Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The vagina (from Latin, literally “sheath” or “scabbard”) serves as the portal to the internal female reproductive tract and a route of egress for the fetus during delivery. The viscera contained within the female pelvis minor include the pelvic colon, urinary bladder and urethra, uterus, uterine tubes, ovaries, and vagina. These structures surround the vagina and interact with it in the clinical setting. Therefore, the vagina also provides a convenient portal to understanding the female pelvic viscera.
The vagina is a thin-walled, distensible, fibromuscular canal, covered by specialized epithelium, which extends from the vulva inward to the cervix and uterus. Under normal circumstances, the vagina is a potential space that is larger in the middle and upper thirds, giving it an inverted pear- or T-shape when viewed perpendicular to its long axis. The walls of the vagina are normally flattened in the anteroposterior diameter, giving the appearance of the letter H in cross section.
In its distal extreme, the vagina opens to the vulva at the hymenal ring, opening at the caudal end of the vulva, behind the opening of the urethra. When upright, the vaginal tube points in an upward–backward direction with the axis of the upper portion of the vagina in close to the horizontal plane, curving toward the hollow of the sacrum. In most women, an angle of at least 90° is formed between the vagina and the uterus. The cervix is directed downward and backward to rest against the posterior vaginal wall. The spaces between the cervix and attachment of the vagina are called fornices, with the posterior fornix considerably larger than the anterior fornix.
Although there is wide variation, the length of the vagina is approximately 6 to 9 cm (2.5 to 3.5 in.) along the anterior wall and 8 to 12 cm (3 to 4.5 in.) along the posterior wall. During sexual arousal, the upper portion of the vagina elongates and widens through a relative upward movement of the uterus and cervix. This is thought to facilitate capture and retention of sperm to enhance the chance of conception.
Throughout most of its length, the vagina lies directly on top of the descending rectum, separated by the rectovaginal septum. The upper one-fourth of the vagina is separated from the rectum by the rectouterine pouch (posterior cul-de-sac). The urethra and base of the urinary bladder lie above the anterior vaginal wall separated by the thin layers of endopelvic fascia. As they enter the bladder, the ureters pass forward and medialward close to the lateral fornices.
The vagina is held in position by the surrounding endopelvic fascia and ligaments: The lower third of the vagina is surrounded and supported by the urogenital and pelvic diaphragms. The levator ani muscles and the lower portion of the cardinal ligaments support the middle third of the vagina, whereas portions of the cardinal ligaments and the parametria support the upper third.
The vagina is supplied by an extensive anastomotic network of vessels that surround its length. The vaginal artery originates either directly from the uterine artery or as a branch of the internal iliac artery arising posterior to the origin of the uterine and inferior vesical arteries. There is an anastomosis with the descending cervical branch of the uterine artery to form the azygos arteries. Branches of the internal pudendal, inferior vesical, and middle hemorrhoidal arteries also contribute to the interconnecting network from below. These can be a significant source of bleeding with obstetric lacerations. They are also important in the development of vaginal transudate during sexual arousal, when the vagina produces lubrication to aid in penetration.
