Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ultrasound is an excellent modality to evaluate head and neck masses in children because of its accessibility and lack of ionizing radiation.
Lesion location is an important factor to consider in evaluation of head and neck masses in children.
Common head and neck masses in children are related to lymph nodes, thyroglossal duct anomalies, branchial anomalies, inclusion cysts, and vascular anomalies.
Use of spectral Doppler waveforms can aid in identification of arterial components in vascular anomalies to narrow the differential diagnoses.
The goal in pediatric imaging is to perform a study with the lowest possible radiation exposure and least sedation to adequately answer the clinical question. Sonography, given a lack of ionizing radiation and its noninvasive approach, is almost invariably the initial imaging modality in a child with a neck lesion. Sonography is cost-effective, widely available, and portable. Ultrasound can illustrate normal cervical anatomy, evaluate vascular structures and lesions with spectral Doppler imaging, and delineate pathology with regard to location, size, and presence of calcification. Cystic masses are common pediatric neck lesions, and sonography is also exceptional at distinguishing solid from cystic lesions and assessing compressibility of lesions. Transducers with frequencies of 7.5 to 10 MHz are excellent for examination of the neck.
Limitations of ultrasound technique include the inability to evaluate bone, small field of view, and degree of soft tissue contrast. Because of these limitations, magnetic resonance imaging (MRI) and computed tomography (CT) are excellent adjunct modalities that can provide additional soft tissue and bone detail and further delineate extent of disease.
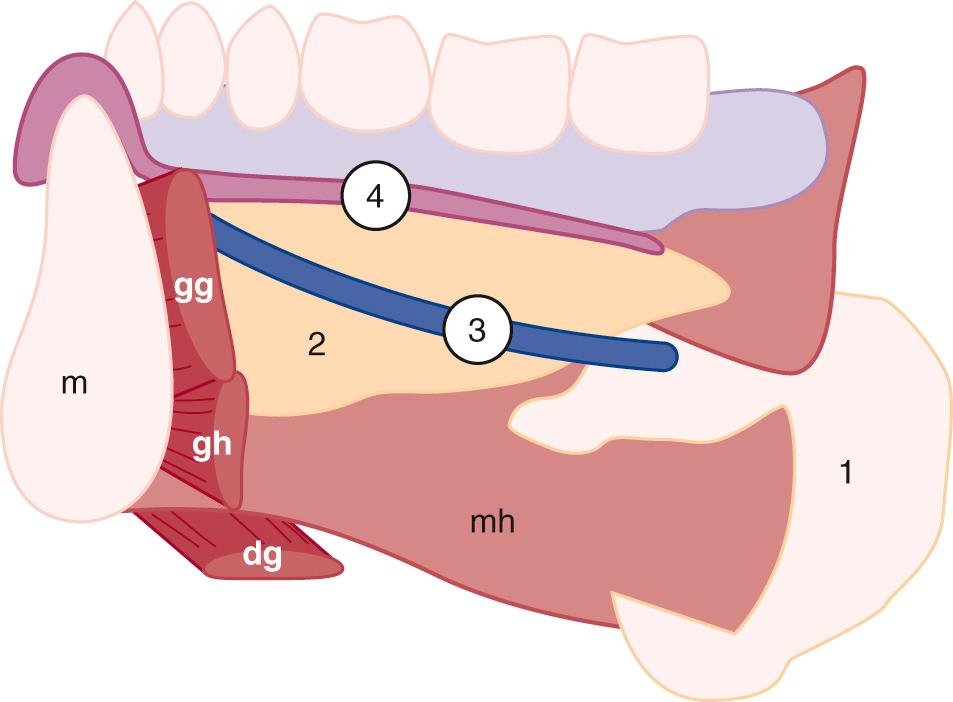
An understanding of normal cervical anatomy is essential to appropriately evaluate head and neck pathology. The soft tissues of the neck can be separated into boundaries of the superficial and deep spaces. The superficial fascia is primarily composed of subcutaneous fat. The platysma muscle, subcutaneous lymph nodes, and nerves lie within the superficial space. The deep cervical fascia, encircled by the superficial tissues, contains the major structures of the neck ( Fig. 48.1 ). The deep cervical fascia includes superficial, middle, and deep layers ( Fig. 48.2 ).
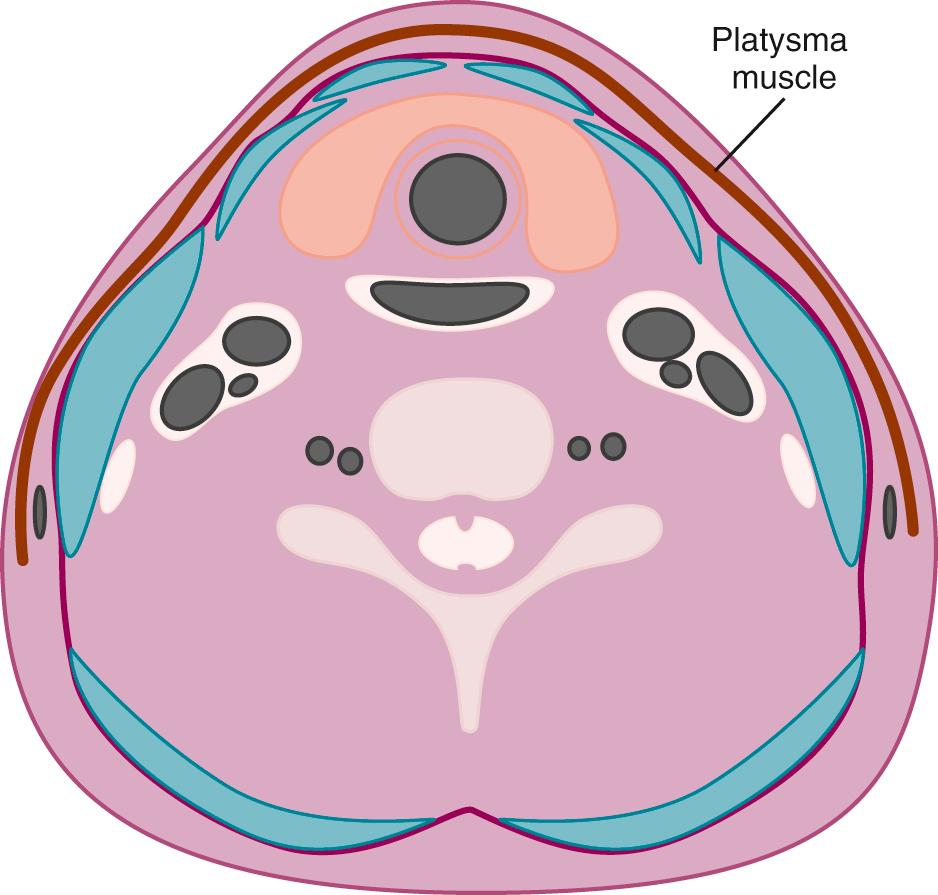
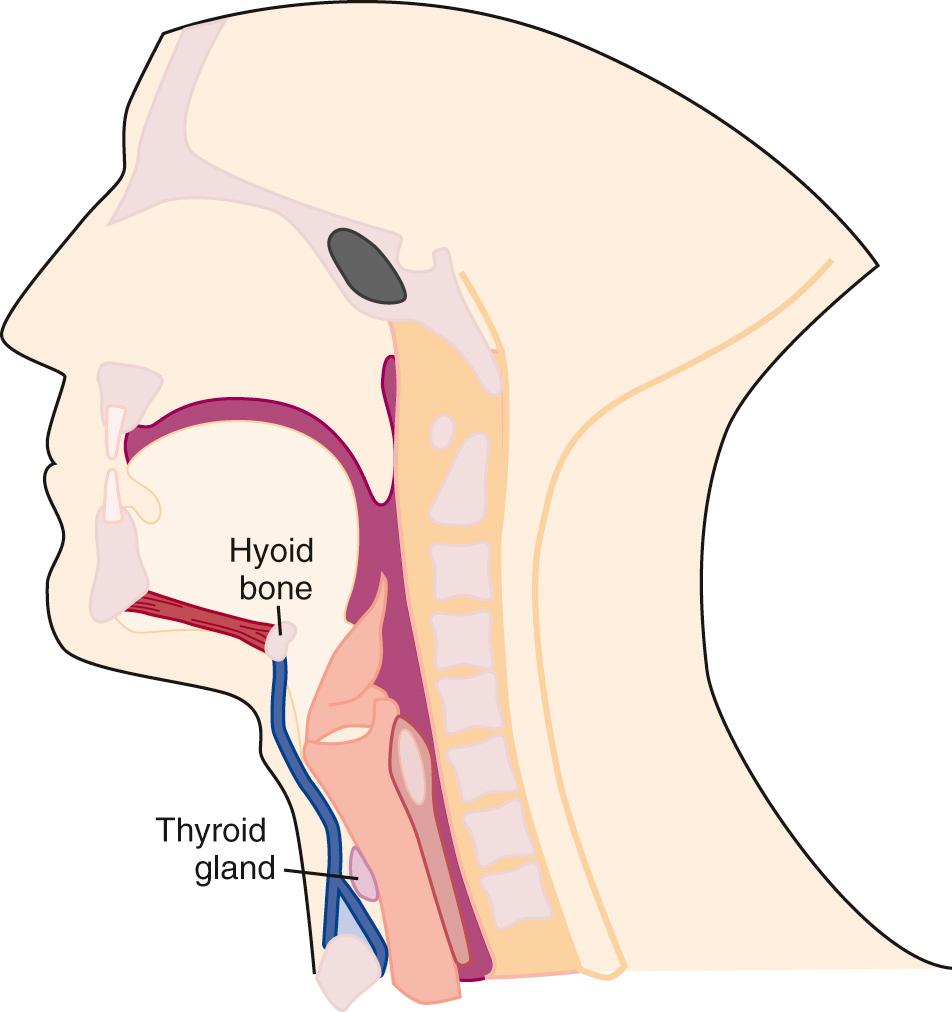
Simplifying the deep cervical fascial anatomy is best achieved by dividing the neck into suprahyoid and infrahyoid locations. The suprahyoid space includes the areas of the neck between the skull base and hyoid bone. The infrahyoid space is the area of the neck between the hyoid bone and clavicles. Some deep fascial planes and lesions extend within both the infrahyoid and the suprahyoid space . These spaces and lesions are described as “lacking definition by the hyoid.” Using this organization, with the knowledge of the normal anatomic elements in each space, the appropriate differential diagnosis for a mass can be proposed.
The suprahyoid space includes the superficial, middle, and deep layers of the deep cervical fascia ( Fig. 48.3 ). The superficial layer envelops three spaces. The parotid space includes the parotid gland, intraparotid facial nerve, retromandibular vein, lymph nodes, and external carotid artery. The masticator space includes muscles of mastication and the posterior body of the mandible. The submandibular space includes the sublingual and submandibular glands, as well as the adjacent lymph nodes, muscles of the tongue, and hypoglossal nerve. Ultrasound is most useful in evaluation of the spaces in the superficial fascial layer.
