Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
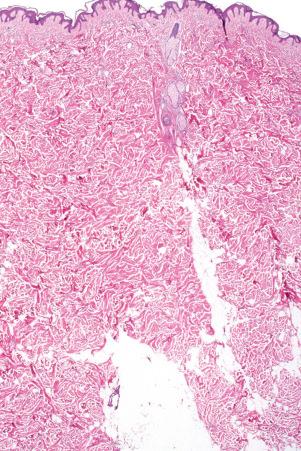
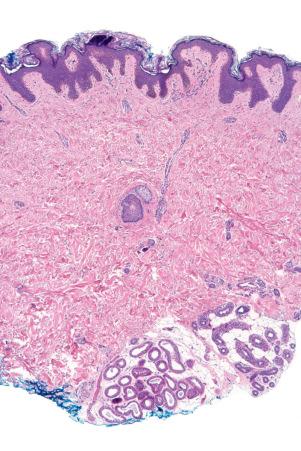
Skin is a double-layered membrane covering the exterior of the body and consists of a stratified cellular epidermis and an underlying dermis of connective tissue. In adults, the skin weighs over 5 kg and covers a surface area approaching 2 m 2 . The epidermis is mainly composed of keratinocytes and is typically 0.05–0.1 mm in thickness. The dermis contains collagen, elastic tissue and ground substance and is of variable thickness, from 0.5 mm on the eyelid or scrotum to more than 5 mm on the back ( Fig. 1.1 ).
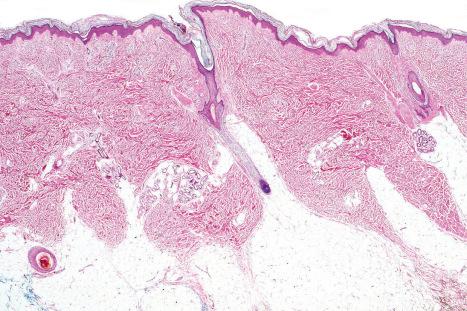
The dermis is subdivided into a more superficial component (the papillary dermis) which is bounded inferiorly by the superficial vascular plexus and an underlying much thicker reticular dermis. Below the dermis is a layer of subcutaneous fat which is separated from the rest of the body by a vestigial layer of striated muscle.
A key role of skin is to provide a mechanical barrier against the external environment. The cornified cell envelope and the stratum corneum restrict water loss from the skin while keratinocyte-derived endogenous antibiotics (defensins and cathelicidins) provide an innate immune defense against bacteria, viruses, and fungi. The epidermis also contains a network of about 2 × 10 9 Langerhans cells which serve as sentinel cells whose prime function is to survey the epidermal environment and to initiate immune responses against microbial threats. Melanin, which is mostly found in basal keratinocytes, provides some protection against DNA damage from ultraviolet radiation. An important function of skin is thermoregulation. Vasodilatation or vasoconstriction of the blood vessels in the deep or superficial plexuses helps regulate heat loss. Eccrine sweat glands are found at all skin sites and are present in densities of 100–600/cm 2 ; they play a role in heat control and aspects of metabolism. Secretions from apocrine sweat glands contribute to body odor. Skin lubrication and waterproofing is provided by sebum secreted from sebaceous glands. Subcutaneous fat has important roles in cushioning trauma as well as providing insulation and a calorie reserve. Fat also has an endocrine function and contributes to tissue remodeling and phagocytosis. Nails provide protection to the ends of the fingers and toes as well as being important in pinching and prising objects. Hair may have important social and psychological value. Skin also has a key function in synthesizing various metabolic products, such as vitamin D.
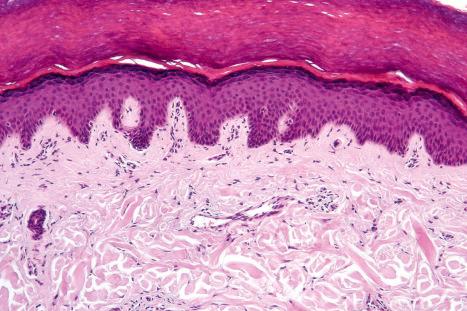
Although the basic structure is relatively constant at various skin sites, there are often clear differences which enable one to determine the site of origin. The epidermis consists of four clearly defined layers or strata:
Basal cell layer (stratum basale)
Prickle cell layer (stratum spinosum)
Granular cell layer (stratum granulosum)
Corneocyte layer (stratum corneum)
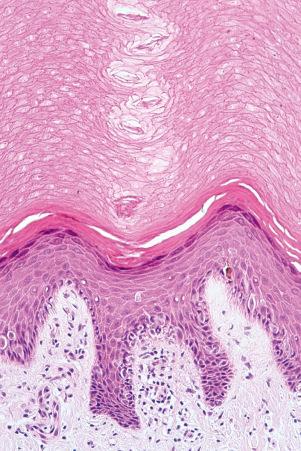
An eosinophilic acellular layer known as the stratum lucidum is sometimes seen in skin from the palms and soles ( Fig. 1.2 ).
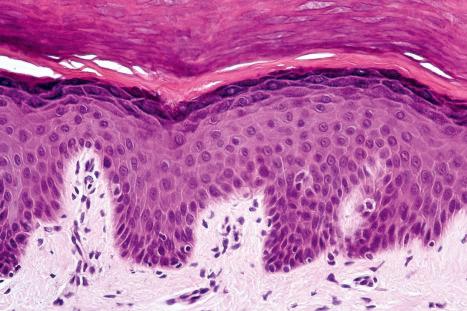
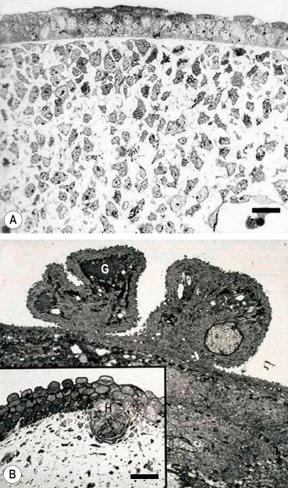
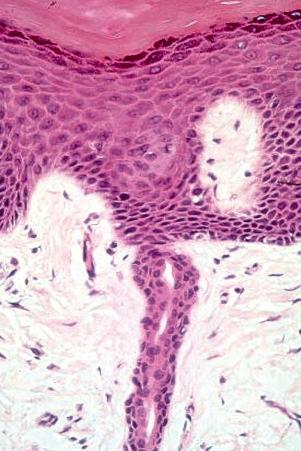
Basal cells are cuboidal or columnar with a large nucleus typically containing a conspicuous nucleolus. Small numbers of mitoses may be evident. Clear cells are also present in the basal layer of the epidermis; these represent melanocytes. Cells with clear cytoplasm seen in the stratum spinosum represent Langerhans cells. Very occasional Merkel cells may also be present but these are not easily identified in hematoxylin and eosin stained sections. Histologically, prickle cells are polygonal in outline, have abundant eosinophilic cytoplasm and oval vesicular nuclei, often with conspicuous nucleoli. Keratohyalin granules typify the granular cell layer ( Fig. 1.3 ). The granular cells typically form three tightly packed layers, with individual cells adopting a tetrakaidecahedron shape. Skin integrity is maintained by a mobile layer of tight junctions that translocate from granular layer cell surface to base as individual keratinocytes transition to the uppermost granular cell layer. Thus the main impermeability plane in skin is between the first and second granular cell layers. Further maturation leads to loss of nuclei and flattening of the keratinocytes to form the plates of the corneocyte layer (stratum corneum). Adjacent cells are united at their free borders by intercellular bridges (prickles), which are most clearly identifiable in the prickle cell layer and in disease states of the skin where there is marked intercellular edema (spongiosis) ( Fig. 1.4 ).
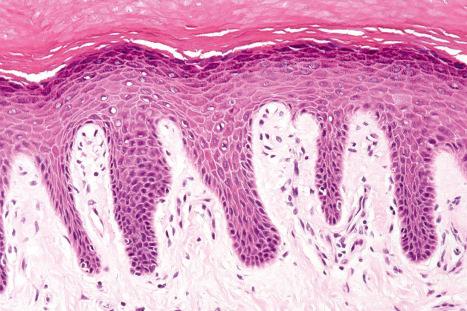
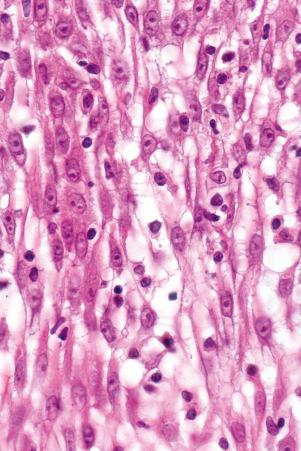
Toker cells represent an additional clear cell population, which may be found in nipple epidermis of both sexes in up to 10% of the population. Toker cells are large, polygonal or oval and have abundant pale staining or clear cytoplasm with vesicular nuclei often containing prominent, albeit small, nucleoli. The cytoplasm is mucicarmine and Periodic acid-Schiff negative. The cells may be distributed singly but more often they are found as small clusters, not uncommonly forming single layered ductules. They are located along the basal layer of the epidermis or suprabasally and are also sometimes seen within the epithelium of the terminal lactiferous duct.
Toker cells are of particular importance as they may be mistaken by the unwary as Paget cells. They are thought to be the source of mammary Paget disease in those exceptional cases where an underlying ductal carcinoma is absent. Toker cells express CK7, AE1/AE3, CAM 5.2, epithelial membrane antigen (EMA), cerbB2, estrogen, and progesterone receptors. They do not express p53 or CD138. Carcinoembryonic antigen (CEA) may also be present, albeit weakly. Paget cells by way of contrast are often negative for estrogen and progesterone receptors and are p53 and CD138 positive.
