Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Under normal conditions, the red blood cell (RBC) mass in humans is tightly controlled and remains relatively constant in a given individual. The numbers of senescent RBCs lost daily are replaced by newly formed ones by a carefully controlled network of growth factors, progenitor and precursor cells. Erythropoiesis can be augmented by a variety of stimuli that increase the delivery of oxygen to tissues. This delicate balance can be disturbed by various pathologic conditions and can result in either reduced numbers of RBCs (anemia) or excessive numbers of RBCs (polycythemia). Hematocrit values over 49% in males and 48% in females are abnormal and require further evaluation to determine if the patient has an absolute increase in their RBC mass and if investigation of its cause should be pursued. The RBC mass is increased if it is greater than 125% above that expected for sex and body mass. The measurement of the RBC mass is a diagnostic study that is now available at a dwindling number of tertiary care centers, making other diagnostic studies pivotal in evaluating patients with elevated hematocrit levels. Polycythemic states can be due to a variety of disorders that can be attributed to several pathophysiologic mechanisms. Determination of the etiology of an individual's polycythemia is a critical step in defining the patient’s appropriate prognosis and treatment plan. Primary polycythemias are the result of innate abnormalities involving hematopoietic progenitors and stem cells that lead to constitutive overproduction of RBCs, accompanied by low erythropoietin (EPO) levels. By contrast, secondary polycythemias are the consequence of a number of conditions that lead to increased EPO production, which acts on normal progenitors to overproduce RBCs. In a small number of patients, the cause of erythrocytosis cannot be determined; these patients are classified as having idiopathic erythrocytosis.
RBC production can be influenced by numerous factors, including nutrients, growth factors, numbers and function of bone marrow (BM) progenitor and precursor cells, and cellular receptors and transcription factors ( Chapter 27 ). EPO is considered to be the physiologic regulator of the terminal phases of erythropoiesis. Alterations in its production are followed by adjustments in the rate of formation of RBCs. In humans, EPO production is controlled by the relative supply of oxygen to the kidneys, the major site of EPO production. In states of severe hypoxia, EPO production can be increased up to 1000-fold. In a healthy person after phlebotomy, EPO production increases, and an inverse logarithmic relationship between hematocrit and EPO production rates exists. Patients with secondary erythrocytosis caused by chronic hypoxia have either normal or increased basal EPO values, but they also have increased values after reduction of the hematocrit to normal levels by phlebotomy. By contrast, EPO excretion is invariably subnormal in patients with polycythemia vera (PV), which demonstrates that this disorder is not a result of excessive EPO production.
Under normal conditions, EPO production is mediated by a reduced oxygen content, termed hypoxemia , which leads to decreased oxygen delivery to tissues ( Chapter 27 ). Regulation of oxygen homeostasis is critical to survival. In humans, oxygen sensing occurs at many levels, leading to both acute and chronic adaptation. The acute reduction of the availability of oxygen leads to the initiation of a cascade of adaptive events that sets in place compensatory events to correct the lack of oxygen supply. Low oxygen levels, or hypoxia (60 mm Hg), in humans causes oxygen-sensing chemosensory cells to undergo rapid membrane depolarization within seconds, leading to the production of action potentials, influx of calcium ions, and release of the neurotransmitters that result in stimulation of the brain stem that controls the respiratory and cardiovascular systems. These chemosensory cells are found within the glomus cells of the carotid body located at the bifurcation of the internal and external carotid arteries. The released neurotransmitters activate the nerve endings of the carotid body sensory nerve to convey to the CNS signals that command ventilation to fight hypoxia, resulting in an increase of the ventilation rate and restoration of normal oxygen tension to vital organs. In addition, there are changes in blood pressure and heart rate to maximize oxygen delivery. The carotid body is the organ with the greatest blood flow within the body. Activation of the carotid body results in the sensation of breathlessness experienced by individuals at high altitudes. During chronic hypoxia when the carotid body is permanently active, there is marked enlargement of the carotid body because of an increase in capillaries and a marked reduction in the mean distance from the capillaries to the edge of the chemoreceptor cells.
In response to chronic hypoxia, multiple compensatory mechanisms come into play over several days within the kidneys, the major site of EPO production. Lack of oxygen activates the cellular signaling pathway of the hypoxia-inducible transcription factors (HIF) that has three isoforms, hypoxia-inducible factor (HIF)-1, HIF-2, HIF-3, regulating expression of several thousand genes. Within the kidney, specialized interstitial cells are the source of EPO. These cells employ the HIF pathway, a pathway that is otherwise present in essentially all cells of the body, to regulate EPO production in an oxygen-sensitive manner. Hypoxic stimulation results in production of HIF-1/HIF-2, the major factors responsible for transcriptional activation of the EPO gene. The HIF transcriptional system is a master regulator of the hypoxic response controlling a large number of genes in multiple cell types. HIF-1 is a heterodimeric protein consisting of HIF-1/HIF-2α and HIF-1β, which is required for normal development of the heart, blood vessels, and blood cells. As the key mediator of cellular oxygen maintenance, HIF-1 facilitates body oxygen delivery and responses to oxygen deprivation by regulating the expression of gene products that are involved in cellular energy metabolism and glucose transport, angiogenesis, erythropoiesis and iron metabolism, pH regulation, apoptosis, cell proliferation, and cell–cell and cell–matrix interactions. Classic HIF target genes include phosphoglycerate kinase, glucose transporter-1, vascular endothelial growth factor (VEGF), and EPO. The HIF proteins are members of the Per–ARNT–Sim family of heterodimeric basic helix–loop–helix transcription factors ( Fig. 70.1 ).
