Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pericardium is an important structure in the evaluation of patients with cardiovascular disease. Understanding pericardial anatomy and the complex hemodynamics associated with pericardial pathology is critical in assessing the pericardium and its impact on cardiovascular function. Unfortunately, clinical evaluation through history and physical examination, clinical laboratory tests, and electrocardiograms (ECG) may be nonspecific and overlap with other clinical syndromes in patients with pericardial disease. Advanced noninvasive imaging, including echocardiography, cardiac chest computed tomography (CT), and cardiovascular magnetic resonance (CMR), is vital in the evaluation and diagnosis of pericardial disease. When combined with a high degree of clinical suspicion, these modalities help advance our understanding pericardial pathophysiology and, ultimately, guide clinicians to optimal treatment and therapies.
Several noninvasive imaging modalities are available to assess the pericardium, with each modality having its strengths and limitations. As a result of the complex nature of the pericardium and its impact on cardiovascular hemodynamics, multimodality imaging is often required to completely evaluate the pericardium.
Chest x-radiography is often the first imaging modality that may suggest pericardial disease and prompt further testing. An enlarged cardiac silhouette may suggest the presence of a pericardial effusion, although this finding has limited sensitivity and poor specificity. Additionally, the chest x-ray (CXR) may detect pericardial calcifications and raise the possibility of constrictive pericarditis; however, calcification is not necessary for pericardial constriction and, in fact, is frequently absent in clinical presentations of constriction.
Direct visualization of the heart through transthoracic echocardiography (TTE) is the most common noninvasive cardiovascular imaging modality for the evaluation of the heart and pericardium. Two-dimensional (2D) TTE with Doppler examination is an excellent, readily available, portable tool for the identification and assessment of pericardial disease. Real-time TTE can detect abnormal cardiac function and hemodynamic compromise secondary to underlying pericardial disease, including pericardial effusions and tamponade physiology. However, patient body habitus, chest trauma, and/or poor acoustic windows attributed to underlying lung disease (e.g., emphysema) may result in suboptimal views and limit TTE in visualizing the entire pericardium. Pericardial thickness is also not reliably assessed by TTE. Although transesophageal echocardiography (TEE) may be more accurate, it is moderately invasive, and the field of view remains limited to the available acoustic windows.
Cardiac CT and CMR provide a larger field of view than echocardiography, enabling visualization of the entire pericardium and the surrounding structures, providing excellent anatomic definition without limitation to specific windows. Although optimal imaging of the pericardium with CT and CMR requires ECG gating to minimize motion artifact, standard chest CT and nongated magnetic resonance imaging (MRI) performed for other indications often reveal most pericardial abnormalities, particularly pericardial effusions or calcifications. Cardiac CT and CMR may be useful when TTE findings are nondiagnostic, particularly for assessment of pericardial thickness and evaluation of pericardial constriction, loculated pericardial effusions, hematomas, and/or pericardial masses.
A specific advantage of CT (as compared with CMR) is its ability to identify pericardial calcification, a frequent, but not obligatory, finding in constrictive pericarditis. Cardiac CT with multidetector scanners and ECG synchronized data acquisition is now widely available, offering the advantage of very short scan times with limited cardiac and respiratory motion artifact. Additionally, CT provides excellent spatial resolution, as a result of the use of multidetector, high-resolution volumetric acquisition. Retrospective ECG-gated CT may also provide reasonable temporal resolution to assess interventricular septal motion in the evaluation of constrictive physiology, although this is better assessed by TTE or real-time CMR.
Disadvantages of CT include use of ionizing radiation, the need for intravenous iodinated contrast for adequate blood–tissue contrast, and limited hemodynamic assessment. Additionally, CT images may not adequately discriminate pericardial fluid from thickened pericardial tissue.
