Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Human primary immunodeficiencies (PIDs) are inborn errors of the immune system and comprise over 260 different genetic disorders. PIDs often result in predisposition to infection and tend to present early in life, with high morbidity and high mortality if left untreated. In recent years, it has become apparent that PIDs are no longer restricted to the risk for developing infections, but increasingly they are associated with immune dysregulation, autoimmunity, autoinflammation, allergy, and increased risk for developing malignancies, in particular lymphomas. PIDs are caused by a sole gene defect (monogenic), not necessarily with complete clinical penetrance, and in the majority of cases tend to cause loss of function (LOF) irrespective of being autosomal dominant (AD), recessive (AR), or X-linked (XR). In recent years, gain-of-function mutations have also been described, predominantly in the AD group. However, a great variability in clinical phenotypes (i.e., time of onset, severity, and clinical presentation) can be observed even within the same family due to additional factors that come into play besides the specific characteristic of the causal mutation(s) involved, such as gene dosage, differential allelic expression, copy number variations and other modulatory influences, gene modifier (epigenetic changes), as well as age, sex, and environmental factors.
In the last 10 years, the number of PIDs has increased significantly largely due to the introduction in 2008 of next-generation sequencing (NGS), expanding the traditional approaches in classical genetics that used cytogenetic tools such as linkage analysis, positional cloning, and candidate gene approach with Sanger sequencing as the gold standard. These new technologies, in particular whole-exome sequencing (WES), which detects mutations in protein-coding and RNA-coding genes, and whole-genome sequencing (WGS), which also analyzes intronic sequences, have also brought new challenges with the detection of a large number of mutations in normal individuals, rendering it even more imperative to validate candidate genes with functional studies and in vivo models. Recently, in addition to the more traditional approach using transfection experiments with knockdown of candidate genes in primary cells and cell lines, new approaches using iPSC and gene editing with CRISPR/Cas9 have provided additional and more sophisticated tools to understand the role of candidate genes in the appropriate cellular context and to assess its relevance to the phenotype under study.
As pathologists, it is important to be aware of these disorders and their pathologic features since an accurate and early diagnosis may contribute to the identification of the disease, point our colleagues in the right direction, and, ultimately, have an impact on outcome and treatment. It is also important to be aware of the systemic nature of these disorders. Besides the frequent involvement of not only immune organs (thymus, lymph nodes, bone marrow, and spleen), they also have wide-ranging effects on target organs such as lung, gastrointestinal tract, skin, and central nervous system, either due to infections (bacterial, fungal, viral, parasitic), autoimmunity, allergic and inflammatory processes (e.g., vasculitis), or neoplasias. In some of these disorders, there are morphologic and phenotypic features that are either unique or are intrinsically related to the underlying genetic defect that lead us to a diagnosis; in other instances, the histologic changes may be non-specific, but they can help in ruling out any of these syndromes. Lastly, understanding PIDs and their pathophysiology is important to give us clues about the functional aspects of the immune system, innate and adaptive and their intricate relationship, which will ultimately lead to design and implement a more targeted therapeutic approach.
The classification of molecularly defined PIDs is updated every 2 years by the International Union of Immunological Societies (IUIS) Expert committee for Primary Immunodeficiency and provides clinical and immunologic synopsis, genetic defect when known, and mode of inheritance in table format. Depending on the genetic defect, when known, or predominant symptom, it is divided into eight broad categories: combined immunodeficiencies (disorders of B cells and T cells), combined syndromic immunodeficiencies, antibody deficiencies, diseases of immune dysregulation, phagocytic disorders, defects of innate immunity, autoinflammatory syndromes, and complement deficiencies. Depending on the prevalent defect and symptoms, some of the inherited disorders are listed under multiple headings. In the most recent version (2014), a separate table of “phenocopies” PID has been introduced. This new category refers to patients (syndromes) that present with characteristics that are similar to inherited PID but are not due to a germline mutation, but rather arise from acquired mechanisms such as somatic mutations (see the discussion on somatic mutations in section on autoimmune lymphoproliferative syndrome” later in the chapter) or autoantibody production against cytokines, or immunologic factors leading to their depletion with subsequent development of PID-like symptoms. It is beyond the scope of this chapter to discuss the entire field of PIDs; the focus will be on a limited number of PIDs with particular emphasis on their histopathologic features and association with lymphoproliferation and lymphomas.