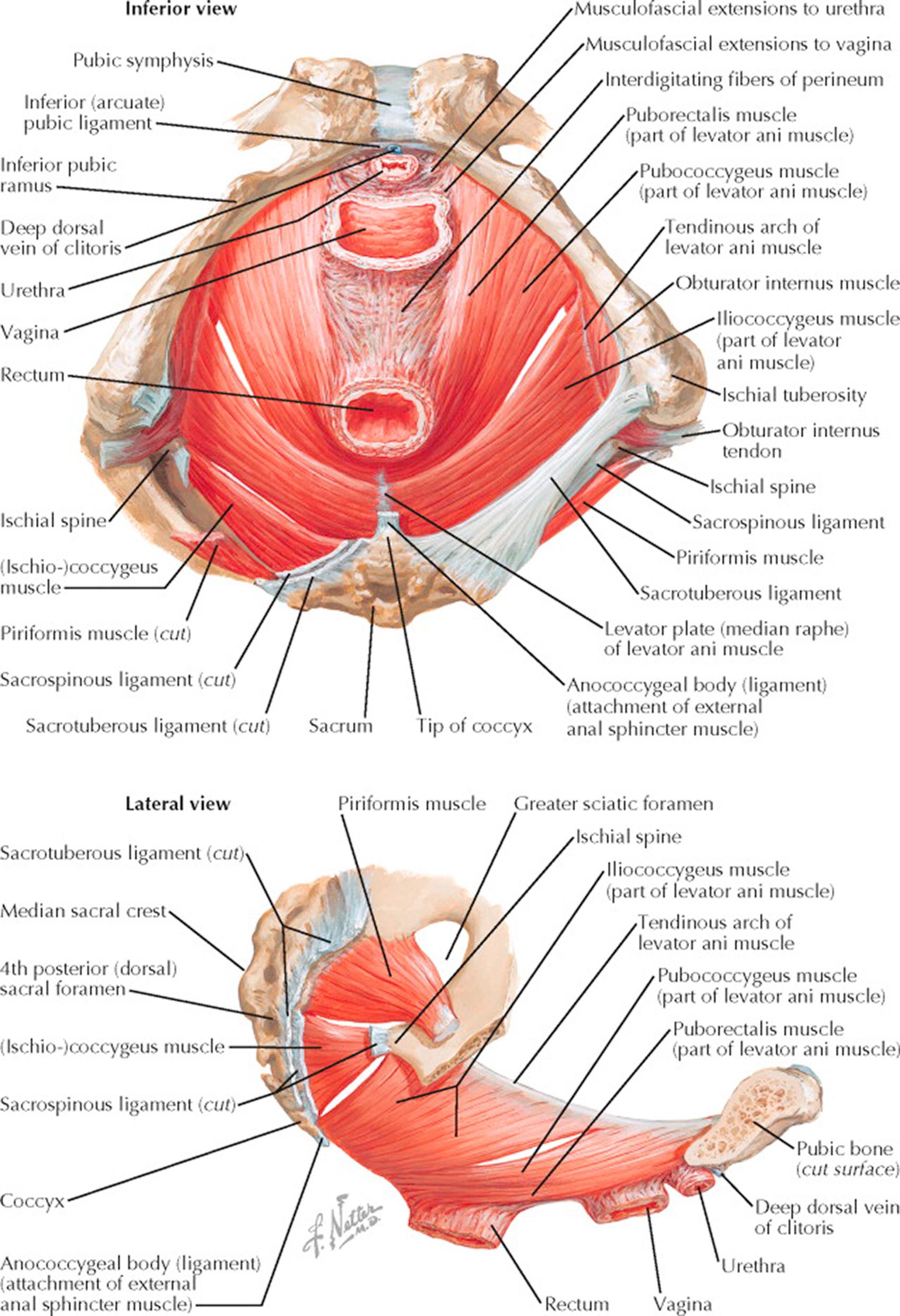
Removing the superficial muscles and fasciae of the pelvic floor, the pelvic diaphragm, viewed from below, forms a hammock of muscle from the pelvic brim, investing the urethra, vagina, and rectum and attaching posteriorly to the sacrum and coccyx. The principal muscles of this group are the levatores ani, consisting of both medial and lateral components on each side and supplied by the pudendal nerve. The larger medial component, the pubococcygeus, arises from the posterior surface of the superior ramus of the pubis adjacent to the symphysis, whence the fibers pass downward and backward around the lateral walls of the vagina, with some fibers reaching the coccyx, some terminating in the fascia forming the central tendinous point of the perineum, and others blending with the longitudinal muscle coats of the rectum. The pubococcygei are separated medially by the interlevator cleft through which pass the dorsal vein of the clitoris, the urethra, vagina, and rectum. These organs are supported by musculofascial extensions from the pubococcygei, their inferior fascia being continuous with the superior fascia of the urogenital diaphragm.
The lateral component of the levatores ani, the iliococcygeus, arises from the ischial spine and from the tendinous arch, a condensation of the parietal pelvic fascia covering the inner surface of the obturator internus muscle, which extends from the posterior surface of the pubis to the spine of the ischium. The iliococcygeus inserts in the last two segments of the coccyx, but some elements cross the midline anterior to the coccyx to unite with those from the opposite side in a raphe, where they are joined at a more superficial level by fibers from the sphincter ani externus and the transverse perineal muscles.
Posteriorly, the main pelvic diaphragm is nearly completed by the triangular coccygeus muscle. The apex of the coccygeus is attached to the spine of the ischium and the sacrospinous ligament, which it directly overlies; the base is attached to the lower portion of the lateral sacrum and the coccyx. This is best seen in the lateral view. In addition to supporting the pelvic viscera, the muscles of the pelvic diaphragm aid in the constriction of the vagina during coitus, in parturition, micturition, and in defecation. The obturator internus and piriformis muscles round out the posterior pelvis before passing through the lesser and greater sciatic foramina, respectively, to insert on the femur. These muscles lie close to the lateral walls of the pelvis.
The obturator internus arises from the circumference of the obturator fossa by fibrous attachments directly to the bone and, to a lesser extent, from the obturator membrane, the tendinous arch, and the obturator fascia, which covers the inner surface of the muscle. The fibers pass downward and backward, forming tendinous bands as they near the lesser sciatic notch and then, passing through this notch, they insert outside the pelvis on the medial surface of the greater trochanter of the femur.
The piriformis, best seen in the lateral view, arises from the lower portion of the sacrum and the sacrotuberous ligament, with its fibers covering a large part of the greater sciatic notch, through which it passes out of the pelvis to attach in the superior portion of the greater trochanter of the femur. The piriformis is supplied by sacral nerves 1 and 2; the obturator internus by sacral nerves 1, 2, and 3. They aid in external rotation and abduction of the hip and are not directly concerned with support of the pelvic floor. However, the fascia covering these muscles is continuous with the pelvic diaphragm and with the endopelvic fascia.
