Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Thrombus plays a major role in the pathophysiology of acute and chronic ischemic coronary syndromes.
Thrombus is a marker of active, unstable atherosclerotic plaques.
Plaque rupture, plaque erosion, and calcified nodules are prominent histopathologic components causing coronary thrombosis.
The necrotic core of the atherosclerotic plaque and its lipid content, especially the morphologic changes and dynamic movement of embedded cholesterol crystals, affect thrombus formation.
The main constituents of thrombus are fibrin, platelets, red blood cells and vasoactive compounds.
Understanding the architecture and structural components of the thrombus and proper burden assessment are essential for tailoring appropriate revascularization strategies.
Enhanced capabilities of thrombus-defining diagnostic modalities and incorporation of thrombus classifications can increase the effectiveness of treatment.
Thrombus has a profound impact on percutaneous coronary intervention (PCI), acting as a cause and a sequala of complicated interventions.
Useful American College of Cardiology/American Heart Association guidelines for the management of patients with acute coronary syndromes are readily available, yet developing precise PCI algorithms for the management of thrombus-containing lesions remains a challenging task owing to the complex characteristics of the coronary thrombus, the presence of underlying atherosclerotic plaque, and the anatomic characteristics of the host vessels.
The main contemporary technologies for thrombus removal are manual aspiration, power-sourced mechanical devices, and embolic protection systems.
Experience with thrombus-dedicated technologies differs widely; therefore current treatment approaches to thrombus-containing lesions diverge considerably.
The development of new pharmaceuticals and technologies is critical to ensure further improvement in the outcomes of interventions for thrombus-containing lesions in acute coronary syndromes and other thrombotic cardiovascular conditions.
Thrombus and thrombotic lesions occupy the daily agenda of interventional cardiologists all over the world. Accordingly, since coronary artery disease is the leading global cause of mortality and morbidity, the pathophysiology and management of thrombus-containing lesions are of paramount clinical importance. Based on the reports of the American Heart Association (AHA), the overall prevalence of myocardial infarction in the United States is at 7.6 million and that of stroke at 6.8 million. The incidence and pathologic interrelationship between coronary plaque and associated thrombosis are displayed in Table 28.1 .
a Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation . 1995:92:1355–1374.
b Davies MJ, Thomas AC. Plaque fissuring—the cause of acute myocardial infarction, sudden ischemic death and crescendo angina. Br Heart J . 1985;53:363–373.
c Yahagi K, Davis HR, Arbustini E, et al. Sex differences in coronary artery disease: pathological observations. Atherosclerosis . 2015;239:260–267
d Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol . 2013;62:1748–1758.
The identification of thrombus-containing lesions during coronary angiography and intervention is ominous. These lesions represent active, unstable, and complex vascular atherosclerotic conditions that require specific and often expeditious management. Although significant progress has been made in the field as percutaneous coronary intervention (PCI) increasingly targets complex lesions with a remarkable high success rate, thrombus-containing lesions continue to serve as markers of PCI-associated major adverse coronary events (MACEs), larger myocardial damage, stent thrombosis, increased rates of in-hospital complications, 6-month recurrent myocardial infarction (MI), and death. Overall, there is a persistent impression that thrombus-containing lesions remain a considerable challenge to the performance of safe and efficacious revascularizations. Consequently thrombus should be seen as a dual biovascular factor that, on the one hand, participates in the pathophysiology of coronary syndromes and, on the other, serves as an initiator of PCI-related complications.
Since the publication of the previous (seventh) edition of the Textbook of Interventional Cardiology in 2014, new research and unique discoveries pertaining to the physical characteristics and biologic features of thrombus have emerged. This development has been accompanied by new insights into the understanding of thrombus-containing lesions, especially regarding the presence and the cardinal role of cholesterol in the pathophysiology of atherothrombotic lesions. Accordingly, this chapter reviews updated concepts related to thrombus involvement in the pathophysiology of acute coronary syndromes (ACSs), describes the structural architecture of thrombus, and delineates its impact on interventions and outcomes. Specific imaging modalities for thrombus identification, dedicated pharmacotherapy, and thrombus removal strategies are delineated. A series of challenging thrombus-containing lesions and their corresponding management options are presented.
