Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Calcium plays a critical role in many cellular processes, including hormone secretion, muscle contraction, nerve conduction, exocytosis, and the activation and inactivation of many enzymes. As described in Chapter 3 , calcium also serves as an intracellular second messenger by carrying information from the cell membrane into the interior of the cell. It is therefore not surprising that the body very carefully regulates the plasma concentration of free ionized calcium, the physiologically active form of the ion, and maintains plasma ionized calcium concentration within a narrow range.
Phosphate is no less important. Because it is part of the ATP molecule, phosphate plays a critical role in cellular energy metabolism. It also plays crucial roles in the activation and deactivation of enzymes. However, unlike calcium, the plasma phosphate concentration is not strictly regulated, and its levels fluctuate throughout the day, particularly after meals.
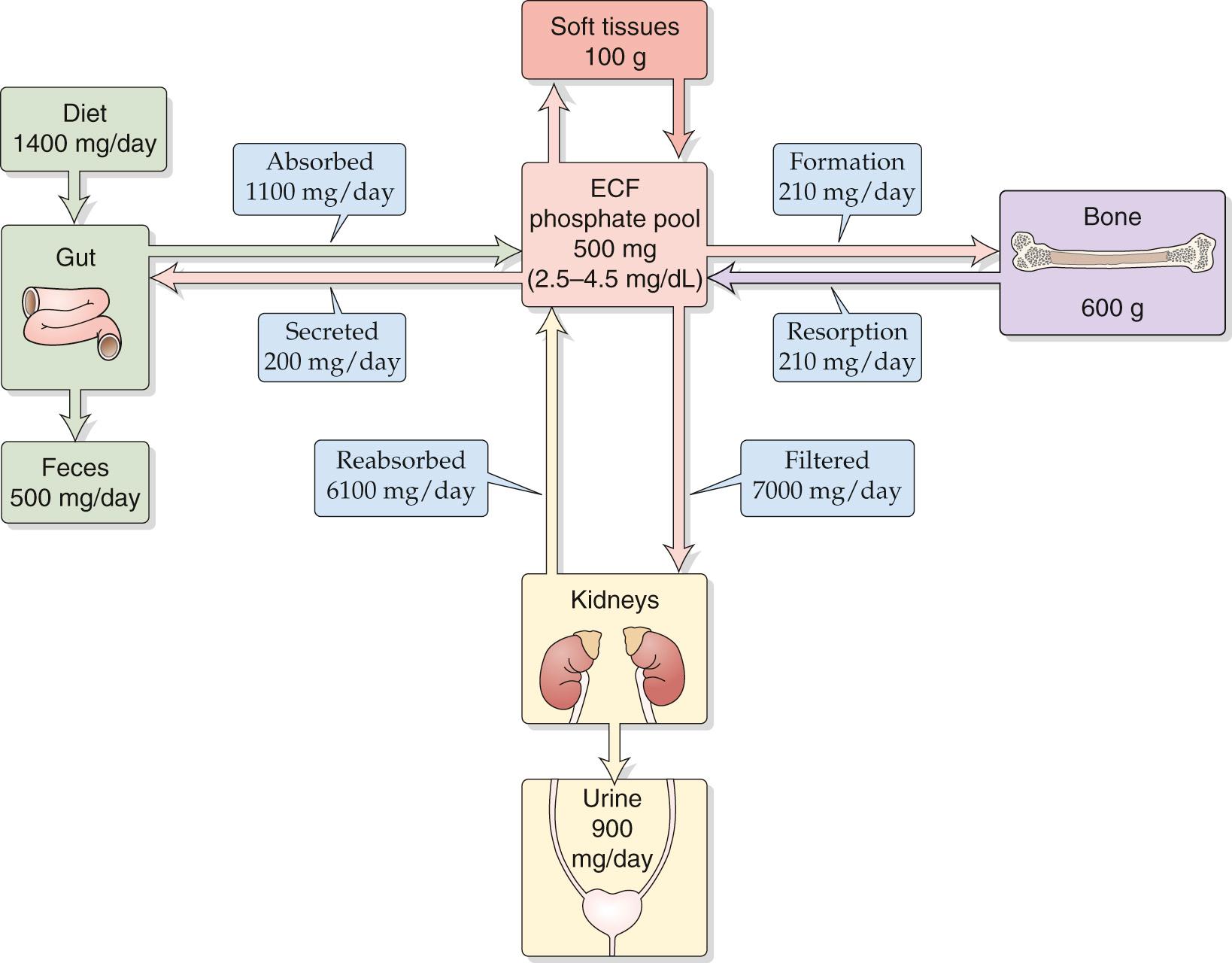
Calcium and phosphate homeostasis are intimately tied to each other for two reasons. First, calcium and phosphate are the principal components of hydroxyapatite crystals [Ca 10 (PO 4 ) 6 (OH) 2 ], which by far constitute the major portion of the mineral phase of bone. Second, they are regulated by the same hormones, primarily parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D (calcitriol) and, to a lesser extent, the hormone calcitonin. These hormones act on three organ systems—bone, kidneys, and gastrointestinal (GI) tract—to control the levels of calcium and phosphate in plasma. However, the actions of these hormones on calcium and phosphate are typically opposed in that a particular hormone may elevate the level of one ion while lowering that of the other. Figures 52-1 and 52-2 depict the overall daily balance of calcium and phosphate for an individual in a steady state.
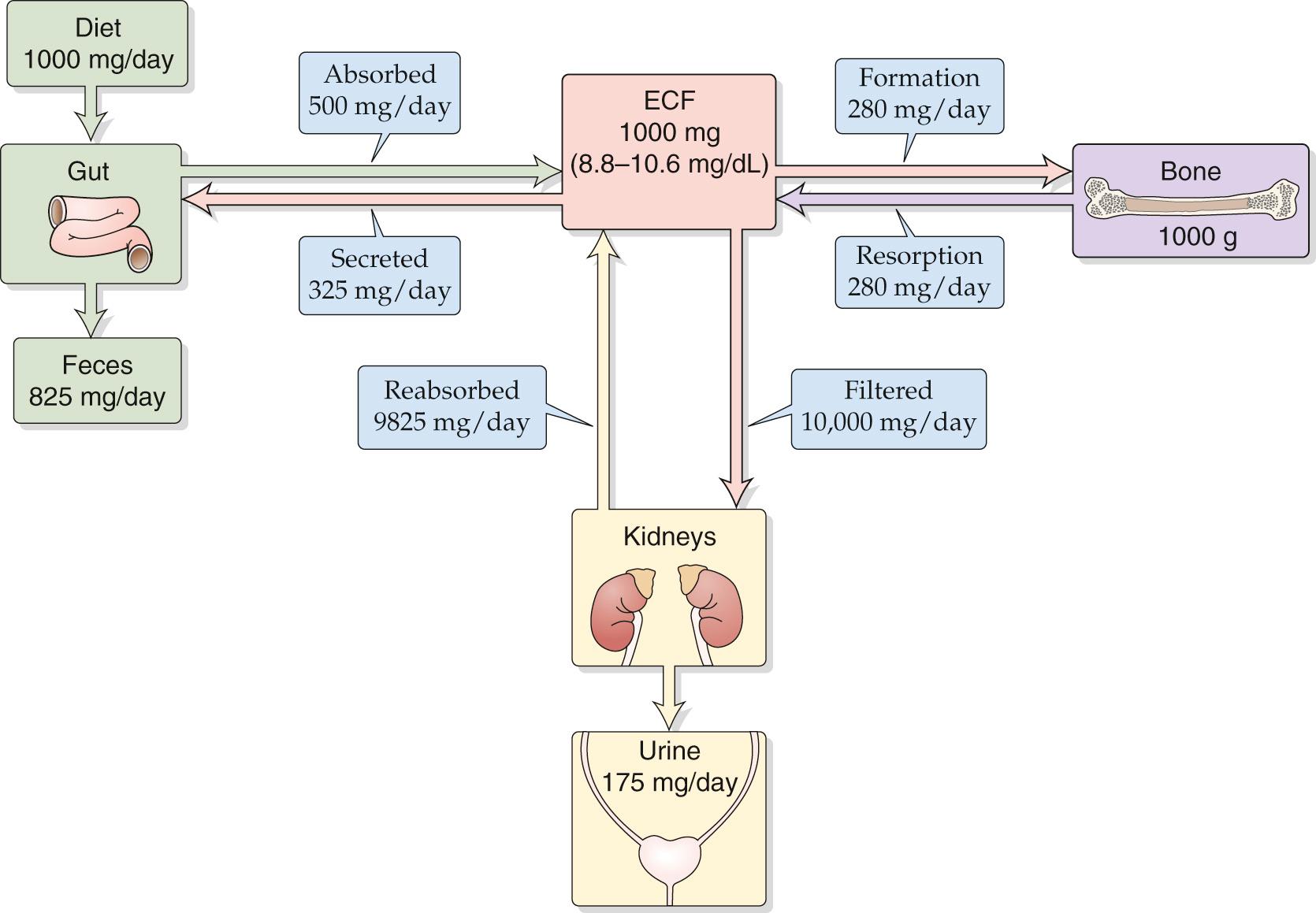
In plasma, calcium exists in three physicochemical forms: (1) as a free ionized species (Ca 2+ ), (2) bound to (more accurately, associated with) anionic sites on serum proteins (especially albumin), and (3) complexed with low-molecular-weight organic anions (e.g., phosphate, citrate, and oxalate). The total concentration of all three forms in the plasma is normally 2.2 to 2.6 mM (8.8 to 10.6 mg/dL). In healthy individuals, ~45% of calcium is free, 45% is bound to protein, and 10% is bound to small anions. The body tightly regulates the ionized form of Ca 2+ between 1.0 and 1.3 mM (4.0 and 5.2 mg/dL). The ionized form is the most important with regard to regulating the secretion of PTH and is involved in most of the biological actions of calcium.
Most total-body calcium is located within bone, ~1 kg (see Fig. 52-1 ). The total amount of calcium in the extracellular pool is only a tiny fraction of this amount, ~1 g or 1000 mg. The typical daily dietary intake of calcium is ~800 to 1200 mg. Dairy products are the major dietary source of calcium. Although the intestines absorb approximately one half the dietary calcium (~500 mg/day), they