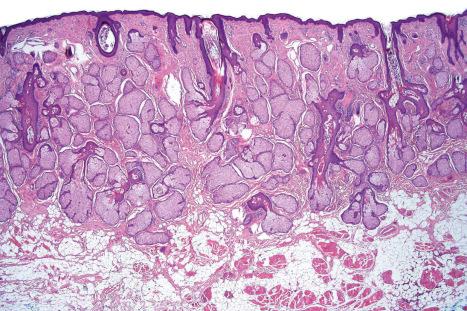
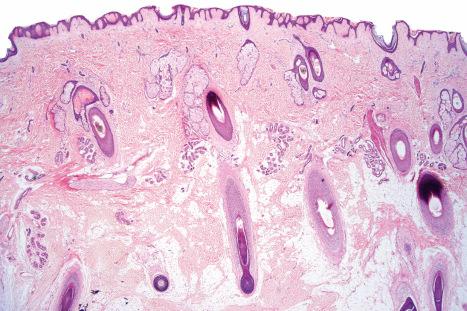
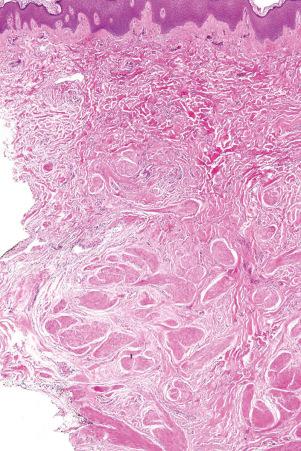
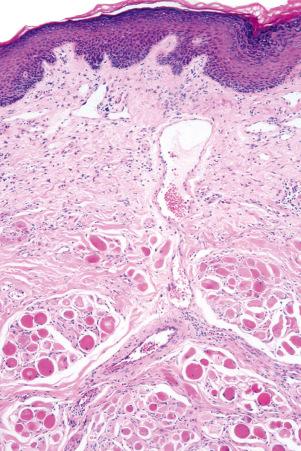
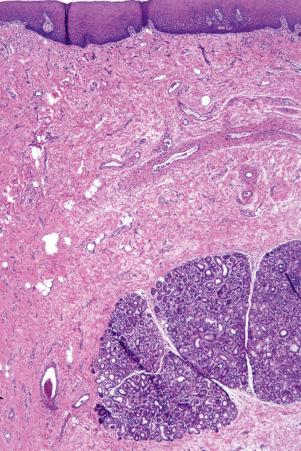
There are two main kinds of human skin: glabrous skin (nonhairy skin) and hair-bearing skin. Glabrous skin is found on the palms and soles. It has a grooved surface with alternating ridges and sulci giving rise to the dermatoglyphics (fingerprints). Glabrous skin has a compact, thick stratum corneum, and contains encapsulated sense organs within the dermis but no hair follicles or sebaceous glands. In contrast, hair-bearing skin has both hair follicles and sebaceous glands but lacks encapsulated sense organs. Hair follicle size, structure and density can vary between different body sites. For example, the scalp has large hair follicles that extend into subcutaneous fat whereas the forehead has only small vellus hair-producing follicles although sebaceous glands are large. The number of hair follicles does not alter until middle life but there is a changing balance between vellus and terminal hairs throughout life. In hair-bearing sites, such as the axilla, there are apocrine glands in addition to the eccrine sweat glands. Sebaceous glands are active in the newborn, and from puberty onwards, and the relative activity modifies the composition of the skin surface lipids. The structure of the dermal–epidermal junction also shows regional variations in the number of hemidesmosomal-anchoring filament complexes (more in the leg than the arm). In the dermis, the arrangement and size of elastic fibers varies from very large fibers in perianal skin to almost no fibers in the scrotum. Marked variation in the cutaneous blood supply is found between areas of distensible skin such as the eyelid and more rigid areas such as the fingertips.
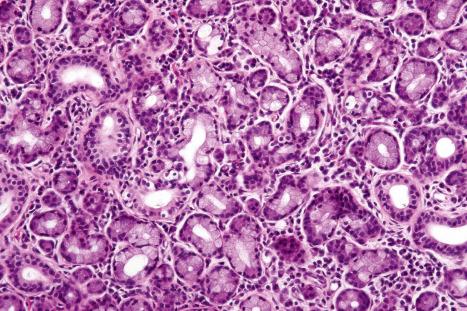
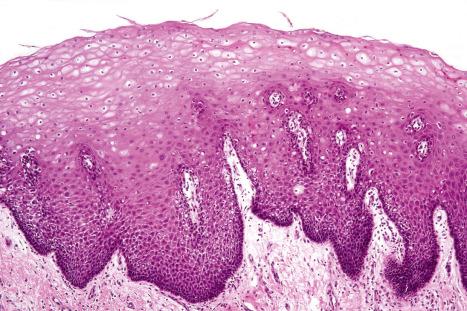
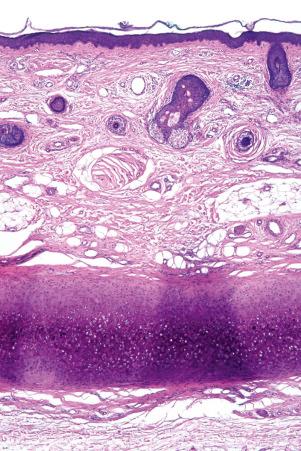
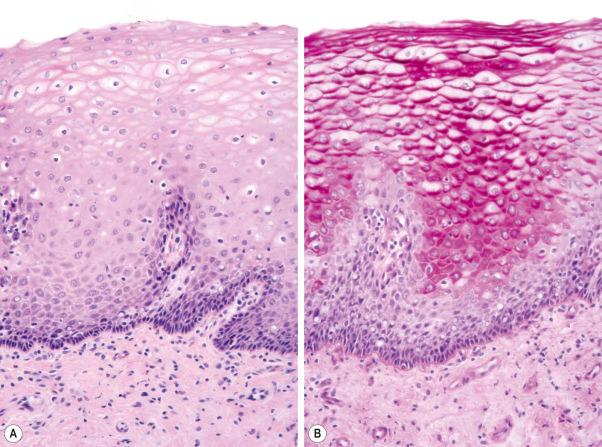
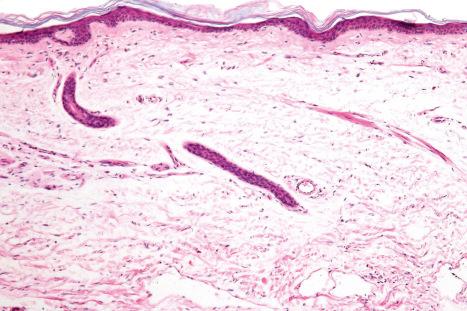
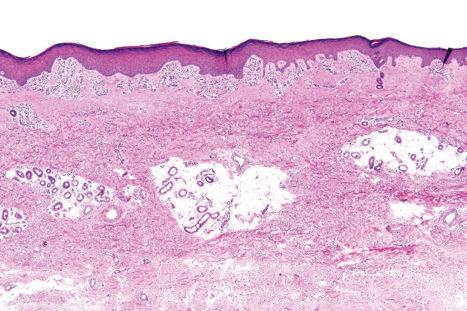
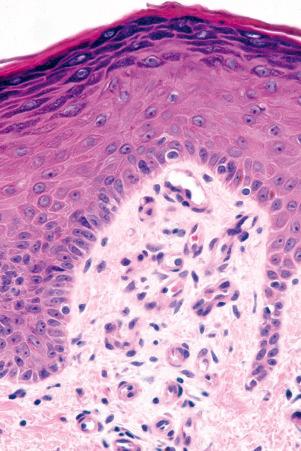
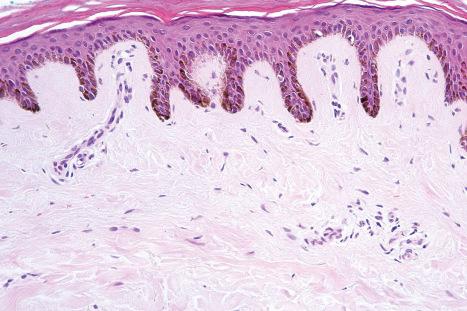
Regional variation in skin structure is illustrated in Figs 1.5–1.20 .
Two major embryological elements juxtapose to form skin. These comprise the prospective epidermis that originates from a surface area of the early gastrula, and the prospective mesoderm that comes into contact with the inner surface of the epidermis during gastrulation. The mesoderm generates the dermis and is involved in the differentiation of epidermal structures such as hair follicles. Melanocytes are derived from the neural crest. After gastrulation, there is a single layer of neuroectoderm on the embryo surface: this layer will go on to form the nervous system or the skin epithelium, depending on the molecular signals (e.g., fibroblast growth factors or bone morphogenic proteins) it receives. The embryonic epidermis consists of a single layer of multipotent epithelial cells which is covered by a special layer known as periderm that is unique to mammals. Periderm provides some protection to the newly forming skin as well as exchange of material with the amniotic fluid. During pregnancy, periderm is present until 24 weeks' gestation whereupon it is shed into the amniotic fluid. One key transcription factor in generating both an embryonic epidermal monolayer as well as a differentiated epidermis is p63. Developmental signals induced by p63 occur before any keratin is generated. The embryonic dermis is at first very cellular and at 6–14 weeks three types of cell are present: stellate cells, phagocytic macrophages and granule-secretory cells, either melanoblasts or mast cells ( Fig. 1.21 ). From weeks 14 to 21, fibroblasts are numerous and active, and perineural cells, pericytes, melanoblasts, Merkel cells and mast cells can be individually identified. Hair follicles and nails are evident at 9 weeks. Sweat glands are also noted at 9 weeks on the palms and the soles. Sweat glands at other sites and sebaceous glands appear at 15 weeks. Touch pads become recognizable on the fingers and toes by the sixth week and development is maximal by the 15th week. The earliest development of hair occurs at about 9 weeks in the regions of the eyebrow, upper lip and chin. Sebaceous glands first appear as hemispherical protuberances on the posterior surfaces of the hair pegs and become differentiated at 13–15 weeks. Langerhans cells are derived from the monocyte–macrophage–histiocyte lineage and enter the epidermis at about 12 weeks. Merkel cells appear in the glabrous skin of the fingertips, lip, gingiva and nail bed, and in several other regions, around 16 weeks. Although some cells of the dermis may migrate from the dermatome (venterolateral part of the somite) and take part in the formation of the skin, most of the dermis is formed by mesenchymal cells that migrate from other mesodermal areas. These mesenchymal cells give rise to the whole range of blood and connective tissue cells, including the fibroblasts and mast cells of the dermis and the fat cells of the subcutis. In the second month, the dermis and subcutis are not discernible as distinct skin layers but collagen fibers are evident in the dermis by the end of the third month. Later, the papillary and reticular layers become established and, at the fifth month, the connective tissue sheaths are formed around the hair follicles. Elastic fibers are first detectable at 22 weeks.