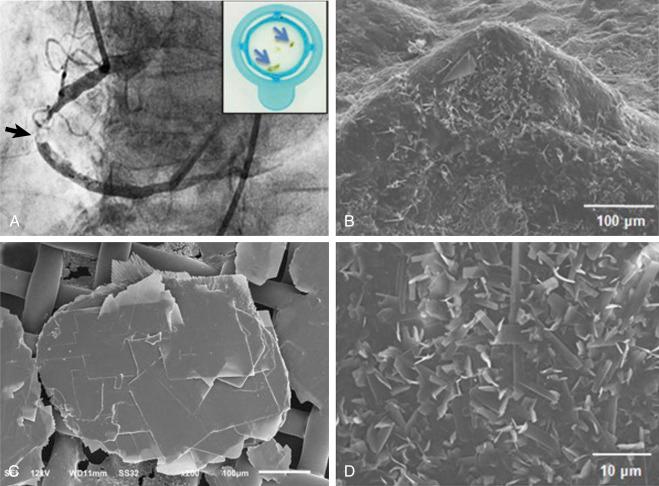
Understanding the processes of thrombus formation in an atherosclerotic plaque and familiarity with the unique structural components of thrombus and their physical characteristics is crucial for the lesion-centered revascularization of atherosclerotic lesions and vessels. Thrombotic lesions are formed when the fibrous cap of an atherosclerotic plaque develops structural defects. Significant morphologic changes in the plaque become manifest by rupture, erosion, or fissure. Such weakening of the plaque’s integrity exposes its necrotic core, an event that enables the movement of plaque material into the arterial lumen. The seminal research of Abela and colleagues has demonstrated the critical role played by cholesterol deposits within the plaque. They elegantly demonstrated that cholesterol crystals within the plaque exhibit unique physical properties including dynamic movement that leads to perforation of the tunica intima, which in turn triggers further disruption ( Fig. 28.1 ). These researchers clearly identified the cholesterol crystals as independent predictors of thrombus formation and subsequent adverse coronary and cerebrovascular clinical events. The ensuing contact of exposed, disrupted, and highly thrombogenic subendothelial matrix and plaque with circulating platelets and white blood cells activates the coagulation cascade. The resultant platelet adhesion and aggregation leads to thrombus formation ( Fig. 28.2 ). Furthermore, released tissue factor from the arterial injury directly activates the extrinsic coagulation cascade and promotes fibrin formation. Activated platelets release powerful promoters of vasoconstriction and aggregation, including serotonin, adenosine diphosphate, thromboxane A2, oxygen-derived free radicals, endothelin, and platelet activating factor. Importantly, plaque rupture should be distinguished from plaque fissures. Plaque rupture is usually surrounded by an apparent luminal thrombus, whereas plaque fissure, in most instances, involves intraluminal thrombus composed of fibrin and platelets with interspersed erythrocytes. The thrombus related to fissure is most commonly small. The delicate vessels within the plaque (i.e., the vasa vasorum) are a major cause of intraplaque hemorrhage. The mechanism behind this phenomenon is the disruption of microvessels lined by discontinuous endothelium without supporting pericytes. Moreover, plaque erosion is generally associated with negative remodeling, whereas plaque rupture is associated with positive remodeling. Also, erosions are frequently the cause of distal microembolization and resultant microvascular obstruction as compared with lesions constituting plaque rupture (71% vs. 42% respectively). At the site of rupture or erosion, the thrombus is predominantly made up of aggregated platelets, whereas a propagated thrombus is red and consists of fibrin and red cells. As the thrombus accumulates to form a critical obstacle ( Fig. 28.3 ), impaired flow dynamics along and distal to the thrombotic lesion develop, frequently accompanied by vasoconstriction and resultant clinical ischemic coronary events. During PCI, thrombus frequently exhibits structural variability as it adheres either loosely or sometimes quite firmly to the underlying plaque and the vessel’s wall. Consequently and unpredictably, it exhibits either marked friability or unyielding rigidity in response to the mechanical forces generated by balloon and stent deployment. These contradictory physical properties stem from the cumulative effects of an assortment of the intra thrombus components.