CMR provides a comprehensive evaluation of the pericardium, both anatomically and hemodynamically, without ionizing radiation. CMR has the ability to obtain morphological, functional, and hemodynamic information in a single examination, which is frequently required when assessing pericardial disease and its impact on cardiovascular function. Balanced steady-state free precession (bSSFP) cine imaging is used to evaluate left ventricular (LV) and right ventricular (RV) motion and can assess the impact of pericardial disease on overall cardiac function. CMR also provides advantages over CT and echocardiography in its ability to characterize the contents of pericardial effusions and pericardial masses through the use of T1-weighted and T2-weighted imaging, first-pass perfusion, and late gadolinium enhancement (LGE). Additionally, real-time imaging by CMR may give vital hemodynamic assessments regarding cardiac motion and inflow patterns while the patient is free-breathing, allowing for the identification of constrictive and/or tamponade physiology. Multiplanar and multisequence CMR can be used to characterize the extent and composition of pericardial abnormalities and real-time CMR sequences allow for functional assessment, including chamber compression caused by ventricular interdependence and interventricular septal motion. Administration of conventional extracellular gadolinium (Gd)-based contrast agents may assist in detecting of pericardial inflammation or enhancing masses.
The pericardium is an elastic dual-layered fibroserous membrane that surrounds nearly the entire heart and extends superiorly to the origins of the great vessels. It consists of an outer fibrous component and a dual-layered inner serous sac. The tough fibrous outer pericardium is loosely attached to the sternum and costal cartilage (anteriorly) and the proximal great vessels (superiorly), and is more firmly attached to the central tendon of the diaphragm (inferiorly). The serous pericardium consists of an outer parietal layer, which is intimately adherent to the inside of the fibrous pericardium, and commonly referred to as the pericardium. The inner visceral serosal layer is adjacent to the heart (but not directly attached), covers the epicardial fat and vessels, and typically referred to as the epicardium. A film of clear pericardial fluid (~15–50 mL) normally separates the two serosal surfaces. The small amount of fluid minimizes the friction between the two layers as the heart expands and contracts. The serosal layers, consisting of the visceral and parietal pericardium, merge at two complex lines of reflection: one at the base of the aorta and pulmonary trunk, and the other at the insertions of the superior and inferior vena cavae and the four pulmonary veins. The entire pericardium generally lies between variable amounts of epicardial and pericardial adipose tissue.
The pericardial cavity is a complex space consisting of the pericardial cavity proper and interconnecting cul-de-sacs known as sinuses, which are further subdivided and connected to multiple recesses. Because of the merging of the serosal layers at two distinct points, the reflections of the serous pericardium become arranged as two complex tubes; one enclosing the base of the aorta and main pulmonary artery and the other enclosing the vena cavae and the four pulmonary veins. The transverse sinus is the space between these two pericardial tubes and lies superior to the left atrium (LA) and posterior to the ascending aorta and main pulmonary artery. The transverse sinus connects to the following four recesses: superior aortic, inferior aortic, left pulmonic, and right pulmonic. The cul-de-sac delineated by the inferior border of the tube, surrounding the venous structures, is the oblique sinus. The oblique sinus is an inverted U-shaped space which lies posteriorly to the LA and includes the posterior pericardial recess. The pericardial cavity proper includes the postcaval, left pulmonary venous, and right pulmonary venous recesses. The transverse sinus is the space between these two pericardial tubes and lies superior to the LA and posterior to the ascending aorta and main pulmonary artery. The transverse sinus connects to the following four recesses: superior aortic, inferior aortic, left pulmonic, and right pulmonic recesses. These recesses are usually linear in appearance and become more band-like and rounded as they accumulate with fluid. Appreciating the normal appearance of these sinuses and recesses is important because these spaces can be misinterpreted as adenopathy, dissection, or an abnormality of an adjacent mediastinal structure.
Although the pericardium is not required for normal cardiac function, it has several homeostatic roles. These include (1) limitation of intrathoracic cardiac displacement; (2) maintenance of normal ventricular compliance; (3) balancing RV and LV output over several cardiac cycles through diastolic and systolic interactions; (4) buffering of changes in chamber filling and output; (5) assisting atrial filling via more negative pericardial pressure during ventricular ejection; (6) limitation of acute dilatation; (7) minimizing friction between cardiac chambers and surrounding structures; and (8) providing an anatomic and immunologic barrier to inflammation and infection from contiguous structures such as the lungs. However, the presence of an intact pericardium may be detrimental in clinical situations in which fluid rapidly fills the pericardial space, resulting tamponade physiology.