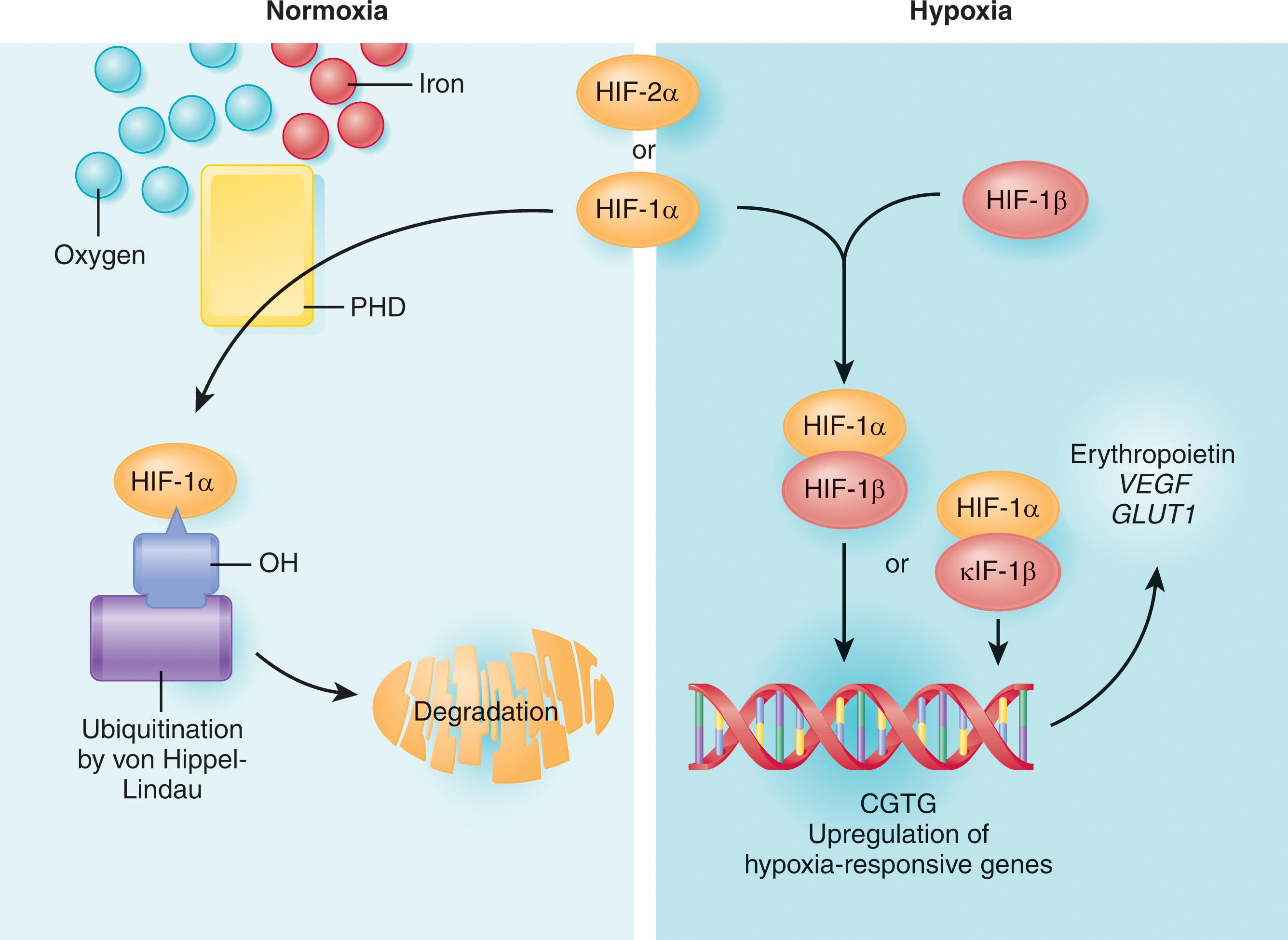
In contrast to the constitutively expressed, HIF-1β subunits, HIF-1α is an oxygen-labile protein that becomes stabilized in response to hypoxia. HIF-1α mRNA and protein levels are induced by hypoxia, and HIF-1α protein levels decay rapidly with return to normoxia. The posttranslational regulation of HIF-1α protein accounts for the majority of the regulation of this gene. Normoxia-induced, ubiquitin-mediated degradation of the HIF-1α protein is the major regulator of HIF-1α levels, thereby reducing the stimulus for additional EPO production. The targeting and subsequent polyubiquitination of HIF-1α require the von Hippel-Lindau (VHL) protein, oxygen, and three different iron-requiring proline hydroxylase (PHD) enzymes (see Fig. 70.1 ). The PHD proteins exist in three isoforms, PHD1, PHD2, and PHD3. HIF-1α is hydroxylated by all three isoforms but primarily by PHD2. The prolyl hydroxylation of HIF-1α is necessary for the binding of HIF-1α to VHL, which is the substrate-recognition subunit of an E3 ubiquitin-protein ligase. Different parts of the HIF-1α chains have different functions. The N-terminus part of HIF-1α is involved in DNA binding and dimerization, and the C-terminus portion has regulatory functions. One domain at the C-terminus influences transcriptional activity without affecting HIF-1α protein levels, and the other region, termed the oxygen-dependent degradation domain (ODD), affects protein abundance. The VHL protein physically interacts with the ODD of HIF-1α, targeting it for ubiquitination and destruction by the proteasome. Iron-chelating drugs can also block the interaction of HIF-1α with the VHL protein, suggesting a role for iron in the degradation of HIF-1α.
Under normoxic conditions, hydroxylation of HIF-1α/HIF-2α is essential for HIF proteolytic degradation by promoting interaction with the VHL tumor-suppressor protein through hydrogen bonding to the hydroxy proline-binding pocket in the VHL-β domain. As oxygen levels decrease, hydroxylation of HIF decreases, and HIF-1α/HIF-2α then no longer binds VHL. As a result, HIF-1α/HIF-2α become stabilized, dimerize with HIF-1β, and activate transcription of target genes. The activity of PHDs depends on the availability of molecular oxygen, which qualifies these enzymes as oxygen sensors. In addition, these dioxygenases require 2-oxyglutarate which acts as a cosubstrate with vitamin C to keep nonheme iron in the ferrous state. Although PHD-2 appears to be the hydroxylase that is essential for HIF-1α/HIF-2α degradation under normoxic conditions, PHD-3 is important for hydroxylation of HIF-1α/ HIF-2α during reoxygenation. Different effects of individual PHDs on HIF-1α and HIF-2α hydroxylation indicate that the stability of individual HIF-1α subunits and their target gene expression might be affected by tissue- and cell-type differences in PHD expression and activity levels. The activity of PHDs can be modulated by mitochondrial reactive oxygen species, implicating mitochondria in oxygen sensing. HIF-2α under hypoxic conditions dimerizes with HIF-1β and activates the transcription of a set of target genes that overlap with the target genes regulated by HIF-1α/HIF-1β heterodimers. HIF-1α is expressed by all nucleated cells, but HIF-2α is expressed by specific cell types, including vascular endothelial cells, renal interstitial cells, hepatocytes, cardiomyocytes, and astrocytes. HIF-2α appears to play a critical role in regulating EPO production in adult mammals and HIF-1α is important during yolk sac erythropoiesis. HIF-1 also controls the absorption and delivery of iron to the BM by repressing of hepcidin and activation of genes encoding transferrin and the transferrin receptor.
HIF3α is another HIF protein which is an oxygen-dependent transcription factor which activates a distinct transcriptional response to hypoxia. Mammalian HIF-3 genes use different promoters, different transcription initiation sites, and alternative splicing to transcribe a large number of mRNA variants. While some short HIF-3α variants act as dominant-negative regulators of HIF-1/2 actions, other HIF-3 variants can inhibit HIF-1/2 actions by competing for the common HIF-β. It is thought that HIF3 acts as a negative regulator of HIF-1 and HIF-2. Similar to HIF1 and HIF2, PHD1 , PHD2 , PHD3 , and VHL also cause HIF-3 protein degradation. Further studies of HIF-3α variants will provide new and important insights into HIF biology.
