Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
When deconstructed to its basic structure, the placenta is an active (“drug handling”) barrier between two separated systems (maternal, fetal) with placental drug disposition driven by differences in concentration-time profiles between both systems, and the maternal and fetal blood flow to and from the filter. This setting is somewhat similar to hemodialysis or extra-corporeal membrane oxygenation, where diffusion will be driven by concentration gradients, characteristics of the membrane, and flows. However, the placenta is an active barrier and transporter. The rate and extent of drug exposure to embryo or fetus are determined by numerous variables, including the drug-related as well as physiology-related characteristics listed in Table 17.1 .
| Transfer Feature | Characteristics | |
|---|---|---|
| Drug | Maternal-Placental-Fetal Unit | |
| Rate | Lipid solubility | Placental structure, size, and function |
| Molecular weight | Maternal, placental, and fetal blood flow | |
| Structural characteristics | Thickness of placental membranes | |
| Type of transfer | Passive diffusion, facilitated or active transport, or pinocytosis | |
| Protein binding | Maternal, placental, or fetal protein binding (albumin, α1 acid glycoprotein) | |
| Extent | Degree of ionization (p K a ) | Maternal-fetal pH gradient |
| Type of transfer | Passive diffusion, facilitated or active transport, or pinocytosis | |
| Protein binding | Maternal, placental (metabolism, passive diffusion, facilitated or active transport, pinocytosis), and fetal drug handling | |
| Both | Blood flows | Maternal arterial uterine blood flow, cardiac output Fetal umbilical blood flow, cardiac output |
The placenta is of fetal origin and acts as interface between the maternal and fetal compartments. The major functions of the placenta are to transfer nutrients and oxygen from mother to fetus and to assist in the removal of waste products from fetus to mother. In addition, it plays an important role in the synthesis of hormones (e.g., β-human chorionic gonadotropin, estrogens), peptides, prostaglandins, or steroids that are all vital for a successful pregnancy. The placenta hereby allows tailored transport of nutrients, enables elimination of waste products, and facilitates gas exchange between mother and fetus. Related to these functions, evidence has accumulated that essentially all pharmacologic agents, but also other exogenous substances, are transferred to the embryo and fetus, regardless of whether this transfer is intentional (e.g., medical treatment of the fetus) or unintentional, with possible teratogenic or toxic fetal effects, including aspects such as drug tolerance.
It is too simplistic to consider the placenta as an absolute and protective barrier, and the concept of a passive filter function is also not sufficiently sophisticated. This is based on the fact that the placenta is not just an innocent bystander, but an active regulator of drug transport (efflux and influx transporters) and metabolism (metabolizing enzymes). , The “barrier” function of the placenta includes passive diffusion, facilitated diffusion, active transport, and pinocytosis or endocytosis. Besides physicochemical and structural characteristics (such as size) that determine placental permeability, it is important to realize that the placenta is also an active organ (drug accumulation, metabolism, and/or transporters). Placenta drug metabolism can also modify fetal drug exposure.
As concentration gradients also matter, fetal drug exposure may vary according to maternal exposure in addition to placental transfer characteristics. Because of the physiologic modifications occurring during pregnancy, maternal concentration-time profiles commonly differ from nonpregnant women. As a simple illustration, if renal elimination clearance increases significantly during pregnancy, maternal exposure will decrease and so will placental transfer during pregnancy, when the same dose is used for drugs (almost) exclusively cleared by the renal route. Along the same line, fetal drug disposition (metabolism, renal elimination, and/or accumulation) also drives exposure as it may affect the maternal-to-fetal drug gradient.
Finally, exposure will also be determined by maternal arterial uterine blood flow and fetal umbilical blood flow. The rate of blood flow increases 12-fold from 10 weeks’ gestation until term (beyond 37 weeks), accounting for about 80% of uterine perfusion at that time. This is still a somewhat underexplored aspect of placental transfer, as maternal (pregnancy-related hypertension; preeclampsia) or fetal (arrhythmia, such as supraventricular tachycardia or heart block; reversed fetal umbilical blood flow) also results in altered maternal or fetal placental blood flow, respectively.