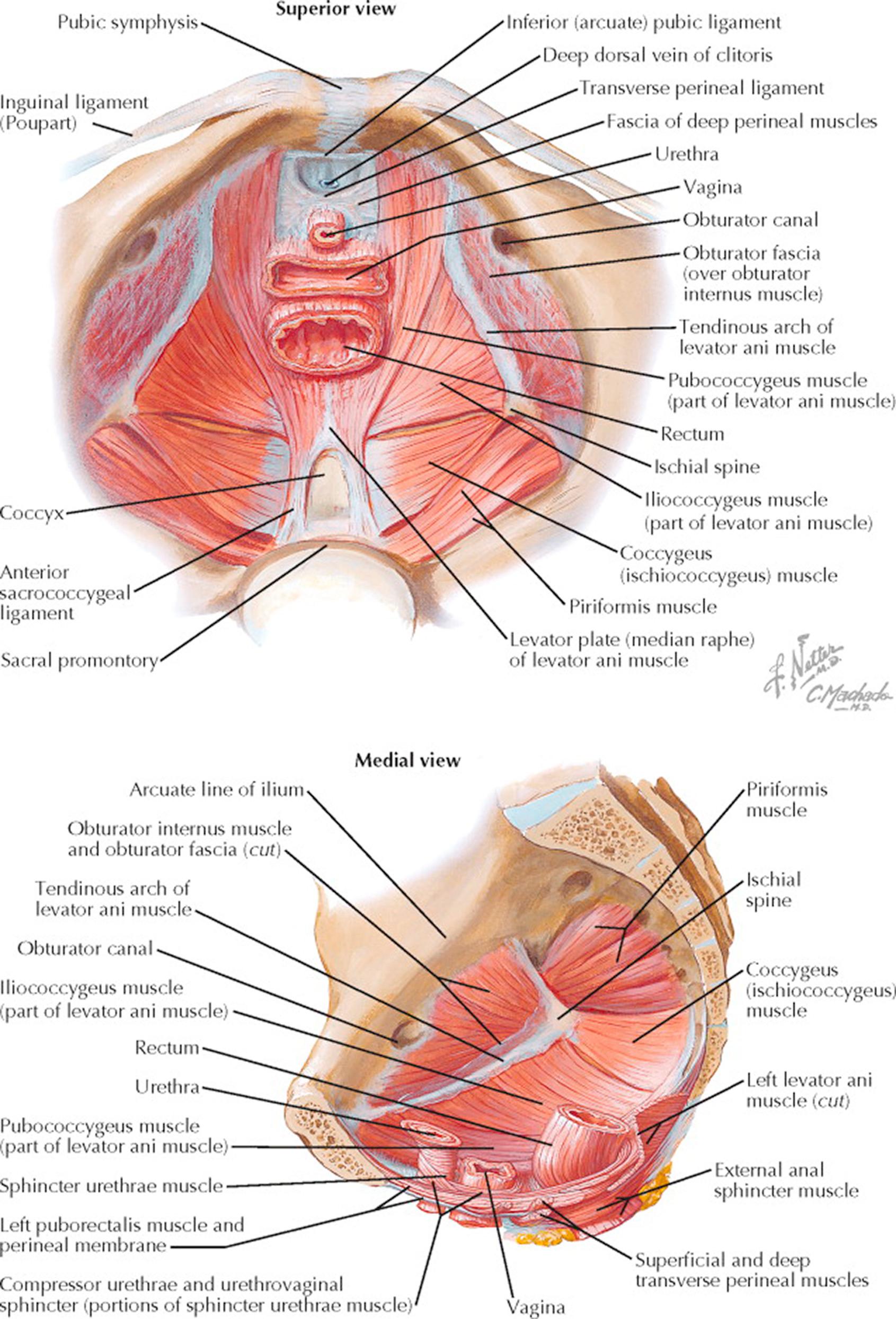
The pelvic diaphragm forms a musculotendinous, funnel-shaped partition between the pelvic cavity and the perineum and serves as one of the principal supports of the urethra, vagina, rectum, and pelvic viscera. It is composed of the levator ani and coccygeus muscles, sheathed in a superior and inferior layer of fascia. The muscles of the pelvic diaphragm extend from the lateral pelvic walls downward and medially to fuse with each other and are inserted into the terminal portions of the urethra, vagina, and anus. Anteriorly, they fail to meet in the midline just behind the pubic symphysis, exposing a gap in the pelvic floor, which is completed by the urogenital diaphragm. This gap is partially filled by the subpubic ligament that is pierced by the dorsal vein of the clitoris. In this area, the inferior fascia of the pelvic diaphragm fuses with the superior fascia of the urogenital diaphragm.
The levator ani muscles may be subdivided into an anterior pubococcygeus and a posterior iliococcygeus portion. They originate on each side at the posterior aspect of the pubis, the tendinous arch, and the ischial spine. They are inserted into the coccyx, the anococcygeal body, the lower end of the anal canal, the central point of the perineum, the lower vagina, and the posterolateral surface of the urethra. The levator ani muscles are primarily supporting structures, but they also contribute a sphincteric action on the anal canal and vagina. These muscles and their investing fascia are critical to maintaining support for the vagina and bladder. Rupture or stretch of this support system following pregnancy or childbirth is one of the major causes of pelvic support defects (hernias) and the attendant problems of urinary incontinence and fecal retention. It is to the tendinous arch (arcus tendineus) that some transabdominal approaches to the treatment of cysto-urethroceles provide anchorage or reattachment. The levator sling is also the plate on which pessaries must rest to provide mechanical support to prolapsing pelvic organs.
The pectineal ligament (also known as the inguinal ligament of Cooper) is an extension of the lacunar ligament that runs on the pectineal line of the pubic bone, seen as a ridge on the superior ramus of the pubic bone and forming part of the pelvic brim. Lying across it are fibers of the pectineal ligament and the proximal origin of the pectineus muscle. This fibrous line has been used clinically as an anchor point for incontinence procedures such as the Marshal-Marchetti-Krantz and Burch procedures.
The coccygeus muscles are triangular in shape, arise from the ischial spine, and are inserted into the lateral borders of the lower sacrum and upper coccyx. They lie on the pelvic aspect of the sacrospinous ligaments.
The fasciae of the pelvic diaphragm are continuous with the fascial layers of the perineal compartments—the endopelvic fascia, the obturator fascia, the iliac fascia, and the transversalis fascia of the abdomen.
Aside from the muscles of the pelvic diaphragm, two muscles—the obturator internus and the piriformis—cover the walls of the true pelvis. The piriformis is triangular and lies flattened against the posterior wall of the pelvis minor. It originates from three or more processes lateral to the first, second, third, and fourth anterior sacral foramina and leaves the pelvis through the greater sciatic foramen above the ischial spine to be inserted by a rounded tendon into the upper border of the greater trochanter of the femur. The obturator internus muscles are fan-shaped and cover the side walls of the pelvis.