VHL syndrome is a hereditary cancer syndrome that is associated with exaggerated responses to hypoxia caused by posttranslational abnormalities in HIF. VHL syndrome is characterized by a propensity for developing clear-cell renal carcinomas, retinal hemangioblastomas, cerebellar and spinal hemangiomas, pancreatic and renal cysts, islet cell tumors of the pancreas, and pheochromocytomas. The tumors result from somatic mutations that cause a loss of heterozygosity (LOH) of the VHL gene. VHL inactivation leads to aberrant stabilization and accumulation of HIF-2α, which drives tumor cell growth. VHL disease affects approximately 1 in 35,000 individuals and is transmitted in an autosomal dominant manner. Individuals with VHL disease carry one wild-type (WT) VHL allele and one inactivated VHL allele. This inactivation can occur by somatic mutation or hypermethylation. Tumor or cyst development is linked to somatic inactivation or loss of the remaining WT VHL allele. Approximately 20% to 37% of VHL patients have large or partial germ-line deletions, 23% to 27% have nonsense or frame-shift mutations, and 30% to 35% have missense mutations. More than 150 different VHL mutations linked to VHL disease have been reported. The tumors linked to VHL inactivation are often highly vascular and can produce angiogenic factors such as VEGF. In addition, renal cell carcinoma, cerebellar hemangioblastomas, and pheochromocytomas have been associated with paraneoplastic erythrocytosis caused by overproduction of EPO. Overproduction of HIF-inducible mRNAs is the hallmark of VHL protein defective cells. Genotype–phenotype correlates in VHL disease suggest that VHL has functions independent of HIF regulation that might play a role in tumor formation. VHL protein has other binding partners, including atypical protein kinase C and a family of deubiquitinating enzymes called VHL-interacting deubiquitinating enzymes 1 and 2. In addition, VHL has been involved in numerous cellular processes, including regulation of extracellular matrix, cytoskeleton stability, cell cycle control, and differentiation. VHL disease is not associated with erythrocytosis.
This growing understanding of intracellular responses to hypoxia has led to the development of new therapeutic strategies for treating solid tumors which was recognized in 2019 by the Nobel Prize in Physiology and Medicine being awarded to William G. Kaelin, Jr., Sir Peter J. Ratcliffe, and Gregg L. Semenza. Many cancers contain areas of intra-tumoral hypoxia, and primary tumors with low oxygen levels are associated with an increased risk of metastasis and patient mortality. Increased HIF-1 levels are associated with increased risk of mortality in patients with many human cancers, including those involving the bladder, brain, breast, colon, esophagus, head/neck/oropharynx, liver, lung, pancreas, skin, stomach, and uterus, as well as in acute lymphocytic and myeloid leukemia. By contrast, in colon carcinoma and clear cell renal cell carcinoma HIF-2 overexpression is associated with disease progression and mortality.
The possibility that HIF family member inhibitor therapy might be used to treat solid tumors was hypothesized by many and recently confirmed by the FDA approving belzutifan. Belzutifan is an oral HIF-2α inhibitor used to treat adults with VHL syndrome who require therapy for associated renal cell carcinoma, central nervous system tumors, hemangioblastomas, or pancreatic neuroendocrine tumors not requiring immediate surgery. In a phase I study of patients with clear cell renal cell carcinoma reduced EPO levels were observed at all doses administered. The confirmed objective response rate was 25% (all partial responses), and the median progression-free survival was 14.5 months. The most common grade ≥ 3 adverse events were anemia (27%) and hypoxia (16%). Belzutifan was well tolerated and demonstrated preliminary anti-tumor activity in heavily pre-treated patients, suggesting that HIF-2α inhibition might offer an effective treatment for clear cell renal cell carcinoma.
Interaction of EPO with the EPO receptor (EPOR) present on the erythroid progenitor and precursor cells leads to its homodimerization, resulting in (1) stimulation of cell division, (2) differentiation by induction of erythroid-specific gene expression, and (3) prevention of erythroid progenitor and precursor cell apoptosis. EPOR is a member of the type I cytokine receptor superfamily. Signal transduction through the receptor is initiated by ligand binding, which induces dimerization of EPOR monomers. The predominant signaling cascade activated by EPOR and other cytokine receptors is the Janus-activated kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. JAK tyrosine kinases are constitutively associated with the membrane proximal regions of cytokine receptor cytoplasmic domains and are activated by receptor dimerization. The EPOR associates predominantly with JAK2. JAK2 binds to the EPOR in the endoplasmic reticulum and acts as a chaperone protein transporting the EPOR to the plasma membrane. Immediately after EPO binding, JAK2 phosphorylates itself and the EPOR on multiple tyrosine residues in its cytoplasmic domain, thus creating a platform for the recruitment and activation of multiple key signaling regulators. One such protein is STAT5. JAK2–STAT5 signaling plays an essential role in EPO/EPOR-mediated regulation of erythropoiesis. Consistent with their essential roles in erythropoiesis, EPO-, EPOR-, and JAK2-deficient mice die embryonically from severe anemia.
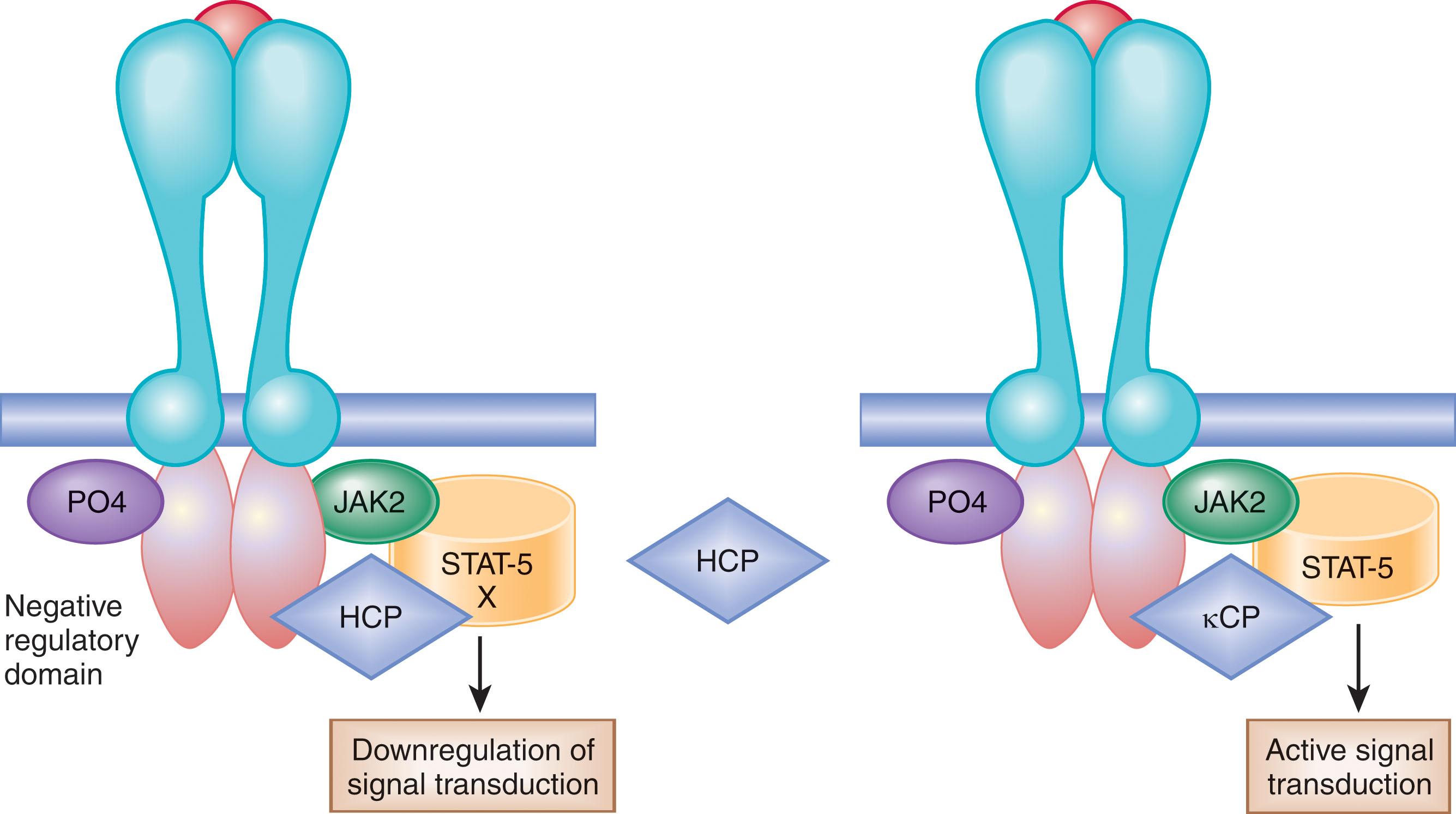
The C-terminal cytoplasmic portion of the EPOR also possesses a negative regulatory domain. Hematopoietic cell phosphatase (HCP; also known as SHP-1 or PTP N6 ) interacts with this portion of the EPOR and downregulates signal transduction by promoting dephosphorylation ( Fig. 70.2 ). Inactivation of the HCP-binding site leads to prolonged phosphorylation of JAK2–STAT5. Another negative regulator of erythropoiesis, suppressor of cytokine signaling-3 (SOCS-3), binds to the cytoplasmic portion of the EPOR and suppresses EPO-dependent JAK2–STAT5 signaling. Thus, deletion of the distal C-terminal cytoplasmic portion of the EPOR abolishes negative regulatory elements and results in increased proliferation of erythroid progenitor cells. Mutations in the EPOR gene have been observed in some patients with primary familial and congenital polycythemia (PFCP) and are occasionally found in erythroleukemia (see Fig. 70.2 ). Such secondary growth factors as insulin-like growth factor-1 (IGF-1) and the components of the renin–angiotensin system (RAS) may also influence the production of RBCs.