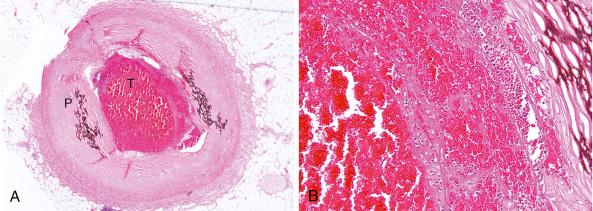
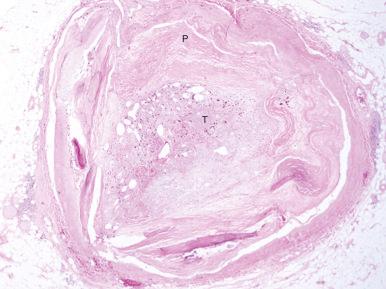
Structurally, the thrombus is held by a scaffolding or platform made of fibrin fibers. Two distinct types of branching fibers are organized in a three-dimensional (3D) network within the thrombus. Dense, thin fibers resist deforming mechanical forces and are poorly dissolved by thrombolytic agents. Thick fibers, on the other hand, are susceptible to the effects of external mechanical forces and are readily dissolved by thrombolytic therapy. Recent discoveries have illuminated the major role that the clot’s structure and function play in the pathogenesis of atherosclerosis, serving as novel risk factors for arterial and venous thrombosis and thromboembolism. Platelets, red blood cells, vasoconstrictors, and procoagulant compounds are anchored to the matrix of the crisscrossing fibers within the clot ( Fig. 28.4 ). Platelet dynamics also play a crucial role in the pathogenesis of atherosclerotic ischemic conditions. Abnormalities in platelet function can persist and predict clinical events following PCI. In patients presenting with ACS, the clot-adhering platelets typically effect a significant increase in the clot’s contractile force, leading to increased platelet aggregation and causing the entire thrombus to exhibit a greater elastic modulus. The platelets sustain and amplify the coagulant response at the plaque site and release procoagulant platelet-derived microparticles.

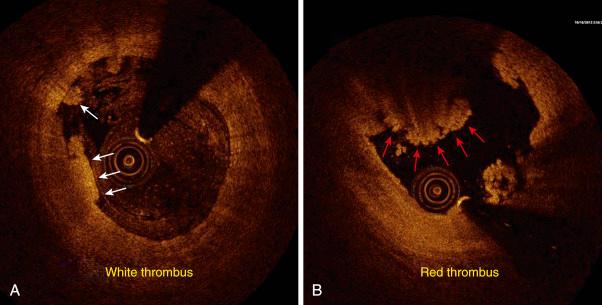
Various biochemical processes and interactions between activated platelets, red blood cells, fibrinogen, vasoconstrictors, atherosclerotic material and the vessel’s wall all have a substantial impact on the fibrin network. These constituents account for the thrombus’s level of activity, stability or instability, and for the overall “aggressiveness” of the thrombus as encountered during intervention. The presence and ratio of the previously mentioned components lead to the formation of distinct types of thrombi, each with unique rheolytic and mechanical properties. The two most prominent are the red and white thrombi. They can be detected by angioscopy and their angiographic characteristics correlate with the histology of extracted thrombi. The red thrombus has a dense surface and a loose inner core. Transmission or scanning electron microscopy demonstrates loosely packed fibrin and many interspersed red blood cells. The white thrombus consists of a dense structure lacking loose inner spaces, yet it contains a high concentration of platelets with fibrin and only few red blood cells ( Fig. 28.5 ). Based on histopathologic analysis of aspirated thrombotic contents, erythrocyte-rich (red) thrombus is found in about 35% of patients, mainly in those presenting with a low thrombosis in myocardial infarction (TIMI) flow. The platelet-rich (white) thrombus is identified in 65% of cases, especially in the early course of acute myocardial infarction (AMI). Indeed, Silvan and colleagues found that the platelet and fibrin contents of the occlusive thrombi in acute ST-elevation myocardial infarction (STEMI) evolve rapidly; here ischemic time was found to have a high impact on the composition of thrombi, resulting in a positive correlation with intracoronary thrombotic fibrin content ( r = .38, P = .01) and a negative correlation with platelet content ( r = –.34, P = .02). Even within a population of patients treated with aggressive antithrombotic regimens, ischemic time was a key determinant of thrombus composition. Sebben and colleagues analyzed the influence of the histopathologic features of coronary thrombi on the clinical outcomes in patients with STEMI. Among 331 patients who underwent thrombus aspiration, they identified a group of 199 whose aspirate was available for analysis. Recent thrombus was identified in 116 patients (58%) and old thrombus in 83 patients (42%). Recent thrombi had greater infiltration of red blood cells than old thrombi ( P = .02), but there were no statistically significant differences between other clinical, angiographic, laboratory, or histopathologic features or medications in both group of patients. The clinical outcomes were similar in both groups.