Pregnant women take drugs, and women who take drugs might become pregnant. A relevant number of over-the-counter drugs are taken as self-medication by women who may be unaware of a still-early pregnancy or of possible adverse effects to the fetus. Consequently, requests for public and reliable information are mounting. , Similarly, young women with medical conditions (e.g., post-transplant, autoimmune disease, hematologic/oncologic diseases, human immunodeficiency virus [HIV], psychiatric diseases, addiction) want to become pregnant. An appreciation of these considerations is important in designing meaningful toxicokinetic and drug disposition studies. , , Therefore, a reasonably accurate prediction of placental transfer before prescription to pregnant women is needed. To conduct such (in vivo, or computer simulation) studies, the earlier mentioned parameters (see Table 17.1 ) that drive the extent (total amount) and rate (amount over time) of placental transfer of a specific compound in the human mother-fetus dyad should be considered, to design a study that will generate robust information.
Physiology-based pharmacokinetic (PBPK) models can integrate these different types of information, such as expression data, ontogeny information, and observations obtained from the ex vivo cotyledon perfusion experiments, combined with flow characteristics and the physicochemical characteristics of a given compound. Such a mechanistic modeling framework may leverage the available information and make more reliable predictions on pharmacotherapy during pregnancy (maternal and fetal). , , The aim of this chapter is to discuss the physiology of placental drug transfer, but it will commence with a short overview on methods and modeling systems currently applied to investigate placental drug transfer.