The RAS regulates fluid and electrolyte homeostasis and blood pressure, and has been hypothesized to also play a role in the regulation of erythropoiesis. The primary function of angiotensin during development is the regulation of tissue growth and differentiation. Angiotensin II (Ang II) is a ligand for two distinct receptors, type 1 and type 2 (AT1 and AT2). AT1 appears to play a major role in the regulation of cell proliferation.
The RAS was first postulated to influence erythropoiesis in the 1980s after the use of angiotensin-converting enzyme (ACE) inhibitors to treat hypertension was shown to result in anemia. In animals, increased blood levels of renin (a major regulator of Ang II synthesis) were found to result in elevated serum EPO levels and erythrocytosis. In humans, the infusion of Ang II in healthy volunteers increased serum EPO levels by 35% or higher via activation of the Ang II type I receptor, and ACE inhibitors significantly decrease plasma EPO levels by as much as 20% to 30%. The pathway underlying Ang II-driven EPO secretion is unknown. However, some investigators have suggested that Ang II modulates renal EPO production through changes in renal perfusion and sodium reabsorption. This hypothesis is based on the presumption that reduced oxygen pressure in the kidneys triggers HIF-1α/HIF-2α to induce release of EPO. Ang II also directly stimulates proliferation of hematopoietic progenitors in vitro, and inhibition of this effect with ACE inhibitors induces apoptosis of erythroid progenitors in renal transplantation patients. ACE-1 knockout mice develop a normocytic anemia that can be reversed by infusion of Ang II. ACE-related anemia is most pronounced in patients with renal insufficiency or end-stage renal disease and in patients who have received a renal allograft. The pathogenesis of this anemia is not clear, but reduced levels of circulating EPO are not solely responsible, suggesting that there might be other contributing factors. The AT1 receptor is present on erythroid progenitors, and its ligand, Ang II, augments EPO stimulation of erythropoiesis. The involvement of JAK2 kinase in Ang II signaling suggests that this signal transduction pathway mediated by EPO and Ang II might overlap. Postrenal transplant erythrocytosis likely can be accounted for by activation of the RAS.
The term polycythemia is a literal translation from Greek, meaning “too many cells in the blood,” and refers to an increase in the RBC mass; it is frequently used interchangeably with the term erythrocytosis . Polycythemia may be due to a myriad of causes ( Table 70.1 ). The polycythemias can be classified as relative and absolute. Relative polycythemia is a disorder in which the patient characteristically has a modest elevation of the hematocrit level without an elevated RBC mass but rather because of contraction of the plasma volume. The absolute polycythemias are accompanied by an actual increase in the circulating RBC mass. Polycythemias can also be classified according to the responsiveness of their erythroid progenitor cells to growth factors or the circulating levels of such growth factors. Primary polycythemias are characterized by increased sensitivity of the erythroid progenitors to regulatory growth factors as a result of acquired somatic or inherited germ-line mutations expressed by hematopoietic progenitor cells (HPCs). In contrast, secondary polycythemias are characterized by an increase in regulatory growth factors, primarily EPO, and normal responsiveness of their erythroid progenitors to these growth factors. These conditions can usually be distinguished by in vitro assays of erythroid progenitor cells, quantitation of serum EPO levels, and detection of somatic JAK2 mutations. In a small number of patients, the cause of erythrocytosis cannot be determined; these patients are classified as having idiopathic erythrocytosis.
| Relative or Spurious Polycythemia |
|
| Absolute Polycythemia |
|
Individuals with a modestly increased venous hematocrit level that is not accompanied by an increased RBC mass are frequently thought to be polycythemic by imprecise yet widely accepted medical practice. Frequently, these individuals are thought to be polycythemic owing to the lack of appreciation by a clinician of what constitutes the upper limit of normal values for a hematocrit (49% in males and 48% in females). Such individuals frequently prove not to have an absolute polycythemia as defined by an actual increase in the measured RBC mass. Relative or spurious polycythemia is a term used to describe an elevation of the hematocrit level either caused by an acute transient state of hemoconcentration associated with intravascular fluid depletion or a chronic sustained relative polycythemia caused by contraction of the plasma volume (see Table 70.1 ).
Transient polycythemias may be a result of acute depletion of the plasma volume from a variety of disorders, including protracted vomiting or diarrhea, plasma loss from external burns, sudden cold exposure or protracted exercise, insensible fluid loss from fever, sepsis, diabetic ketoacidosis, or acute ethanol intoxication. These elevations of hematocrit can be easily corrected by appropriate replacement of intravascular fluids.
Gaisböck syndrome, first described in 1905, is a condition observed mainly in obese, hypertensive, middle-aged, and male smokers. Alcohol, diuretics, obesity, hypoxia, psychologic stress, and excess catecholamine secretion have been identified as possible causes of relative polycythemia. Such individuals can have a chronic modest-to-moderate elevation of the hematocrit level associated with a normal RBC mass and low plasma volume, which has been attributed to reduced venous compliance, or they can have a high normal RBC mass with either a normal or slightly decreased plasma volume. The primary significance of the identification of a patient with relative polycythemia is the recognition of the increased risk of developing thrombotic vascular events likely caused by excessive smoking, hypertension, and obesity associated with this disease. Treatment is generally directed at correction of the patient's underlying cardiovascular risk factors.