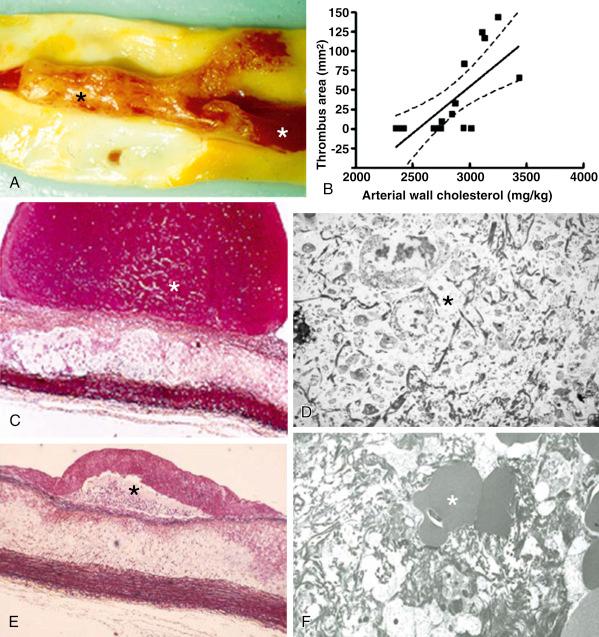
In many patients the occlusive thrombus is a mixture of red and white clot and the frequent resistance encountered in attempts to extract the thrombus suggests that certain clots contain layers upon layers of one thrombus type interspersed with the other. Calcified nodules are an important albeit the least frequent cause of coronary thrombosis (5% prevalence). These lesions reside in highly calcified, tortuous arteries; they are characterized by a disrupted luminal surface and many calcified nodules with overlying thrombus and no necrotic core or only a minimal one. From the perspective of the clinical management of coronary artery disease, the presence of major risk factors—such as smoking, male gender, and hypercholesterolemia—in patients with ACSs is known to influence plaque morphology adversely and is associated with a higher frequency of thrombus. Once the thrombus has formed, the size of the culprit lesion correlates with larger infarcts and worse left ventricular function. The size of the thrombus also relates to PCI-induced distal embolization. This was demonstrated in a pathologic analysis of aspirated embolized debris where the larger the thrombus at a culprit lesion was, the larger were the dimensions of the debris collected inside a filter. In turn, the dimensions of the debris predicted arteriolar occlusion. A list of factors contributing the formation of high-grade thrombus in atherosclerotic lesions is given in Table 28.2 .
Myocardial Infarction–Related
Lesion-Related
Vessel-Related
Percutaneous Coronary Intervention-Related
|
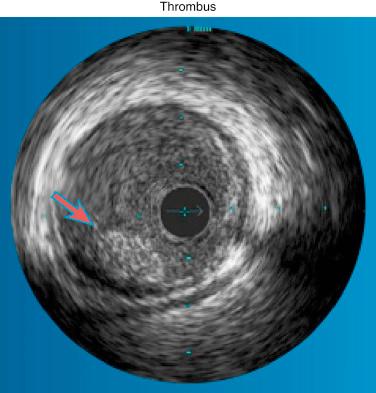
Various invasive imaging modalities are available for the diagnosis of intracoronary thrombus. Numerous studies have demonstrated the poor sensitivity of angiography, although its specificity approaches 100% when multiple angiographic views are obtained for verification and strict definitions. Nevertheless, angiography remains the practical gold standard for the recognition of thrombus, demonstrating the classical findings of reduced contrast density, staining, haziness, irregular lesion contour, “filling defect,” or a smooth convex meniscus at the site of a total thrombotic occlusion. When thrombus is suspected but not apparent angiographically, the optical coherence tomography (OCT) imaging modality is the tool of choice ( Fig. 28.6 ). Because of its user-friendly application and remarkable imaging quality, this technology has rapidly gained recognition and popularity among interventionalists. Recently Negishi et al. demonstrated the usefulness of OCT in the evaluation of thrombi to determine whether a distal protection device should be applied in the setting of STEMI. Investigating the unique no-reflow phenomenon that affects filter protection devices, they found, through OCT analysis, that the length of the plaque’s lipid pool plus the length of the thrombus served as an independent predictor of the filter protection no-reflow phenomenon. Thrombus also appears to be involved in the long-term processes that determine plaque repair in the presence of an ACS. Souteyrand and colleagues used OCT to decipher the mechanisms of plaque complications causing ACS. They discovered that in the months following a successfully dissolved acute thrombosis, OCT revealed that the cavities of ruptured fibrous cap plaques persist and are bordered by a smooth neointima, whereas intact fibrous cap plaques exhibited partial incorporation of the deepest layers of thrombus within the plaque. Other technologies for the detection of thrombi, which today are less commonly used, include intravascular coronary ultrasound ( Fig. 28.7 ) and, in some centers, percutaneous angioscopy.