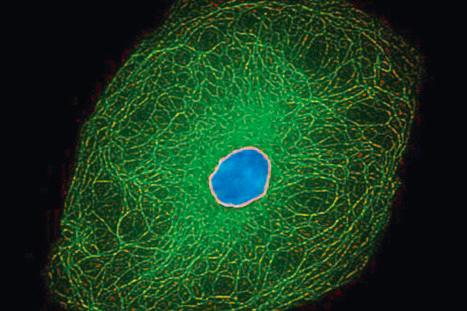
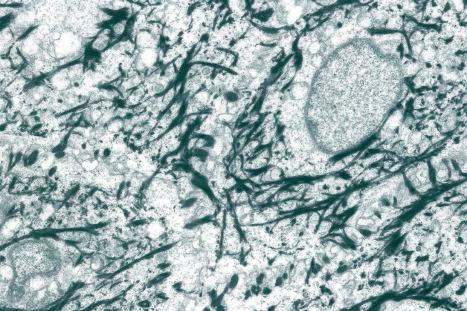
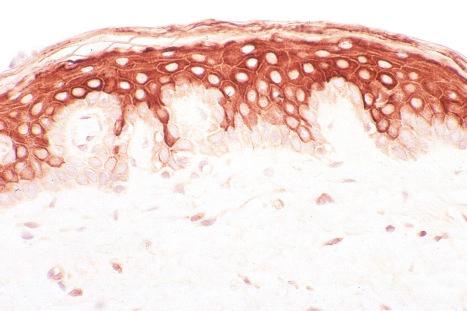
The cytoskeleton of all mammalian cells, including epidermal keratinocytes, comprises actin containing microfilaments ≈7 nm in diameter, tubulin containing microtubules 20–25 nm in diameter, and filaments of intermediate size, 7–10 nm in diameter, known as intermediate filaments. There are six types of intermediate filaments of which keratins are the filaments in keratinocytes ( Figs 1.22 , 1.23 ). The human genome possesses 54 functional keratin genes located in two compact gene clusters, as well as many nonfunctional pseudogenes, scattered across the genome. Keratin genes are very specific in their expression patterns. Each one of the many highly specialized epithelial tissues has its own profile of keratins. Hair and nails express modified keratins containing large amounts of cysteine which forms numerous chemical cross-links to further strengthen the cytoskeleton. The genes encoding the keratins fall into two gene families: type I (basic) and type II (acidic) and there is coexpression of particular acidic–basic pairs in a cell- and tissue-specific manner. Keratin heterodimers are assembled into protofibrils and protofilaments by an antiparallel stagger of some complexity. Simple epithelia are characterized by the keratin pair K8/K18, and the stratified squamous epithelia by K5/K14. Suprabasally, keratins K1/K10 are characteristic of epidermal differentiation ( Fig. 1.24 ). K15 is expressed in some interfollicular basal keratinocytes as well as keratinocytes within the hair-follicle bulge region at the site of a population of multipotent stem cells. K9 and K2 expression is site restricted in skin: K9 to palmoplantar epidermis and K2 to superficial interfollicular epidermis.
Apart from their structural properties, keratins may also have direct roles in cell signaling, stress responses and apoptosis. In epidermal hyperproliferation, as in wound healing and psoriasis, expression of suprabasal keratins K6/K16/K17 is rapidly induced.
Currently, 21 of the 54 known keratin genes have been linked to monogenic genetic disorders, and some have been implicated in more complex traits, such as idiopathic liver disease or inflammatory bowel disease. The intermediate filament database has now recorded over 40 diseases and more than 400 mutations involving keratins ( www.interfil.org ). The first genetic disorder of keratin to be described was epidermolysis bullosa simplex, which involves mutations in the genes encoding K5 or K14 ( Fig. 1.25 ). About half of the keratin genes are expressed in the hair follicle, and mutations in these genes may underlie cases of monilethrix as well as hair and nail ectodermal dysplasias.
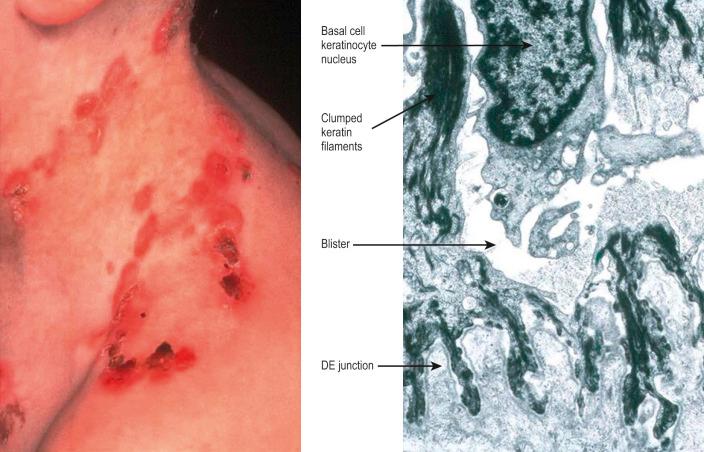
To maintain, repair and regenerate itself, the skin contains stem cells which reside in the bulge area of hair follicles, the basal layer of interfollicular epidermis and the base of sebaceous glands ( Fig. 1.26 ). Stem cells are able to self-renew as well as give rise to differentiating cells. It is not clear, however, whether every basal keratinocyte or only a proportion of cells is a stem cell. Two possible hypotheses have emerged. One theory divides basal keratinocytes into epidermal proliferation units, which comprise one self-renewing stem cell and about 10 tightly packed transient amplifying cells, each of which is capable of dividing several times and then exiting the basal layer to undergo terminal differentiation. This unit gives rise to a column of larger and flatter cells that culminates in a single hexagonal surface. The process of division of basal cells in this model is viewed as a symmetric process in which equal daughter cells are generated with the basal cells progressively reducing their adhesiveness to the underlying epidermal basement membrane, delaminating and committing to terminal differentiation. The alternative theory is that some basal cells (perhaps up to 70% of cells in murine studies) can undergo asymmetric cell division, shifting their spindle orientation from lateral to perpendicular. Asymmetric cell divisions provide a means of maintaining one proliferative daughter while the other daughter cell is committed to terminal differentiation. Asymmetric cell divisions, therefore, can bypass the need for transient amplifying cells.
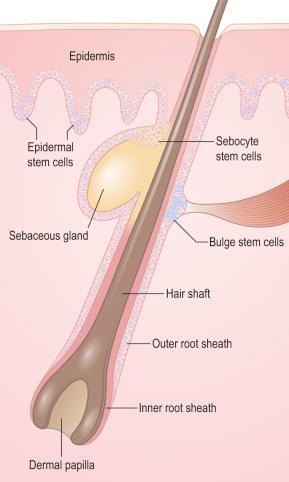
Hair follicle stem cells are found in the bulge regions below the sebaceous glands ( Fig. 1.27 ). The bulge area stem cells generate cells of the outer root sheath (ORS), which drive the highly proliferative matrix cells next to the mesenchymal papillae. After proliferating, matrix cells differentiate to form the hair channel, the inner root sheath (IRS) and the hair shaft. Hair follicle stem cells can also differentiate into sebocytes and interfollicular epidermis. Despite this multipotency, however, the follicle stem cells only function in pilosebaceous unit homeostasis and do not contribute to interfollicular epidermis unless the skin is wounded.
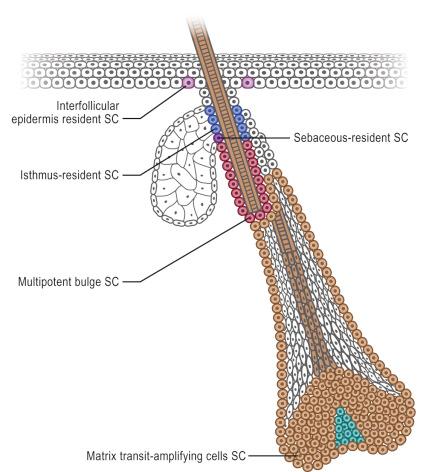
Apart from stem cells in the hair follicles, sebaceous glands and interfollicular epidermis, other cells in the dermis and subcutis may have stem cells properties. These include cells that have been termed skin-derived precursors (SKPs), which can differentiate into both neural and mesodermal progeny. In addition, a subset of dermal fibroblasts can have adipogenic, osteogenic, chondrogenic, neurogenic and hepatogenic differentiation potential. Skin wounding can also induce mobilization and recruitment of inflammatory and noninflammatory cells (including epithelial progenitors) from bone marrow.
A major function of the epidermis is to form a barrier against the external environment. To achieve this, terminal differentiation of keratinocytes results in formation of the cornified cell envelope. This physical barrier is rendered highly insoluble by the formation of glutamyl-lysyl isodipeptide bonds between envelope proteins, catalyzed by transglutaminases. Several different proteins contribute to construction of the cornified cell envelope, including involucrin, and the family of small proline-rich proteins (SPR1), including cornifin or SPR1 and pancornulins. Other envelope proteins include skin-derived anti-leucoproteases (SKALP)/elafin and keratolinin/cystatin. Some precursors of the cornified envelope are delivered by granules: small, smooth, sulfur-rich L granules contain the cysteine-rich protein loricrin, and accumulate in the stratum granulosum. Loricrin is the major component of the cornified envelope. Profilaggrin in F granules may make a minor contribution to the envelope. Membrane-associated proteins that contribute to the cornified envelope include the plakin family members, periplakin, envoplakin, epiplakin, desmoplakin as well as plectin. Formation of the cornified cell envelope is triggered by a rise in intracellular calcium levels. This leads to cross-link formation between plakins and involucrin catalyzed by transglutaminases. Other desmosomal proteins are then also cross-linked, forming a scaffold along the entire inner surface of the plasma membrane. Ceramides from the secreted contents of lamellar bodies are then esterified onto glutamine residues of the scaffold proteins. The cornified cell envelope is reinforced by the addition of a variable amount of SPRs, repetin, trichohyalin, cystostatin α, elafin and LEP/XP-5 (skin-specific protein). Although most desmosomal components are degraded, keratin intermediate filaments (mostly K1, K10 and K2) may be cross-linked to desmoplakin and envoplakin remnants.