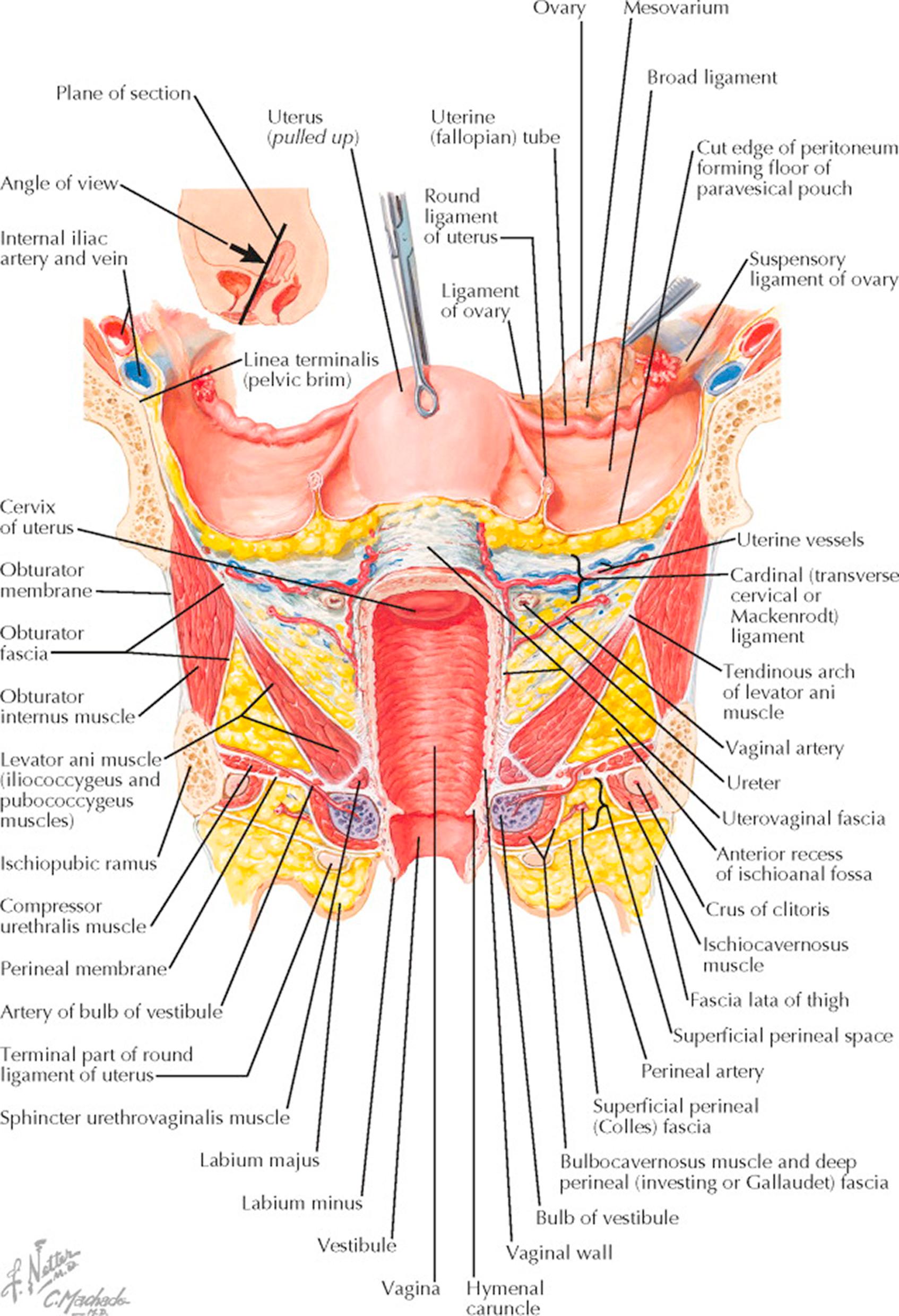
To clarify the relationships of muscles and fasciae in supporting the pelvis, with particular reference to the vagina and internal female genitalia, the uterus, in the accompanying picture, has been elevated upward and backward. The plane chosen for the section (small upper diagram) runs from a point anterior to the body of the uterus down through the anterior vaginal fornix and along the longitudinal axis of the vagina to the perineum. At this level, the large iliac vessels run close to the superior pubic rami which form the lateral pelvic walls. These pubic rami are connected to the ischiopubic rami across the obturator foramen by the obturator membrane, the obturator internus muscle, and the obturator fascia. The broad ligaments begin at the lateral pelvic walls as double reflections of the parietal peritoneum, forming large wings, which divide to include the uterus and separate the pelvic cavity into anterior and posterior compartments. They are continuous with the peritoneum of the bladder anteriorly and the rectosigmoid posteriorly. The broad ligaments contain fatty areolar tissue, blood vessels, and nerves, and at their apices invest the round ligaments, which are condensations of smooth muscle and fibrous tissue holding the uterus forward and inserting below and anterior to the fallopian tubes. The left ovary has been lifted up to demonstrate the uteroovarian and infundibulopelvic ligaments, the latter containing the ovarian blood supply. The bladder peritoneal reflection has been detached from the uterus, revealing the endopelvic or uterovaginal fascia, which runs laterally to the pelvic wall as the cardinal ligament, and with the associated blood vessels, nerves, and fat forms the parametrium. The uterine arteries and veins extend medially from their origins in the hypogastric vessels to the lateral vaginal fornices. The ureters (cross-sectioned) at this point pass beneath the uterine vessels and then continue in the uterovaginal fascia medially and anteriorly across the upper vagina into the bladder. The close proximity of the ureters to the uterine blood supply and vagina explains why they may easily be injured during hysterectomy and in operations to repair lacerations of the endopelvic fascia.