Angiography remains the most practical imaging modality for the identification and quantification of intracoronary and peripheral arterial thrombi during the diagnostic and intervention portions of cardiac and vascular catheterization. The classification systems for thrombi and related scoring systems are useful for the quantification and qualification of the thrombus burden. Then these classifications provide a clinical/angiographic correlation that affects management decisions prior to, during, and after intervention.
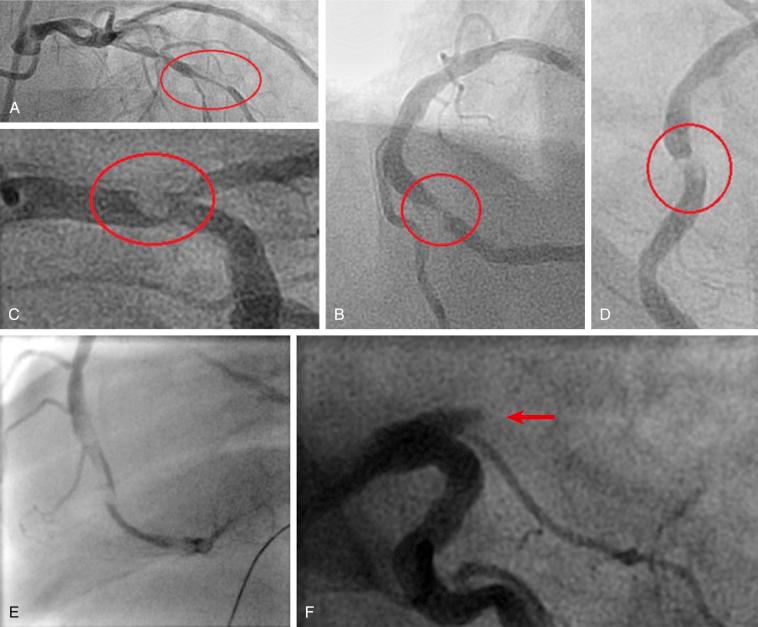
The widely used TIMI grading classification was published in 1985 by the TIMI study investigators. This pioneering scale was based on the recognition that the yield of thrombolytic therapy for evolving AMI was defined by the varying sizes and load of angiographically evident intracoronary thrombi. Relying on visual assessment of angiographic views demonstrating the thrombus size as compared with the diameter of the host vessel, this classification defined thrombus as follows: grade 0 means the absence of angiographic characteristics indicating thrombus; grade 1—determined by reduced contrast density, haziness, irregular lesion contour, or a smooth convex meniscus at the site of total occlusion—is suggestive but not diagnostic of thrombus; grade 2 means that thrombus is definitely present, its greatest dimension being half the vessel’s diameter; grade 3 also means that thrombus is present, its greatest dimension being between half the vessel’s diameter and less than two vessel diameters; grade 4 indicates the presence of thrombus with its largest dimension being more than twice the vessel’s diameter; and grade 5 indicates that the vessel is completely occluded. Table 28.3 details the parameters of the classic TIMI thrombus grading scale.