In the upper stratum spinosum and stratum granulosum lipid is synthesized and packaged into lamellated membrane-bound organelles known as membrane-coating granules, lamellar granules or Odland bodies ( Fig. 1.28 ). They are found adjacent to the cell membrane with alternating thick and thin dense lines separated by lighter lamellae of equal width, consistent with packing of flattened discs within a membrane boundary. These granules contain phospholipids, glycolipids and free sterols and move towards the plasma membrane as the cells move through the granular layer where they cluster at the cell membrane. They fuse with the plasma membrane, dispersing their contents into the intercellular space. Polar lipids from the lamellar granules are remodeled into neutral lipids in the intercellular space between corneocytes, thereby contributing to the barrier.

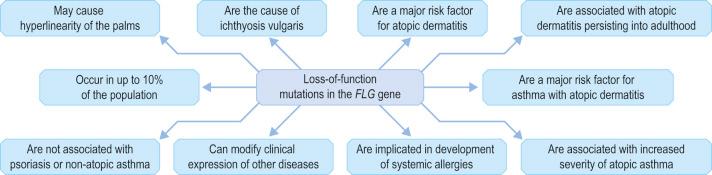
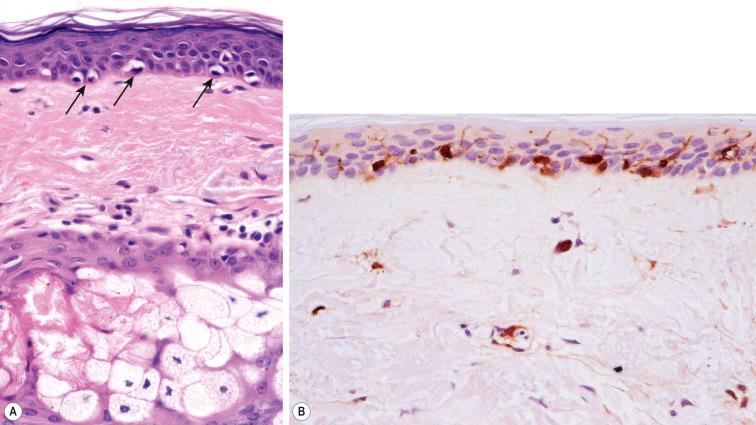
Within the granular layer of the epidermis, the main keratinocyte proteins are keratin and filaggrin, which together contribute approximately 80–90% of the mass of the epidermis and are ultrastructurally represented by the keratohyalin granules ( Fig. 1.29 ). Filaggrin is initially synthesized as profilaggrin, a ≈500-kDa highly phosphorylated, histidine-rich polypeptide. During the post-translational processing of profilaggrin, the individual filaggrin polypeptides, each ≈35 kD, are proteolytically released. These are then dephosphorylated, a process that assists keratin filament aggregation and explains the origin of the name ‘filaggrin’ ( fil ament aggr egating prote in ) ( Fig. 1.30 ). Typically, there are 10 highly homologous filaggrin units, although the number of filaggrin repeat units is variable and genetically determined, with duplications of filaggrin repeat units 8 and/or 10 in some individuals. Fewer filaggrin repeats leads to dryer skin. Loss-of-function mutations in filaggrin are very common, occurring in up to 10% of the European population. These mutations lead to reduced or absent keratohyalin granules, and are the cause of ichthyosis vulgaris as well as constituting a major risk factor for atopic dermatitis ( Fig. 1.31 ).
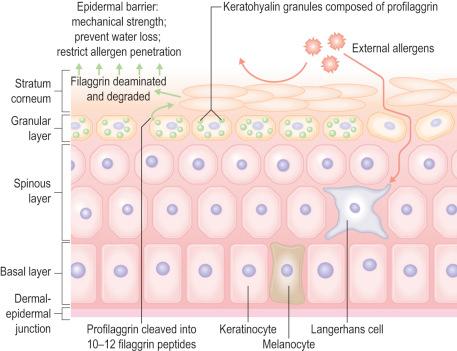
Within the stratum corneum, the main intercellular adhesive structures are the corneodesmosomes. These complexes become modified from desmosomes at the most superficial layer of the stratum granulosum, the main difference being the presence of corneodesmosin in the extracellular portion. Corneodesmosomes are structurally different from desmosomes in that they are homogeneously electron-dense and their attachment plaques are integrated into the cornified cell envelopes. When the extracellular regions of corneodesmosomes are fully degraded, desquamation occurs. The degradation process of corneodesmosomes is carefully controlled by a number of proteases and their inhibitors. The most important proteases are the kallikrein-related peptidases, which are physiologically regulated by the lympho-epithelial Kazal-type related inhibitor. Other proteolytic regulators include matriptase, meprin and mesotrypsin. Mutations in some of these proteases or their inhibitors underlie different forms of ichthyosis, often with skin barrier impairment as well as hypotrichosis.
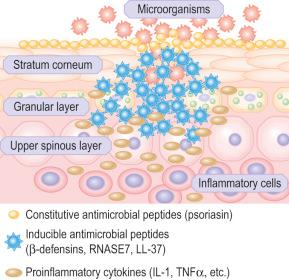
Skin possesses both innate and adaptive immune responses to defend against microbial pathogens and thereby prevent infection. Nevertheless, skin also encourages colonization by certain microorganisms to promote a cutaneous ecosystem known as the microbiome. This skin microbial community, which varies considerably with body region, interacts with host immunity to create homeostasis, with dysbiosis contributing to the pathogenesis of some skin diseases, including atopic dermatitis and rosacea. One of the primary immune responses of skin is the synthesis, expression and release of antimicrobial peptides ( Fig. 1.32 ). There are dozens, possibly hundreds, of antimicrobial peptides in the skin, including cathelicidins, β-defensins, substance P, regulated on activation, normal T cell expressed and secreted (RANTES), Ribonuclease (RNase) 2, 3, and 7, and S100 calcium-binding protein A7 (S100A7). Many of these peptides have antimicrobial action against bacteria, viruses, and fungi. In the stratum corneum there is an effective chemical barrier maintained by the expression of S100A7 (psoriasin). This antimicrobial substance is very effective at killing Escherichia coli . Subjacent to this in the skin there is another class of antimicrobial peptides, such as RNASE7, which is effective against a broad spectrum of microorganisms, especially enterococci. Below this in the living layers of the skin are other antimicrobial peptides including the β-defensins. The antimicrobial activity of most peptides occurs as a result of unique structural characteristics that enable them to disrupt the microbial cell membrane while leaving human cell membranes intact. The antimicrobial peptides can have immunostimulatory and immunomodulatory capacities as well as being chemotactic for distinct subpopulations of leukocytes and other inflammatory cells. Some peptides have additional roles in signaling host responses through chemotactic, angiogenic, growth factor and immunosuppressive activity. These peptides are known as alarmins. Alarmins may also stimulate parts of the host defense system, such as barrier repair and recruitment of inflammatory cells.
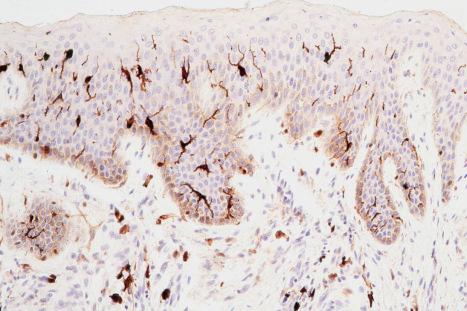
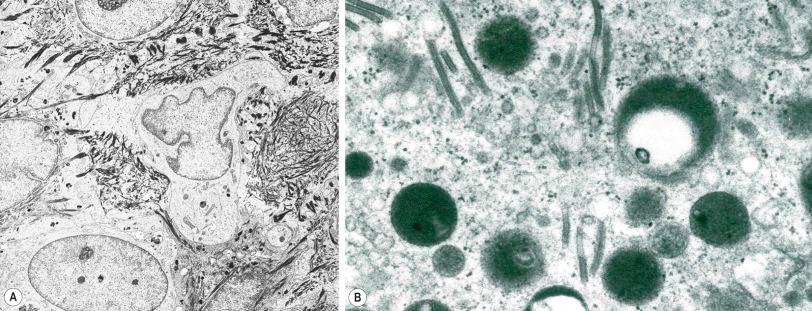
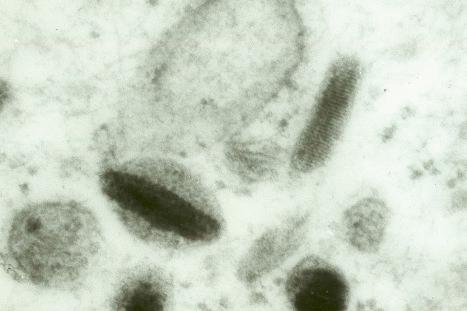
Skin immunity is also provided by a distinct population of antigen presenting cells in the epidermis known as Langerhans cells ( Fig. 1.33 ). These are dendritic cells that were first described by Langerhans, who demonstrated their existence in human epidermis by staining with gold chloride. Without stimulation, Langerhans cells exhibit a unique motion termed ‘Dendrite Surveillance Extension And Retraction Cycling Habitude (dSEARCH)’. This is characterized by rhythmic extension and retraction of dendritic processes between intercellular spaces. When exposed to antigen, there is greater dSEARCH motion and also direct cell-to-cell contact between adjacent Langerhans cells which function as intraepidermal macrophages, phagocytosing antigens among keratinocytes. Langerhans cells can be found throughout the epidermis, extending superficially to within the granular cell layer. Stimulated Langerhans cells then leave the epidermis and migrate via lymphatics to regional lymph nodes. In the paracortical region of lymph nodes the Langerhans cell expresses protein on its surface to present to a T lymphocyte that can then undergo clonal proliferation. Langerhans cells, in combination with macrophages and dermal dendrocytes, represent the skin's mononuclear phagocyte system. By electron microscopy, Langerhans cells have a lobulated nucleus, a relatively clear cytoplasm and well-developed endoplasmic reticulum, Golgi complex and lysosomes. They also possess characteristic granules which are rod or racquet-shaped ( Fig. 1.34 ). These ‘Birbeck’ granules represent subdomains of the endosomal recycling compartment and form at sites where the protein Langerin accumulates.