It is also important to emphasize that overfilling of blood collection vacuum tubes can result in pseudopolycythemia, pseudothrombocytopenia, and pseudoleukopenia as a result of inadequate sample mixing. Careful attention to such a seemingly trivial detail can help avoid expensive, unnecessary diagnostic workups.
This is an autosomal dominant disorder. Although PFCP is uncommon, it is more prevalent than polycythemia caused by high-oxygen–affinity hemoglobin mutants or a 2,3-biphosphoglycerate (2,3-BPG) deficiency. Unlike patients with PV, patients with PFCP lack splenomegaly and do not progress to acute leukemia. It is not unusual for these patients to present with headaches, dizziness, epistaxis, and exertional dyspnea that resolve with normalization of the hematocrit level. An increased incidence of cardiovascular events and premature morbidity and mortality has been reported in some affected members, but many appear to have a benign clinical course. Although clinical symptoms are relieved by phlebotomy, the increased risk of cardiovascular morbidity is not corrected by maintaining a normal hematocrit. Characteristic laboratory findings are (1) an increased hematocrit and RBC mass without an increased leukocyte or platelet count, (2) an absence of an activating mutation of JAK2 , (3) a normal hemoglobin–oxygen dissociation curve, (4) low serum EPO levels, and (5) in vitro hypersensitivity of erythroid progenitors to EPO. Even though PFCP is present at birth, many affected patients are incidentally diagnosed later in life after the performance of routine blood counts or when evaluated in the context of multiple family members having polycythemia. It is of interest that one individual so affected was an accomplished cross-country skier who had won medals at the Olympic Games. Numerous mutations of the EPOR associated with PFCP have been described, leading to a loss in the negative regulatory domain of the EPOR.
The physiologic basis for EPO-mediated activation of erythropoiesis is as follows: EPO activates its receptor by conformational changes of its dimers, leading to initiation of an erythroid-specific cascade of events. The first signal is initiated by the binding of a tyrosine kinase to the EPOR and its phosphorylation and activation of a transcription factor, STAT5, which regulates erythroid-specific genes. This “on” signal is negated by dephosphorylation of the EPOR by HCP, that is, the “off” signal. Truncation of the EPOR leads to a loss in the negative regulatory domain of the EPOR, a binding site for HCP, leading to a gain-of-function mutation of the EPOR (see Fig. 70.2 ). In addition, the negative regulation of erythropoiesis by SOCS-3, cytokine-inducible SH2 domain containing protein (CIS), and Src homology region 2 domain-containing phosphatase-1 (SHP-1) is presumed to contribute to the underlying cause of PFCP.
Alternative explanations for the increased sensitivity of erythroid progenitors to EPO of patients with PFCP have been proposed. EPOR downregulation provides another mechanism by which EPO desensitization can occur. EPOR downregulation is a complex process that involves EPOR-induced internalization or ubiquitination and degradation by proteasomes. EPO-induced receptor internalization is an efficient means of rapidly reducing EPO responsiveness. This process is mediated by binding of the EPOR to the p85 subunit of phosphatidylinositol 3-kinase (PI3K) but does not involve its kinase activity as the PI3K inhibitor wortmannin does not impair EPOR internalization. All of the truncated mutants associated with PFCP are associated with failure to internalize the EPOR, contributing to prolonged signaling through the EPOR. The EPOR degradation process removes all of the phosphorylated tyrosine residues in the intracellular domain of the receptor, thereby preventing further signal transduction. The remaining part of the EPO–EPOR complex is then internalized and degraded by lysosomes. The E3 ligase B-transducin repeat containing protein-1 (B-Trcp-1) is responsible for EPOR ubiquitination and degradation. Mutations in B-Trcp-1 abolish EPOR ubiquitination and degradation, making cells expressing the EPORs hypersensitive to EPO. Each of the PFCP mutations involving the EPOR results in loss of the binding site for B-Trcp-1. These findings suggest that the EPO hypersensitivity in PFCP might not be attributable to a failure to recruit negative regulators such as phosphatases to inactivate JAK2, or that these mutant receptors are defective in EPO-induced receptor downregulation.
The effect of a truncated EPOR is not always predictable. Some patients who inherit an EPOR mutation are not polycythemic. This observation suggests that undefined environmental or genetic factors may mask the development of polycythemia. Also, the heterogeneity of the polycythemic phenotype observed in a PFCP animal model appears to be strain dependent. This indicates that gene modifiers or epigenetic factors may mask the development of the full PFCP phenotype. Four different rearrangements of the EPOR have been observed in Philadelphia chromosome-like (Ph-like) acute lymphoblastic leukemia (ALL) B cells ( Chapter 36, Chapter 37 ). Normal B cells do not express the EPOR, but the EPOR has been described in ETV6-RUNX1–positive ALL blast cells. These rearrangements are different from those observed in PFCP but result in truncation of the cytoplasmic tail of the EPOR at residues similar to those mutated in PFCP, with preservation of the proximal tyrosine essential for receptor activation and loss of distal regulatory residues. This leads to dysregulated EPOR expression, hypersensitivity to EPO stimulation, and heightened JAK–STAT activation. Expression of truncated EPOR in murine B-cell progenitors leads to the development of ALL in vivo (see Chapter 36, Chapter 37 ). Several observations suggest that the EPOR mutations in human leukemic cells in Ph-like ALL are driver mutations that are acquired during early stages of leukemogenesis and therefore JAK inhibitor therapy can be used in combination with current ALL treatment strategies to treat patients with this type of leukemia.
Secondary polycythemias can be either congenital or acquired (see Table 70.1 ). Conditions leading to hypoxia, such as high altitude, cyanotic heart disease, obstructive sleep apnea or chronic lung disease, may result in physiologic polycythemia mediated by increased levels of EPO. There are marked variations in EPO levels and subsequent erythroid responses in the face of chronic hypoxia, suggesting that some of these factors may be genetically determined. The same degree of renal tissue hypoxia may induce substantially different levels of EPO production in response to high altitude. It is likely that these individual variations are a function of genetic differences in hypoxia sensing and the hypoxia response pathways. For purposes of simplicity and clinical diagnostic usefulness, the secondary polycythemic disorders are divided into those that are acquired and those that are congenital. It should be kept in mind that this division, although useful for purposes of differential diagnosis, is artificial. Patients with inherited germ-line mutations, for instance, can develop an EPO-secreting pheochromocytoma or renal cell cancer, and a patient with PV can smoke and have chronic obstructive pulmonary disease (COPD). In other instances, polycythemia caused by a germ-line mutation can be masked by an acquired environmental factor or another gene-modifying mutation.