The pelvic diaphragm is quite thin in cross section, contrasting sharply with its breadth. Although some of the fibers of the levators come directly from the pelvic brim, the main portion of the muscle originates from the tendinous arch formed by a condensation of the fascia of the obturator internus. The levators here are passing around the posterior vagina and enclosing the upper two-thirds of that organ. Below the levators and separated from them laterally by the upward extension of the ischiorectal fossa is the urogenital diaphragm or triangular ligament, containing at this level the deep transverse perineal muscle and the artery of the clitoris. The lower third of the vagina lies superficial to the pelvic diaphragm, and its opening into the vestibule is bounded by the hymen and farther laterally by the vestibular bulb and its covering bulbocavernosus muscle. Close to the ischiopubic rami at the margin of the bony outlet of the pelvis are the crura of the clitoris, covered medially by the ischiocavernosus muscles and the fat pad in the superficial perineal compartment, which is limited below by Colles fascia. The labia (majora and minora) lie superficial to Colles fascia and between the thighs. The muscles and fasciae below the triangular ligament are concerned chiefly with coital function and play no part in the support of the pelvic viscera. This plate demonstrates the surgical implications of either the abdominal or vaginal approach to reconstruction of the elaborate supporting framework of the pelvic floor.
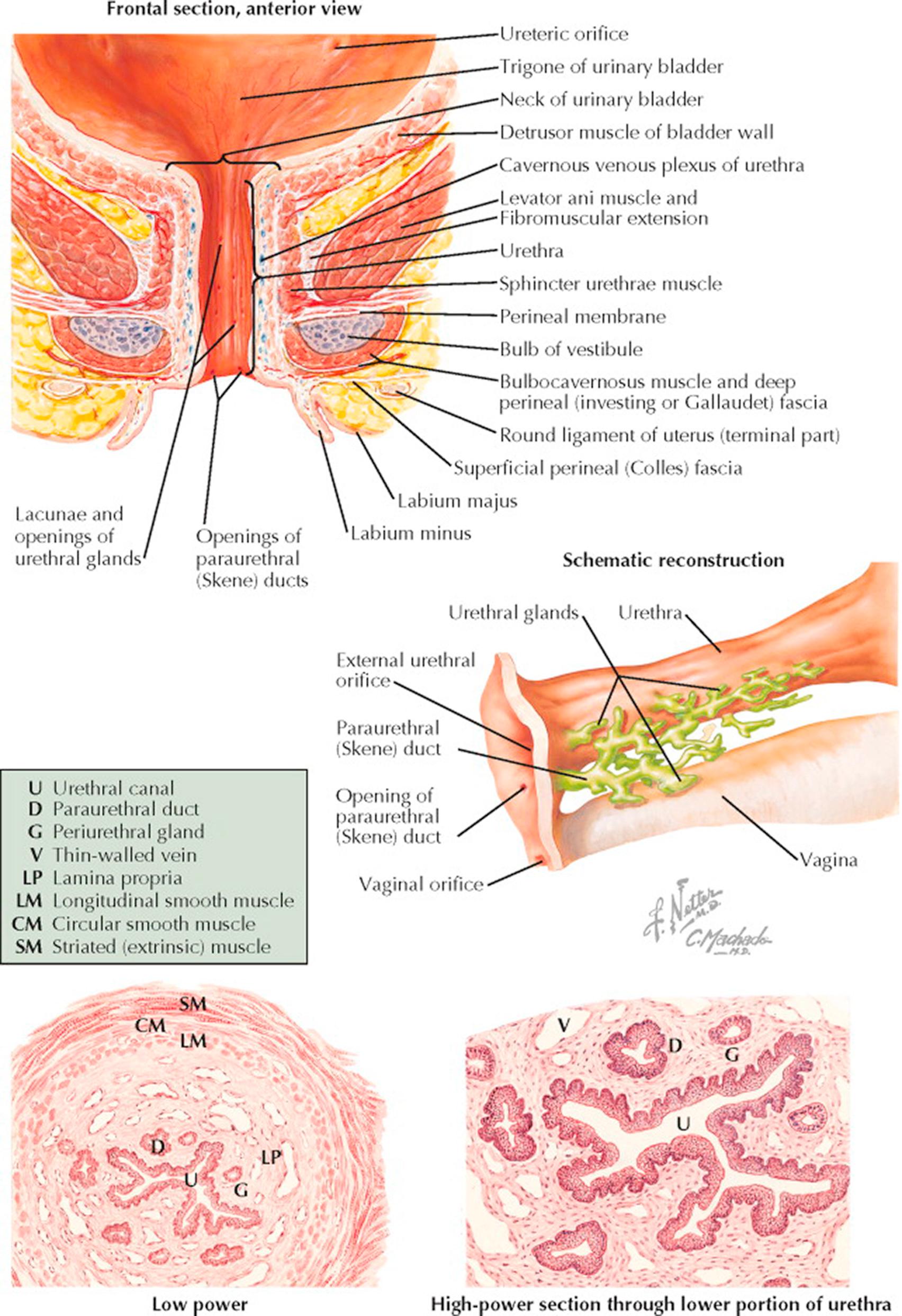
The urethra, situated at the lowest portion of the bladder and passing downward and forward beneath the symphysis, varies from 3 to 5 cm in length and averages about 6 mm in diameter. The angle formed by the internal urethral orifice and the bladder at the bladder neck and surrounded by the intrinsic sphincter is critical to maintaining normal urinary continence; to withstand the hydrostatic pressure of the bladder, this area is further supported by the fascia and tensing muscles of the pelvic diaphragm. Its mucosal surface is thrown into longitudinal folds by the constricting action of the external supporting structures. The most prominent of these longitudinal folds, situated on the posterior aspect of the urethra, is sometimes referred to as the urethral crest. The endopelvic fascia that covers the bladder is continuous over the entire urethra just below the mucosal layer, and contiguous to it is a thin layer of erectile tissue formed by the cavernous venous plexus. The muscular coats that surround the bladder also cover the urethra but become thinner as it passes downward toward the external meatus. The upper two-thirds of the urethra lie behind the symphysis pubis and are referred to as the intrapelvic urethra. It is this portion that passes through the musculofascial attachments forming the interlevator cleft. The perineal portion extends from the superior fascia of the urogenital diaphragm to the meatus. As it passes through the urogenital diaphragm, the urethra is surrounded by the sphincter urethrae membranaceae, the homologue of the muscle of the same name in the male but a far weaker and less important structure. Near the external meatus, the urethra is adjacent to the upper ends of the vestibular bulbs and the surrounding bulbocavernosus muscles. At its meatus, the urethra lies in the anterior vaginal wall between the folds of the labia minora 2 to 3 cm below the clitoris. Along its entire length, but especially in its perineal portion, the urethra is perforated by the openings of numerous small periurethral glands, the homologues of the prostatic ducts in the male.