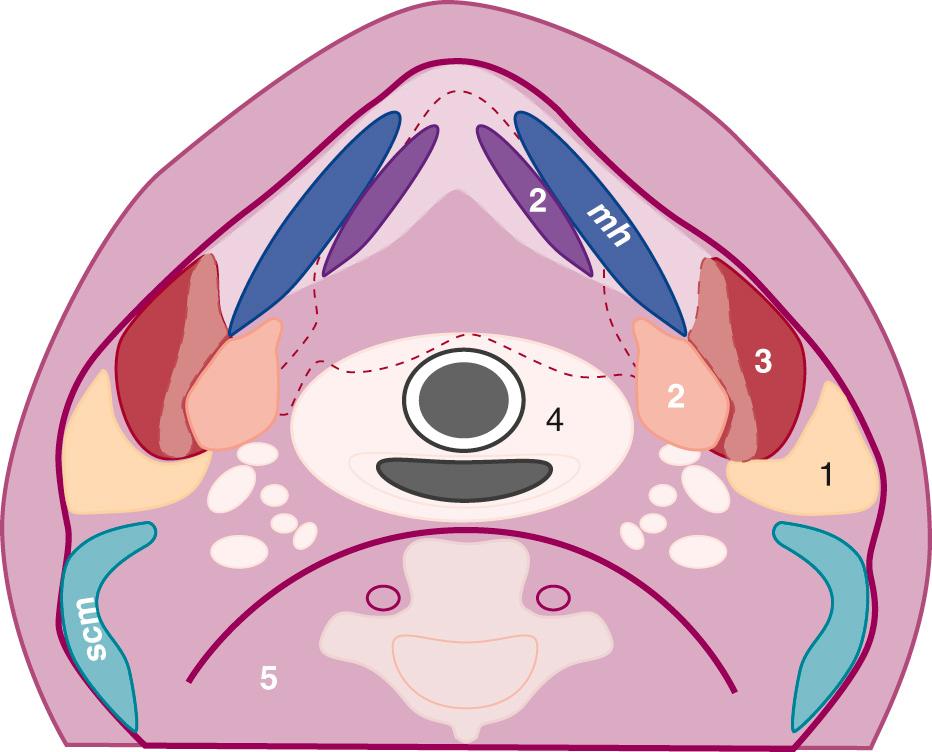
The middle territory of the suprahyoid space lies between fascial layers and includes the parapharyngeal and pharyngeal spaces. This middle layer, which includes lymphoid tissue, minor salivary glands, and fat encircling the pharynx, is difficult to visualize with ultrasound and is not discussed separately. The carotid space is formed from fibers of the middle and deep layers of cervical fascia. The retropharyngeal space is contained by layers of the middle cervical space anteriorly and deep cervical layers posteriorly. However, because the retropharyngeal space and prevertebral layer, a deep cervical space, cannot be separated, it is easiest to consider these layers together in the deep cervical facial layer as the retropharyngeal space. The carotid space and retropharyngeal space extend above and below the hyoid and are discussed later.
Because the parotid and submandibular areas primarily contain salivary tissue and thus have similar pathology, these two spaces are considered together. The major salivary glands that occupy these spaces include the parotid glands, the submandibular glands, and the sublingual glands.
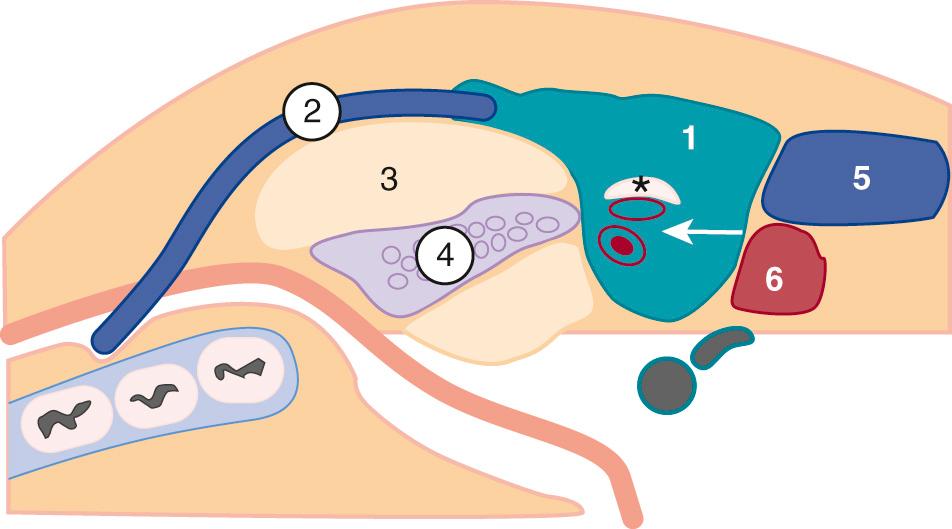
The parotid gland is the largest salivary gland. This encapsulated gland, containing lymphoid tissue, vessels, and nerves, wraps around the angle of the mandible anterior to the mastoid tip ( Fig. 48.4 ). Most parotid tissue lies superficial to the masseter muscle. In 20% of patients, an accessory parotid gland, which is a nodule of salivary tissue separate from the main parotid, can be identified on the masseter muscle. The parotid gland contains acini that drain through the Stensen duct, a structure that extends anteriorly to exit above the upper second molar. The duct lies approximately 1 cm below the inferior margin of the zygomatic arch. The facial nerve travels within the parotid gland, lying lateral to the retromandibular vein and posterior belly of the digastric muscle, which is the muscle deep to the mastoid tip. The facial nerve acts a boundary to divide the parotid gland into superficial and deep components. The deep lobe of the parotid, which accounts for 20% of the gland, lies adjacent to the parapharyngeal space and beneath the angle of the mandible.
Imaging of the parotid should be performed with a high-frequency transducer. Usually, linear transducers of 5 to 12 MHz are used. The entire gland should be evaluated in two perpendicular planes. Because the facial nerve is poorly delineated on ultrasound, the retromandibular vein, which lies directly deep to the nerve and lateral to the external carotid artery, is an excellent landmark to separate the superficial and deep lobes. Typically, the deep lobe can only be partially visualized, concealed by acoustic shadowing of the mandibular ramus. On sonography, the parotid gland is generally homogeneous and hyperechoic with regard to the adjacent muscle ( Fig. 48.5 ). The degree of echogenicity of the gland depends on the amount of intraglandular fatty tissue; the greater the fat content, the higher the echogenicity.
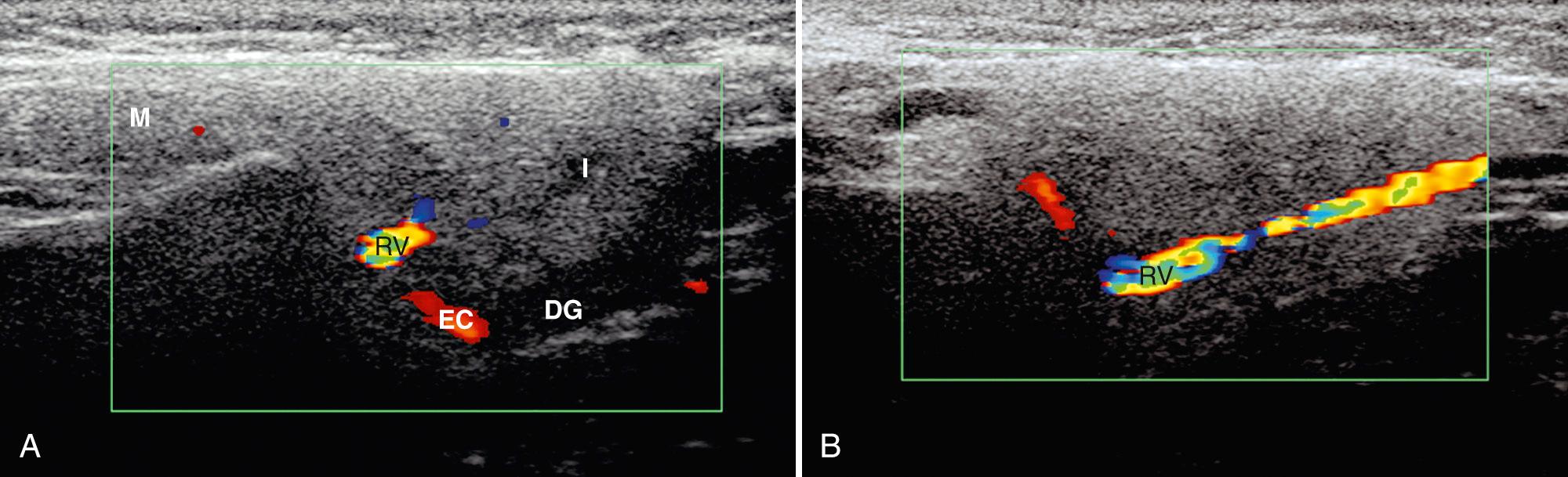
Normal intraparotid ducts may be visualized as linear reflective structures. The Stensen duct is usually not visible in the absence of dilation. In the parotid parenchyma, normal lymph nodes may be observed. Most nodes are identified at the upper or lower pole, are oval in shape, measure 5 to 6 mm in the short axis, and appear hypoechoic with a hyperechoic central hilum. Ultrasound is especially useful in differentiating intraparotid lesions from extraparotid masses and separating solid and cystic lesions in this location.
The submandibular space contains both the submandibular glands and the sublingual glands in addition to submandibular lymph nodes. The submandibular glands lie within the posterior submandibular space, whereas the sublingual glands are anterior ( Fig. 48.6 ). The submandibular glands are bordered by the mandible laterally and the mylohyoid muscle superiorly and medially. A small portion of the gland may pass posterior to the mylohyoid muscle to lie within the sublingual area. The space anterior to the submandibular gland contains lymph nodes. The torturous facial artery typically crosses the parenchyma laterally. The anterior facial vein can often be identified along the anterosuperior aspect of the gland. Medially, the lingual artery and vein may be evident. The Wharton duct, the excretory duct for the gland, extends along the mylohyoid and medial part of the sublingual gland to its orifice at the floor of the mouth. On sonography, the submandibular glands should be examined in two perpendicular planes, usually long and transverse, with a high-frequency linear transducer. The glands are triangular, homogeneous, and more echogenic than muscle but less echogenic than the parotids ( Fig. 48.7 ). On high-resolution sonography, fine linear streaks can be observed, representing intraglandular ductules. The Wharton duct may be visualized as a thin echogenic wall medial to the sublingual gland. Movement of the patient's tongue may aid in visualization of the duct.
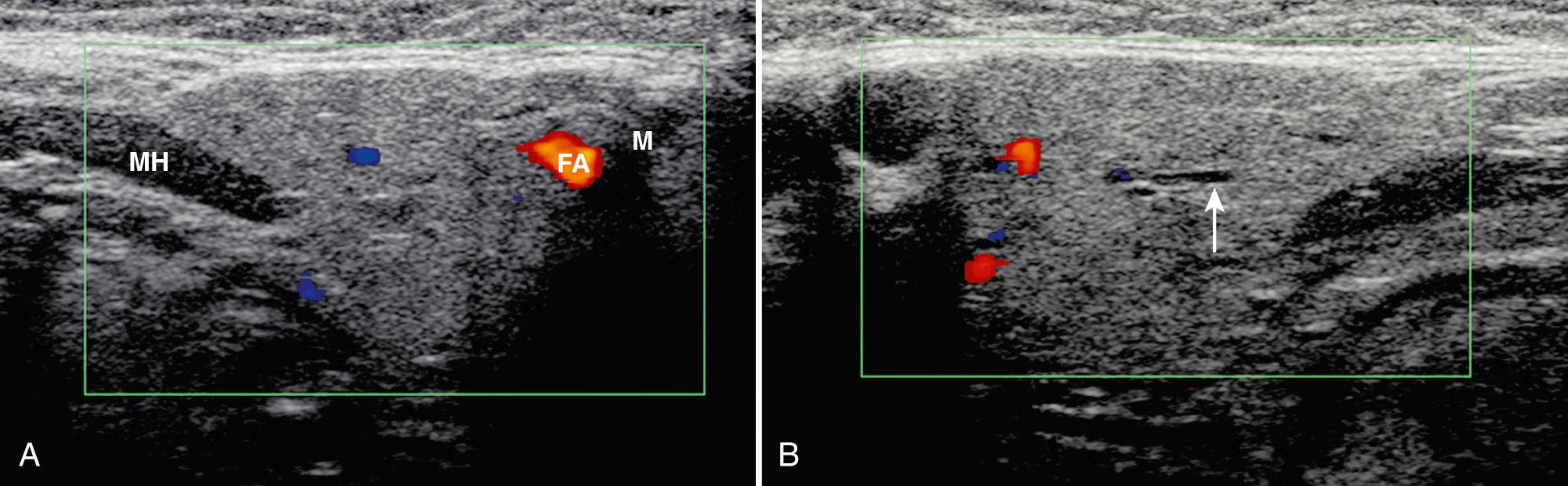
The sublingual gland lies on the mylohyoid muscle between the mandible and genioglossus muscle. In addition to the gland, the sublingual area includes the submandibular duct and sublingual vessels, which lie medial to the gland. Scanning of the gland should be performed with a high-frequency linear transducer submental in two perpendicular planes with the patient supine and the head retroflexed. A standoff pad can be helpful in accessing the anatomy. On transverse imaging the gland is oval, whereas on long sections the gland has a lentiform shape ( Fig. 48.8 ). The echogenicity of the sublingual gland is similar to the parotid and submandibular glands.