Patients with cyanotic heart disease and pulmonary disease frequently have arterial hypoxemia, leading to increased production of EPO and polycythemia. Excessive EPO production occurs when the PaO 2 is sustained below 67 mmHg as a result of severely impaired pulmonary mechanics. Because patients with severe pulmonary disease and secondary erythrocytosis frequently have elevated plasma volumes, the degree of elevation of the hematocrit level may be modest. Hematocrit levels as high as 65% or rarely 75% have, however, been reported. Moderate elevations of hematocrit have been estimated to occur in 20% of patients with COPD. Polycythemia in this setting can contribute to pulmonary hypertension, pulmonary endothelial cell dysfunction, reduced cerebral blood flow, hyperuricemia, gout, and an increased risk of venous thromboembolic disease.
Why some patients with pulmonary disease and congenital heart disease develop polycythemia but others do not remains unclear. Increased oxygen-carrying capacity may improve oxygen delivery; however, it is not obvious at what hematocrit level the resultant elevation in blood viscosity impairs blood flow to the tissues, leading to a reduction in oxygen uptake. In addition, oxygen uptake to the tissues is markedly influenced by whole blood volume. Thus, whereas the optimal hematocrit level for oxygen delivery is about 45% in normovolemic subjects, it rises to over 60% in hypervolemic states, likely as a result of engorgement of the vascular bed and a decrease in peripheral resistance. Furthermore, chronic exposure to hypoxia leads to respiratory alkalosis that in turn promotes the synthesis of 2,3-BPG, facilitating increased oxygen delivery to tissues.
The practical relevance of an elevated hematocrit level in this clinical situation is whether and at what level it is harmful or beneficial. An extremely elevated hematocrit level may be detrimental to optimal oxygen delivery. Extreme but not moderate polycythemia caused by chronic hypoxia may affect systemic vascular function by altering blood viscosity, vessel wall shear stress, reduced endothelial cell–derived nitric oxide release, and increasing the secretion of endothelin. Although it is widely accepted that polycythemic pediatric patients with cyanotic heart disease are at an increased risk for developing cerebrovascular accidents, the literature provides conflicting data as relates to the prevalence of such events among adults. A 10% to 13.6% prevalence of stroke and transient ischemic attacks (TIAs) has been reported in a cohort of adult patients with cyanotic heart disease, but others have claimed that such events are rare. Iron deficiency occurs in more than 30% because of the total depletion of iron stores to support erythropoiesis. Microcytic RBCs are, however, rarely found, and despite the iron deficiency, these patients frequently have normal mean corpuscular volumes and high mean corpuscular hemoglobin concentrations, which might maximize the amount of hemoglobin within an individual RBC, thereby maximizing oxygen delivery. In the past, compensatory erythrocytosis was thought to lead to an increased plasma viscosity, leading to a compromised microcirculation, resulting in symptoms such as headache, sluggish mentation, dizziness, blurry vision, muscle weakness, or paresthesias. In reality, such symptoms are rare in patients with chronic compensated secondary erythrocytosis, and the secondary erythrocytosis is viewed as a physiologically desirable response to chronic hypoxia. The symptoms delineated above are likely attributable to decreased tissue oxygen delivery rather than hyperviscosity.
The treatment of hyperviscosity secondary to erythrocytosis in cyanotic heart disease with prophylactic phlebotomy is rarely used. In fact, phlebotomy has been reported to have harmful rather than beneficial effects in adults with cyanotic congenital heart disease. Because almost one-third of these patients are iron deficient even though their RBC indices do not reflect this, routine assessment of the patient’s iron status is suggested with gradual supplementation with sufficient iron to attain appropriate compensatory levels of erythropoiesis but avoiding excessive sudden increases in the degree of erythrocytosis. The present evidence indicates that prophylactic phlebotomy promotes the development of iron deficiency, decreases exercise tolerance, and increases the number of cerebrovascular events. Currently, experts in this field recommend that phlebotomy should be restricted to individuals with symptoms with extreme erythrocytosis (hematocrit >65%) and preoperatively to improve hemostasis. Clinical data to justify these recommendations are lacking. Phlebotomy should be followed by the infusion of an equal volume of fluids to maintain intravascular volume and blood flow, as well as to provide a dilutional effect to reduce the hematocrit level. Hydroxyurea (HU) therapy has been used occasionally to reduce erythropoiesis in this situation to reduce the need for phlebotomy, but little evidence exists for this approach. The superiority of HU therapy versus phlebotomy therapy has not been documented. HU might act not only by suppressing RBC production but also by promoting macrocytic RBC formation, thereby increasing RBC deformability and decreasing RBC adhesiveness.
Chronic oxygen therapy in patients with severe COPD has resulted in relief of hypoxia and a modest reduction in hematocrit levels. Pharmacologic interventions, including theophylline, inhaled nitric oxide, sildenafil, or antagonism of the renin–angiotensin pathway with losartan, may also reduce the degree of pulmonary hypertension or secondary erythrocytosis. In a retrospective study, hematocrit levels were found to be significantly lower in 50 COPD patients with erythrocytosis receiving theophylline as compared to levels in the 61 patients not receiving theophylline. Since the levels of oxygen saturation were similar in both groups, the normal hematocrit levels in the theophylline treated group could not be explained by improved oxygen availability. Serum EPO levels, however, declined in 70% of theophylline treated patients. Theophylline has previously been shown to reduce the production of EPO in both normal subjects and patients with erythrocytosis after renal transplantation and is likely acting in a similar fashion to reduce the degree of erythrocytosis in COPD patients.
Obstructive sleep apnea syndrome is characterized by repetitive episodes of partial or complete obstruction of airflow during sleep. Common symptoms include loud snoring and breathing pauses observed by a bed partner, feelings of nonrefreshing sleep, and excess daytime sleeping. Although the evidence is largely anecdotal and somewhat controversial, secondary polycythemia is a recognized complication of long-standing sleep apnea. The factor that seems to have the strongest prediction for polycythemia is nocturnal mean oxygen saturation. On the other hand, most studies demonstrate that the apnea-hypopnea index does not reliably associate with polycythemia except in severe cases. The mechanism by which sleep apnea can cause polycythemia is unclear. Differences in EPO levels between normoxic and hypoxemic patients referred for suspected sleep apnea have not been documented. Obstructive sleep apnea is also associated with an increased risk of developing cardiovascular diseases, including systemic hypertension, pulmonary hypertension, cardiac arrhythmias, atherosclerosis, ischemic heart disease, and stroke. Intermittent hypoxia is thought to be a major cause of cardiovascular complications. These patients undergo repeated episodes of hypoxia and normoxia. The hypoxia leads to ischemia, and the reoxygenation causes a sudden increase of oxygen. This reoxygenation phase results in the production of reactive oxygen species and the promotion of oxidative stress, leading to an inflammatory response and the development of vascular complications. Nguyen and Holly analyzed 1604 consecutive veterans undergoing sleep evaluations for suspected obstructive sleep apnea to identify the incidence and causes of erythrocytosis. They reported four important findings: (1) Clinically significant erythrocytosis (HCT >48%) was relatively uncommon in those with suspected or confirmed OSA occurring in only 8.0% of cases with only 1.6% having clinical erythrocytosis as defined as Hct ≥51% in males, Hct ≥48% in females. (2) The severity of obstructive sleep apnea as measured by Apnea Hypopnea Index was not associated with higher hematocrit levels or absolute erythrocytosis. (3) Both nocturnal hypoxemia and hypoxemia occurring while individuals were awake were independent predictors of erythrocytosis. (4) The severity of obstructive sleep apnea was predictive of nocturnal hypoxemia. These authors concluded that factors other than obstructive sleep apnea should initially be evaluated for unexplained secondary erythrocytosis, although nocturnal oximetry might be of diagnostic use. They concluded that in patients with unexplained nocturnal hypoxemia and erythrocytosis, an obstructive sleep apnea evaluation using a formal sleep study was warranted. Conversely, PV may induce central sleep apnea by decreasing cerebral blood flow to diencephalic respiratory centers, and patients so affected can have complete resolution of their sleep disorder with normalization of their blood counts.
