Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Burn patients, with or without inhalation injuries, commonly exhibit a clinical picture that is largely produced by systemic inflammation. The term systemic inflammatory response syndrome (SIRS) was introduced to designate the signs and symptoms of this condition. SIRS has a continuum of severity ranging from physiologic alterations such as tachycardia, tachypnea, fever, and leukocytosis to refractory hypotension and, in its most severe form, shock and multiple organ system dysfunction. In thermally injured patients, the most common cause of SIRS is tissue damage caused by the burn itself. Sepsis, characterized by SIRS in the presence of infection, is also common in burn patients and is a significant cause of morbidity and mortality. Starting from a local infection at the burn wound, an infected catheter tip, or pulmonary infection, the spread of microbes and their toxins can further potentiate systemic inflammation. Pathological alterations of metabolic, cardiovascular, pulmonary, renal, gastrointestinal, and coagulation systems occur as a result of the hyperactive immune system. This chapter will review current understanding of SIRS and the associated immunological, cardiovascular, and pulmonary dysfunction that occurs following trauma and thermal injury.
The term systemic inflammatory response syndrome (SIRS) was recommended by the American College of Chest Physicians/Society for Critical Care Medicine (ACCP/SCCM) consensus conference in 1992 to describe a systemic inflammatory process, independent of its cause. The proposal was based on clinical and experimental results indicating that a variety of conditions, both infectious and noninfectious (i.e., burns, ischemia–reperfusion injury, multiple trauma, pancreatitis), induce a similar host response. Two or more of the following conditions must be present for the diagnosis of SIRS to be made:
Body temperature of greater than 38°C or less than 36°C;
Heart rate of greater than 90 beats/min;
Respiratory rate of greater than 20/min or a Pa co 2 of less than 32 mm Hg;
Leukocyte count of greater than 12,000/µL, less than 4000/µL, or more than 10% immature (band) forms.
All of these pathophysiologic changes must occur as an acute alteration from baseline in the absence of other known causes. This definition is very sensitive and nonspecific, and most of the SIRS criteria are also addressed in other scoring systems of injury-induced physiologic derangement, such as the Acute Physiology and Chronic Health Evaluation (APACHE), Mortality Probability Model (MPM), and Simplified Acute Physiology Severity (SAPS) systems. Several investigators have criticized the definition of SIRS as being too sensitive and encompassing the majority of ICU patients and certainly the vast majority of patients suffering extensive thermal injury. The initial definition of SIRS also did not address the continuum of disease severity defined for sepsis. Criteria for the diagnosis of severe sepsis included the additional derangements of organ dysfunction, hypotension, and hypoperfusion. Evidence of hypoperfusion included, but was not limited to, the presence of lactic acidosis, oliguria, and altered mental status. Septic shock was characterized by hypotension and hypoperfusion in patients who were adequately volume resuscitated or required treatment with catecholamines or other vasoactive drugs to support cardiovascular function. Muckart and Bhagwangee, in an effort to define a continuum of severity for SIRS, later proposed the categories of severe SIRS and SIRS-associated shock. These conditions were defined by the same criteria as severe sepsis and septic shock in the absence of demonstrable infection. In its most severe form, SIRS can induce organ injury and subsequent multiple organ dysfunction syndrome (MODS).