The overall prevalence of monogenic PIDs is low; however, they vary greatly depending on ethnicity, consanguinity, and specific disorder. For instance, it ranges from 1 : 600 for selective IgA antibody deficiency to 1 : 100,000 for severe combined immunodeficiency (SCID) or 1 : 1,000,000 to 2 : 1,000,000 for XLP. Interestingly, the introduction of newborn screening for the analysis of T-cell receptor excision circles (TRECs), a measure of thymic output, in 11 screening programs across the United States has provided more accurate data on SCID prevalence of 1 : 58,000. This screening is now implemented in 26 states in the United States.
Severe combined immunodeficiency (SCID) is an extreme form of T-cell deficiency. Depending on the gene defect, they are associated with the absence or presence of B cells and NK cells, and, in some instances, they are associated with non-immunologic manifestations such as radiosensitivity and skeletal or neurologic abnormalities. It is a very heterogeneous group caused by several different genes involved in T-cell development with deleterious mutations involving VDJ recombination ( RAG1 and RAG2, DCLRE1C, PRKDC, NHEJ, and LIG4 ) and with other severe maturation defects ( ADA, AK2, and PNP ). In addition, profound genetic T-cell lymphopenias (combined immunodeficiency) may be considered within the spectrum of SCID due their similar clinical presentation, and include mutations involving cytokine signaling ( IL2RG, IL7R, and JAK3 ) and T-cell receptor signaling ( CD3D, CD3E, and CD3Z ) or motility (failure of thymic egression) (CORO1A). Leaky SCIDs are due to hypomorphic mutations of some of these genes (including RAG1-2 ), which allow some degree of T-cell development due to residual activity, but retain impaired T-cell-mediated immunity. In typical SCID, the diagnosis is based on absence or a very low number of autologous T cells (<300/mL) and very low T-cell function (PHA stimulation <10%), frequently with T cells of maternal origin present (maternal engraftment); TRECs levels are also undetectable or extremely low at birth. Leaky SCID and some of the profound T-cell lymphopenias may have normal or even an increased number of circulating T cells associated with severe immune dysfunction leading to a similar clinical presentation, but often with a delayed onset and unusual symptoms such as autoimmunity, granulomatous inflammation, skin disease, and increased risk for lymphoproliferative malignancies.
Infants with SCID, although normal at birth, present early in life with respiratory tract infections ( Pneumocystis jirovecii, cytomegalovirus, adenovirus, parainfluenza type 3, respiratory syncytial virus, chronic respiratory syncytial virus), which are often severe, prolonged, and complicated, or persistent bronchiolitis. Other symptoms include diarrhea, failure to thrive, or thrush. On physical examination, no lymph nodes are palpable, and imaging studies reveal a lack of thymus shadow. Newborn screening for SCID adopting TREC analysis (originally used in HIV patients to monitor new T-cell output) was implemented in order to detect these disorders before symptoms develop so patients can receive appropriate medical treatment, avoid live vaccines and non-irradiated blood products, and ultimately reconstitute their immune system with allogeneic hematopoietic stem cell transplantation (HSCT).
In general, TREC analysis is very successful in identifying typical SCID and most of the profound T cell dysfunctions, but exceptions exist, including leaky SCID, for which the diagnosis may be delayed.
Histologic and immunophenotypic characterization of the thymus in these severe PIDs give us clues to understand their pathophysiology. Various histologic patterns (i.e., dysplastic depleted, dysplastic non-depleted, and non-dysplastic non-depleted) have been described based on the distribution of thymic epithelial cells (TECs), thymocytes, and their functional subsets including nTregs, dendritic cells, and macrophages and correlate to the underlying genetic defect affecting T-cell development ( Fig. 54-1 ).