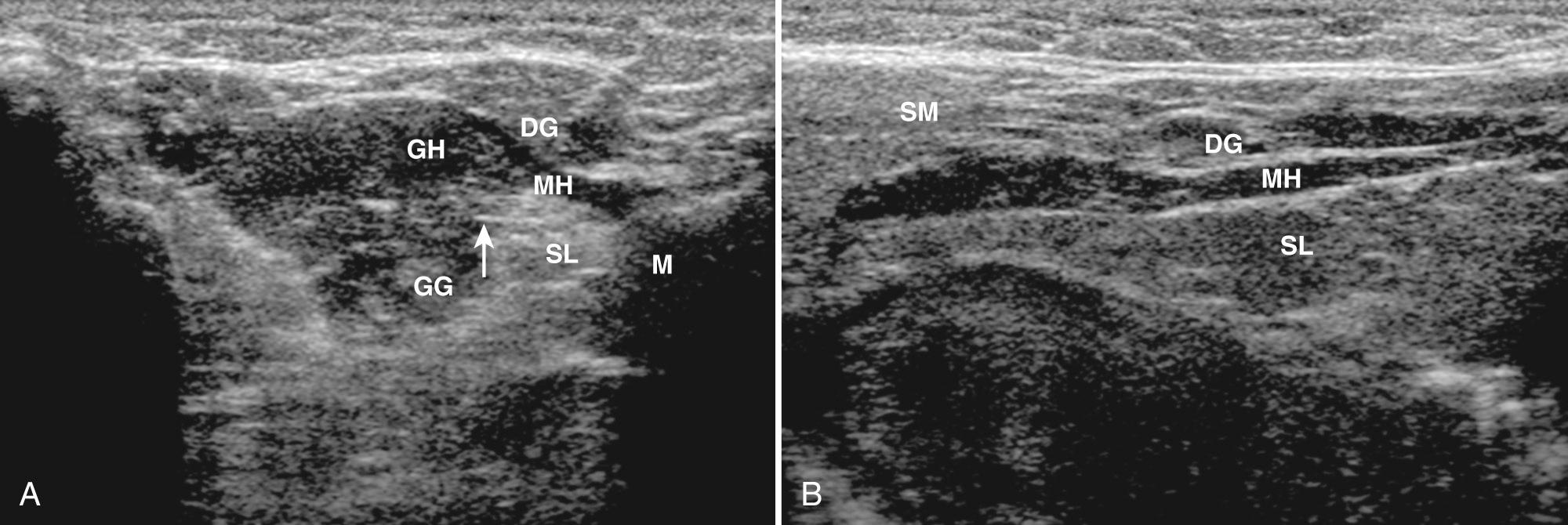
Most salivary gland pathology in children is caused by inflammatory lesions, which can be acute or chronic. The acute form may be secondary to viral or bacterial etiologies. The chronic form includes a long differential diagnosis (e.g., human immunodeficiency virus [HIV], Sjögren syndrome, sialoadenitis, sarcoidosis, other granulomatous processes ).
In children, viral salivary gland infections are the most common cause of acute inflammation. Endemic viruses, including mumps, mononucleosis, and cytomegalovirus (CMV), are the most common viral causes, causing painful unilateral or bilateral swelling of the salivary tissue. In 85% of cases, the parotid gland is involved. Although less prevalent with the advent of immunization, mumps is still the most common cause of parotitis. Mumps, a highly contagious worldwide infection spread by airborne droplets, is typically encountered in the winter and spring and primarily affects children younger than 15 years. The incubation period for this virus is 14 to 21 days, but the infection is contagious 3 days before the onset of swelling until the resolution of the swelling. With ultrasound, viral salivary infections show a diffusely enlarged gland that may have a normal, heterogeneous and/or hypoechoic echotexture with increased vascularity ( Fig. 48.9 ). Frequently there is bilateral involvement, although unilateral involvement may be seen in up to one-third of patients.
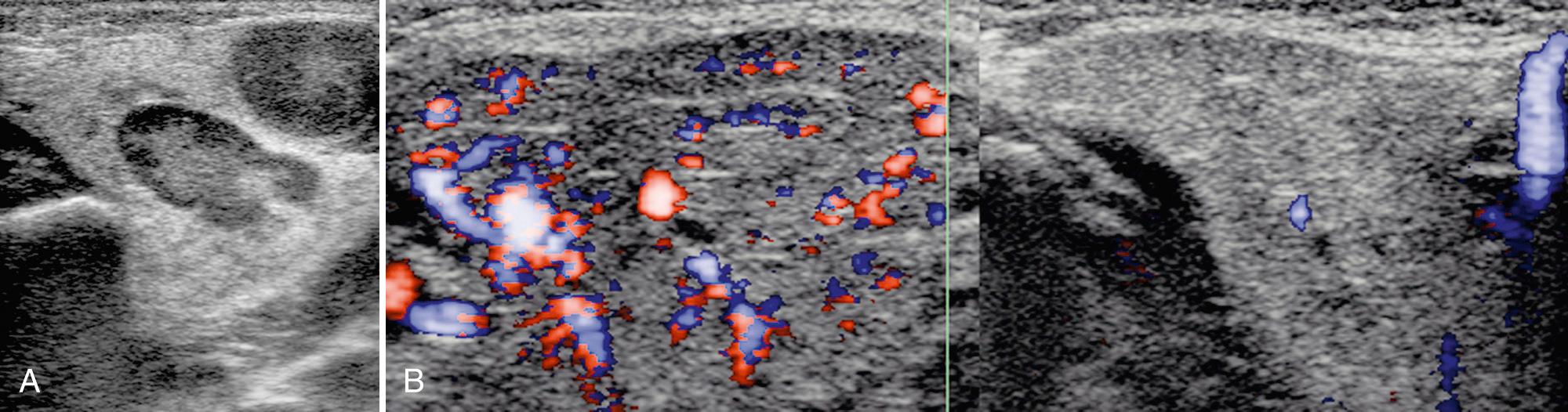
Bacterial infection is rare in children, primarily affecting the parotid gland. Staphylococcus aureus is the most common cause. Children younger than age 1 year, especially premature infants (35%-40% cases), immunosuppressed patients, and children with severe dental and gingival disease are particularly vulnerable. Infection is typically unilateral and associated with fever, dehydration, and gland pain and edema. Proposed etiologies include infection in the mouth or stasis of salivary flow through the ducts. Using ultrasound, the parotid gland appears enlarged and heterogeneous in echotexture with discrete, hypoechoic nodules representing enlarged intraparotid lymph nodes ( Fig. 48.10 ). There may be dilation of the central parotid ducts. Adjacent cervical lymphadenopathy is common. In patients with uncomplicated parotitis, treatment is primarily supportive with administration of intravenous (IV) antibiotics. In the presence of severe dehydration and especially in neonates 7 to 14 days old with bacterial infection, an intraparotid abscess may develop. These collections typically appear hypoechoic or anechoic with posterior acoustic enhancement and occasionally a hyperechoic halo ( Fig. 48.11 ). Internal debris may be noted, and in some patients, hyperechoic foci consistent with gas bubbles. Ultrasound-guided drainage is useful when abscess is present. Recurrence is uncommon.
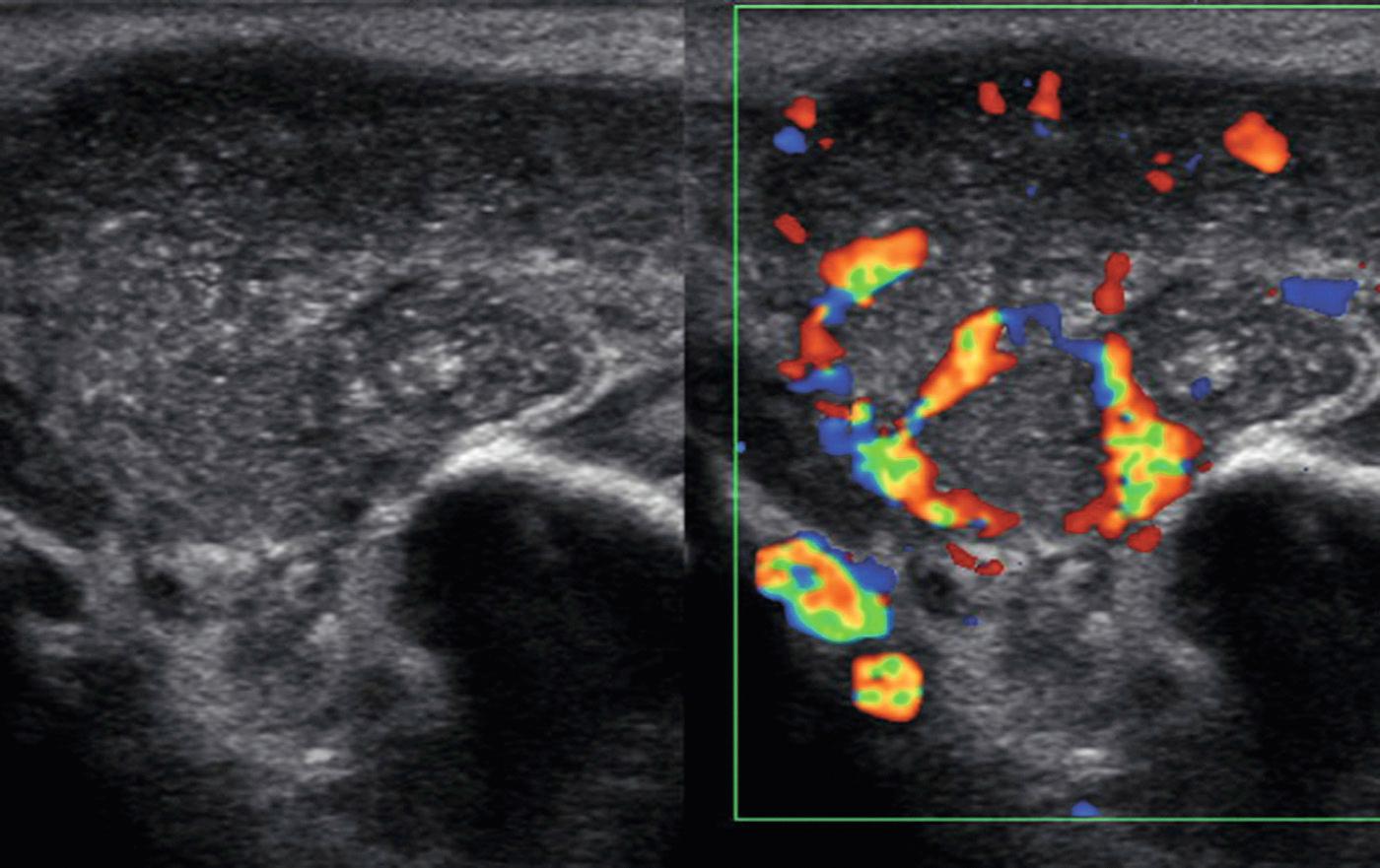
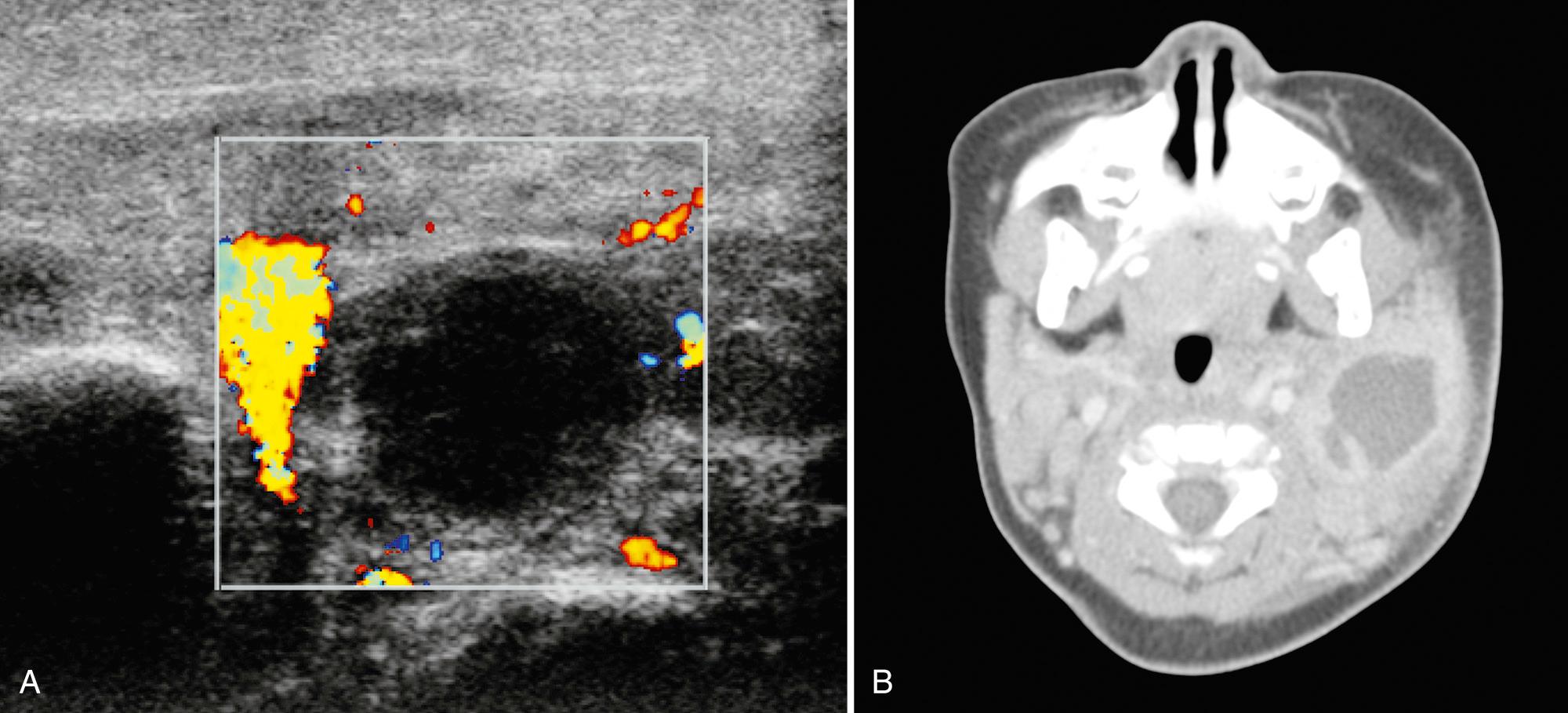