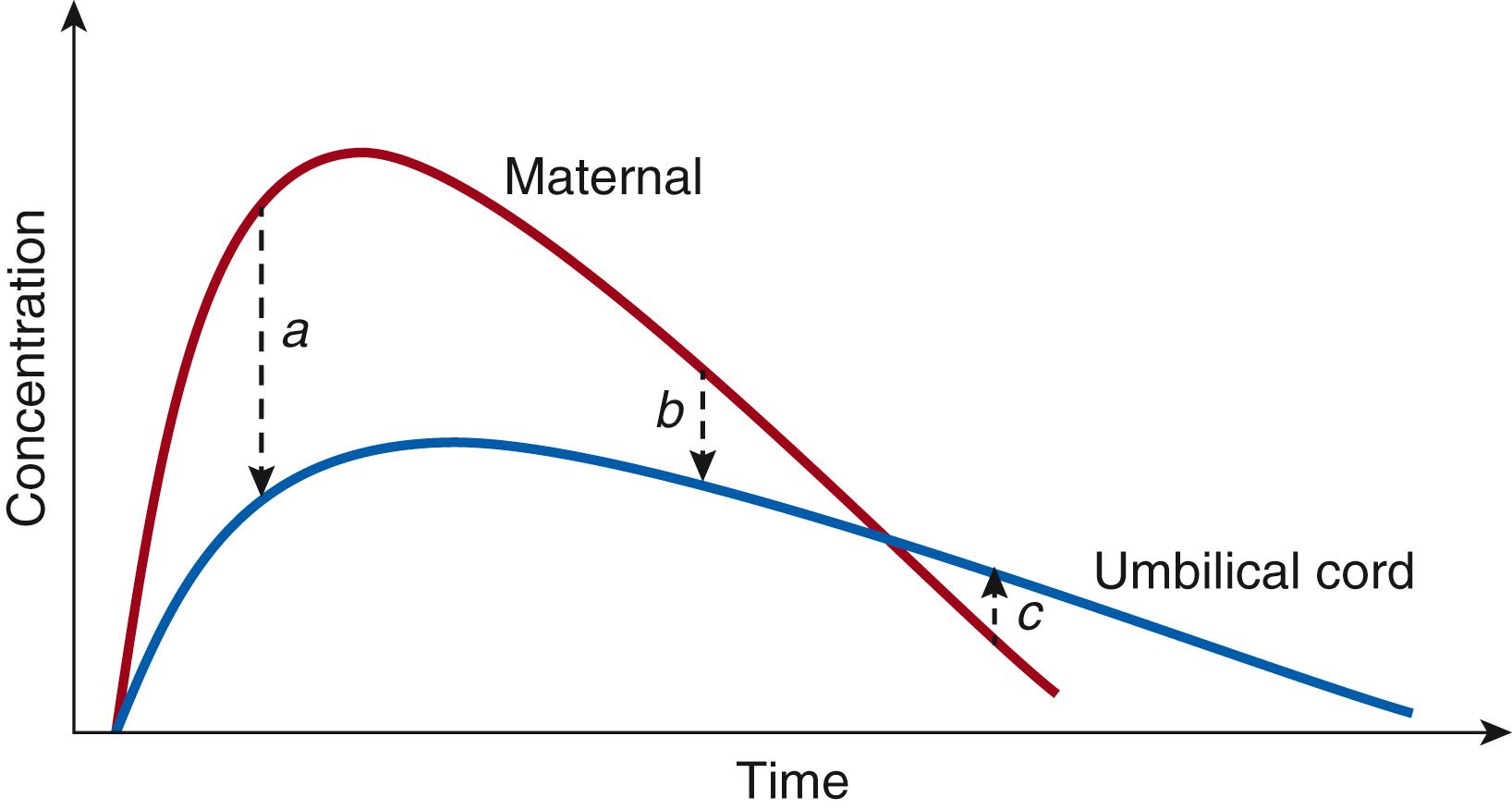
Taking the ethical constraints into account, in vivo quantification of the disposition of clinically indicated drugs in maternal compartments is feasible. In contrast, human fetal sampling is restricted to specific procedures. The most commonly reported approach is paired sampling (umbilical cord and maternal blood) at delivery. There are limitations using this approach, since—depending on the timing of paired sampling—the maternal/fetal ratio will be different, so that a ratio based on the area under the curve (AUC maternal/fetal) is likely more accurate ( Fig. 17.1 ). However, this necessitates pooled data within a broad time interval. Other opportunities to collect samples are ultrasound-guided transfusion, fetal surgery, or termination of pregnancy following timed maternal drug exposure. Serial fetal sampling in the human setting is at present not possible, while in vivo transplacental kinetics are derived from different subjects studied at various times. , Besides fetal blood, coelocentesis in the first trimester, amniotic fluid (both more commonly used for genetic diagnoses), or quantification of drugs and metabolites in meconium or hair samples (more commonly used for toxicology) after delivery are other matrices to consider. However, these methods were initially developed for developmental (environmental, qualitative exposure) toxicology, and cannot simply be applied in kinetic models to extrapolate quantitative drug transfer to the fetus and estimate subsequent risks. Therefore, we need additional in vivo or in vitro models to estimate an s kinetic model (distribution between maternal and fetal circulation).