Despite the limitations in the definitions of SIRS and sepsis, most clinicians and investigators generally adopted the SIRS concept. However the initial definition and criteria were not felt to be optimized. To address these issues, a second consensus conference was assembled in 2001. The goal of this conference was to revisit the previously defined criteria for SIRS and sepsis as well as to determine whether revision of these criteria was indicated. The consensus was that the concepts of sepsis and SIRS are useful, but the diagnostic criteria are overly sensitive and nonspecific. The participants added additional criteria that defined metabolic, biochemical, and functional alterations associated with SIRS and sepsis. Among these were hyperglycemia, edema, elevated plasma C-reactive protein (CRP) concentration, coagulation abnormalities, thrombocytopenia, ileus, and hyperbilirubinemia. The group further proposed a staging system for sepsis and SIRS that could be used to stratify patients for prediction of outcome. This staging system, termed PIRO, defined several criteria, including the predisposition of patients to a poor outcome as determined by premorbid conditions and possible genetic factors. Other factors include the severity and type of insult, the host response to injury, and the presence of organ dysfunction. The participants proposed that this model could be used to generate more specific criteria for defining the SIRS phenomenon. Although the validity of this approach remains to be established, Rubulotta recently reported that the PIRO criteria have predictive value regarding mortality in septic patients. More recent studies have also validated the PIRO model. Macdonald and colleagues reported that the PIRO model performed better than SOFA or MEDS scores in predicting mortality in patients presenting to the emergency room with severe sepsis or septic shock. Some practitioners advocate for the validity and value of the PIRO system, but the system is not widely used in clinical practice.
Although inflammation is certainly present in patients with burn injuries, sepsis, and other severe injuries, the contribution of SIRS to the pathogenesis of those conditions is under question. The most recent consensus conference assembled to define clinical criteria for sepsis dropped SIRS from the defining criteria. The conference participants defined sepsis as “life threatening organ dysfunction due to a dysregulated host response to infection” and noted that SIRS criteria have poor predictive validity among patients who are infected.
Another pitfall in the designation of SIRS is the difficulty of applying the initial criteria to children. Some of the criteria, particularly those for heart and respiratory rates, fall within the normal physiologic range for young children. In 2002, a consensus conference was assembled to define criteria for sepsis and SIRS in children. The participants defined six age groups based on clinical and physiological characteristics ( Table 19.1 ). SIRS was defined as the presence of at least two of the following four criteria, one of which must be abnormal temperature or leukocyte count:
temperature of greater than 38.5°C or less than 36°C;
tachycardia, defined as a mean heart rate of greater than 2 SD above normal for age or for children younger than 1 year;
bradycardia, defined as a heart rate of less than 10% of normal for age;
mean respiratory rate of greater than 2 SD above normal for age or requirement for mechanical ventilation;
leukocyte count elevated or depressed for age.
| Newborn 0 day to 1 week Neonate 1 week to 1 month Infant 1 month to 1 year Toddler or preschool 2 to 5 years School age 6 to 12 years Adolescent 13 to 18 years |
Severe sepsis was defined as sepsis plus one of the following: cardiovascular dysfunction, acute respiratory distress syndrome, or two or more organ dysfunctions (respiratory, renal, neurologic, hematologic, or hepatic).
The definition of septic shock is problematic in children because children can maintain blood pressure until they become severely ill. Therefore hypotension is not a useful criterion for diagnosing shock in children. This group proposed criteria for septic shock that included the presence of tachycardia in conjunction with signs of decreased peripheral perfusion, such as decreased capillary refill, decreased peripheral pulses, decreased urine output, altered mental status, and cold/mottled extremities.
Several studies have been conducted with the goal of determining the prognostic value of the SIRS designation. In the acute setting, SIRS has been demonstrated in the majority of critically injured patients, and the intensity of the response correlates directly with the severity of injury. The presence of SIRS within the first 24 hours after severe injury has not served as a reliable predictor of mortality in trauma or burn patients. However, the presence of shock is an important predictor of poor outcome, particularly when associated with MODS. In addition, the presence of more than two of the SIRS criteria in the setting of acute injury has correlated with increased morbidity and mortality. A study by Rangel-Frausto et al. showed that trauma patients who did not meet SIRS criteria had a mortality rate of 3%, compared to 6% in those with two SIRS markers. Patients with three or four SIRS criteria had mortality rates of 10% and 17%, respectively, whereas those with culture-negative shock had a 46% death rate. Haga et al. have shown that the persistence of SIRS for more than 3 days in surgical patients is a harbinger of complications and is associated with increased morbidity. Talmor et al. reported that persistence of SIRS to the second postoperative day in high-risk surgical patients correlated with an increased incidence of MODS. Additional studies have shown that persistence of SIRS criteria for more than 3 days in trauma and burn patients is associated with worse outcome. Therefore, three important factors appear to determine the effect of SIRS on the host. The first is the severity of the initial inflammatory response. This response is proportional to the severity of injury, and the presence of shock or MODS within the first 24 hours after injury carries a poor prognosis. The second determinant is the persistence of SIRS beyond the first few days of injury. Specifically prolongation of SIRS beyond the second day after severe trauma or thermal injury is associated with an increased complication rate. Factors that appear to be important in reducing the incidence of a prolonged inflammatory state include adequate fluid resuscitation within the first 24 hours after injury, aggressive excision of necrotic tissue, and enteral feeding. A third factor is the adaptive capacity of the host. Results of several studies have shown that extremes of age and the presence of coexisting disease will diminish the adaptive capacity of the host and predict a worse prognosis for any given severity of injury. As noted earlier in this chapter, the actual contribution of SIRS to the pathogenesis of sepsis and other forms of systemic injury are in question.