Sialolithiasis is uncommon in children, with 90% in the submandibular gland and 10% in the parotid. In 25% of patients, stones are often multiple and intraglandular or intraductal in location. Submandibular glands are prone to calculi because of the alkaline nature and high viscosity of their secretions. The Wharton duct is long with an upward course and thus also has a higher propensity for stone formation. Clinically, recurrent swelling during eating and superimposed infection may result from partial or complete obstruction of the duct by a stone. Sonography is as sensitive as 94% in the detection of salivary calculi. Features include hyperechoic foci with acoustic shadowing representing stones ( Fig. 48.12 , Video 48.1 ). With duct occlusion, hypoechoic tubular areas are typically consistent with dilated ducts. About 50% of patients have inflammation of the gland in conjunction with the calculus, and the gland will demonstrate a heterogeneous architecture. Although 80% of submandibular and 60% of parotid stones are radiopaque on x-ray examination, when more information is needed beyond ultrasound, a CT scan provides the best detail.
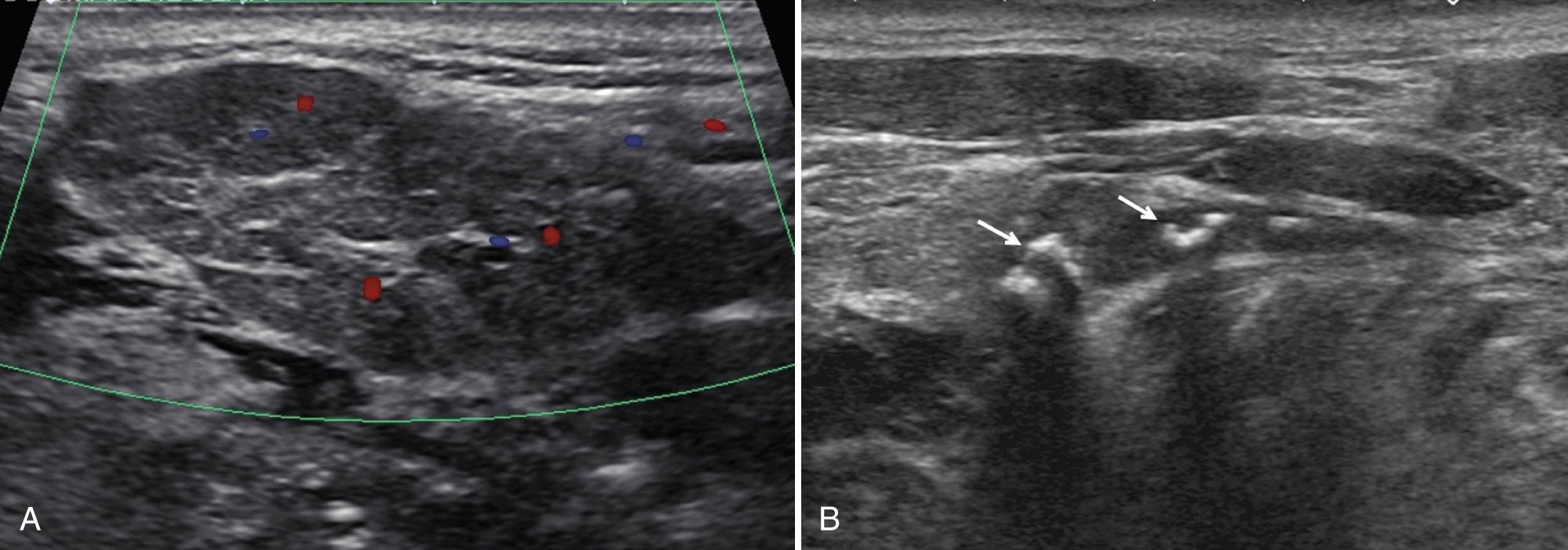
Nodal enlargement in the parotid is often present in association with cervical adenopathy caused by infection or neoplasm. Parinaud oculoglandular syndrome presents with conjunctivitis and ipsilateral intraparotid or periauricular adenopathy and can result from Chlamydia infection or cat-scratch disease. In the submandibular space, sonography is useful to differentiate nodal from glandular disease.
Juvenile (recurrent) parotitis is the most common cause of childhood parotid swelling in developed countries. This disorder manifests with intermittent pain, fever, and unilateral or bilateral parotid swelling. The submandibular and sublingual glands are not affected, and there is no known cause for the recurrent parotitis. Differential diagnosis includes mumps or suppurative parotitis, which is excluded by lack of pus from the parotid duct. Age at presentation is typically 3 to 6 years, and the episodes tend to cease near puberty or in late adolescence. Boys are more often affected than girls. Some patients acquire superimposed acute infection, typically with Streptococcus viridans.
Ultrasound is the favored imaging approach, demonstrating enlarged parotid glands containing multiple round, hypoechoic areas measuring 2 to 4 mm in diameter, likely representing peripheral sialectasis and lymphocytic infiltration ( Fig. 48.13 ). Some glands may be hypervascular, secondary to acute inflammation. Treatment is generally supportive with analgesics and antibiotic administration.
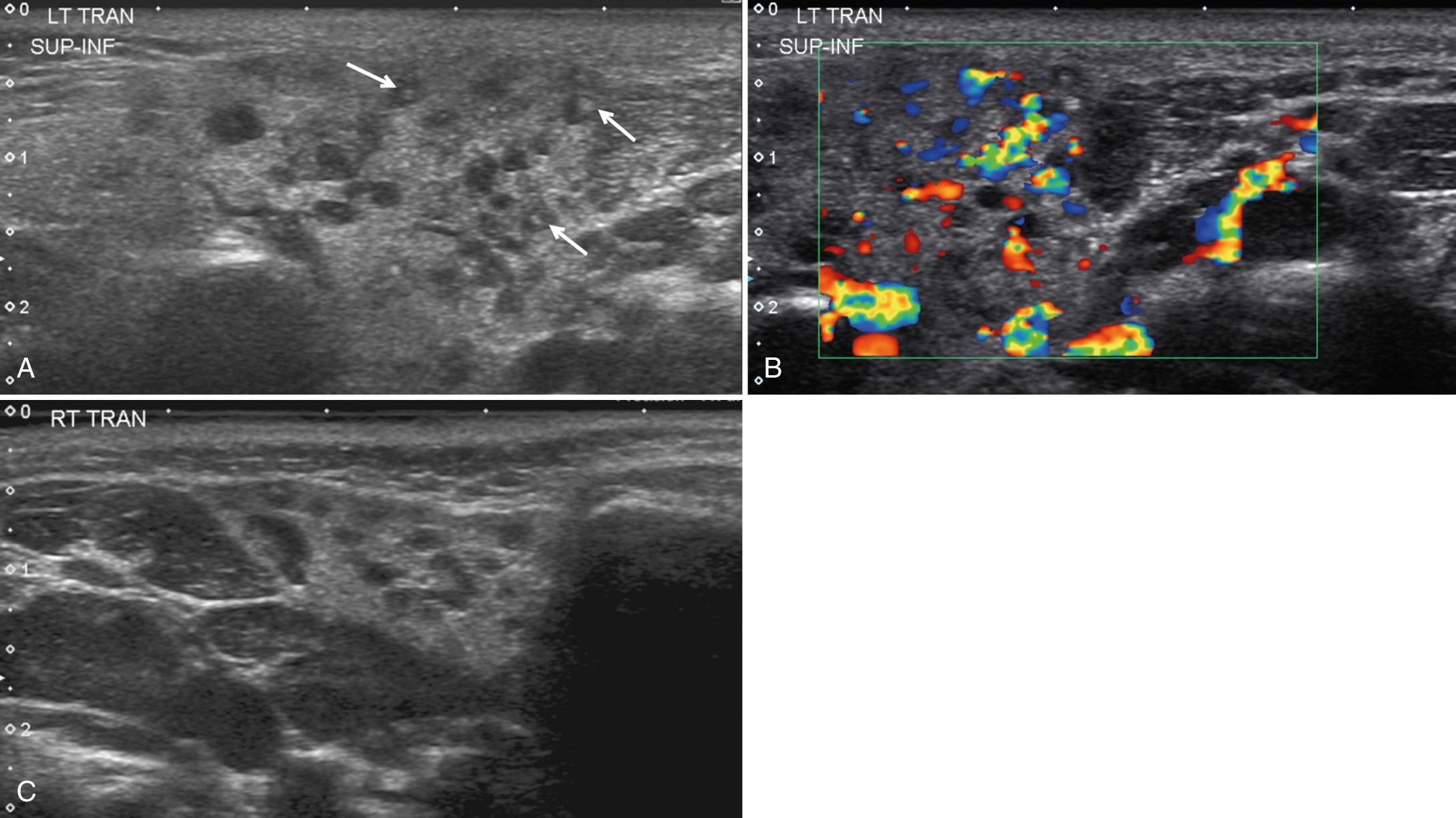
Chronic sialadenitis is caused by an inflammatory process that damages the acini, altering the drainage system of the gland. The etiology may be infectious or noninfectious. Clinically, patients have gland swelling and pain, particularly postprandial. Patients with chronic sialadenitis on sonography often demonstrate a heterogeneous gland with small, punctate, echogenic areas or with multiple hypoechoic areas ( Fig. 48.14 ). Punctate areas are believed to represent mucus in the dilated ducts or walls of the dilated ducts. The hypoechoic areas likely represent edema and sialectasis. Increased vascularity can be demonstrated in areas of abnormal echotecture. Findings may be bilateral and associated with intraglandular or adjacent lymph node involvement.