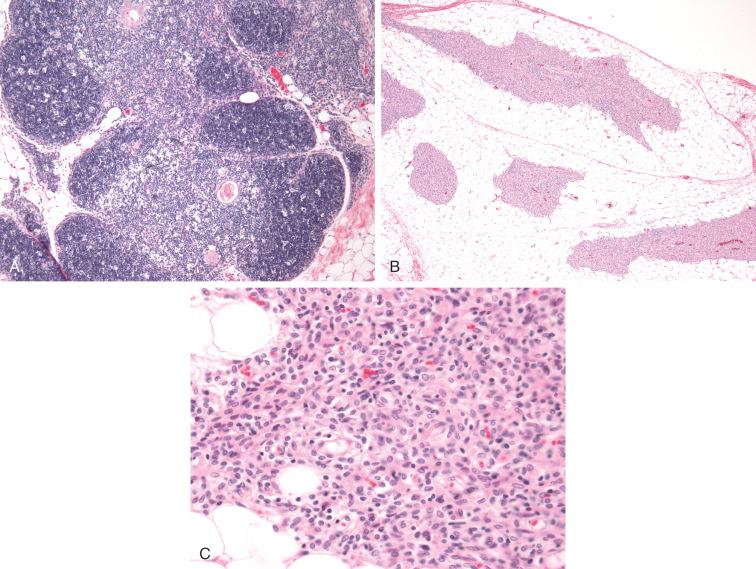
One of the exceptions that can be missed by TREC analysis is Omenn's syndrome (OS), in which T-cells can be in normal numbers or even elevated due to hypomorphic mutations (leaky defect) that occur in a variety of genes involved in VDJ recombination ( RAG1, RAG2, DCLRE1C, LIG4, RMRP, and ADA ). They allow some degree of maturation of a very limited number of thymic T cells with a limited repertoire reflected in the oligoclonal expansion in the periphery. OS was originally described by Gilbert Omenn in 1965 as a syndrome characterized by a profound immunodeficiency with severe erythroderma, lymphadenopathy, and eosinophilia. OS shares a similar clinical presentation with typical SCID (i.e., presents in infancy, pneumonitis, chronic diarrhea, and failure to thrive); however, the presence of adenopathy, hepatosplenomegaly, and generalized erythroderma associated with increased IgE levels and eosinophilia are distinguishing features. In contrast to typical SCID, the persistent presence of inflammation leads to an increased number of circulating T cells with an activated phenotype and inability to proliferate in response to mitogens. Morphologically, lymph nodes show complete effacement of the architecture with a depleted look and increased number of dendritic cells and eosinophils; they usually lack primary and secondary B follicles. Phenotypically, CD3-positive T cells express CD45RO, CD4, and also CD30 with a cytokine profile consistent with a Th2-type response.
One of the protective effects of this type of response against persistent inflammation is the upregulation of natural T-regulatory cells (nTregs); however, these are severely reduced due to thymic dysplasia with profound abnormalities of TEC differentiation affecting central tolerance. All of these factors contribute to autoimmunity and inflammation in OS.
Class-switch recombination deficiencies or hyper IgM syndromes are characterized by normal or elevated serum IgM and low or absent serum levels of other Ig classes. In the adaptive immune system, antibody-mediated immune responses are either T-cell independent, in which B cells proliferate upon encountering specific antigens and secrete IgM, or T-cell dependent and in order to generate high affinity antibodies B-cell undergo clonal expansion, affinity maturation through somatic hypermutation (SHM), and class-switch recombination (CSR). These latter events take place within the germinal centers in secondary lymphoid organs upon T-cell–B-cell interaction mediated through CD40-ligand and CD40. Germline mutations can affect CD40/CD40L and CD40 signaling pathways as well as enzymes involved in double-stranded DNA breaks and repair that occur during CSR.