The schematic reconstruction of this duct system shows that although the ducts of the small glands may enter the urethra independently, the majority of them form an interdependent conducting system terminating in the large paraurethral (Skene) ducts, which open on either side of the midline, posterior to the urethral meatus. These are vestigial remnants that serve no specific purpose but are important in that their position predisposes them to infection, especially by the gonococcus, and that their relatively poor drainage increases the risk of a chronic infection.
Cross sections through the lower urethra show the mucosal folds and the immediate supporting structures: The submucosal lamina propria is a loose network of fibrous and elastic tissue containing a prominent venous system, the cavernous plexus or corpus spongiosum, which accounts for the extreme vascularity in the area. The muscle coats consist of an inner longitudinal and an outer circular layer, both quite thin and mutually interdependent. A thin layer of striated muscle referred to as the external sphincter and supplied by the pudendal nerve also surrounds the lower urethra, but these distal muscle groups have little to do with micturition. Under high-power microscopy, it can be seen that the epithelium of both the urethra and the periurethral ducts are of the stratified squamous type. The epithelium of the intrapelvic portion of the urethra, as it approaches the bladder neck, tends to be transitional. The glandular epithelium, on the other hand, is of the columnar type, not infrequently stratified. The submucosal connective tissue is relatively poor in cells.
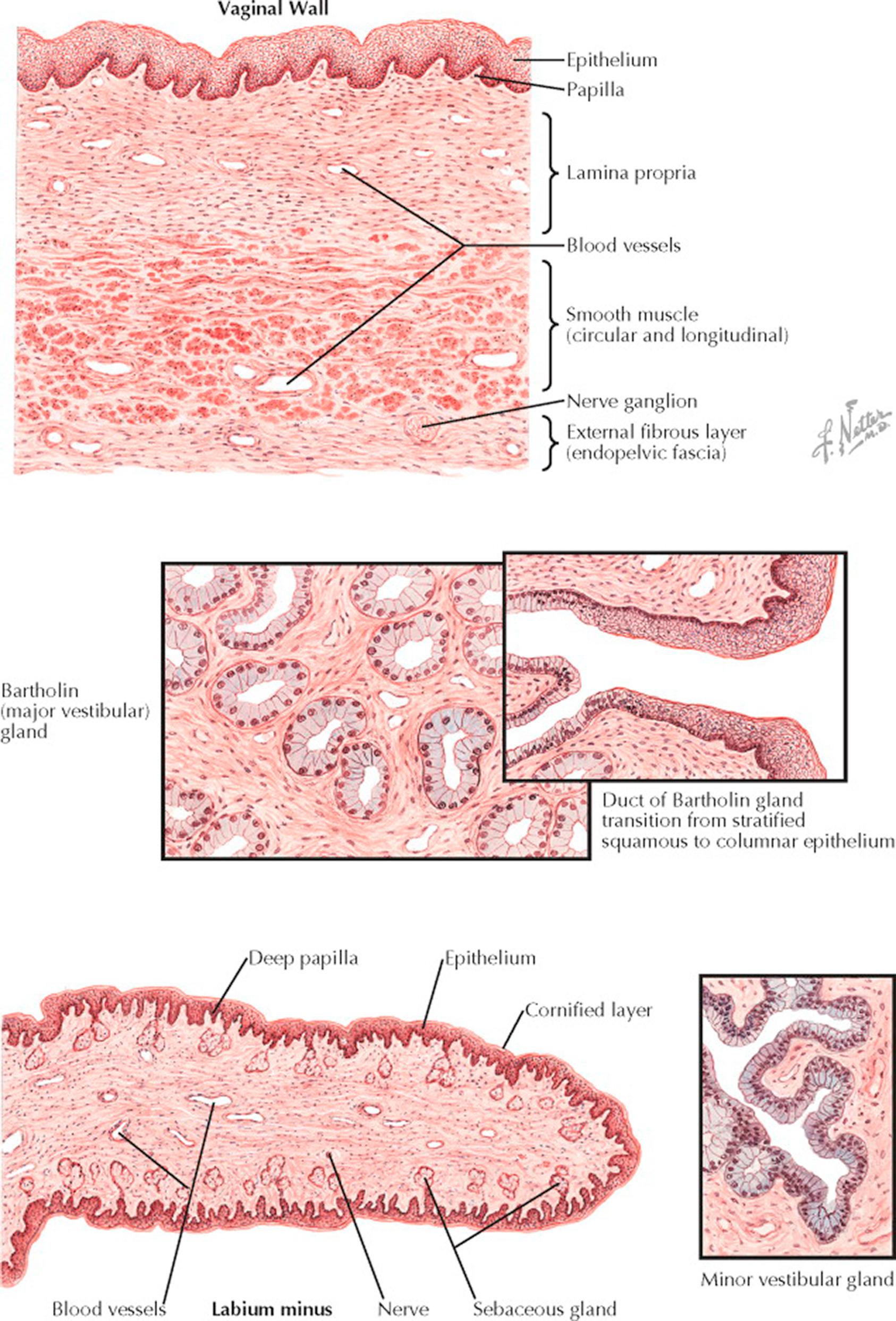
The vagina is lined by squamous epithelium and capable of dilation and constriction as a result of the action of its supporting muscles and erectile tissue. The three principal layers are easily recognized in the cross section through the vaginal wall. The epithelial surface is composed of stratified squamous epithelium divided into basal cell, transitional cell, and spinal or prickle cell layers, also referred to as basalis, intraepithelial, and functionalis. The superficial cells contain keratin but normally show no gross cornification in women of reproductive age. The epithelium is slightly thicker than the corresponding structure in the cervix and sends more and larger papillae into the underlying connective tissue, giving the basement membrane an undulating outline. These papillae are more numerous on the posterior wall and near the vaginal orifice. Beneath the epithelium, which has a thickness of 150 to 200 μm, a dense connective tissue layer known as the lamina propria is supported by elastic fibers crossing