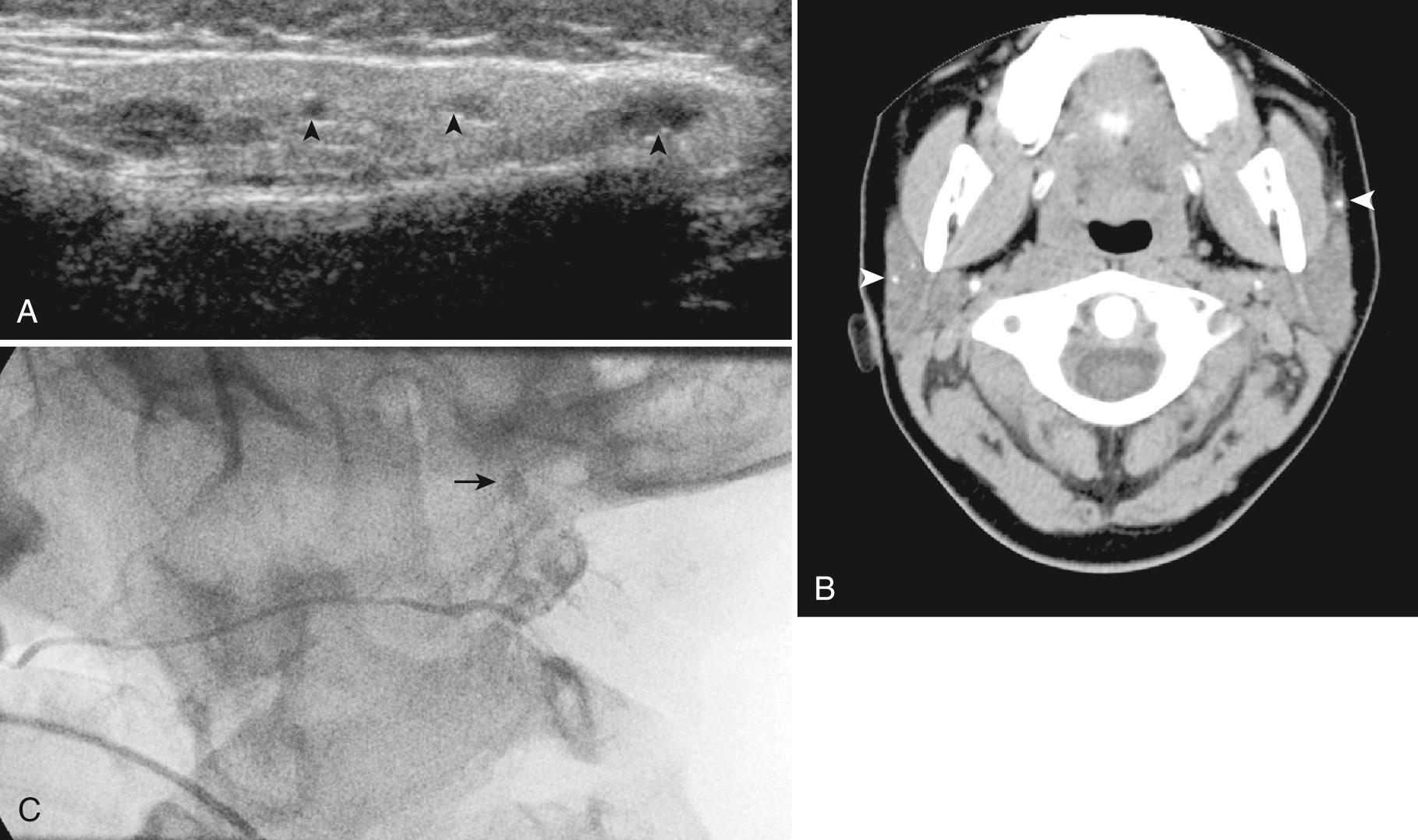
Infectious etiologies include granulomatous diseases such as mycobacterial, actinomycosis, and histoplasmosis. Primary tuberculosis of the salivary gland is rare; however, nontuberculous salivary gland infection, typically secondary to Mycobacterium avium-intracellulare, is more common, usually manifesting at age 16 to 36 months. It is important to remember that tuberculosis and actinomycosis can mimic neoplasm because as the disease progresses, the lesions may appear as hypoechoic masses with poorly defined margins. On color Doppler sonography, these infectious lesions, unlike neoplasms, show no color flow. Complications with actinomycosis and mycobacteria include draining sinuses and fistulas. Treatment is typically with antimicrobial therapy, although surgery may be necessary.
The most common noninfectious salivary gland inflammatory processes include autoimmune disease, sarcoid, and recurrent sialolithiasis (see Fig. 48.14 ). Sjögren syndrome is an autoimmune disorder that results in inflammation and destruction of the exocrine glands, primarily lacrimal and salivary tissue. These patients are usually monitored with ultrasound because of increased risk for lymphoma. Sarcoid is an idiopathic granulomatous disease that is uncommon in children. Parotid involvement, noted in 30% of patients, may be the only initial finding. Heerfordt disease is the combination of parotid involvement, uveitis, and facial paralysis. Calcified hypoechoic lesions may be present. Treatment is primarily with steroids.
Salivary glands can also be involved in the spectrum of immunoglobulin G4 (IgG4)–associated disease (IgG4-associated disease), a systemic disease characterized by abundant infiltration of IgG4-positive plasma cells and lymphocytes with associated fibrosis resulting in multiorgan dysfunction. There can be multiple sites of involvement in the head and neck. Mikulicz disease and chronic sclerosing sialadenitis (Küttner tumor) are the two manifestations of IgG4-associated disease in the salivary glands. Both these conditions are more common in elderly men, but pediatric cases of Küttner tumor have been reported. Mikulicz disease is characterized by painless swelling of the parotid, submandibular, and sublingual salivary glands, as well as the lacrimal glands. Küttner tumor is a hard masslike enlargement frequently involving the submandibular glands, either unilateral or bilateral. In Mikulicz disease, sonography reveals multiple hypoechoic areas in enlarged salivary glands. On sonography Küttner tumor often shows diffuse involvement of the salivary gland with heterogeneous echogenicity, dilated ducts, calculi, and prominent intraglandular vessels. Focal salivary gland lesions show hypoechoic heterogeneous regions with a radial branching vascular pattern demonstrated by Doppler.
Kimura disease is a rare, possibly autoimmune inflammatory disorder with slow-growing painless soft tissue masses frequently in the subcutaneous tissues over the parotid, periauricular, and submandibular regions. There can be contiguous extension of the lesions into the parotid gland. These lesions are frequently described in adolescent and young adult Asian males. There may be associated intraparotid, submental, and submandibular lymphadenopathy, with normal node morphology. On sonography the soft tissue masses are found to be heterogeneous hypoechoic centrally with echogenic hypervascular rim. Lymph nodes show exaggerated hilar and capsular vascularity.
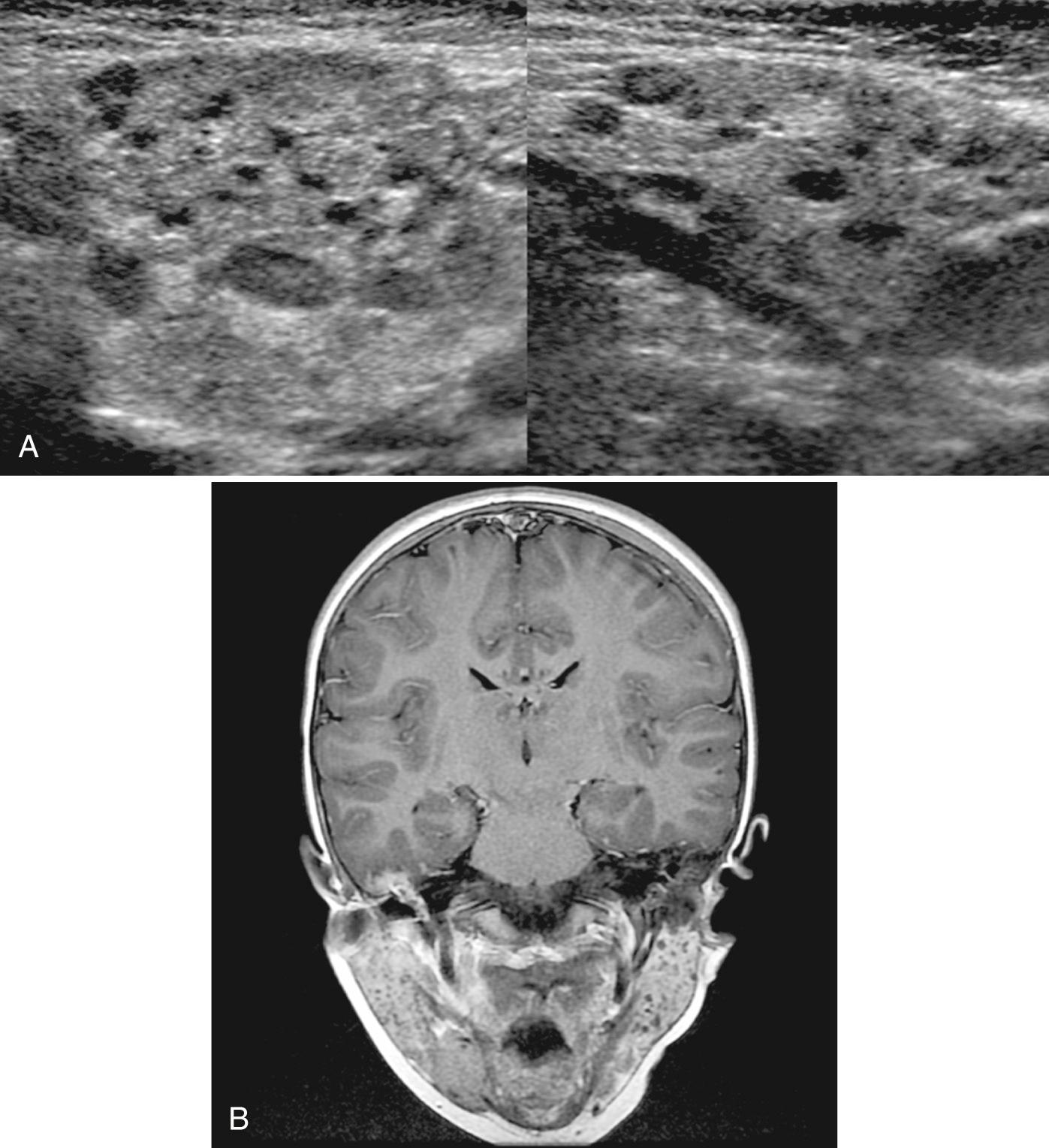
Human immunodeficiency virus (HIV) infection can affect all salivary glands, but primarily the parotid. The patient may demonstrate bilateral parotid swelling and lung disease from lymphocytic interstitial pneumonitis. Infection is characterized by benign lymphoepithelial lesions, consisting of lymphoid hyperplasia accompanied by an intranodal cyst lined by epithelial cells. Prevalence of salivary gland abnormalities in pediatric HIV can be as high as 58%, and they can be present even in the absence of a history of parotid enlargement. Bilateral cystic enlargement of the parotid glands is a widely recognized cause of parotid gland swelling in patients with HIV infection but can be difficult to differentiate from recurrent parotitis, Sjögren syndrome, lymphoma, and Warthin tumor. In 70% of patients, on sonography an enlarged gland is noted containing small, hypoechoic areas without acoustic enhancement and with thick septations consistent with lymphoid infiltration ( Fig. 48.15 ). In 30% there are large, anechoic areas consistent with lymphoepithelial cysts replacing the gland. From 40% to 70% of HIV patients have associated symmetrical cervical adenopathy and enlarged adenoids.