CD40L deficiency (XL) or Hyper IgM type 1 defect involves CD40L, which is expressed on activated T cells in a tightly controlled manner, but it is not lethal to T cells. Originally thought to be a defect in isotype switching, it is now known to be a defect of T-cell priming, help, and function leading to impaired class switching, best classified as combined immunodeficiency rather than predominantly antibody deficiency. About 200 cases have been described in the literature. It presents with recurrent upper and lower respiratory tract involvement due to opportunistic infections ( P. jirovecii , cytomegalovirus, Cryptococcus , histoplasmosis, and Candida ), diarrhea when infectious often due to Cryptosporidium parvum (80%), and neutropenia before 2 years of age. Subsequent complications involving the biliary tree and liver are often related to Cryptosporidium - and Giardia -persistent infections of the biliary system leading to sclerosing cholangitis, hepatitis, cirrhosis, and increased gastrointestinal malignancies including cholangiocarcinoma. Immunologic features include very low serum levels of IgG and IgA with normal to increased IgM, decreased number of memory B cells, and limited to un-switched memory B cells (CD27 positive, IgM positive, IgD positive) in keeping with defects in CSR. Although the number and distribution of T-cell subsets is not affected, CD40L deficiency affects their costimulatory functions for T-cell–B-cell interaction, also with monocytes expressing CD40 leading to poor interferon gamma and interleukin-12 production. Dendritic cell signaling is also affected. Lymph nodes are usually small with IgM/IgD-positive B cells in the far cortex lacking secondary B follicles with minimal to absent dendritic meshworks, and well-preserved T-cell areas ( Fig 54-2 ). CD40 deficiency (AR) hyper IgM type 3 is similar in all respects to HGM1.
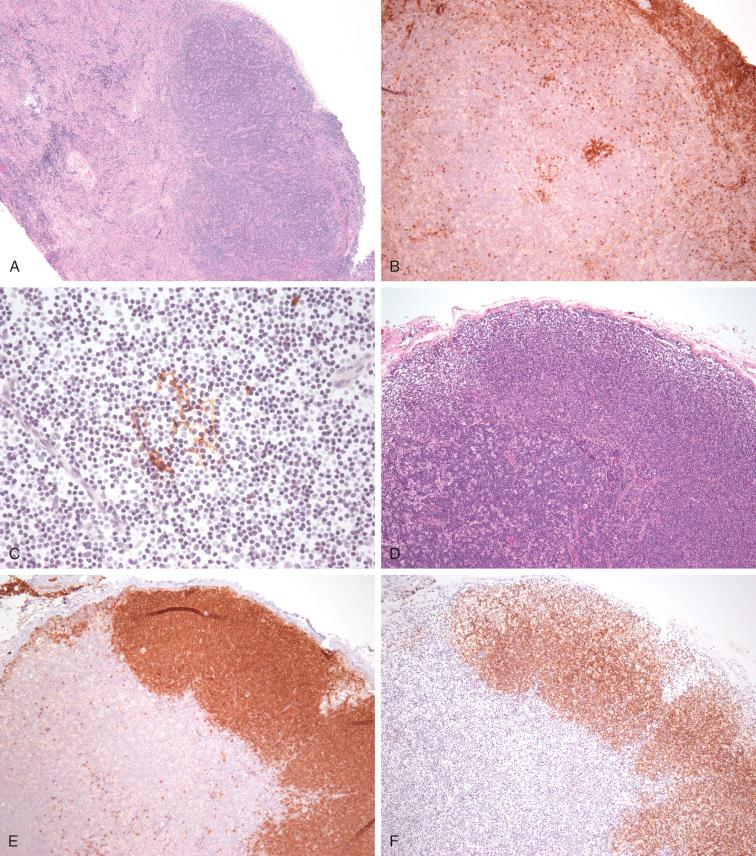
Patients with activation-induced cytidine deaminase ( AICDA gene) AID deficiency (AR) (HIGM2) present with lymphadenopathy, enlarged tonsils, and recurrent bacterial sinopulmonary infections, but no opportunistic infections. Morphologically the lymph nodes are characterized by florid follicular hyperplasia with large, expanded germinal centers. Similar to the other IGHM12, they lack switched memory B cells and show profound defects also in SHM, reflecting the underlying genetic defect involving AID, which participates in both the CSR and SHM process; however, not all AID mutants are associated with defects in SHM; AID C terminal defect (AD) is an example. These observations lead to a better understanding of the complexity of the CSR mechanism. More recently, AID has been implicated not only in B-cell development through CSR and gene activation through demethylation, but also as a candidate gene in inducing genomic instability in other systems.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here