| Definition a | |
|---|---|
| Grade 0 | No angiographic characteristics of thrombus |
| Grade 1 | Possible angiographic features of thrombus include decreased density of contrast, haziness, irregular lesion contour, or a smooth convex meniscus at the site of total occlusion suggestive but not diagnostic of thrombus |
| Grade 2 | Definite thrombus present in multiple angiographic views: markedly irregular lesion contour with a significant filling defect. The thrombus’s greatest dimension is half of the vessel’s diameter |
| Grade 3 | Definite thrombus in multiple views with its greatest dimension ranging from one-half to two vessel diameters |
| Grade 4 | Definite large thrombus with greatest dimension larger than two vessel diameters |
| Grade 5 | Complete thrombotic occlusion of the vessel: a convex margin that stains with contrast and persists for several cardiac cycles |
a Gibson CM, de Lemos JA, Murphy SA, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction—a TIMI 14 sub-study. Circulation . 2001;103:2550–2554.
Specific angiographic images representing the classic TIMI thrombus grading scale are demonstrated in Fig. 28.8 .
Although this classification is user friendly and universally accepted, the accuracy of both the score’s lowest level (i.e., grade 0) and the highest level (i.e., grade 5) is considerably suboptimal. With its hallmark characteristic of TIMI 0 flow, the ischemia or infarct-related vessel containing a thrombus grade 5 is totally occluded. Consequently the actual ratio between the volume of the underlying plaque and the associated thrombotic volume is unknown, yet the assumption of the TIMI score is that this grade represents the greatest thrombotic burden. Therefore interventionalists from the Thoraxcenter, Rotterdam, in the Netherlands, introduced an important step of restratification for the evaluation and management of grade 5 thrombus as detailed in Table 28.4 .
| Intervention aimed at crossing the thrombotic occlusion in order to restore antegrade flow. Once a measure of flow has been gained, the size of the exposed underlying thrombus is stratified as follows: |
| Small thrombus—grades 1–3 |
| Large thrombus—grade 4 |
a Sianos G, Papafaklis MI, Daemen J, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol . 2007;50:572–583.
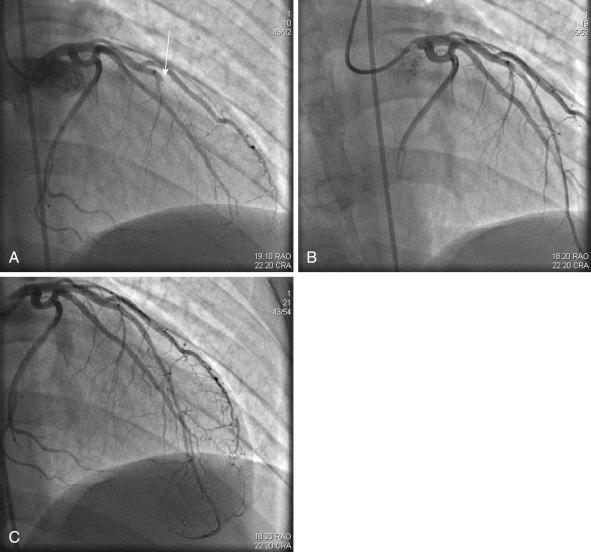
The first step of this process involves the advancement of a PCI guidewire or a 1.5-mm balloon aimed at crossing the occlusive thrombus ( Fig. 28.9 ). This intervention is expected to recanalize the thrombus and restore antegrade flow. Accordingly a newly exposed thrombus scored as grade 1, 2, or 3 is a relatively small thrombus; one scored as grade 4 is a relatively large thrombus. Therefore additional revascularization devices can be applied. From a practical stand point, a stratified small residual thrombus can be successfully managed with an aspiration catheter and adjunctive stenting. Adjunctive pharmacotherapy can be chosen as well. For postrecanalization removal of a large thrombus, especially when resistance is encountered during initial aspiration, utilization of a power-based mechanical thrombectomy device may be required. Among the useful devices for this task are rheolytic thrombectomy, excimer laser, and the X-Sizer thrombectomy device. When stenting is required in the presence of a large thrombotic load, the choice of a dedicated thrombus-capturing stent is a valid management option.