The use of animal models is helpful, but their relevance may be limited because of difficulties in extrapolating results to humans: only humans have human placentas . , , Placental structures differ extensively between species. Based on the macroscopic characteristics, a classification of chorioallantoic placentas has been suggested in (1) zonary (carnivore like dogs or cats), (2) diffuse (horses, pigs), (3) cotyledonary (ruminants such as cows, sheep or goats), or (4) discoid (primate and rodents). , , Interspecies differences are also defined according to the Grosser classification, which focuses on placental barrier structure and degree of maternal tissue erosion. This classification distinguishes four types in eutherian mammals: syndesmochorial, epitheliochorial, endotheliochorial or hemochorial (human, primates, but also rodents) types. Hemochorial placentation can subsequently be subdivided in three subtypes, based on the number of trophoblastic cells at the villous surface: mono- (human), and di- or trichorial (rodent). , ,
In vitro models can also contribute to the understanding of placental drug transfer. Such models can mimic the bicompartmental structure by culturing cells—commonly derived from choriocarcinoma cell lines—on a porous filter in a multiwall culture plate. BeWo cell lines hereby result in a cellular monolayer with tight junctions. , Zhang and colleagues used Caco-2 cell monolayer permeability patterns to predict placental transfer, a model more commonly used to predict intestinal drug permeability. , A microphysiologic model of the human placental barrier (“placenta on a chip”) with co-culture of human trophoblast cells and fetal endothelial cells in a physiologically accurate spatial arrangement has been reported. ,
Human placenta obtained at birth provides an obvious approach to investigate the human placenta using in vitro perfusion studies. , , The technique of dual perfusion of the human placental lobule provides potential valuable information on the extent and rate of net transfer of drugs, including placental drug metabolism. Both maternal-to-fetal clearance and fetal-to-maternal clearance can be standardized by comparison with antipyrine clearance, and maternal or fetal flow can be modulated. These tissues can also be used to quantify placental drug metabolism or active transporter processes. , , Hutson and colleagues provided a systematic review on performance of the single placental lobule perfusion model to predict placental drug transfer. In their hands, the fetal-to-maternal drug concentration ratios matched well with in vivo samples, commonly taken as single umbilical cord samples at delivery. Once modeling for differences in maternal and fetal/neonatal protein binding and blood pH was established, the perfusion results were able to accurately predict in vivo transfer at steady state ( r = 0.92). Of the 70 different compounds evaluated, 49 (70%) showed placental transfer (fetal/maternal at least 0.1) in both models, 9 (13%) showed limited transfer (fetal/maternal <0.1), and for 12 (17%) there were discrepancies between in vitro and in vivo observations.
There are also obvious limitations of these models: the experimental period is limited to a few hours; the material is human placenta at delivery (either term or preterm) but not at early gestation, and the model in itself does not fully allow for observation of physiologically relevant parameters, such as blood flow (important for rapidly transferred compounds), plasma protein binding (affects the free concentration), and fluctuations of concentrations in the maternal and fetal compartments. , , To further illustrate this, it seems that the equilibrium between the maternal and fetal compartment happens faster in vivo than in the in vitro perfusion model (e.g., morphine 5 minutes versus 120 minutes; bupivacaine 2 to 5 minutes versus 60 minutes). These differences can likely be explained by differences in perfusion rates and differences in the villus surface area, and may reflect some of the limitations of this model.
There are two aspects that need more consideration in future placental studies to better reflect the relevant maternal-fetal setting of women receiving chronic pharmacotherapy. First, placenta tissue accumulation has been described in these in vitro models, for example, tacrolimus or sildenafil in “naïve” unexposed placenta, so that the results may not be simply extrapolated to a chronic setting. , Second, maternal diseases such as preeclampsia in themselves may affect the permeability characteristics of the placenta (as documented for sildenafil) in addition to maternal blood flow characteristics. ,
Regardless of the model used to estimate placental transfer, integration of the different pieces of information (the system pharmacology approach, PBPK ) is the way forward to secure further progress. One hereby integrates available in vitro or in vivo observations to guide rational study design or to support dose adjustment for pregnant women. , , , By integrating all physiologic, preclinical, and clinical data, anticipated changes during pregnancy (maternal, fetal, placental) can be quantified using PBPK. , , The growing success based on this integrated approach is illustrated in Table 17.2 . , The different compounds reflect different clearance pathways (glucuronidation, cytochrome mediated metabolism, renal route), with different approaches to model the placental barrier (ex vivo cotyledon, cell line observations, or estimated permeability based on the physicochemical characteristics), while all were subsequently validated based on umbilical cord blood observations, commonly matched with maternal data. This stresses the relevance of high-quality clinical observational data.