also secrete calcium for removal from the body (~325 mg/day), and therefore, the net intestinal uptake of calcium is only ~175 mg/day. The second major organ governing calcium homeostasis is bone, which in the steady state deposits ~280 mg/day of calcium and resorbs an equal amount. The third organ system involved, the kidney, filters ~10 times the total extracellular pool of calcium per day, ~10,000 mg/day. The kidneys reabsorb ~99% of this Ca 2+ , so that the net renal excretion of Ca 2+ is ~1% of the filtered load (see Fig. 36-16 ). In a person in Ca 2+ balance, urinary excretion (~175 mg/day) matches net absorption by the GI tract.
The concentration of total phosphate in adult plasma—predominantly inorganic phosphate in the form of  and
and  —ranges from 0.8 to 1.5 mM, a variation of 80%. It is ~50% higher in children. Laboratories report total plasma phosphate concentration as elemental phosphorus (range in adults, 2.5 to 4.5 mg/dL). Between 85% and 90% of the circulating inorganic phosphate is filterable by the kidneys, either ionized (50%) or complexed to Na + , Ca 2+ , or Mg 2+ (40%); only a small proportion (10% to 15%) is protein bound.
—ranges from 0.8 to 1.5 mM, a variation of 80%. It is ~50% higher in children. Laboratories report total plasma phosphate concentration as elemental phosphorus (range in adults, 2.5 to 4.5 mg/dL). Between 85% and 90% of the circulating inorganic phosphate is filterable by the kidneys, either ionized (50%) or complexed to Na + , Ca 2+ , or Mg 2+ (40%); only a small proportion (10% to 15%) is protein bound.