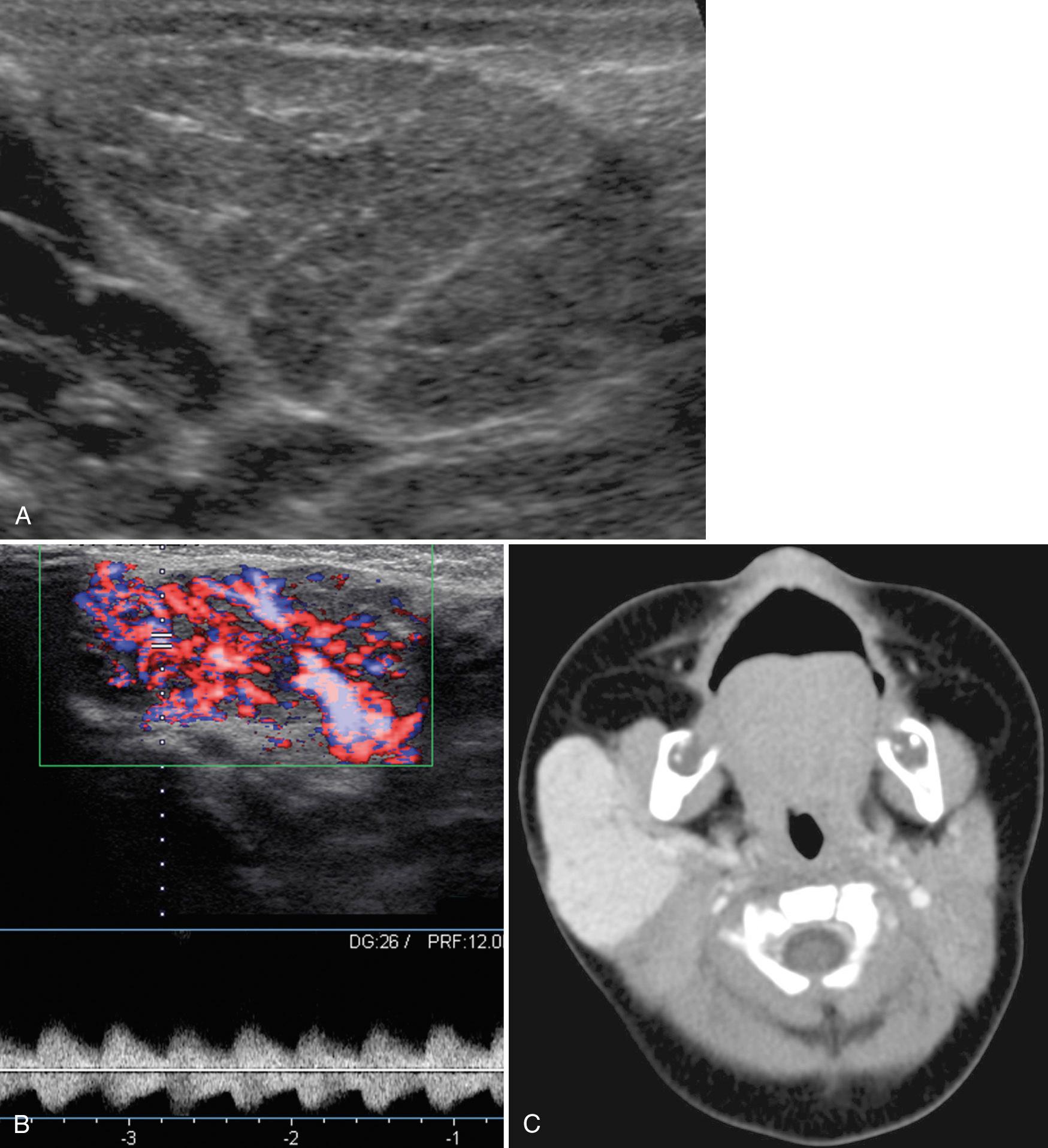
The common childhood salivary gland vascular masses include vascular neoplasms such as hemangiomas, and vascular malformations such as venous or lymphatic malformations. Hemangiomas represent 60% of all salivary gland neoplasms. About 80% arise in the parotid, and 18% are present in submandibular tissue. On ultrasound, the lesions are infiltrative or well circumscribed, usually hypoechoic relative to the glandular tissue, and may involve all or part of the gland ( Fig. 48.16 ). In the proliferative phase, on color Doppler sonography there is a high vessel density, with both increased arteries and increased veins. Arterial spectral Doppler demonstrates high flow velocity and low resistive index (RI) with spectral broadening. Because most infantile hemangiomas spontaneously regress, medical management with propranolol, laser therapy, or surgical excision is used only when symptoms warrant. Lymphatic malformations may involve the parotid gland or may infiltrate the submandibular space. On ultrasound, a lymphatic malformation within the parotid gland shows cystic spaces of varying sizes, with or without solid elements, septations, and internal layering debris ( Fig. 48.17 ). Venous malformations can be small, involving only a portion of the salivary gland, or may be diffuse, involving multiple spaces of the neck. These lesions contain multiple cystic areas, ectatic venous channels, and often phleboliths, which aid in sonographic diagnosis.
Tumors of the salivary glands account for only 1% of all pediatric tumors. About 8% of primary tumors of the head and neck in children originate within the salivary glands ; 90% to 95% occur in the parotid and the rest in the submandibular and sublingual glands. Most masses of the salivary gland are benign and have a vascular etiology, with only 13% reported to be solid salivary gland tumors. Pediatric epithelial salivary masses tend to occur in children older than 10 years, with 23% to 50% of them reported to be malignant. Most malignant tumors in children are of lower grade compared with adults, although children younger than 10 years tend to have higher grade tumors.
In children, sonography is the first step, especially for palpable lesions because the majority of space-occupying lesions in the salivary glands are well delineated. There are no specific sonographic findings differentiating malignant from benign salivary gland neoplasms, although ill-defined tumor margins and adjacent lymphadenopathy are suspicious features. For further definition of a lesion, MRI is the method of choice because it provides precise information with regard to the nature of the mass as well as its extent, especially the presence of perineural invasion.
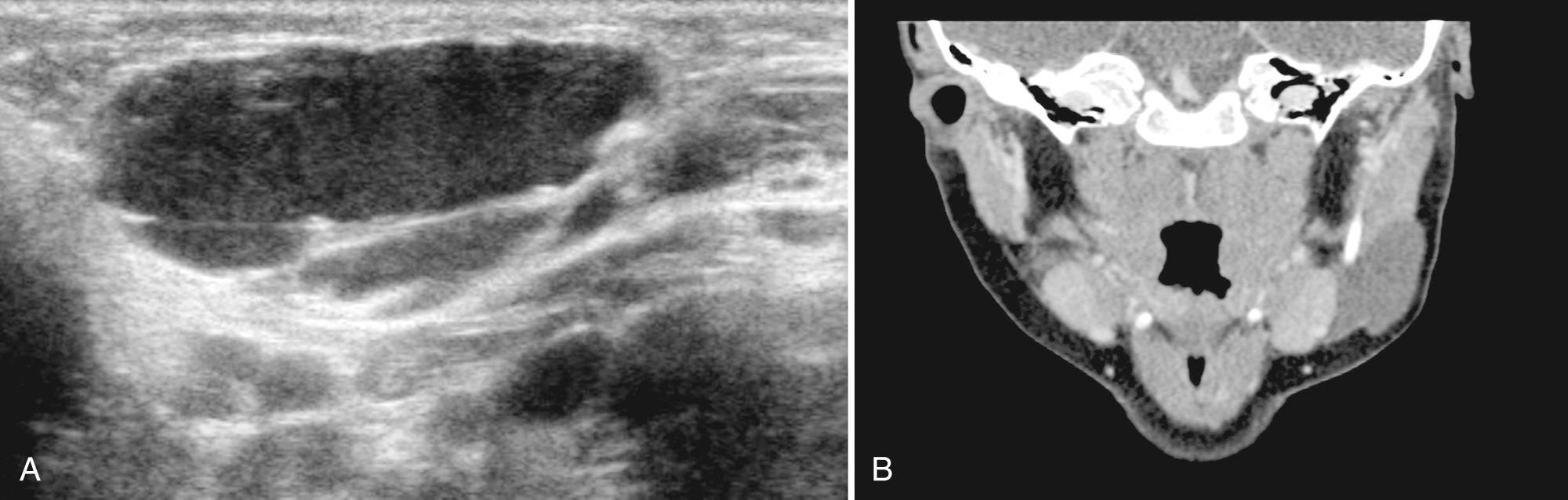
Pleomorphic adenomas, also referred to as benign mixed-cell tumors, contain both mesenchymal and epithelial cell lines. These neoplasms are the most common benign salivary gland tumor in childhood and occur in all pediatric age groups (median, 15 years). Most occur as a solitary, hard, painless, slow-growing mass. Approximately 60% to 90% of these lesions originate in the parotid gland and 10% to 30% in the submandibular gland. These lesions can recur (1%-50%) as multiple masses or rarely can develop late metastatic deposits. On sonography, the lesion appears lobulated, well defined, and hypoechoic or isoechoic (with respect to normal salivary tissue) ( Fig. 48.18 ). Cystic areas and hyperechoic shadowing foci representing small calcifications may be noted. Typically, pleomorphic adenomas demonstrate peripheral vascularity with a hypovascular center. Treatment includes surgical resection with facial nerve sparing procedures if in the parotid gland.