The normal pericardium is seen as a very thin linear density surrounding the heart. Discrimination of the pericardium from the myocardium by noninvasive imaging requires the presence of interposed epicardial fat or the presence of pericardial fluid with associated enlargement of the pericardial space. The thickness of the epicardial fat is increased in patients with obesity and/or diabetes and has been found to increase the risk of coronary atherosclerosis. The pericardium is best visualized over the right ventricle and, as a result of less epicardial and pericardial fat to provide adjacent tissue contrast, may not be well visualized around the left ventricle. Technical factors, such as spatial and temporal resolution, may affect pericardial thickness measurements because the normal pericardium is a thin irregularly shaped sac that moves with cardiac and respiratory motion, affecting the pericardial recesses to varying degrees. The superior aortic and left pulmonic recesses are less impacted by cardiac motion, whereas the inferior aortic recess, left pulmonary venous recess, and right pulmonic recess border the LA or left ventricle, and are often blurred because of cardiac motion.
The maximum normal pericardial thickness measured through advanced noninvasive imaging is typically 2 mm, which is slightly greater than normal cadaveric pericardial thickness, which measures up 1 mm. This difference is primarily caused by the inherent limitations of spatial and temporal resolution of CT and CMR. A possible pitfall in determining pericardial thickness by CT is that trace pericardial effusions, as well as partial volume effects, may produce the appearance of focal thickening. These imaging effects, along with normal intrinsic inhomogeneity in pericardial thickness, produce significant variations in pericardial thickness, and should not be mistaken for disease. The superior aortic recess of the transverse pericardial sinus, which surrounds the ascending aorta, may be mistaken for an aortic dissection or lymphadenopathy. The oblique pericardial sinus, which is located behind the LA, may simulate abnormalities in the esophagus, descending thoracic aorta, and subcarinal and bronchopulmonary lymph nodes. Recognizing the appearance of these normal structures is important to avoid mistaking them for mediastinal disease.
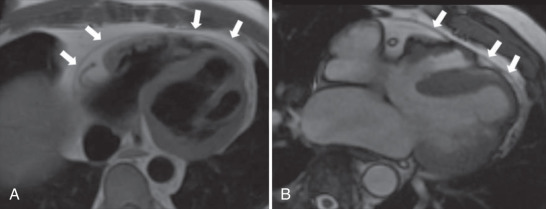
CMR, with its superior tissue contrast, is an excellent modality to evaluate the heart and pericardium. It is able to differentiate between small amounts of pericardial fluid and actual thickening. With CMR, the normal pericardium appears as a linear low-intensity signal between the high-intensity mediastinal and sub-epicardial fat. It is best visualized during systole ( Fig. 46.1 ). bSSFP cine imaging is used to evaluate cardiac motion and can be helpful in detecting pericardial effusions. Black-blood T1-weighted spin echo sequences, such as double inversion recovery fast spin echo, are commonly performed to evaluate the pericardial anatomy thickness and morphology. T2-weighted spin echo sequences, usually with a short tau inversion recovery sequence to null the signal from fat (referred to as a triple inversion spin echo sequence), is used to depict difference between pericardial thickening and small amounts of fluid. Normal pericardium will demonstrate low signal on both T1-weighted and T2-weighted sequences, whereas pericardial fluid will be high signal on the T2-weighted imaging. Pericardial inflammation may also demonstrate increased signal on T2-weighted images, but will commonly exhibit abnormal enhancement on the LGE imaging, helping to differentiate it from a small pericardial effusion. Real-time cine gradient echo imaging in the short axis during dynamic breathing allows for the detection of ventricular coupling in patients with suspected constrictive pericarditis. T1-weighted gradient echo myocardial tagged cine can be useful to detect the presence or absence of epicardial–pericardial slippage in cases with suspected fibrinous adhesions exist between pericardial layers. Phase contrast sequences using velocity encoded gradients can be used to assess for diastolic dysfunction and pulmonary venous flow patterns, which are abnormal in patients with restrictive physiology.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here