from the epithelium to the underlying muscle. These elastic fibers, here and throughout the pelvis, are critical to pelvic support and function. The lamina propria becomes less dense as it approaches the muscle, and in this area it contains a network of large, thin-walled veins, giving it the appearance of erectile tissue. The smooth muscle beneath this layer is divided into internal circular and external longitudinal groups, the latter being thicker and stronger and continuous with the superficial muscle bundles of the uterus. No dividing membrane or fascia separates these two interlacing muscle groups. The adventitial coat of the vagina is a thin, firm, fibrous layer arising from the visceral or endopelvic fascia. In this fascia and in the connective tissue between it and the muscle runs another large network of veins and, in addition, a rich nerve supply.
The Bartholin gland is situated just lateral to the vaginal vestibule and appears in cross section as a collection of small mucus-secreting glands lined by a single layer of columnar epithelial cells with basally placed nuclei. Occasionally, the columnar epithelium is stratified. The small glands tend to be oval and symmetric and are supported in a loose, vascular connective tissue. The main Bartholin duct is lined by columnar epithelium as it runs upward along the side of the vagina, but as it nears its opening in the midportion of the lateral wall of the vestibule, the epithelium takes on the stratified squamous characteristics of the vaginal epithelium. This transition accounts for the fact that malignant tumors of Bartholin gland may be of either the adenomatous or the squamous type.
The minor vestibular glands, situated around the clitoris and urethra and aiding in the lubrication of the vaginal surface, are of a more racemose, branching type than Bartholin glands. The mucus-secreting epithelium of these glands is tall, columnar, and one or two cells deep.
The labium minus has an epithelium more deeply pigmented than that of the vagina. The superficial cells are more markedly keratinized and form a horny (cornified) layer, which is especially prominent in postmenopausal women. The papillae of the lamina propria push deeply into the overlying epithelium, but the surface layer is clearly demarcated from the underlying layers by its basement membrane and often by a thin area of edema. Close to the surface are located numerous small sebaceous glands but no hair follicles or fat cells, in contrast to the labia majora. The connective tissue supporting the labium is acellular but rich in nerves and small vessels. The veins are not so numerous or so large as those in the vagina and cannot be regarded as erectile tissue.