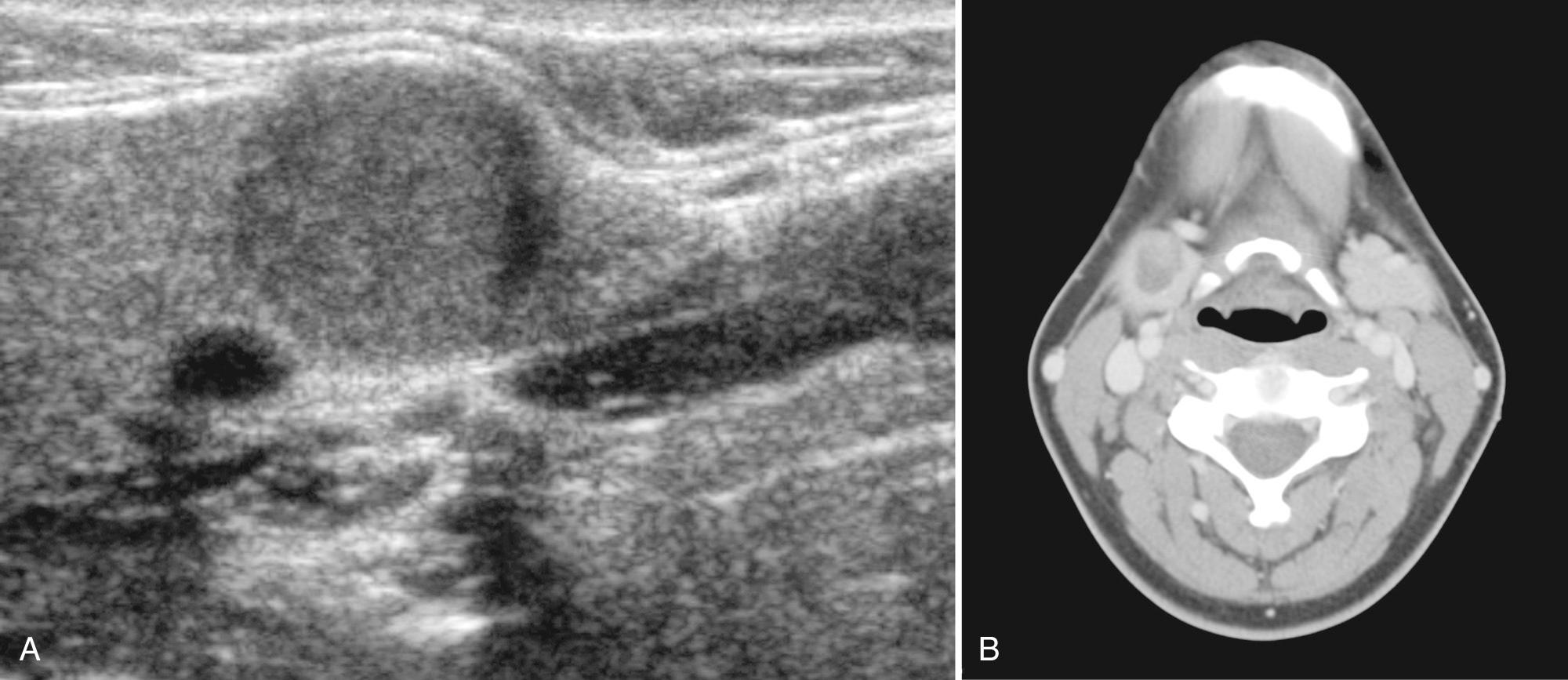
Warthin tumor, also referred to as papillary cystadenoma lymphomatosum or adenolymphoma, is a rare benign salivary gland neoplasm of children and solely identified in the parotid gland. The lesion contains a double layer of epithelial cells resting on a dense lymphoid stroma and is believed to result from incorporation of lymphatic elements and heterotopic ductal epithelium within the parotid lymph nodes. Ultrasound findings include an oval, hypoechoic, well-defined mass containing multiple microcystic or anechoic areas, given the high propensity for cystic change. With color and Doppler sonography, the tumor typically is hypervascular. Technetium 99m scintigraphy shows intense uptake in the tumor but is almost never performed in children. Treatment is surgical resection; however, recurrence is possible from multifocality.
Mucoepidermoid carcinoma, the most common malignant salivary gland neoplasm in children, consists of cords, sheets, or cystic spaces lined by squamous and mucous cells. Of the malignant salivary gland lesions, 60% are diagnosed as mucoepidermoid carcinomas, and about 50% arise from the parotid. Clinically, these lesions may be tender, may grow rapidly, and may cause facial nerve paralysis. Most (76%) mucoepidermoid carcinomas in children are of low grade. Ultrasound characteristics depend on histologic grade. However, increased intratumoral RI and high peak systolic flow velocity (>60 cm/sec) should increase suspicion for malignancy. Sonography should also be performed in the area of the lesion to assess for metastatic-appearing lymph nodes.
Acinic cell carcinoma accounts for approximately 10% of malignant salivary gland tumors, most commonly arising from the parotid gland and less frequently from the submandibular or minor salivary glands. On ultrasound, these lesions are usually irregular in shape with discrete margins, heterogeneous, hypoechoic, and poorly vascularized ( Fig. 48.19 ). Lesions may also demonstrate a mixed cystic and solid appearance and have posterior acoustic enhancement. Importantly, these lesions may be difficult to differentiate from other benign masses. Treatment is usually wide surgical resection with radiotherapy reserved for extraglandular extension, lymph node metastases, or local recurrences. This tumor has the best prognosis of all malignant salivary gland neoplasms.
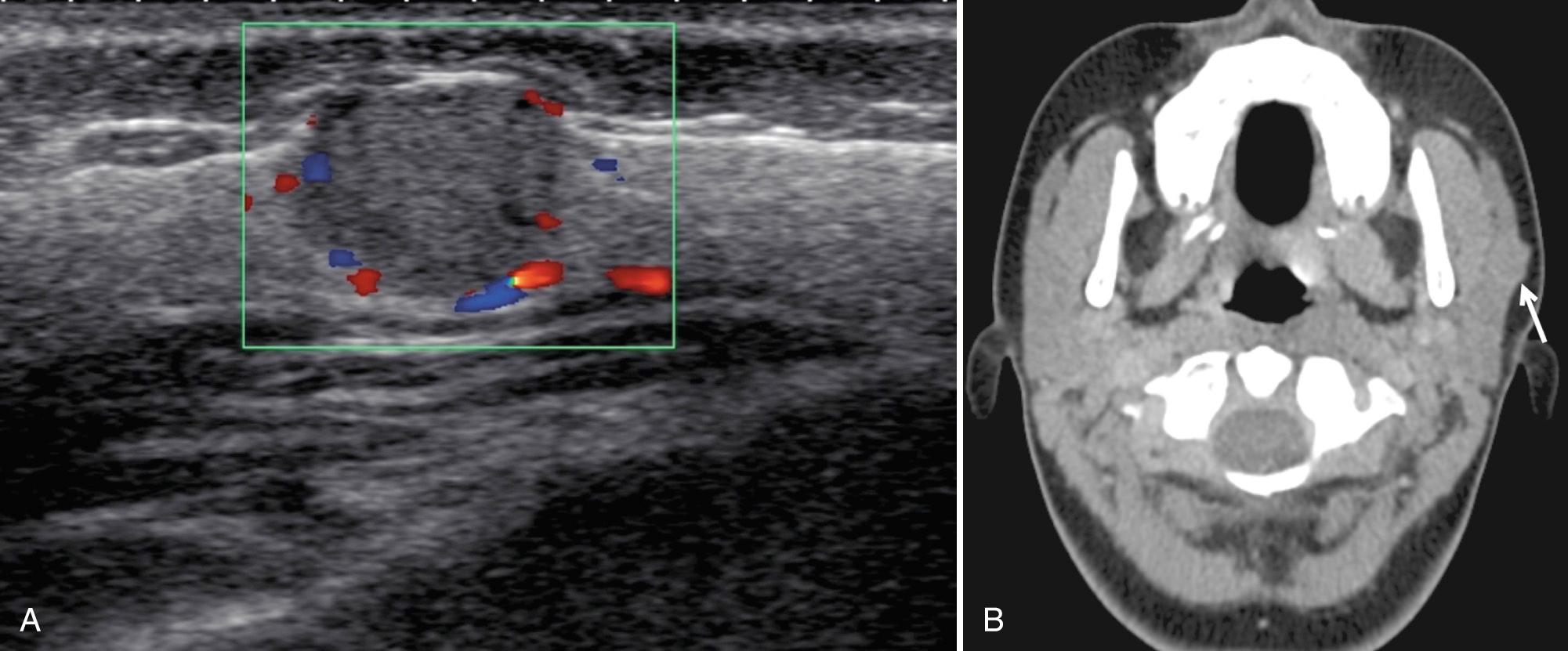
Adenoid cystic carcinoma accounts for approximately 9% of malignant salivary gland tumors in children, almost always in the parotid gland. These are slow-growing tumors but have increased association with perineural invasion. No specific sonographic characteristics are described. Pain and numbness are present in 40% of children with adenoid cystic carcinoma and strongly correlate with perineural invasion.
Sialoblastoma is a rare neonatal neoplasm of the salivary gland, with less than 50 cases described in the literature. This lesion consists of basaloid and myoepithelial cells that are reminiscent of the developing salivary anlage. The lesion is most frequent in the parotid gland and less common in the submandibular gland. This lesion often manifests as a large parotid mass identified in early infancy or childhood, although prenatal and some adult cases have also been described. Sialoblastoma is a focally aggressive tumor, with local recurrences and infrequent distant metastases. Sonographic characteristics are not well described. However, descriptions include that of a multinodular well-defined predominantly hypoechoic salivary gland mass with multiple septations and without increased vascularity. On MRI, heterogeneous appearance is often described, with areas of hemorrhage or necrosis. There are few case reports of combined sialoblastoma and hepatoblastoma.
Lingual thyroid may appear as a homogeneous solid mass in the midline dorsum of the tongue. In 70% of patients, this ectopic tissue is the only functioning thyroid tissue.
Rhabdomyosarcoma occurs 40% of the time in the head and neck and can arise from any muscle. This is the second most common malignancy in the parotid and submandibular spaces in the pediatric population. Salivary gland involvement is frequently by direct extension.
Primary lymphoma of the salivary glands is termed MALToma, denoting origin from mucosa-associated lymphoid tissue. Patients with Sjögren syndrome and other autoimmune disease, including HIV, are at risk for primary lymphoma. Secondary involvement of lymphoma is rare, but more common in the parotid. Ultrasound demonstrates a focal mass or diffuse infiltration with an enlarged gland. Lesions may consist of multiple small, hypoechoic nodules or an irregularly shaped, heterogeneous mass without calcification or cystic degeneration. A reported case of submandibular gland involvement by MALToma showed hypoechoic compartments surrounded by hyperechoic lines, creating a tortoiseshell appearance. Increased color Doppler flow is typical.
Lipomas represent 1% of parotid tumors; 57% of lipomas in the parotid area arise within the gland. These lesions are compressible, oval, and well defined. On ultrasound, lipomas contain striped or feathered internal echoes devoid of color and Doppler flow. In younger children, lipoblastomas are more common.
Leukemic infiltration is rare, with a presentation similar to lymphoma on ultrasound.
Metastatic disease is rare in children but would include neuroblastoma and thyroid cancer.
Peripheral nerve sheath tumors including neurofibroma and schwannoma can occur in the parotid gland, often in relation to the facial nerve. Multiple or plexiform neurofibromas are typical in patients with neurofibromatosis type 1. Sonography demonstrates multiple hypoechoic areas within the gland.
In the parotid space, type I branchial cysts are most common. Simple cysts of the salivary glands are rare and likely caused by mucous retention. On ultrasound, the lesions are well defined and anechoic, with posterior acoustic enhancement and no internal blood flow. In the preauricular region, superficial to the parotid gland, dermoid and epidermoid cysts may also occur.
Branchial apparatus cyst type I
Parotid mucus retention cyst
Tumors with cystic components
Parotid abscess
Lymph node necrosis
Lymphatic malformation
Branchial apparatus cyst type II
Ranulae
Dermoid or epidermoid
Thyroglossal duct cyst
Vallecular cyst
Lymph node necrosis
Lymphatic malformation
In the submandibular space laterally, type II branchial cysts can be identified posterior to the submandibular gland. Lymphatic malformations may also extend into the space. Ranulae (sublingual cysts) are mucous retention cysts resulting from obstruction of the sublingual gland or duct. They present paramidline, in the area of the sublingual gland, as a painless swelling along the floor of the mouth. When large, sublingual cysts can extend below the level of the mylohyoid muscle, termed “plunging ranulae.” Rarely, the lesions are bilateral, and some large lesions extend into the parapharyngeal space. Imaging of a simple ranula on ultrasound demonstrates a unilocular cyst. If infected, the lesion may show internal debris, poorly defined borders, and adjacent inflammation ( Fig. 48.20 ). These lesions are managed with intraoral marsupialization or surgical resection.
