ( Fig. 30.2 ). The first successful example of such an immune approach was the anti-CTLA4 mAb ipilimumab, which was approved by the US Food and Drug Administration (FDA) for use in melanoma. An equally exciting strategy targets the programmed death receptor 1 (PD-1) T-cell coreceptor and its ligands B7-H1/PD-L1 and B7-DC/PD-L2, a pathway that maintains an immunosuppressive tumor microenvironment. In addition to turning on T cells, removing inhibitory signals (KIR-2DL and KIR-3DL) on NK cells could also have clinical potential for both hematologic and solid malignancies. Blocking mAbs (e.g., anti-CD47) have also been effective in unleashing macrophages to phagocytose tumor cells in the absence or presence of mAbs, both in vitro and in vivo, in leukemia and lymphoma as well as in solid tumor models.
In 1997, the anti-CD20 chimeric antibody rituximab became the first mAb approved by the FDA for the treatment of follicular lymphoma (FL). Chemosensitization of rituximab when combined with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) was later demonstrated in diffuse large B-cell lymphoma (DLBCL). Addition of rituximab to induction chemotherapy improved survival, but most patients were not cured and experienced relapse after a median of 4 years. The use of rituximab maintenance did improve progression-free survival (PFS) and overall survival (OS) in a meta-analysis.
For most patients, rituximab was well tolerated. Severe adverse events thought to be secondary to complement activation often occurred with the first infusion, especially if there were high numbers of circulating tumor cells. These infusion-related reactions usually appeared 30 to 120 minutes after mAb injection and could be lethal. B-cell depletion occurred in most patients, although hypogammaglobulinemia appeared in only 14% of all patients, and without clinical morbidity. Late-onset neutropenia following rituximab therapy occurred in 5% to 27% of patients and was correlated with high-affinity FCGR3A polymorphism.
Poor tumor response to rituximab includes low-affinity FcγRIII polymorphic allele in the patient's white cells, low density of CD20 molecules, or the presence of complement inhibitory molecules (CD55 and CD59) on tumor cells. Ofatumumab is a next-generation humanized anti-CD20 antibody with more potent cytolytic potential partly because of membrane proximity of its epitope, and was approved for fludarabine refractory chronic lymphocytic leukemia (CLL) resistant to alemtuzumab or with bulky lymphadenopathy. It also showed efficacy when added to chlorambucil in previously untreated patients with CLL or Waldenström macroglobulinemia and when combined with ibrutinib (an inhibitor of Bruton tyrosine kinase) in patients previously treated for CLL and small lymphocytic lymphoma. However, no survival advantage was found between ofatumumab and rituximab in the setting of autologous stem cell transplantation in patients with relapsed or refractory DLBCL. Ocrelizumab, veltuzumab, GA101 (obinutuzumab), AME-133v, and PRO131921 are all humanized anti-CD20 antibodies with enhanced binding to low-affinity FcR receptor that are currently undergoing clinical testing.
Campath-1H (alemtuzumab), a humanized rat IgG1 anti-CD52 mAb, demonstrated antitumor activity in patients with recurrent B-cell CLL in whom treatment with fludarabine had failed. Grade 3 or 4 infections were reported in 26.9% of patients. Opportunistic infections including bacterial sepsis and viral infections, vasovagal and hypotensive episodes, and marrow aplasia have since been reported. After treatment with alemtuzumab, cytomegalovirus (CMV) antigenemia responsive to antiviral therapy was found in all patients.
Epratuzumab is a humanized IgG1 antibody directed at CD22, a cell surface antigen expressed in B-precursor acute lymphoblastic leukemia (ALL), showing modest activity in patients with non-Hodgkin lymphoma (NHL), as single agent or in combination with rituximab. Most clinical effort has been focused on its immunotoxin or radiolabeled forms. SGN-30 (chIgG1 anti-CD30), XmAb2513 (humanized SGN-30), and MDX-060 were developed for Hodgkin disease, with modest clinical activity as naked mAb. Conjugation of SGN-30 to the antimitotic agent monomethyl auristatin E (MMAE) led to generation of an antibody-drug conjugate, SGN-35 (brentuximab vedotin), which was approved for CD30+ Hodgkin and anaplastic large cell lymphoma.
Blinatumomab, a tandem single-chain variable fragment (scFv) bispecific antibody (bispecific T-cell engaging antibody [BiTE]) retargets cytolytic T cells to kill leukemia cells through classic cytolytic immune synapse. At extremely low doses of 60 µg/m 2 /day given as a continuous intravenous injection, for 4 to 8 weeks, major responses were achieved in 82% of patients with mantle cell lymphoma (MCL), FL, and DLBCL, as well as molecular complete remission (CR) in 80% of patients with adult B-cell acute lymphoblastic leukemia (B-ALL). In pediatric patients with relapsed or refractory ALL, blinatumomab achieved a 39% complete remission within the first two cycles; complete minimal residual disease (MRD) response was seen in 52% of these patients. Blinatumomab dosage for children with relapsed or refractory ALL was 5 µg/m 2 /day for the first 7 days, followed by 15 µg/m 2 /day thereafter. Blinatumomab was approved for adult B-ALL and in pediatric relapsed or refractory B-cell precursor ALL that is Philadelphia chromosome negative. Significant side effects include cytokine release syndrome (CRS; especially during the first treatment days) and reversible central nervous system (CNS) symptoms probably due to neuroinflammation (seizures, convulsions, encephalopathy, confusion, and cerebellar symptoms), both preventable by premedication with dexamethasone and stepwise dose escalation. In myeloid leukemia, the addition of lintuzumab (HuM195, IgG1, anti-CD33) to salvage induction chemotherapy was found to be safe, but it did not result in a statistically significant improvement in response rate or survival in patients with refractory or relapsed acute myeloid leukemia (AML).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here