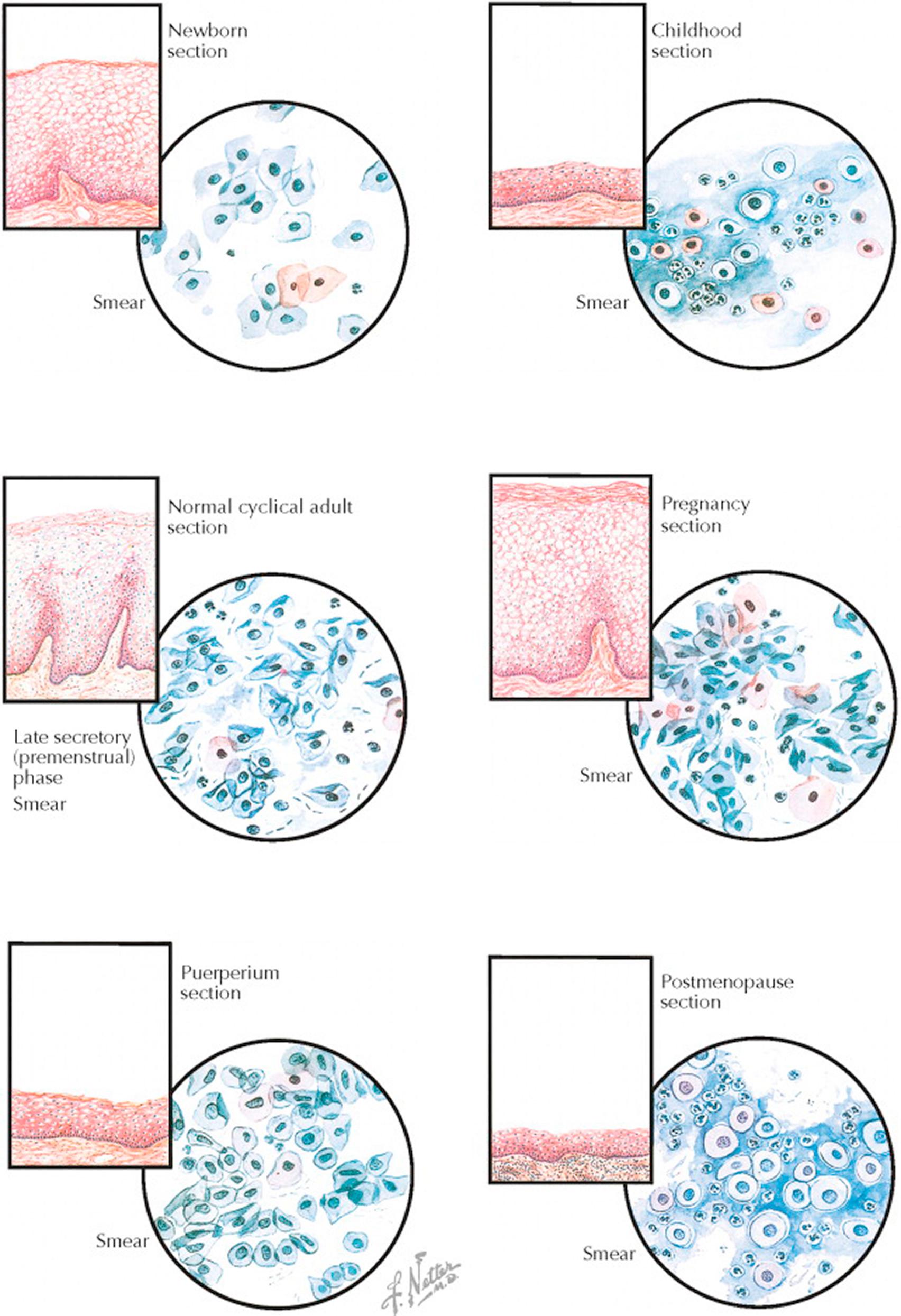
The superficial cells of the vaginal epithelium are under the influence of ovarian hormones, changing in character over the course of a woman's lifetime and the phases of the reproductive cycle. These variations are largely dependent upon the amount of circulating estrogen.
In the newborn, the vaginal epithelium is lush as a result of the transfer of maternal estrogen transmitted across the placenta. The precornified and cornified cells shed from this epithelium are scattered but occasionally form loose clusters. A few polymorphonuclear leukocytes but few, if any, lactobacilli (Döderlein bacilli) are present. After delivery, circulating estrogen levels fall rapidly and the vaginal epithelium quickly transitions to that of childhood.
In childhood, when the circulating estrogen is at a low level, the vaginal epithelium is thin, fragile, and at risk for infection. The cytologic smear is composed chiefly of basal cells, with a background of mucus and polymorphonuclear leukocytes. The basal cells are round or oval, with vesicular nuclei and a large nuclear–cytoplasmic ratio. The cytoplasm is, with only a few exceptions, basophilic. This smear is typical of an atrophic condition and similar to that seen after menopause.
During the reproductive years, the vaginal epithelium thickens and undergoes cyclic change in response to the varying hormone levels of the menstrual cycle. In the past, these changes from more immature, basaloid cells early in the cycle to a predominance of mature precornified cells late in the cycle were used to help assess hormone levels and to provide indirect evidence of ovulation. Lactobacilli help to maintain an acidic environment, stabilizing the vaginal flora and promoting cornification. A few white blood cells are normally present.
In pregnancy, as a result of the high levels of both estrogen and progesterone, the epithelium becomes a thick layer of superficial cells with marked keratinization. Desquamation of superficial precornified cells is associated with marked clumping and folding of the cells, which have elongated or oval vesicular nuclei. A few cornified cells with pyknotic nuclei, polymorphonuclear leukocytes, lactobacilli, and free nuclei are present in varying numbers. Near the 12th week of pregnancy, a change in the vaginal smear occurs; fewer cells are shed, clumping and folding have lessened, precornified cells predominate, and the overall appearance is similar to that of a normal early proliferative phase. As pregnancy advances, the increased progesterone produces progressively smaller precornified cells, which are referred to as “navicular cells.”
In the puerperium, following the sudden drop of steroid hormones prior to recurrence of normal menstrual cycles, the vaginal epithelium is thin. The puerperal vaginal smear is characterized by the presence of a large number of basal cells, a few precornified and cornified cells, and some inflammatory debris in the background, but not to the same extent as in smears from atrophic epithelium. With the return of normal ovarian cycling, the epithelium will again rebound to its normal prepregnancy thickness and character.
In the postmenopausal years, the vaginal epithelium is very thin, smooth, and relatively pale as a result of the cessation of ovarian function and the significant decrease in estrogen levels. The absence of this protection leads to bacterial invasion and a resulting inflammatory reaction in the supporting layers, with a thin layer of edema beneath the basement membrane. The atrophic smear is characterized by almost 100% basal cells dispersed in a thick background of mucus. Numerous polymorphonuclear leukocytes and some lymphocytes are present, but when these occur in excessive numbers, they usually indicate the presence of clinical infection. Most of the cells are basophilic, but a few pink acidophils are found.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here