Like calcium, most total-body phosphate is present in bone, which contains ~0.6 kg of elemental phosphorus (see Fig. 52-2 ). A smaller amount of phosphorus (0.1 kg) resides in the soft tissues, mainly as organic phosphates, such as phospholipids, phosphoproteins, nucleic acids, and nucleotides. An even smaller amount (~500 mg) is present in the extracellular fluid (ECF) as inorganic phosphate. The daily dietary intake of phosphorus is typically 1400 mg, mostly as inorganic phosphate. Again, dairy products are the major source. The net absorption of phosphate by the intestines is ~900 mg/day. In the steady state, bone has relatively small phosphate turnover, ~210 mg/day. The kidneys filter ~14 times the total extracellular pool of phosphate per day (~7000 mg/day) and reabsorb ~6100 mg/day. Hence, the net renal excretion of phosphorus is ~900 mg/day, the same as the net absorption by the GI tract.
Bone consists largely of an extracellular matrix composed of proteins and hydroxyapatite crystals, in addition to a small population of cells. The matrix provides strength and stability. The cellular elements continually remodel bone to accommodate growth and allow bone to reshape itself in response to varying loading stresses. Basically, bone has three types of bone cells. Osteoblasts promote bone formation. Osteoblasts and preosteoblasts are the principal target cells for PTH's action to stimulate bone growth. Osteoclasts promote bone resorption and are found on the growth surfaces of bone. Their activity is increased by cytokines, with RANK ligand being particularly important. Osteocytes are found within the bony matrix and are derived from osteoblasts that have encased themselves within bone. In response to mechanical loading, osteocytes produced both stimulatory and inhibitory cues. These cells stimulate the bone-forming activities of osteoblasts and lining cells by secreting growth factors such as osteocalcin and Wnt ligands. Osteocytes inhibit osteoblast activity by secreting antagonists of Wnt signaling, including sclerostin and dickkopf1. Osteocytes also appear to play a role in the transfer of mineral from the interior of bone to the growth surfaces. Bone remodeling consists of a carefully coordinated interplay of osteoblastic, osteocytic, and osteoclastic activities.