The crucial pathophysiologic event that precipitates systemic inflammation is tissue damage. This can occur both as a result of direct injury to tissues from mechanical or thermal trauma and as a result of cellular injury induced by ischemia and reperfusion. Injury results in the acute release of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, and IL-6. If injury is severe, as in extensive thermal injury, a profound release of cytokines and noncytokine mediators of inflammation occurs, resulting in the induction of a systemic inflammatory reaction. The ability of the host to adapt to this systemic inflammatory response depends on the magnitude and duration of the response as well as the adaptive capacity of the host. If the insult and the host's response to it are beyond the adaptive capacity of the host, or if adequate resuscitation is not promptly initiated, organ injury may ensue during the early post-injury period. Factors that have been implicated in the worsening or prolongation of SIRS include inadequate resuscitation during the acute phase following thermal injury, persistent or intermittent infection, ongoing tissue necrosis, and translocation of bacteria or endotoxin across the bowel.
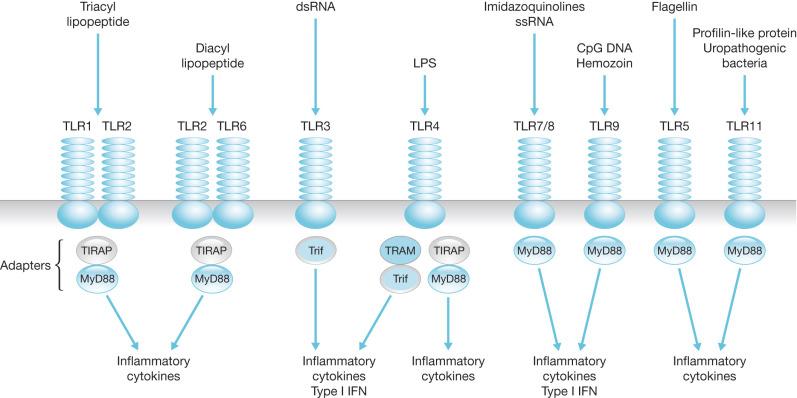
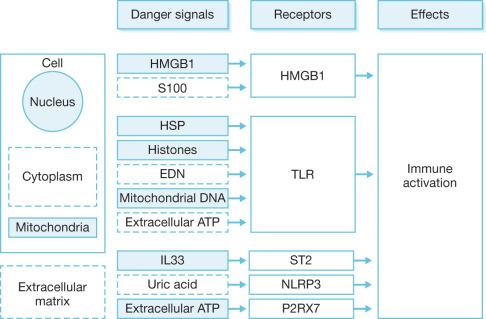
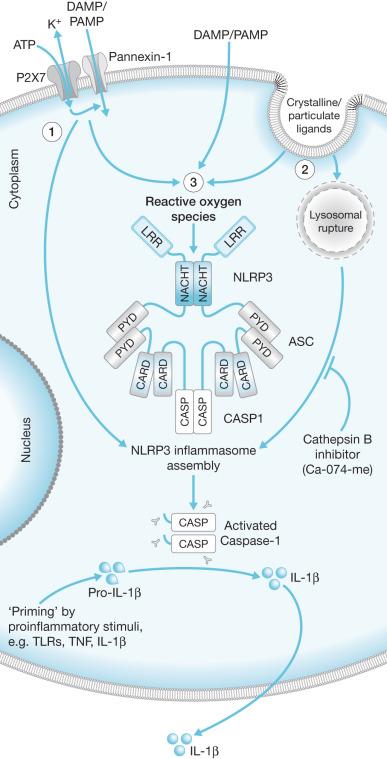
The actual mechanisms that initiate trauma-induced inflammation at the cellular and molecular levels are becoming increasingly understood. Two primary sensing systems have been identified. Toll-like receptors (TLR) respond to endogenous cellular factors that are produced or released following tissue trauma. TLR are expressed by leukocytes and some parenchymal cells. They allow the host to sense the presence of microbes via pathogen-associated molecular patterns (PAMPs) and initiate an innate immune response ( Fig. 19.1 ). TLR also have the ability to respond to endogenous ligands that are leaked from cells upon their destruction by burn injury. These molecules are referred to as damage-associated molecular patterns (DAMPs) and are typically intracellular molecules with normal cellular functions ( Fig. 19.2 ). As cells are damaged by thermal injury, these molecules are released into the extracellular matrix. Due to their normal intracellular localization, these molecules are recognized as danger signals and can bind to the same TLRs that are activated by microbial products. Among the proposed endogenous TLR ligands are heat shock proteins (recognized by TLR4 and TLR2), high-mobility group box 1 (HMGB1; recognized