| Reference | Compound | Placental Model | Validation fetal Compartment |
|---|---|---|---|
| Liu et al. | emtricitabine acyclovir , both renal (GFR + tubular) |
Ex vivo cotyledon perfusion + apparent Caco-2 cell permeability + OSP estimated permeability (physicochemical characteristics) | Model matched with maternal + umbilical cord blood data |
| Mian et al. | acetaminophen (UGT1A1 + sulfation + CYP2E1) | Ex vivo cotyledon perfusion + apparent Caco-2 cell permeability + OSP estimated permeability (physicochemical characteristics) | Model matched with maternal + umbilical cord blood data |
| Freriksen et al. | dolutegravir (UGT1A1 + CYP3A) | Ex vivo cotyledon perfusion. | Model matched with maternal + umbilical cord blood data |
| Atoyebi et al. | thalidomide (hydrolysis + CYP2C19) efavirenz (CYP1A2,2A6,2B6,3A) | A priori assumption of bidirectional passive diffusion (Fick’s law) for both drugs | Model matched with maternal + umbilical cord blood data for efavirenz |
| Zhang et al. | zidovudine (UGT2B7) theophylline (CYP1A1) |
Apparent Caco-2 cell line permeability, verification, passive permeability drugs | Model matched with maternal + umbilical cord blood data for both drugs |
| De Sousa-Mendes et al. | nevirapine (CYP3A4,2D6,2B6) | Ex vivo cotyledon perfusion | Model matched observed umbilical cord blood data |
| De Sousa-Mendes et al. | tenofovir emtricitabine, both renal (GFR + tubular) |
Ex vivo cotyledon perfusion | Model matched with maternal + umbilical cord blood data for both drugs |
Although such integrated PBPK models are useful tools, we should still be aware that fetal concentration-time profiles are crucial biomarkers of fetal outcome, as clinical pharmacology covers both PK (concentration-time) and pharmacodynamics (concentration-effect). Moreover, (side)-effects do not always necessitate fetal exposure, as (side)-effects can be mediated at the level of the placenta with secondary fetal effects.
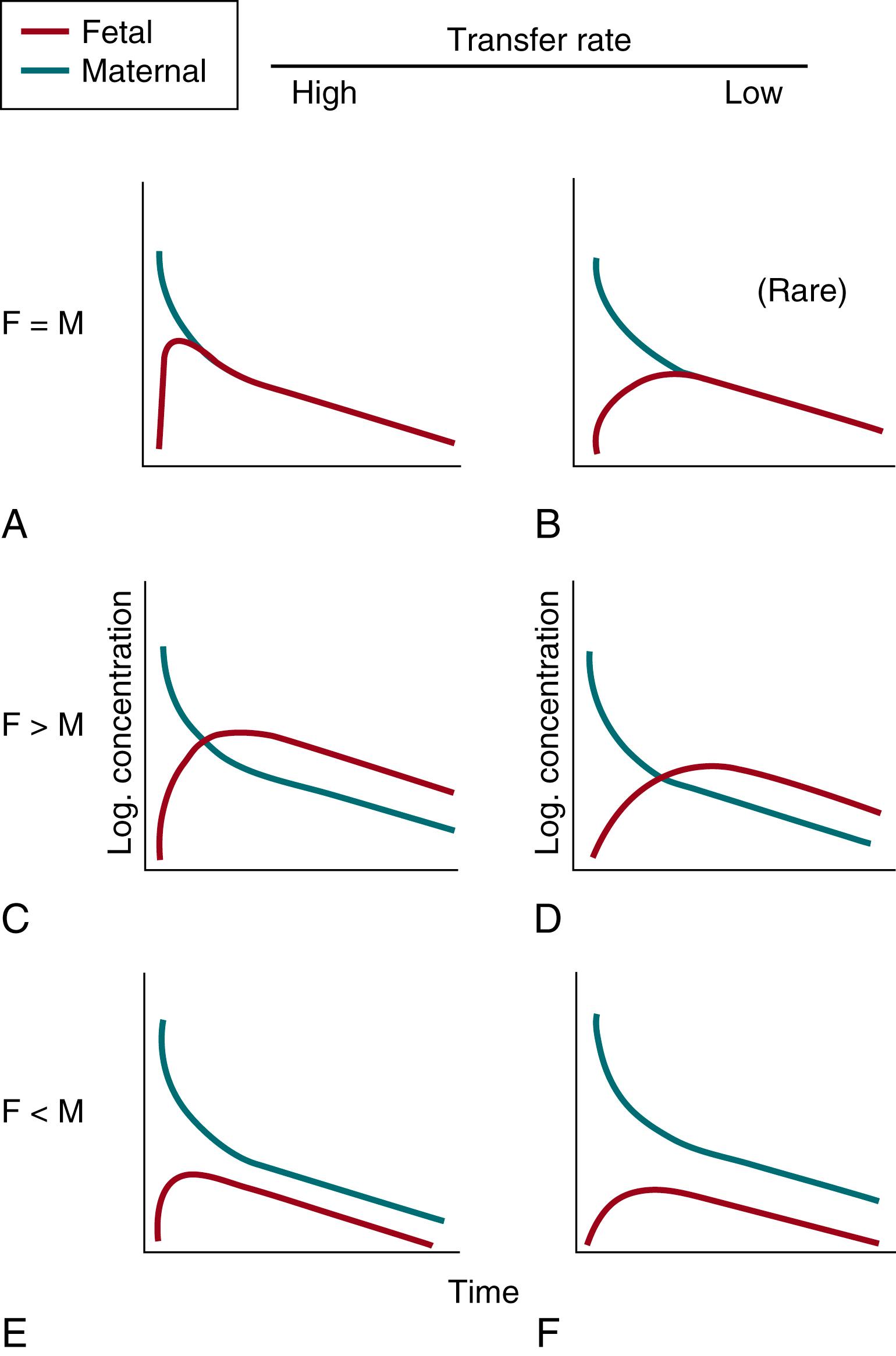