Besides antigen detection and the processing role by epidermal Langerhans cells, cutaneous immune surveillance is also carried out in the dermis by an array of macrophages, T cells and dendritic cells. These immune sentinel and effector cells can provide rapid and efficient immunologic back-up to restore tissue homeostasis if the epidermis is breached. The dermis contains a very large number of resident T cells. Indeed, there are approximately 2 × 10 10 resident T cells, which is twice the number of T cells in the circulating blood. Dermal dendritic cells may also have potent antigen-presenting capacities or the potential to develop into CD1a-positive and Langerin-positive cells. Dermal immune sentinels are capable of acquiring an antigen-presenting mode, a migratory mode or a tissue resident phagocytic mode.
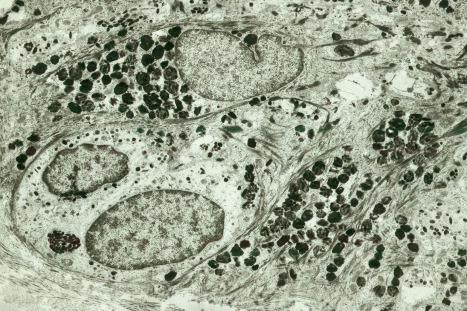
Melanocytes are pigment-producing cells and are found in the skin, inner ear, choroid and iris of the eye. In skin, melanocytes are located in the basal keratinocyte layer. The ratio of melanocytes to basal cells ranges from approximately 1 : 4 on the cheek to 1 : 10 on the limbs. They appear as vacuolated cells in hematoxylin and eosin stained sections ( Fig. 1.35 ). Ultrastructurally, melanocytes have pale cytoplasm and are devoid of tonofilaments, hemidesmosomes, and desmosomes ( Fig. 1.36 ). They are easily recognized by their specific cytoplasmic organelles (melanosomes) which are derived from the smooth endoplasmic reticulum. Melanosomes are believed to represent a specialized variant of lysosome ( Fig. 1.37 ). The function of melanocytes is the production of melanin, a complex of pigmented proteins that vary in color from yellow to red to brown or black and accounts for the various skin colors within and among races. Melanin protects the mitotically active basal epidermal cells from the injurious effects of ultraviolet light, which accounts for individuals with less pigmentation (fair-haired and light-skinned) having a much greater risk of sunburn and developing cutaneous malignancies (squamous cell and basal cell carcinomas, and melanoma). The mechanism involves absorbing or scattering ultraviolet radiation and/or its photoproducts. Other functions of melanin include control of vitamin D 3 synthesis and local thermoregulation.
In skin and hair, two forms of melanin pigment are produced; eumelanin and pheomelanin. Eumelanin is a brown or black pigment and is synthesized from tyrosine; it is particularly found in dark-colored races, whereas, pheomelanin has a yellow-red color and is synthesized from tyrosine and cysteine; it predominates in Caucasian skin.
Melanocytes also possess melanocyte-specific receptors including melanocortin-1 (MC1R) and melatonin receptors. The activation or the inhibition of melanocyte-specific receptors can augment normal melanocyte function, skin color, and photoprotection. Moreover, receptor polymorphisms are known to underlie red hair phenotypes, as well as skin pallor or freckles (ephelides). Hair graying reflects abnormalities in melanocyte signaling. Notably, Notch transcription factor signaling in melanocytes is essential for the maintenance of proper hair pigmentation, including regeneration of the melanocyte population during hair follicle cycling.
Melanin is transferred from melanocytes in melanosomes to neighboring keratinocytes in the epidermis and into the growing shaft in hair follicles and can be identified by silver techniques such as the Masson-Fontana reaction ( Fig. 1.38 ). Transport occurs along the dendritic processes of the melanocytes and the melanosomes are engulfed as membrane-bound (lysosomal) single or compound melanosomes by a group of adjacent largely basally located keratinocytes (epidermal melanin unit) where they are typically seen in an umbrella-like distribution over the outer aspect of the nucleus ( Fig. 1.39 ). A compound melanosome typically contains from three to six single melanosomes. In heavily pigmented skin and dark hair, melanosomes remain solitary and are longer than those seen in melanogenesis in paler races. Other cells that may contain compound melanosomes include macrophages (melanophages), melanoma cells and, occasionally, Langerhans cells, the other type of epidermal dendritic cell. Macromelanosomes (giant melanosomes) measure several microns in diameter and therefore are readily visible in hematoxylin and eosin stained sections ( Fig. 1.40 ). They may be encountered in normal skin, in lentigines, dysplastic nevi, Spitz nevi, in the café-au-lait macules of neurofibromatosis and in albinism. A key protein involved in melanosome assembly is NCKX5, encoded by the gene SLC24A5 . Loss of expression of this gene in mice results in marked changes in skin color with loss of pigment. Mature melanosomes of eumelanin are ellipsoidal in shape, while pheomelanin-producing melanosomes are spherical.
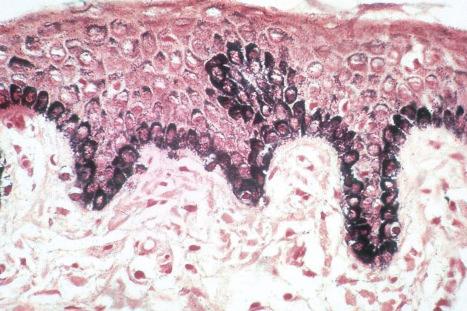
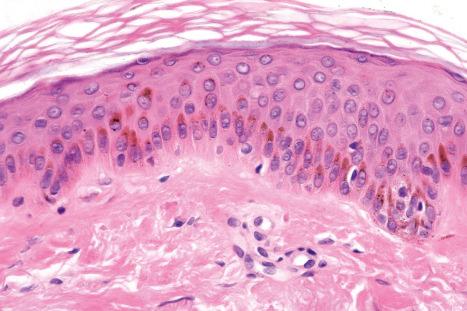
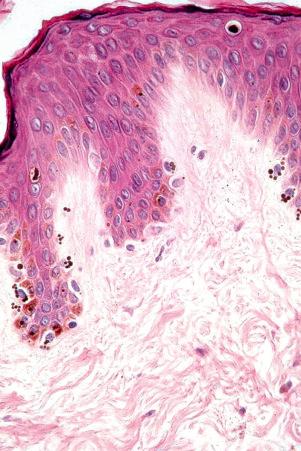
Merkel cells are postmitotic cells scattered throughout the epidermis of vertebrates and constitute 0.2–0.5% of epidermal cells. Merkel cells represent part of the affector limb in cutaneous slowly adapting type-1 (SA1) mechanoreceptors and are therefore particularly concerned with touch sensation. They are located amongst basal keratinocytes and are mainly found in hairy skin, tactile areas of glabrous skin, taste buds, the anal canal, labial epithelium and eccrine sweat glands. In glabrous skin, the density of Merkel cells is ≈50 per mm 2 . Sun-exposed skin may contain twice as many Merkel cells as non-sun-exposed skin. Numerous Merkel cells can be found in actinic keratoses. Merkel cells cannot be recognized in conventional hematoxylin and eosin stained sections. Rather, immunohistochemistry, particularly using antikeratin antibodies (e.g., to keratin 20), or electron microscopy, is necessary for their identification ( Figs 1.41 and 1.42 ).
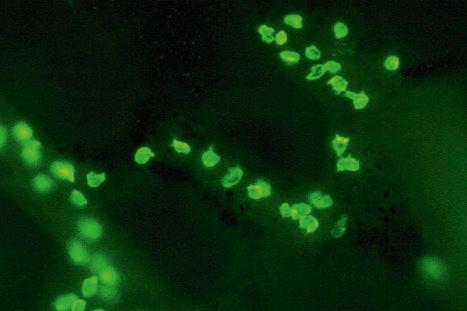
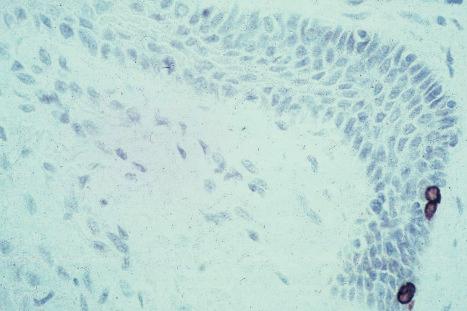
Ultrastructurally, Merkel cells appear oval with a long axis of ≈15 µm orientated parallel to the basement membrane ( Fig. 1.43 ). They also have a large bilobed nucleus and clear cytoplasm which reflects a relative scarcity of intracellular organelles. Merkel cells contain numerous neurosecretory granules, each 50 nm to 160 nm across; these are found opposing the junctions with the sensory nerve ending ( Fig. 1.44 ). Merkel cells contain keratin filaments, particularly keratin filament types 8, 18, 19, and 20, which are characteristic of simple epithelium and fetal epidermis. Immunocytochemically, Merkel cells also express neuropeptides including synaptophysin, vasoactive intestinal peptide (VIP) and calcitonin gene-related polypeptide (CGRP). They contain neuron-specific proteins including neuron-specific enolase (NSE) and protein gene product (PGP) 9.5. In addition, Merkel cells express desmosomal proteins, membranous neural cell adhesion molecule and nerve growth factor receptor. Merkel cells show a positive uranaffin reaction. Merkel cells form close connections with sensory nerve endings and secrete or express a number of these peptides. The close contact between Merkel cells and nerve fibers represents a Merkel cell–neurite complex, but there is no clear evidence of synaptic transmission, although numerous vesicles can be identified in neurons apposed to Merkel cells.