by TLRs 4 and 2), histones (recognized by TLR4), and mitochondrial DNA (recognized by TLR9). Activation of TLR signaling pathways results in the transcription of genes associated with inflammatory responses. A second sensing system is comprised of cytoplasmic NOD-like receptors (NLRs) that sense endogenous and exogenous ligands during loss of cellular compartmentalization or membrane integrity and cause activation of inflammasomes ( Fig. 19.3 ). DAMPs that activate NLRs, either directly or via adapter molecules, include uric acid, endogenous DNA, and adenosine triphosphate (ATP). Inflammasome assembly ultimately results in activation of inflammatory caspases such as caspase-1. Activated caspase-1, also known as IL-1-converting enzyme, causes cleavage of pro-IL-1 and pro-IL-18 into active mature proteins. The IL-1 and IL-18 produced by this interaction are released from cells and facilitate the pro-inflammatory response.
Inflammatory responses are important for the early initiation of tissue repair and activation of innate immune cells in response to local infection. Cytokines such as IL-6, TNF-α, and IL-1 recruit and activate macrophages and neutrophils at sites of infection. To prevent tissue damage caused by prolonged or excessive inflammation, local anti-inflammatory responses are initiated to resolve the early inflammatory response. In the case of large burn injuries, the inflammatory response is massive and becomes systemic. A compensatory anti-inflammatory response referred to as the counter anti-inflammatory response syndrome (CARS) may be mounted in an attempt to restore homeostasis and control SIRS. Increased production of anti-inflammatory cytokines such as IL-10 and transforming growth factor-β (TGFβ) and leukocyte apoptosis are part of this response. A prolonged or excessive anti-inflammatory response predisposes the patient to infection. The pathogenesis of injury-induced immunosuppression is discussed in detail elsewhere in this book. Nevertheless there is likely great heterogeneity in the host response to injury and infection. A recent report by Davenport and colleagues showed that there is great interindividuality in the peripheral blood transcriptomic response during sepsis but that patients exhibiting a signature reflective of immunosuppression, endotoxin tolerance, and T-cell exhaustion had a worse prognosis. They further noted that the immunosuppressed phenotype can develop early during sepsis and may render the septic host unable to adequately mount an adequate response to clear the primary infection.