The rate and extent of drug exchange to embryo or fetus are determined by numerous variables (see Table 17.1 ), rarely resulting in a “mirror” pattern of concentration-time profiles in both mother and fetus. The combination of these variables results in different archetypical patterns of PK concentration-time profiles in the maternal and fetal compartments ( Fig. 17.2 ). Considering these archetypical patterns is a prerequisite for rational interpretation of a dataset. Although “rich” datasets on placental transfer are rare, attempts have been made to assign the kinetics of some drugs, on a tentative basis, to the patterns defined in Fig. 17.2 . Examples of drugs with transplacental pharmacokinetics according to the different patterns are listed in Box 17.1 .
| Pattern A or B | Pattern C | Pattern D | Pattern E | Pattern F |
|---|---|---|---|---|
| Some barbiturates Thiopental Pentobarbital Secobarbital Antipyrine Promethazine Ritodrine Magnesium (sulfate) Thiamphenicol Digoxin Ketamine Dexmedetomidine Acetaminophen Nucleoside reverse transcriptase inhibitors (abacavir, lamivudine, stavudine) Nevirapine |
Some benzodiazepines Diazepam Lorazepam Desmethyldiazepam Oxazepam Valproate Salicylate Nalidixic acid Nicotine Urea Some penicillins Ampicillin Penicillin G Methicillin Azidocillin |
Ascorbate Colistimethate a Furosemide Meperidine Indomethacin Some cephalosporins Cephalothin Cefazolin Cephapirin Cephalexin Some aminoglycosides Gentamicin Kanamycin Amikacin |
Amide-type local anesthetic agents Lidocaine Bupivacaine Some β -adrenoreceptor blockers Propranolol Sotalol Labetalol Dexamethasone Betamethasone Cimetidine Ranitidine Methadone Remifentanil Propofol Some sulfonamides b Protease inhibitors (atazanavir, darunavir) |
Heparin TCDD Quaternary ammonium compounds Tubocurarine Succinylcholine Vecuronium Pancuronium Fazadinium Alcuronium Elemental ions (Cd, Hg) Fenoterol Chlorthalidone Etozolin (ozolinone) Dicloxacillin Erythromycin Clarithromycin Nitrofurantoin Atosiban |
a Polypeptide antibiotic, molecular weight 1200.
b In the first and second trimester. TCDD , 2,3,7,8-Tetracholorodibenzo-p-dioxin.