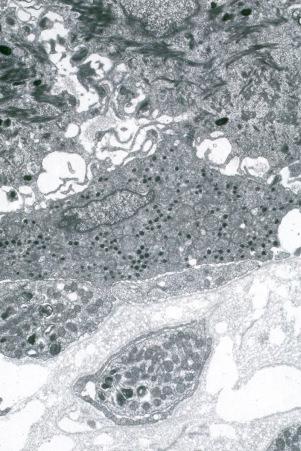
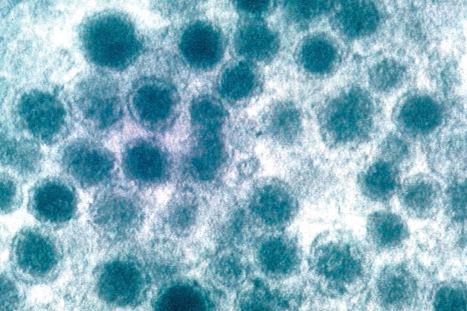
Human skin contains an extensive neural network that contains cholinergic and adrenergic nerves and myelinated and unmyelinated sensory fibers. Moreover, the skin also contains several transducers involved in the perception of touch, pressure, and vibration, including Ruffini organs surrounding hair follicles, Meissner corpuscles, Vater–Pacini corpuscles located in the deep layer of the dermis, and nerve endings which pass through the epidermal basement membrane. Some of these contain Merkel cells which form the Merkel cell–neurite complex, while others are free nerve endings. The cell bodies for all these neurons reside in the dorsal root ganglion. The Merkel cell–neurite complexes are thought to serve as mechanoreceptors and to be responsible for the sensation of touch via Piezo2-dependent transmission channels. They are clustered near unmyelinated sensory nerve endings, where they group and form ‘touch spots’ at the bottom of rete ridges. These complexes are also known as hair discs, touch domes, touch corpuscles, or Iggo discs. The complex is innervated by a single, slowly adapting type 1 nerve fiber. In hairy skin, Merkel cells also cluster in the rete ridges and in the ORS of the hair follicle where the arrector pili muscles attach. The function of Merkel cells in hair follicles is unclear, although they may be involved in the induction of new anagen cycles.
There are two hypotheses for the origin of Merkel cells: one possibility is that they differentiate from epidermal keratinocyte-like cells and the other is that they arise from stem cells of neural crest origin that migrated during embryogenesis, in similar fashion to melanocytes. Merkel cell hyperplasia is a common histologic finding and may accompany keratinocyte hyperproliferation as well as being frequently seen in adnexal tumors such as nevus sebaceus, trichoblastomas, trichoepitheliomas, and nodular hidradenomas. Merkel cell hyperplasia is associated with hyperplasia of nerve endings that occurs in neurofibromas, neurilemomas, nodular prurigo, or neurodermatitis.
Desmosomes are the major intercellular adhesion complexes in the epidermis. They anchor keratin intermediate filaments to the cell membrane and link adjacent keratinocytes ( Fig. 1.45 ). Desmosomes are found in the epidermis, myocardium, meninges and cortex of lymph nodes. Ultrastructurally, desmosomes contain plaques of electron-dense material running along the cytoplasm parallel to the junctional region, in which three bands can be distinguished: an electron-dense band next to the plasma membrane, a less dense band, and then a fibrillar area ( Fig. 1.46 ). Identical components are present on opposing cells which are separated by an intercellular space of 30 nm within which there is an electron-dense midline. There are three main protein components of desmosomes in the epidermis: the desmosomal cadherins, the armadillo family of nuclear and junctional proteins, and the plakins ( Fig. 1.47 ). The transmembranous cadherins comprise mostly heterophilic associations of desmogleins and desmocollins. There are four main epidermis-specific desmogleins (Dsg1–4) and three desmocollins (Dsc1–3). These show differentiation-specific expression. For example, Dsg1 and Dsc1 are found predominantly in the superficial layers of the epidermis whereas Dsg3 and Dsc3 show greater expression in basal keratinocytes. The intracellular parts of the cadherins interact with the keratin filament network via the desmosomal plaque proteins, mainly desmoplakin, plakoglobin and plakophilin.
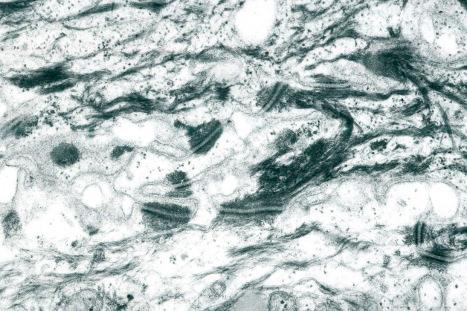

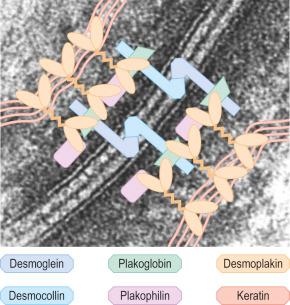
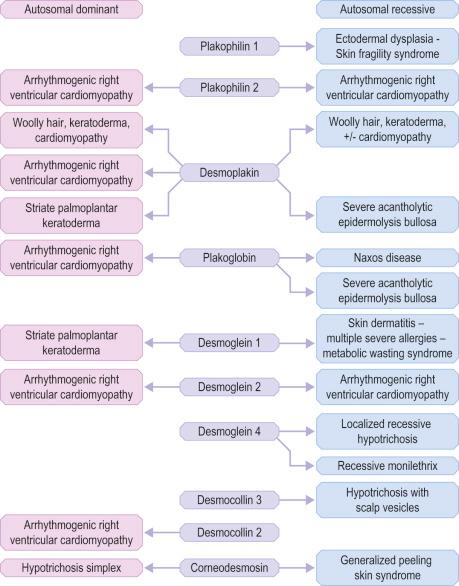
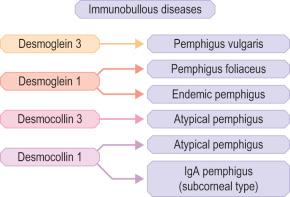
Clues to the biologic function of these desmosomal components have arisen from various inherited and acquired human diseases. Naturally occurring human mutations have been reported in ten different desmosome genes with variable skin, hair and heart abnormalities and several desmosomal proteins serve as autoantigens in immunobullous blistering skin diseases such as pemphigus ( Figs 1.48 and 1.49 ). Antibodies to multiple desmosomal proteins may develop in diseases such as paraneoplastic pemphigus through the phenomenon of epitope spreading. Cleavage of the extracellular domain of Dsg1 has also been demonstrated as the basis of staphylococcal scalded skin syndrome and bullous impetigo.
Adherens junctions are recognized ultrastructurally as electron-dense transmembrane structures, with two opposing membranes separated by approximately 20 nm, that form links with the actin skeleton. They are 0.2–0.5 µm in diameter and can be found as isolated cell junctions or in association with tight junctions and desmosomes. Adherens junctions are expressed early in skin development and contribute to epithelial assembly, adhesion, barrier formation, cell motility and changes in cell shape. They may also spatially coordinate signaling molecules and polarity cues as well as serving as docking sites for vesicle release. Adherens junctions contain two basic adhesive units: the nectin-afadin complex and the classical cadherin complex. The nectins form a structural link to the actin cytoskeleton via afadin (also known as AF-6) and may be important in the initial formation of adherens junctions. The cadherins form a complex with the catenins (α-, β-, and p120 catenin) and help mediate adhesion and signaling. Cell signaling via β-catenin can activate several pathways linked to morphogenesis and cell fate determination.
Inherited gene mutations of the adherens junction proteins plakoglobin and P-cadherin have been reported. Plakoglobin mutations result in Naxos disease (woolly hair, keratoderma, cardiomyopathy). P-cadherin mutations underlie autosomal recessive hypotrichosis with juvenile macular dystrophy as well as ectodermal dysplasia-ectrodactyly-macular dystrophy (EEM) syndrome, in which there is hypotrichosis, macular degeneration, hypodontia and limb defects, including ectrodactyly, syndactyly and camptodactyly.
Gap junctions represent clusters of intercellular channels, known as connexons, which form connections between the cytoplasm of adjacent keratinocytes (and other cells). Formation of a connexon involves assembly of six connexin subunits within the Golgi network. This complex is then transported to the plasma membrane where connexons associate with other connexons (homotypic or heterotypic) to form a gap junction. To date, 13 different human connexins have been described. The formation and stability of gap junctions can be regulated by protein kinase C, Src kinase, calcium concentration, calmodulin, adenosine 3′,5′-cyclic monophosphate (cAMP) and local pH. The connexins are classified into three groups (α, β and γ) according to their gene structure, overall gene homology and specific sequence motifs. Apart from the connexins, vertebrates also contain another class of gap junction proteins, the pannexins, which are related to the innexins found in nonchordate animals. The function of gap junctions is to allow sharing of low molecular mass metabolites (< 1000 Da) and exchange of ions between neighboring cells. Gap junction communication is essential for cell synchronization, differentiation, cell growth and metabolic coordination of avascular organs, including epidermis.
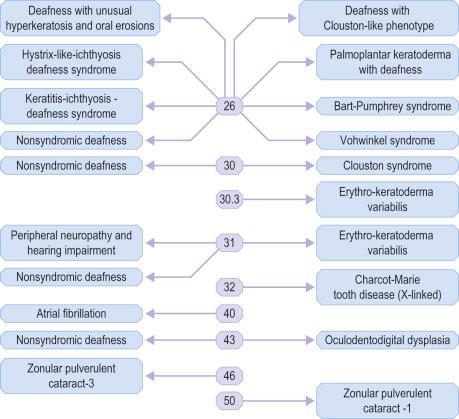
Inherited abnormalities in genes encoding four different connexins (Cx26, 30, 30.3 and 31) have been detected in several forms of keratoderma and/or hearing loss ( Fig. 1.50 ). Nondermatologic disorders can also arise from mutations in some higher molecular weight connexins (Cx32, 40, 43, 46 and 50).