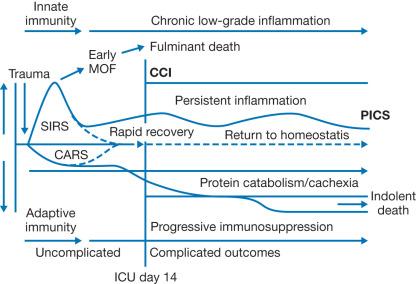
Our understanding of these syndromes has evolved to better describe this response that is shared by burn and severe non-burn trauma patients. For decades, the host response to injury-induced systemic inflammation has been described by the SIRS/CARS paradigm. It was initially believed that SIRS was resolved during CARS due to mutual regulatory effects of the mediators associated with the respective syndromes. However, examination of the transcriptome of leukocytes from trauma patients has revealed that SIRS and CARS exist simultaneously. Specifically, genes associated with both inflammatory and anti-inflammatory pathways were simultaneously up-regulated after injury, and this was described as a “genomic storm.” Genes associated with adaptive immune responses were down-regulated at the same time, suggesting impaired effector functions. The magnitude and duration of these responses correlated with complications after injury. This is supported by earlier studies examining levels of pro-inflammatory IL-6 and anti-inflammatory IL-10 in the circulation of burn patients. Burn patients who eventually developed sepsis during their acute care had substantially higher levels of circulating IL-6 and IL-10 as early as 1 day post-burn. Later development of lethal sepsis was associated with persistently high levels of these cytokines. More recently, a new model has been proposed to characterize the responses and outcomes of patients following severe traumatic injury ( Fig. 19.4 ). This model proposes that patients who rapidly resolve their SIRS and CARS responses return to immune homeostasis and recover from injury without major complications. However, if SIRS and CARS are not resolved, the patient develops persistent inflammation, immunosuppression, and catabolism syndrome (PICS), in which persistent inflammation and immunosuppression are accompanied by severe muscle protein catabolism (described in other chapters). Secondary nosocomial infections are a risk and can exacerbate the existing inflammation, leading to multiorgan failure and death.
Some investigators have described a phenomenon in which the injured host manifests an exaggerated inflammatory response if confronted with a secondary inflammatory stimulus during the post-injury period. This phenomenon has been termed the “two-hit hypothesis.” Although the pathobiology of the two-hit hypothesis is not completely understood, monocytes and macrophages appear to play a central role in mediating the process. For example, the lymphokine α-interferon (IFN-α), produced during the initial injury, might act as the first signal and prime macrophages for a heightened inflammatory response if a second stimulus is encountered. Several changes in macrophage function, including an increase in the transcription rate of mRNA for TNF-α, can be induced by exposure to IFN-α. However, TNF-α protein is not produced in large amounts in response to the first inflammatory insult. If a second stimulus, such as endotoxin exposure, is provided in even a small dose, macrophages are triggered to become fully activated and to secrete large amounts of TNF-α. Studies by Paterson et al. showed that macrophages have increased responsiveness to ligands for TLR2 and TLR4 following burn injury. TLR2 and TLR4 play an integral role as components of receptor complexes for DAMPs and various microbial products such as peptidoglycans, lipoproteins, and lipopolysaccharide. Enhancement of TLR2 and TLR4 responses during the post-injury period may be one mechanism contributing to the two-hit phenomenon.
The exaggerated response to a secondary stimulus seen in severely injured patients appears to have functional consequences. Several studies that focused on organ injury caused by systemic inflammatory processes indicate that a phenomenon comparable to the cellular events just described can occur in severely injured patients. Dehring et al. found more persistent pulmonary hypertension and an exaggerated hyperdynamic response to bacteremia in sheep when a week-old thermal injury preceded systemic bacterial challenge. In a rat model of intestinal ischemia–reperfusion injury and endotoxemia, lung albumin leak and mortality rate increased only if both injuries occurred sequentially. Combined administration of low doses of endotoxin and TNF-α to rats caused hypotension and metabolic effects that are commonly seen after giving a highly lethal dose of either compound alone. These findings are in keeping with the fact that multiple organ damage usually develops over a prolonged period during which several insults might occur. It also emphasizes why it is so important to minimize inflammatory insults such as tissue ischemia or infection, particularly in patients in whom systemic inflammation is already present.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here