Pattern A ( Fig. 17.2 ) is applicable for a drug that crosses the placenta rapidly and subsequently distributes rapidly within a single fetal compartment, to quickly attain equilibrium with the maternal compartment. Fetal concentrations rapidly rise to reach maternal plasma concentrations; thereafter, the fetal and maternal curves overlap. This is commonly based on differences in concentration-driven “back leak” from the fetal to maternal circulation. When maternal-fetal exchange is rapid, two additional patterns are possible: as soon as equilibrium between the maternal and fetal compartments has been attained, fetal concentrations may exceed maternal plasma levels (see curve in pattern C in Fig. 17.2 ), or they may be less than the corresponding maternal plasma levels (see curve in pattern E in Fig. 17.2 ). As discussed later, differential plasma protein binding (total versus free concentration) or a pH gradient in the maternal-to-fetal compartment (pH difference affects ionization) may be responsible for the relatively large (curve in pattern C ) or small (curve in pattern E ) extent of fetal drug exposure after equilibrium between the maternal and fetal compartment is attained.
When the rate of placental transfer is low, pattern D ( Fig. 17.2 ) is often applicable for the maternal-fetal unit. The increase in drug concentration in the fetus is slow. However, because the transport of drug from the fetus back to the mother also is slow, fetal concentrations exceed maternal plasma levels after the crossover point of the two curves. Thus, the fetus can be considered as a “deep” compartment. The same is applicable to the amniotic fluid compartment if fetal renal elimination occurs (e.g., cefazolin PK model). , Protein binding or pH gradient may affect the fetal-maternal concentration gradient once the distribution equilibrium has been reached ( pattern D ) . In pattern F , the fetal concentrations never reach the corresponding maternal plasma values because the plasma protein binding or the pH gradient favors higher maternal than fetal concentrations (e.g., differences in protein binding capacity). Alternatively, efficient fetal clearance (e.g., fetal kidney into amniotic fluid) may be responsible for the relatively low fetal drug levels. In pattern B —rarely observed—drug transport is slow from mother to fetus but rapid from fetus to mother. This implies an active or facilitated transport system (efflux transporters).
Considerations in defining patterns may appear of theoretical interest only. However, these patterns reflect the relevance of cautious interpretation of experimental and clinical studies when, for instance, only single-point paired maternal/fetal samples are available. If samples were collected only before crossover (see pattern C, Fig. 17.2 ), the wrong conclusion (i.e., relatively low fetal exposure) could be made. It might therefore be prudent to vary sampling times—if feasible—in a naïve pooled dataset (“clever sparse opportunistic sampling”) to facilitate concentration-time profiles description. , ,
For an adequate description of fetal exposure, both maternal and fetal concentration-time curves must be defined, because both the transfer rate (high A,C,E, versus low B,D,F) and the extent (ratio fetal-to-maternal, A,B to C,D to E,F) matter if the goal is to describe exposure (AUC concept) (see Figs. 17.1 and 17.2 ).
The placental “barrier” functions include passive diffusion, facilitated diffusion, active transport, and pinocytosis, or endocytosis. Passive diffusion and its mechanisms will be discussed first and will be followed by a section on selective, “non-passive” transporter mechanisms and placental drug metabolism.
Passive diffusion is primarily driven by the concentration gradient between the maternal and fetal compartments, further modulated by maternal, fetal, and placental blood flow. The rate and extent of drug transfer mainly relates to the physicochemical and structural characteristics of the specific compound, as well as to the physiologic characteristics of the maternal-placental-embryonic-fetal unit. a
a References 2, 5, 9, 16, 20, 32–34.
The concept of the placenta as a lipoid membrane is hereby useful to describe and predict the impact of physicochemical characteristics of a specific compound on its placental transfer. For lipophilic drugs, the maternal and fetal blood flow is critical for the rate of placental exchange over the placenta (flow-limited), as opposed to the hydrophilic drugs, which are permeability-limited. In contrast, a drug cannot passively pass the placental barrier in its ionized or charged form. a
b References 2, 5, 9, 16, 20, 32–34.