Tight junctions contribute to skin barrier integrity and maintaining cell polarity, although in simple epithelia they are major regulators of permeability. An important function is to regulate the paracellular flux of water-soluble molecules between adjacent cells. The main structural proteins of tight junctions are the claudins, of which there are approximately 24 subtypes, as well as the IgG-like family of junctional adhesion molecules (JAMs) and the occludin group of proteins. The principal claudins in the epidermis are claudin 1 and 4. These transmembranous proteins can bind to the intracellular zonula occudens proteins ZO-1, ZO-2, ZO-3 which interact with the actin cytoskeleton.
Clinically, abnormalities in tight junction proteins can result in skin, kidney, ear and liver disease. Inherited gene mutations in claudin 1 have been reported in a few pedigrees with diffuse ichthyosis, hypotrichosis, scarring alopecia and sclerosing cholangitis.
There are four classes of pilosebaceous unit: terminal on the scalp and beard; apopilosebaceous in axilla and groin; vellus on the majority of skin; and sebaceous on the chest, back and face. The dermal papilla is located at the base of the hair follicle and is associated with a rich extracellular matrix. Around the papilla are germinative (matrix) cells that have a very high rate of division, and give rise to spindle-shaped central cortex cells of the hair fiber, and the single outer layer of flattened overlapping cuticle cells. A central medulla is seen in some hairs, with regularly stacked condensed cells interspersed with air spaces or low-density cores. The cortical cells are filled with keratin intermediate filaments orientated along the long axis of the cell, interspersed with a dense interfilamentous protein matrix. The cuticular cells are morphologically distinct, with flattened outward-facing cells, with three layers inside the cuticle of condensed, flattened protein granules: endocuticle, exocuticle and ‘a’ layer. Around the cuticle is the IRS, which is composed of three distinct layers of cells that undergo keratinization: the IRS cuticle, the Huxley layer and the outermost Henle layer. Differentiation in the IRS involves the development of trichohyalin granules, with 8–10 nm filaments orientated in the direction of hair growth. The IRS moves up the follicle, forming a support for the hair fiber, and degenerates above the sebaceous gland. The outermost layer is the ORS, which is continuous with the epidermis and expresses epithelial keratins, K5/K14, K1/K10 and K6/K16 in the upper ORS and K5/K14/K17 in the deeper ORS.
Normal growth of the hair fiber is 300–400 µm/day. Hair growth is generated by the high rate of proliferation of progenitor cells in the follicle bulb. There are three phases of cyclical hair growth: anagen, when growth occurs; catagen, a regressing phase; and telogen, a resting phase. The follicle re-enters anagen, and the old hair is replaced by a new one.
Immediately above the basal layer in the hair bulb, cells undergo a secondary pathway of ‘trichocyte’ or hair differentiation, and express a further complex group of keratins, the hard keratins. Two families of hair keratins, types I and II, are present in mammals, which have distinctive amino- and carboxy-terminals with high levels of cysteine residues but lack the extended glycine residues of epidermal keratins. The proteins differ from epithelial keratins in position on two-dimensional gels but form acidic and basic groups. The nomenclature for human keratins and keratin-associated proteins was updated in 2006 and 2012, respectively.
Mutations in hair keratin genes have been found to cause autosomal dominant forms of the human disease monilethrix. More common hair variants, such as curly hair, may be explained by dynamic changes during hair growth. Curvature of curly hair is programmed from the very basal area of the follicle and the bending process is linked to a lack of axial symmetry in the lower part of the bulb, affecting the connective tissue sheath, ORS, IRS and the hair shaft cuticle.
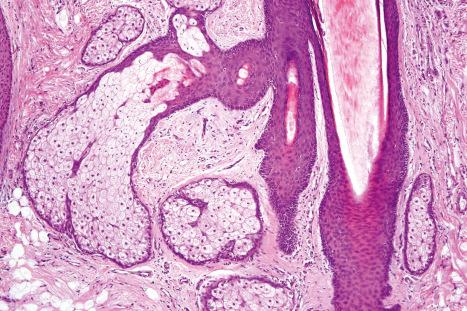
Sebaceous glands usually develop as lateral protrusions from the ORS of hair follicles, but at certain sites, such as the eyelids, lips, areolae, nipples and labia minora, they appear to arise independently and drain directly onto the skin's surface ( Figs 1.51 and 1.52 ). They are widespread in distribution, being found everywhere on the body except on the palms and soles. They are particularly abundant on the face and scalp, in the midline of the back and about the perineum, and are concentrated around the orifices of the body ( Fig. 1.53 ). Those of the eyelid are known as the glands of Zeis and the meibomian glands. Sebaceous glands within the areolae are known as Montgomery tubercles. The largest sebaceous glands are associated with small vellus hairs in specialized pilosebaceous units known as sebaceous follicles (facial pores).
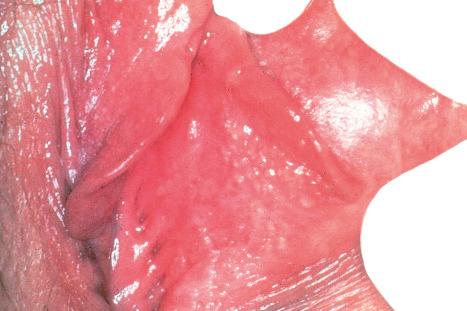
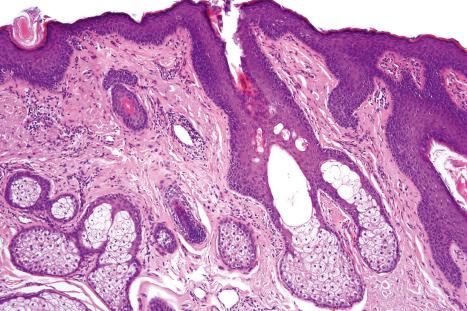
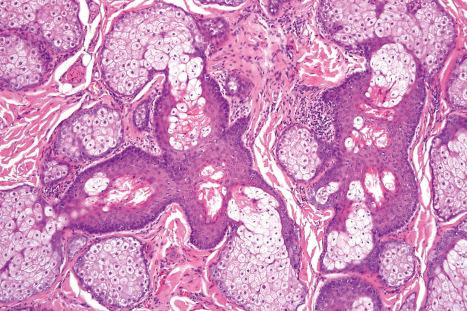
Sebaceous glands consist of several lipid-containing lobules, usually connected to a hair follicle ( Fig. 1.54 ). Each lobule is composed of an outer layer of small cuboidal or flattened basophilic germinative cells, from which arises the inner zone of lipid-laden vacuolated cells with characteristic crenated nuclei ( Fig. 1.55 ). The secretions drain into the sebaceous duct, which joins the hair follicle at the level of the infundibulum ( Fig. 1.56 ). The duct is lined by keratinizing stratified squamous epithelium and is continuous with the external root sheath. The glands are holocrine because their secretions depend on complete degeneration of the acini, with release of all the cells' lipid contents to become sebum.
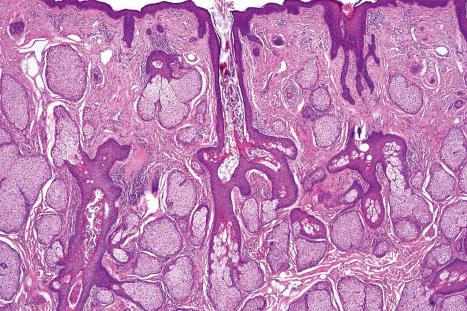
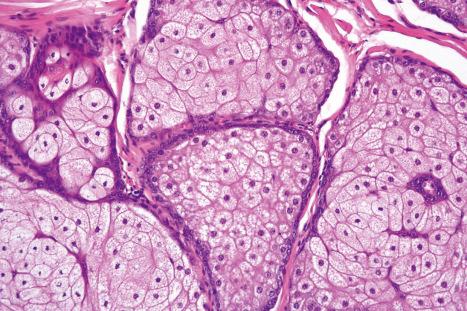
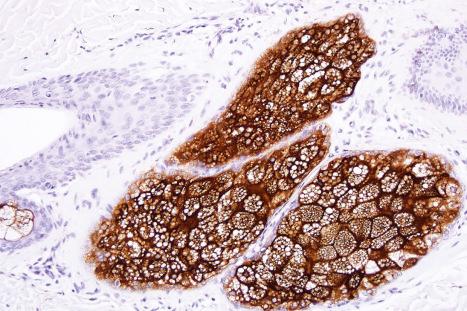
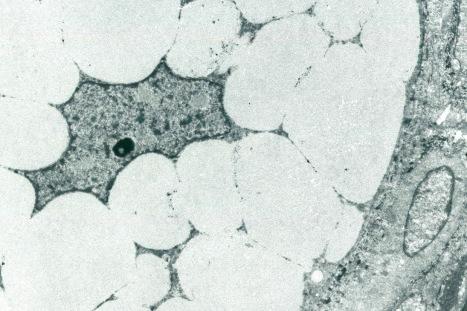
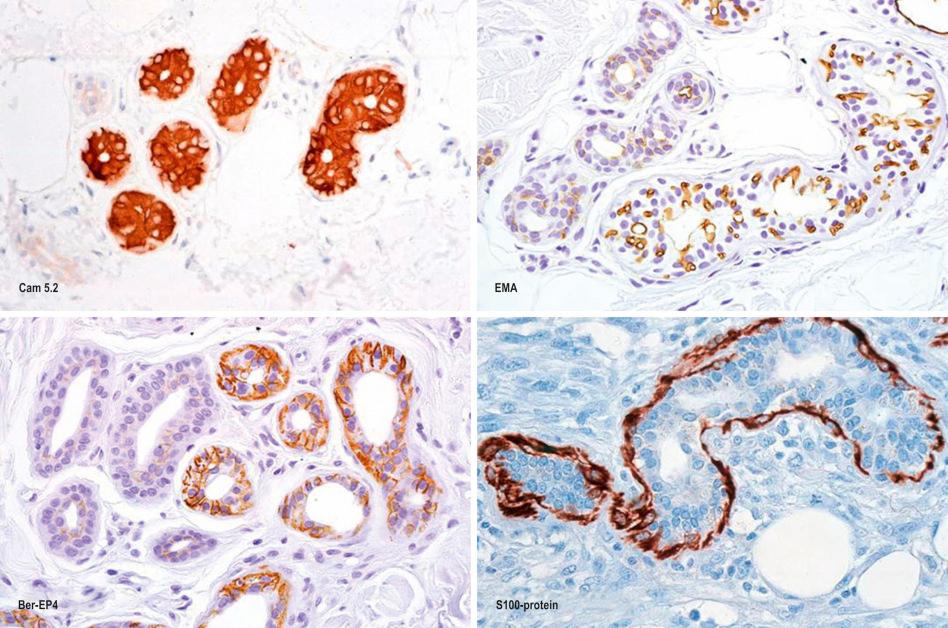
Immunohistochemically, the sebaceous cells label strongly for EMA but they do not express CEA or low molecular weight keratin (CAM 5.2) or S100 protein ( Fig. 1.57 ). Ultrastructurally, the mature sebaceous gland shows gradual accumulation of variably sized, nonmembrane-bound, lipid inclusions in differentiating cells. Numerous mitochondria, ribosomes and membrane-bound vesicles may also be evident. As the cells mature before their disintegration, the lipid droplets completely fill the cytoplasm and compress the centrally located nucleus ( Fig. 1.58 ).