Pickwickian syndrome or obesity–hypoventilation syndrome, seen in morbidly obese individuals, is characterized by chronic hypoxemia and hypercapnia caused by alveolar hypoventilation, with a resultant increase in EPO production, polycythemia, and cor pulmonale. The three principal causes are the high cost of the work of respiration in morbidly obese individuals, dysfunction of the respiratory centers, and repeated episodes of nocturnal obstructive apnea. Effective treatments include surgically induced weight loss, nasal continuous positive airway pressure ventilation, and the respiratory stimulant medroxyprogesterone acetate.
Polycythemia caused by the hypoxic conditions encountered by high-altitude dwellers would appear at first glance to represent a universal adaptive process to altitude. High altitude results in hyperventilation, alkalosis, and shifting of the O 2 dissociation curve to the left, leading to the impaired release of O 2 from hemoglobin and ultimately tissue hypoxia. This tissue hypoxia results in markedly increased EPO production, leading to increased plasma iron turnover, reticulocytosis, and a rising hematocrit level. Residents of the Andes Mountains who live 4200 m above sea level frequently have 30% higher hematocrit levels than individuals living at sea level.
People native to high altitudes (highlanders) live in a hypobaric hypoxic environment characterized by a low ambient partial pressure of oxygen. In response to this environment, they develop alveolar hypoxia, hypoxemia, and polycythemia. Healthy highlanders develop pulmonary hypertension, right ventricular hypertrophy, and an increased amount of smooth muscle cells in the distal pulmonary arterial branches, which leads to increased pulmonary vascular resistance and pulmonary artery pressure compared with individuals living at sea level. The importance of these structural changes in the pulmonary vasculature in highlanders is confirmed by the slow decline of pulmonary artery pressure, which is normalized after living for 2 years at sea level. Despite these adaptive changes, healthy highlanders are able to perform physical activities similar to or often even more strenuous than those living at sea level. In fact, there are differences in ventilation rates between athletes performing at sea level and those at high altitudes. Ventilation rates of athletes increase normally during exercise at sea level, but relative hypoventilation occurs in highlanders. This relative hypoventilation is characteristic of Andean natives and has been attributed to desensitization of the carotid bodies to the hypoxic stimulus. The erythrocytosis observed in individuals who reside at high altitudes for relatively short periods of time (days) can also be attributed in part to excessive water loss and contraction of the plasma volume. Total acclimatization of an individual who moves from sea level to a high altitude may actually require years. Individuals who reside at sea level and are acutely exposed to high altitudes are at increased risks of developing a deep venous thrombosis, pulmonary infarction, retinal hemorrhage, or ischemic digits because of increased blood viscosity. High-altitude climbers frequently combat these problems by intravenous administration of isotonic saline, with considerable success.
The chronic responses of various ethnic and racial groups to high altitudes are quite variable. Andean natives, known as the Quechua and Aymara Indians, experience a gradual increase in their hemoglobin levels with age. In addition, hemoglobin values are almost 10% higher in those living at 5500 m above sea level than in those living at 4355 m above sea level. Curiously, their Tibetan and Ethiopian counterparts living at similar altitudes do not respond to the resultant chronic hypoxia by increasing their hematocrits. It has been suggested that high levels of nitrous oxide in the exhaled breath of Tibetans may improve oxygen delivery by inducing vasodilatation and increasing blood flow to tissues, thus making the compensatory increased RBC volume unnecessary. Interestingly, Tibetans and Ethiopians have lived much longer as mountain dwellers than the Quechua or Aymara Indians, suggesting that extreme elevation of the RBC mass is a maladaptation that Tibetans and Ethiopians have avoided by adopting more physiologic compensatory mechanisms. Many residents of the Tibetan plateau reside at elevations exceeding 4000 m and experience oxygen concentrations that are about 40% lower than experienced at sea level. Human adaptation to a high-altitude environment is believed to be the result of advantageous genetic mutations and selective pressure. These genetic adaptations are shared by common ancestors within East Asian but not Central and South Asian populations and confer characteristics including adaptation to hypoxia, the absence of chronic mountain sickness (CMS), and high offspring survival rates. Polymorphisms in the EPAS1 gene that encodes HIF-2α, and the EGLN1 gene, which encodes PHD2, have been positively selected and have been shown to be associated with the key adaptive features in Tibetans. The putative advantageous haplotypes of EGLN1 and EPAS1 have revealed negative correlations with hemoglobin levels in Tibetans compared with lowlander Han Chinese. A variant in EGLN1, c.[12 C>G; 380 G>C], contributes functionally to the Tibetan high-altitude phenotype. PHD2 triggers the degradation of HIFs. The PHD2 p.[Asp4Glu; Cys127Ser] variant exhibits a lower Km value for oxygen, suggesting that it promotes increased HIF degradation under hypoxic conditions. Whereas hypoxia stimulates the proliferation of WT erythroid progenitors, the proliferation of progenitors with the c.[12 C>G; 380 G>C] mutation in EGLN1 is significantly impaired under hypoxic culture conditions. The c.[12 C>G; 380 G>C] mutation abrogates hypoxia-induced and HIF-mediated augmentation of erythropoiesis, which provides a molecular mechanism for the observed protection of Tibetans from polycythemia at high altitude.
This individual variability of elevation of serum EPO levels in high-altitude dwellers and the resultant increase in RBC mass appears widespread. For example, acclimatization to moderately high altitudes when combined with low-altitude training (so-called living high , training low ) improves sea-level performance in endurance athletes, in part because of the erythropoietic effects of altitude exposure. This substantial individual variability in response to all forms of altitude training correlates with improved athletic performance and with elevation of EPO levels. A large component of this individual variability appears to be related to differences in the peak and rate of decay of the increase in EPO in response to altitude exposure. These observations suggest that genetically determined variables account for individual responses to hypoxia.