Most drugs cross the placental membranes by passive diffusion. The rate of diffusion is directly proportional to the surface area of exchange and the maternal-to-fetal concentration gradient across the membrane, and inversely proportional to the membrane thickness. This rate is governed mainly by physicochemical factors according to Fick’s law a
a References 2, 5, 9, 16, 20, 32–34.
:
where A = area of exchange, d = membrane thickness, Δ c = drug concentration gradient across the membrane (e.g., difference between maternal and fetal plasma drug concentrations), and D = diffusion constant for the drug.
This definition reflects the impact of gestational age (area of exchange, membrane thickness), dose, and maternal disease characteristics (area of exchange, membrane thickness) or treatment modalities (e.g., prenatal lung maturation affects placental growth). b
d References 2, 5, 9, 16, 20, 32–34.
To further illustrate its application, placental diffusion parameters will be upscaled to the size of the placenta from in vitro cotyledon transfer parameters to placental transfer (see Table 17.2 , as reported for emtricitabine, acyclovir, acetaminophen, dolutegravir, nevirapine, tenofovir, emtricitabine). , , From this equation, it is predicted that a larger area of placental exchange (A) , consisting of membranes with limited thickness (d) , favors placental bidirectional drug transfer. A, d, and Δ c can be determined in a model; however, D is far more difficult to predict because it results from the interactions between membrane and molecule. The resistance within the tissue layers interposed between the maternal and fetal circulations (compartments) limits diffusion, significant for hydrophilic molecules.
In the human placenta, two layers (trophoblast, endothelium) contribute to this diffusional resistance. Hydrophilic molecules either have to cross these layers (i.e., the membrane hypothesis ) or find their way through water-filled channels that extend through the trophoblast and communicate with the intracellular channels of the endothelial layer (i.e., the aqueous pores hypothesis ). Faster placental transfer relates to better lipid solubility and low ionization and protein binding of drugs with a molecular weight (MW) of less than 500 Da.
The permeability of lipid-soluble substances is much higher. For these substances, the placental transfer rate is limited mainly by availability of drug at the area of exchange, which is ultimately determined by blood flow. b
c References 2, 5, 9, 16, 20, 32–34.
Therefore the initial maternal-fetal concentration gradient, Δ c, depends on uterine and umbilical blood flow. This mathematical concept has been applied to predict placental barrier permeability using the Quantitative Structure-Activity Relationship (QSAR) method and has been further developed in the meanwhile. Such a QSAR hereby integrates the chemical structures of molecules and the available biologic properties to estimate placental permeability (see Table 17.2 , as reported for emtricitabine, acyclovir, and acetaminophen). ,
A number of studies have noted that as placental thickness and the number of placental layers decreases and the area of exchange increases during gestation, increased placental transfer occurs. b
f References 2, 5, 9, 16, 20, 32–34.
However, in vivo, the placenta barrier cannot fully be described by only anatomic parameters such as area or thickness. Thornburg and Faber found that in rabbit placenta, the fetal endothelium, which is not markedly altered during pregnancy, is the layer defining the transfer rate of many drugs; area of exchange (A) and membrane thickness (d) apparently are of secondary importance. Maternal-to-fetal transfer occurs across the placental barrier, made up of both the syncytiotrophoblast on the maternal side and the endothelial cell layer on the fetal side. The diffusion of drugs across the hemotrichorial placenta (mouse, rat) often is faster than across the hemomonochorial placenta (monkey, human). The earlier mentioned placenta-on-a-chip approaches will likely generate relevant insights in aspects of interspecies “translation” of such findings. ,
Drugs have the greatest chance of crossing the placenta if they are (1) lipid-soluble, (2) weak acids, and (3) of low MW (<500 Da) (see Box 17.1 ). Another important covariate is plasma or tissue protein binding. c
g References 2, 5, 9, 16, 20, 32–34.
As mentioned earlier, modeling approaches based on—for example, the QSAR method—hereby integrate these different aspects to predict the phenotypic placental barrier permeability pattern and have been integrated in PBPK models (see Table 17.2 ). ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here