The usefulness of this reclassification extends beyond its application in all patients with AMI and ischemic coronary disease who exhibit TIMI 0 antegrade flow due to grade 5 thrombus. However, growing experience with dedicated thrombus removal strategies reveals that the Thoraxcenter’s restratification method calls for a modification to correct the inherent erroneous a priori assumption that every grade 5 thrombus can be restratified. In reality, all grade 5 thrombi are not alike, and they certainly exhibit varying responses to the process of restratification. Recognizing these facts, we have recently introduced a new modification to the restratified TIMI score for grade 5 thrombi based on the identification of three distinct types of thrombi (i.e., A, B, and C).
The angiographic features of the new modification are presented in Table 28.5 and illustrated in Fig. 28.10 .
|
a Topaz O, Topaz A. Thrombus classifications: critical tools for diagnostic and interventional cardiovascular procedures. In: Topaz O, ed. Cardiovascular Thrombus: From Pathology and Clinical Presentations to Imaging, Pharmacotherapy and Interventions . Philadelphia: Elsevier; 2018:175–187.
|
a Niccoli G, Spaziani C, Marino M, et al. Effect of chronic aspirin therapy on angiographic thrombotic burden in patients admitted for a first ST-elevation myocardial infarction. Am J Cardiol . 2010:105:587–591.
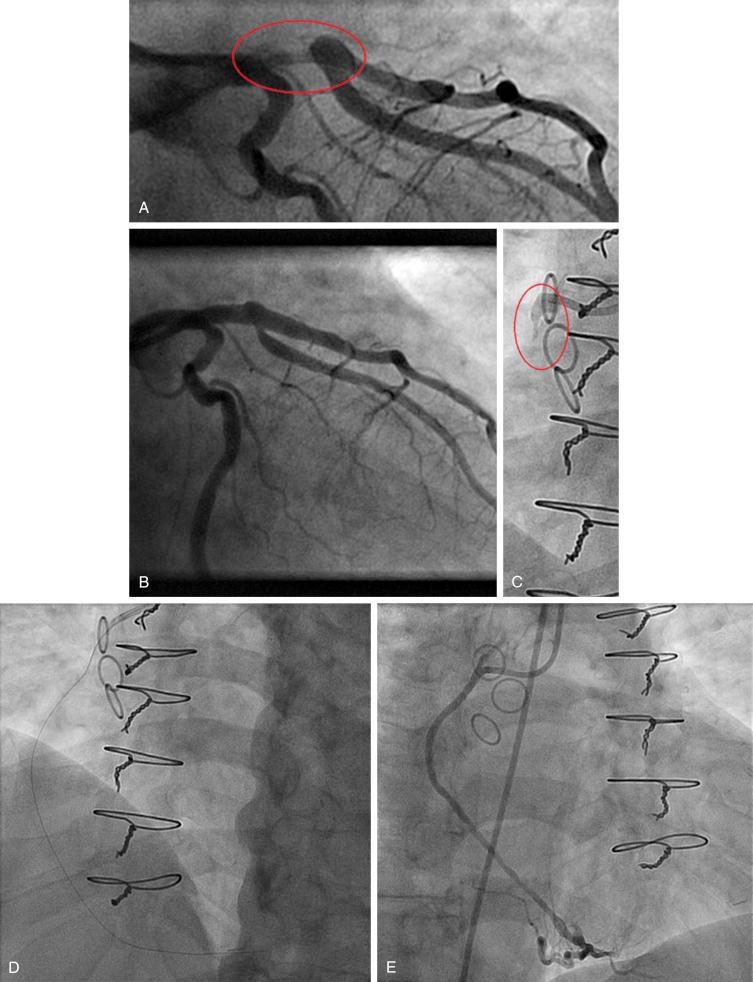
Another innovative classification was introduced by Aleong and colleagues. It relies on the combined analysis of edge detection and video-densitometry-based quantitative coronary angiography for the enhancement of quantitative thrombus assessment. Their experience with this method indicates that it quantifies the thrombotic volume accurately. A different, practical classification by Niccoli is presented in Table 28.6 .