Chronic CMS is a pathological loss of adaptation to altitude. CMS is a clinical syndrome that occurs in a fraction of natives or lifelong residents living above 2500 m. Prevalence of CMS has considerable variability in different high-altitude parts of the world. The prevalence of CMS increases with age and varies among the mountainous regions of the world. CMS is an adult disease, and its prevalence is the lowest in Ethiopians (<1%) and Tibetans (~1%), higher in South Asian Indians (6.2%) and Han Chinese relocated to high altitudes (6%), and highest among South American Andeans (15%). This variability is mostly attributed to differences in ethnicity. Various ethnic groups have had different periods of adaptation to chronic hypoxia. Those living at high altitudes for the longest time are more likely to be better adapted. It appears that native Tibetans and Ethiopians are the most adapted ethnic groups as compared to Andeans and Han Chinese immigrants. CMS is characterized by excessive erythrocytosis (females, Hb >19 g/dL; males, Hb >21 g/dL); severe hypoxemia; and in some cases, moderate or severe pulmonary hypertension that may lead to the development of cor pulmonale and congestive heart failure. The clinical picture of CMS gradually disappears after descending to lower altitudes and reappears after returning to high altitudes. The prevalence of CMS is higher in men than women and increases with altitude, aging, associated lung disease, history of smoking, and air pollution. CMS is a public health problem in those living in mountainous regions of the world above 2500 m. In China alone, 80 million people live above that altitude, while in South America, 35 million people live above 2500 m. The CMS Andean phenotype has been associated with a single-nucleotide polymorphism (SNP) in the Sentrin-specific Protease 1 ( SENP1 ) gene. The SENP1 gene encodes for a protease that regulates the function of hypoxia-relevant transcription factors such as HIF and GATA1 and thus regulates erythropoiesis in individuals with CMS by modulating expression of EPO and the EPOR. The critical role of GATA1 in erythropoiesis is attributed to its ability to drive expression of many erythroid specific genes including enzymes involved in heme biosynthesis, hemoglobin, and the EpoR (see Chapter 27 ). SENP1 has been shown to regulate EPO production by regulating the stability of HIF1α during hypoxia, and indeed SENP1 −/− mice die of anemia during early life. SENP1 also mediates a positive-feedback loop under hypoxic conditions that is responsible for VEGF production and angiogenesis. SENP1-mediated regulation of EpoR expression is likely to occur through GATA-1. Compared with normoxic controls, hypoxic conditions induce a significant downregulation of SENP1 expression in non-CMS cells and an upregulation in CMS cells. Therefore, it is likely that hypoxia in patients with CMS induces a higher expression of SENP1 which favors exaggerated erythropoiesis. Thus Andeans, but not Tibetans and Ethiopians, possess a proerythropoietic SNP rather than SNPs that protect individuals from developing erythrocytosis. The presence of SENP1 C/G or C/C SNPs are significantly associated with more severe forms of CMS. SENP-dependent stabilization of HIF2α would be anticipated to lead to increased Epo expression. Surprisingly, elevated Epo values are uncommon in CMS patients, yet a subgroup of CMS patients do have significantly higher Epo levels which is associated with slightly higher hematocrits. These CMS patients with high and normal EPO levels likely reflects genetic differences between these two populations. The development of CMS is associated with relative alveolar hypoventilation. Healthy highlanders characteristically hyperventilate. A gradual decline in the rate of alveolar ventilation in these individuals leads to progressive loss of adaptation to chronic hypoxia and the development of CMS. The main components of this syndrome include (1) alveolar hypoventilation leading to relative hypercapnia and increasing hypoxemia; (2) excessive erythrocytosis leading to increased blood viscosity and expansion of the total lung blood volume; (3) pulmonary hypertension and right ventricular hypertrophy that may evolve to hypoxic cor pulmonale and heart failure; (4) neuropsychiatric symptoms, including sleep disorders, headache, dizziness, and mental fatigue; (5) proteinuria in spite of a normal glomerular filtration rate; and (6) a contracted plasma volume which might potentially lead to more extreme polycythemia and clinical symptoms.
Physical examination reveals cyanosis of the nail beds, ears, and lips in contrast to the ruddy complexion that is characteristic of a healthy highlander. In some cases, the face is almost black, and the mucosa and conjunctiva are dark red. The fingers are frequently clubbed, and auscultation of the heart reveals an increased pulmonary second sound. The patients are frequently hypertensive and have evidence of heart failure. Chest radiographic and electrocardiographic findings are characteristic of right atrial and right ventricular hypertrophy. Criteria for the diagnosis of CMS have been published and are useful in identifying CMS patients as well as monitoring their response to treatment.
The definitive treatment for CMS is descent to lower altitudes or sea level. The degree of polycythemia decreases after a few weeks or months, and eventually the hematocrit level returns to sea-level values. Pulmonary hypertension and right ventricular hypertrophy gradually resolve and disappear after 1 to 2 years of living at sea level. Phlebotomy or isovolemic hemodilution can reduce the excessive erythrocytosis and hyperviscosity, improve oxygenation, and lead to relief from symptoms. Due to its transient effects phlebotomy is not a long-term treatment for CMS since at high altitude hematocrit reaches or exceeds pretreatment values within a few weeks. A variety of drugs has also been evaluated for the treatment of patients with CMS. Ten weeks of the respiratory stimulant medroxyprogesterone acetate at doses of 60 mg/day was reported to lead to a reduction of hematocrit levels from 60% to 52% and an increase in arterial oxygen saturation from 84% to 90% in 17 highlanders with CMS. Medroxyprogesterone use, however, was associated with a loss of libido in men and therefore is infrequently used in this population. Therapy with almitrine, a respiratory stimulant, or enalapril, an ACE inhibitor (10 mg/day for 30 days), has resulted in even more modest reductions of hematocrit levels. Therapy with acetazolamide is the most useful treatment for CMS. Acetazolamide therapy has been evaluated in two double-blind, placebo-controlled, randomized clinical trials of patients with CMS. Acetazolamide is an inhibitor of carbonic anhydrase and stimulates ventilation by promoting the development of metabolic acidosis. Furthermore, acetazolamide reduces renal EPO production. Patients with CMS were randomized to receive placebo or acetazolamide therapy at a dose of 250 or 500 mg/day for 21 days. Drug therapy at both doses of acetazolamide resulted in reduction of hematocrit levels by 7% and serum EPO levels by 50% to 67%, with an increase in nocturnal oxygen saturation levels of 5%. Presently, 250 mg of acetazolamide daily appears to be an inexpensive, nontoxic, and effective therapy for CMS.
Smoking is the most common cause of secondary polycythemia. Those affected have a carboxyhemoglobin-induced increase in RBC mass or decrease in plasma volume, either of which is reversible with smoking cessation. Excessive carbon monoxide exposure can also be attributed to exposure to industrial emissions and automobile exhaust. Carbon monoxide binds to hemoglobin with a more than 200-times greater affinity than oxygen, resulting in not only occupation of one of the heme groups of hemoglobin but also an increase in the O 2 affinity by the remaining heme group. Individuals smoking even one pack of cigarettes a day frequently have elevated hematocrit levels. These patients characteristically have normal blood gases and elevation of carboxyhemoglobin levels, resulting in a reduction in P 50 O 2 . The elevation of the hematocrit level is reversed with interruption of the smoking behavior. Increased hematocrit levels have been observed in 3% to 5% of heavy smokers. Although these patients are not immune to thrombotic complications, the number of thromboembolic events is lower than in patients with PV. Polycythemia has also been reported to be associated with hookah use. A hookah is an oriental pipe containing tobacco often mixed with molasses and fruit flavors connected by a long flexible tube that draws the smoke to the bowl of water. Hookah use exposes the user to generous amounts of carbon monoxide, resulting in erythrocytosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here