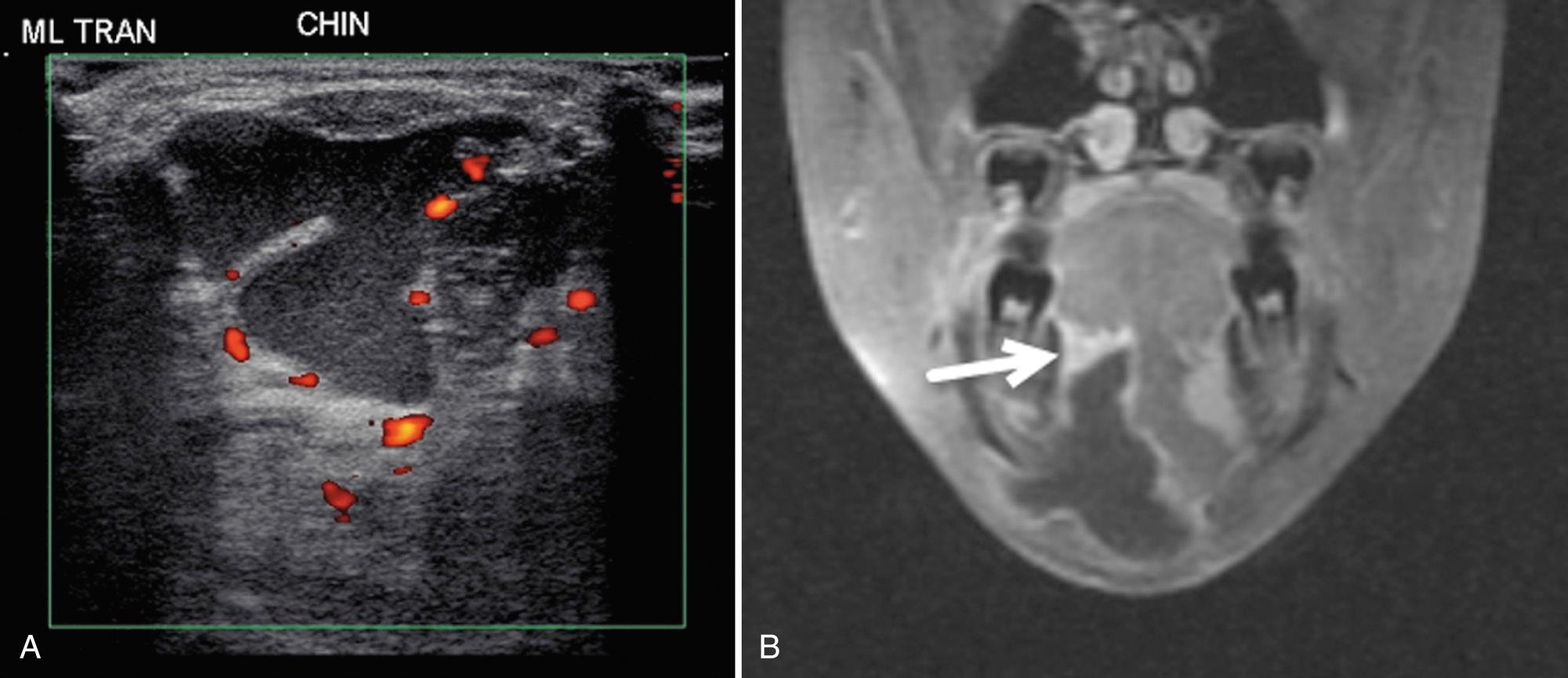
Midline lesions in the suprahyoid area include dermoid or epidermoid tumors and thyroglossal duct cysts. A cyst of the vallecula results from retained secretions in the mucous glands of the pharyngeal wall. These cysts can be congenital or acquired and can cause upper airway obstruction resulting in death. The lesions are midline and on sonography appear as a hypoechoic or an anechoic cystic mass, behind and below the tongue ( Fig. 48.21 ).
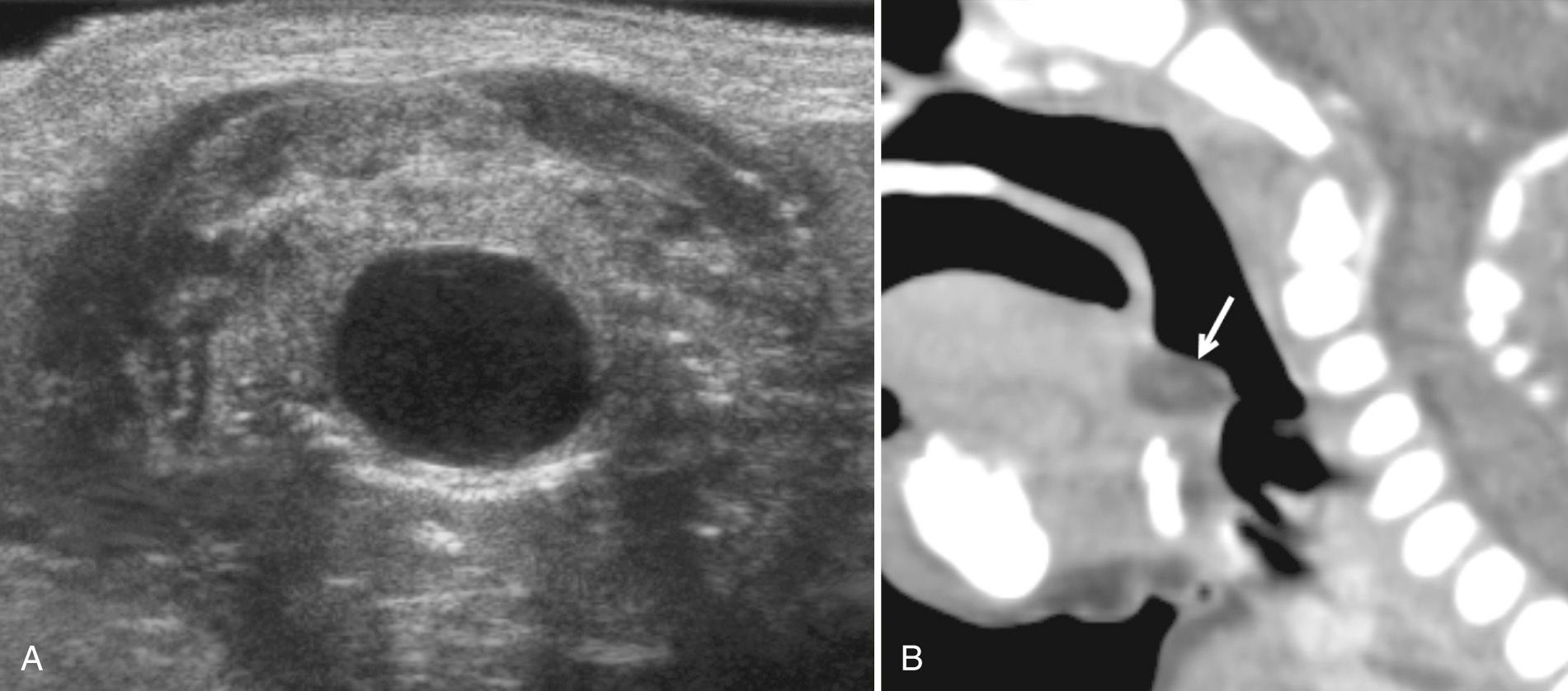
Ultrasound is helpful in evaluating pathology in the masticator space as well as excluding parotid gland involvement. The two most common soft tissue masses to consider in the masticator space are sarcomas and vascular malformations. It is important to remember that the masseter and pterygoid muscles are often involved in venous malformations. An uncommon homogeneous mass to consider is benign hypertrophy of the masseter muscle. However, tumor infiltration of the muscle from leukemia or lymphoma can mimic hypertrophy; thus, clinical correlation is essential ( Fig. 48.22 ). In the presence of trauma, a hematoma appearing as a hypoechoic area may be demonstrated. Inflammatory lesions also may develop, including secondary or primary myositis or osteomyelitis of the mandible, often in the presence of an infected tooth. Cellulitis and soft tissue abscesses may accompany these infections.
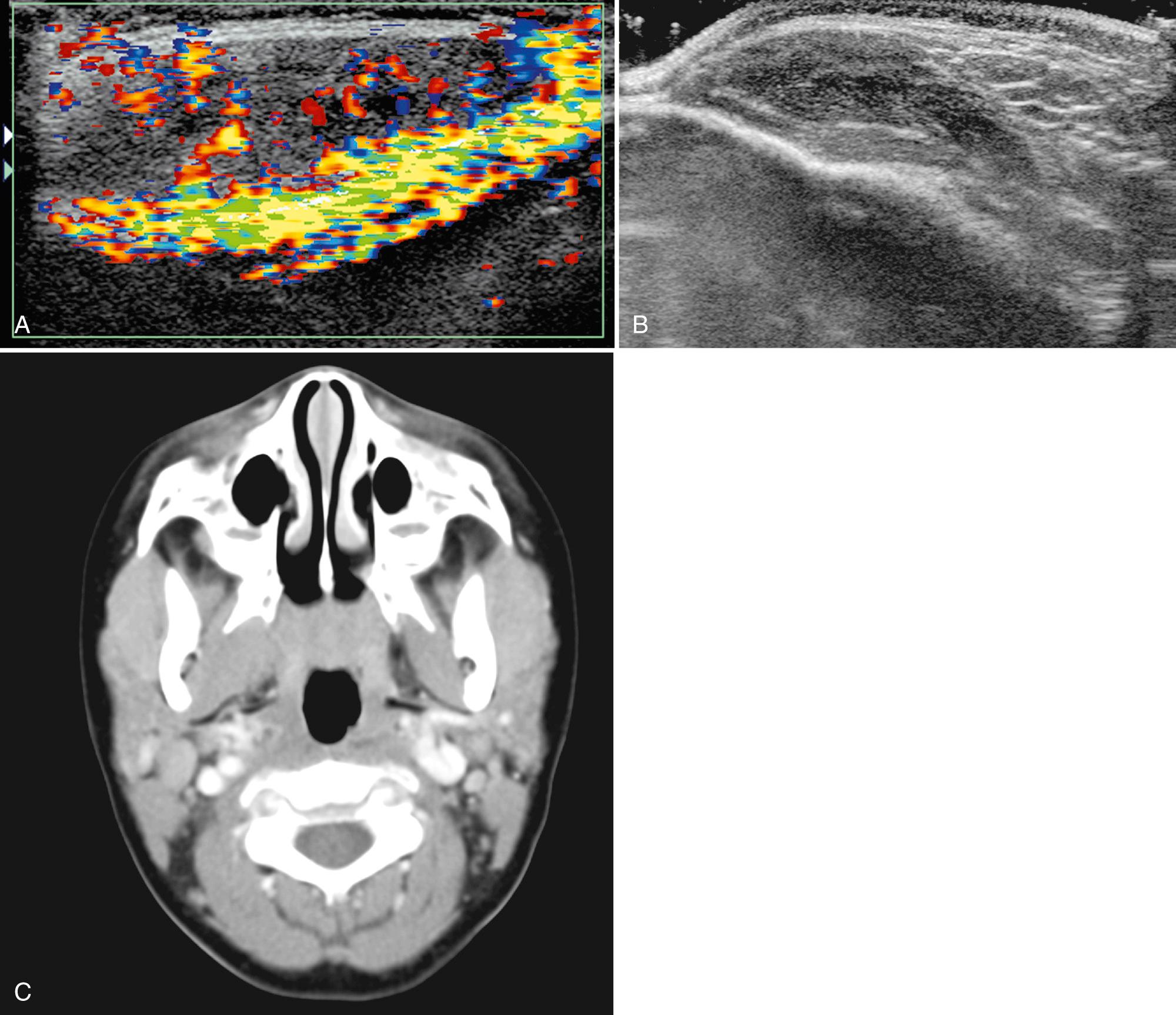
The infrahyoid deep cervical fascia is split into superficial, middle, and deep compartments ( Fig. 48.23 ). The superficial layer at this level is the suprasternal space, lying above the sternum and anterior to the sternothyroid and sternohyoid muscles. The visceral space is delineated by the middle layer of deep cervical fascia. The visceral space contains the thyroid, parathyroid, trachea, esophagus, paraesophageal lymph nodes, and recurrent laryngeal nerve.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here