Altogether, while the usefulness and merits of the contemporary angiography-based classifications of thrombus scores are highly valuable, some limitations must be recognized. First, reliance on the visual interpretation of angiography implies an inherent observer bias that can decrease accuracy. Indeed, underestimation of presence and size of thrombus occurs when angiographic assessments are compared with more accurate imaging methods such as OCT, coronary ultrasound, and angioscopy. Moreover, the current classifications fall short of differentiating between types of thrombus (i.e., white vs. red) and do not define the thrombotic content within chronic total occlusions. Third, thrombus classifications neither describe nor take into account the underlying morphology and severity of the accompanying atherosclerotic plaque. This is of a concern especially when heavily calcified lesions may also contain thrombus. Nevertheless, it should be recognized that angiography remains the most user friendly and least expensive imaging modality currently available for thrombus-related management decisions during PCI. As such, angiographic thrombus classifications continue to serve as highly relevant clinical tools. In that regard, it is intriguing to realize that the Syntax scoring system —which is a useful scoring system serving as a differentiator for the outcome of patients undergoing PCI for three-vessel and unprotected left main coronary disease—gives only one point for the presence of thrombus without any further classification.
The indications for targeted thrombus revascularization are displayed in Table 28.7 .
| Clinical Stable and unstable angina, acute coronary syndromes: ST-elevation myocardial infarction and non–ST-elevation myocardial infarction when the clinical condition is associated with a need to
Pathologic
|
For patients with a suspected or small thrombus (corresponding to TIMI grades 1 to 2), the aspiration catheter is considered to be the tool of choice. However, management of a significant (TIMI grade 3) or heavy thrombus burden (TIMI grades 4 to 5) is challenging due to the common occurrence of friable thrombotic material dislodged by the balloon and stent. Embolization of fragmented thrombotic particles obstructs flow within the distal arterial segments, side branches, and myocardial microvessels. Overall, in patients with ACS who undergo standard PCI, distal embolization occurs in the range of 6% to 15% for all lesion types and is associated with up to a sevenfold increase in the rate of periprocedural MI. In most instances resultant severe ischemia, microinfarctions, an inflammatory response, and contractile dysfunction reduce coronary reserve, causing deleterious effects on recovery and salvage. Moreover, the optimal myocardial blush score of grade 3 is gained in only 28% to 35% of PCI patients who had a final TIMI 3 epicardial flow, thus attesting to the limited yield of standard PCI in thrombus-containing lesions. This condition is classically termed the “illusion of reperfusion,” which succinctly presents the marked discrepancy between primary PCI-gained TIMI 3 flow versus the disappointing suboptimal degree of subsequent myocardial salvage. The main cause is the prevalent intracoronary thrombus and related distal embolization, microvessel obstruction, no reflow, and myocardial necrosis. Clearly patients who develop procedure-related no reflow tend to sustain larger infarct sizes, significantly worse left ventricular function, and a greater risk of adverse cardiac events and death. A recent Australian study by Mazhar and colleagues identified the phenomenon in a series of 189 patients in whom multivariate analysis identified thrombus scores ≥4, symptom-to-balloon time >360 minutes, and age above 60 years as independent predictors of no reflow. Similarly, a recent Chinese study determined the factors related to the no-reflow phenomenon in 203 patients with STEMI who underwent direct PCI, finding five risk factors including high thrombus burden. Indeed, cumulative evidence shows that these complications are especially pronounced among patients who undergo primary PCI for AMI. The Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS) project studied the sequelae of angiographically visible distal embolization in 883 STEMI PCI patients on triple antiplatelet therapy. The aspirated thrombus was larger in patients with evidence of angiographic embolization versus those without ( P = .002), and it more often contained erythrocytes (50% vs. 15.7%, P < .001, respectively). Those who sustained angiographically evident embolization had significantly worse outcomes than patients without, as indicated by a lower myocardial blush grade, impaired ST-segment resolution and a higher level of myocardial enzyme leakage. As compared with the revascularization of thrombus-free lesions, embolization significantly increased the need for emergency bypass surgery and the procedure-related death rate. At 1-year follow-up, reinfarction occurred in 8.9% of the TAPAS patients versus only 3.0% in patients without embolization ( P = .018). Thus the thrombus’s effect on the development of adverse coronary events and suboptimal outcomes should be taken into consideration and dealt with promptly before, during, and after PCI. The overall impact of thrombus on PCI is presented in Table 28.8 .
|
The principles governing the mechanisms of action and interaction with targeted thrombus that involve specific thrombus treatment strategies are described in Table 28.9 .
|
|
|
|
|
|
|
|
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here