The secretion of sebaceous glands is sebum, an exceedingly complicated lipid mixture that includes triglycerides (57%), wax esters (26%) and squalene (12%). Its function includes waterproofing, control of epidermal water loss, and a protective function, inhibiting the growth of fungi and bacteria. Secreted sebum undergoes significant changes due to the presence of Propionibacterium acnes (triglyceride hydrolysis) within the pilosebaceous canal and Staphylococcus epidermidis (cholesterol ester formation) on the perifollicular skin. Skin surface lipid is composed of a mixture of sebum and epidermal lipids.
Human sweat glands are generally divided into two types: eccrine and apocrine. The eccrine gland is the primary gland responsible for thermoregulatory sweating in humans. Eccrine sweat glands are distributed over nearly the entire body surface. The number of sweat glands in humans varies greatly, ranging from 1.6 to 4.0 million.
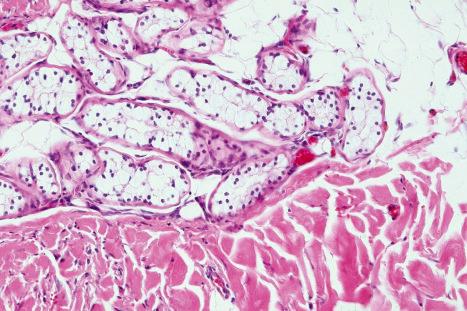
Histologically, eccrine sweat glands are divided into four subunits: a highly vascularized coiled secretory gland, a coiled dermal duct, a straight dermal duct, and a coiled intraepidermal duct (the acrosyringium) ( Fig. 1.59 ). The secretory coil is located in the lower dermis, and the duct extends through the dermis and opens directly onto the skin surface ( Figs 1.60 , 1.61 ). The active sweat glands are present most densely on the sole, forehead and palm, somewhat less on the back of the hand, still less on the lumbar region, and the lateral and extensor surfaces of the extremities, and least on the trunk and the flexor and medial surfaces of the extremities. The uncoiled dimension of the secretory portion of the gland is approximately 30–50 µm in diameter and 2–5 mm in length. The size of the adult secretory coil ranges 1–8 × 10 –3 mm 3 . The secretory component lies in the lower reaches of the reticular dermis or around the interface between the dermis and subcutaneous fat and is surrounded by a thick basement membrane and loose connective tissue often rich in mucin. It embodies an outer discontinuous layer of contractile myoepithelial cells and an inner layer of secretory cells comprising two cell types: large clear pyramidal cells, which appear to be responsible for water secretion, and smaller, darkly staining mucopolysaccharide-containing cells (probably secreting a glycoprotein), which are much less commonly seen. Between adjacent cells are canaliculi, which open into the lumen of the tubule (see below). Sometimes the secretory lobules show striking clear cell change due to glycogen accumulation ( Fig. 1.62 ). The myoepithelial cells contract in response to cholinergic stimuli. They have spindled cell morphology and are distributed in a spiral, parallel array along the long axis of the secretory tubule. On the basis of their expression of keratin filaments, they appear to be of ectodermal rather than mesenchymal derivation. They do not label for vimentin. Myoepithelial cells therefore develop from the epithelial cells of the tip of the secretory coil and not, as might be expected, from adjacent mesenchymal cells. The dermal duct components consist of a double layer of cuboidal basophilic cells. The duct is not merely a conduit, but has a biologically active function, modifying the composition of eccrine secretion and, particularly, the reabsorption of water. The intraepidermal portion of the sweat duct opens directly onto the surface of the skin. A myoepithelial layer is absent.
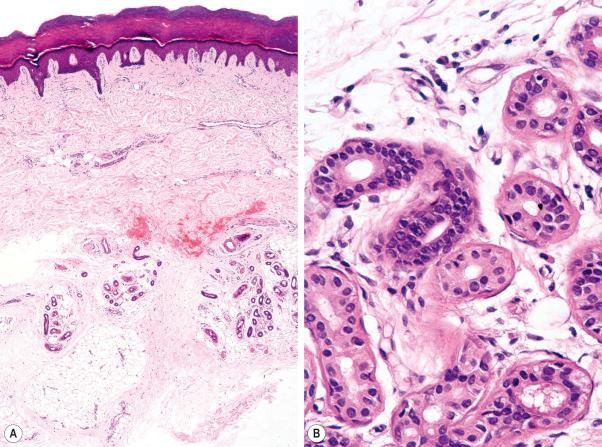
The secretory unit is strongly labeled by CAM 5.2 (both cytoplasmic and membranous) and Ber-EP4 and there is luminal accentuation ( Fig. 1.63 ). The ductal component is completely negative. EMA can be detected along the luminal aspect of the secretory unit and outlining the intercellular canaliculi. It is also present around the luminal border of the duct, and is often present in large quantities within the lumen. CEA is present in a similar distribution to EMA although secretory labeling tends to be rather focal and somewhat weaker while the ductal lumen is more strongly outlined. The myoepithelial cells can be identified by antibodies to S100 protein, desmin and smooth muscle actin. The eccrine glands show strong activity for the enzymes amylophosphorylase, leucine aminopeptidase, succinic dehydrogenase and cytochrome oxidase. Weak or no activity is seen for Nicotinamide adenine dinucleotide (NADH) diaphorase, esterase and acid phosphatase.
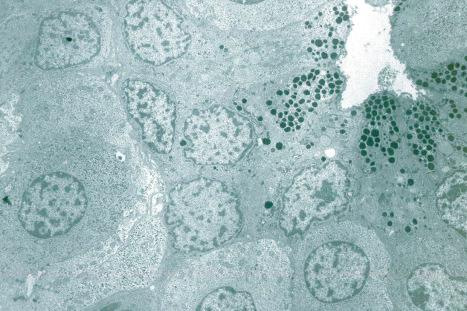
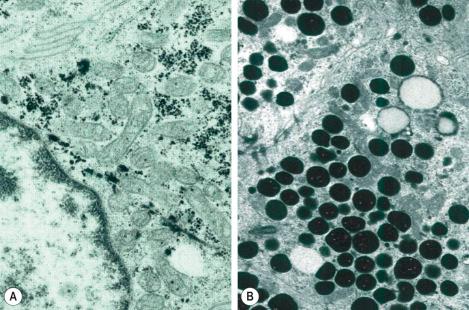
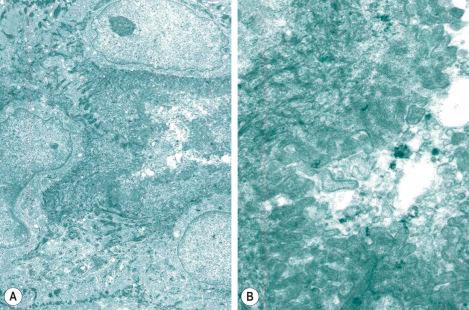
With electron microscopy, the serous cells are characterized by abundant intracytoplasmic glycogen granules and numerous mitochondria ( Figs 1.64 , 1.65 ). Adjacent cell membranes, which show marked interdigitations, may separate to form microvilli-lined intercellular canaliculi. The mucous cells contain numerous electron-dense lipid droplets and lysozymes. Myoepithelial cells are present at the periphery of the secretory coil within the eccrine basal lamina (lamina densa) and contain abundant myofilaments with characteristic dense bodies. The sweat duct lumen is bordered by conspicuous microvilli ( Fig. 1.66 ). The cytoplasm contains numerous clear vesicles. Tonofilaments are characteristically orientated in a circumferential manner deep to the plasma membrane, the so-called cuticle of light microscopy. This is particularly well developed in the acrosyringium.
Human perspiration is classified into two types: insensible perspiration and active sweating. Insensible perspiration involves water loss from the respiratory passages, the skin, and gaseous exchanges in the lungs. Heat, exercise and carbon dioxide can all induce active sweating in human beings. Active sweating may be classified into two types: thermal and mental/emotional. Thermal sweating plays an important role in keeping the body's temperature constant and involves the whole body surface. The secretory nerve fibers innervated in human sweat glands are sympathetic, which appear to be cholinergic in character as sweating is produced by pilocarpine and stopped by atropine. VIP coexisting in the cholinergic nerve fibers has been suggested as a candidate neurotransmitter that may control the blood circulation of the sweat glands. Acetylcholine is the primary neurotransmitter released from cholinergic sudomotor nerves and binds to muscarinic receptors on the eccrine sweat gland, although sweating can also occur via exogenous administration of α- or β-adrenergic agonists. The initial fluid released from the secretory cells is isotonic and similar to plasma although it is devoid of proteins. As the fluid travels up the duct towards the surface of the skin, sodium and chloride are reabsorbed, resulting in sweat on the surface being hypotonic relative to plasma. When the rate of sweat production increases, however, for example during exercise, ion reabsorption mechanisms can be overwhelmed due to the large quantity of sweat secreted into the duct, resulting in higher ion losses. The sodium content in sweat on the skin's surface, therefore, is greatly influenced by sweat rate.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here