As shown in Figure 52-3 , bone consists of two types of bone tissue. Cortical (also called compact or lamellar ) bone represents ~80% of the total bone mass. Cortical bone is the outer layer (the cortex) of all bones and forms the bulk of the long bones of the body. It is a dense tissue composed mostly of bone mineral and extracellular matrix elements, interrupted only by penetrating blood vessels and a sparse population of osteocytes nested within the bone. These osteocytes are interconnected with one another and with the osteoblasts on the surface of the bone by canaliculi, through which the osteocytes extend cellular processes. These connections permit the transfer of Ca 2+ from the interior of the bone to the surface, a process called osteocytic osteolysis. Dense cortical bone provides much of the strength for weight bearing by the long bones.
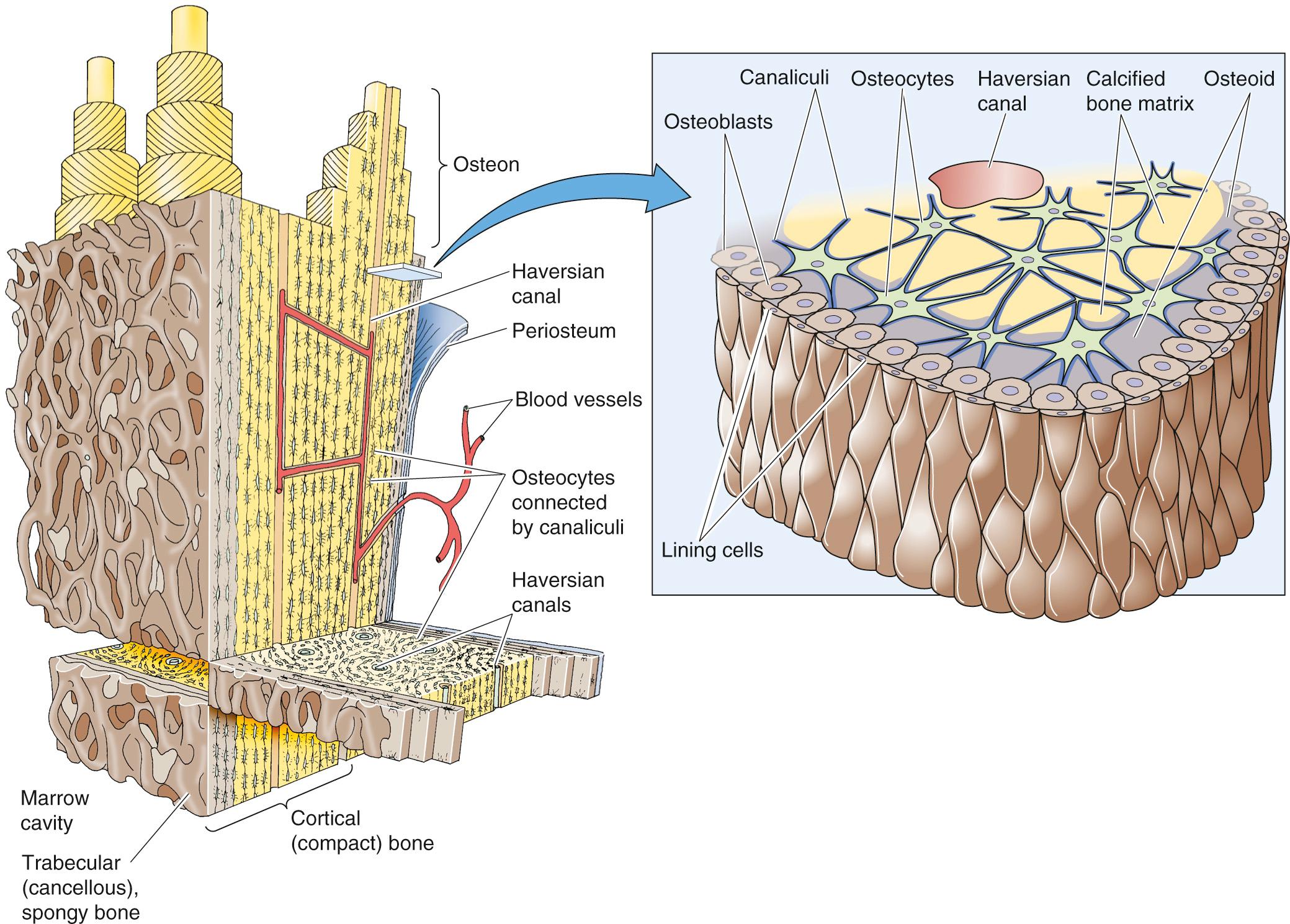
Trabecular (or cancellous or medullary ) bone constitutes ~20% of the total bone mass. It is found in the interior of bones and is especially prominent within the vertebral bodies. It is composed of thin spicules of bone that extend from the cortex into the medullary cavity (see Fig. 52-3 , inset). The lacework of bone spicules is lined in many areas by osteoblasts and osteoclasts, the cells involved in bone remodeling. Trabecular bone is constantly being synthesized and resorbed by these cellular elements. Bone turnover also occurs in cortical bone, but the fractional rate of turnover is much lower. When the rate of bone resorption exceeds that of synthesis over time, the loss of bone mineral produces the disease osteoporosis.
Collagen and the other extracellular matrix proteins that form the protein matrix of bone are called osteoid. Osteoid provides sites for the nucleation of hydroxyapatite crystals, the mineral component of bone. Osteoid is not a single compound, but a highly organized matrix of proteins synthesized principally by osteoblasts. Type I collagen accounts for ~90% of the protein mass of osteoid. It comprises a triple helix of two α1 monomers and one α2 collagen monomer. While still within the osteoblast, monomers self-associate into helical structures. After secretion from the osteoblast, helices associate into collagen fibers; cross-linking of collagen occurs both within a fiber and between fibers. Collagen fibers are arranged in the osteoid in a highly ordered manner. The organization of collagen fibers is important for the tensile strength (i.e., the ability to resist stretch or bending) of bone. In addition to providing tensile strength, collagen also acts as a nidus for nucleation of bone mineralization. Within the collagen fibers, the crystals of hydroxyapatite are arranged with their long axis aligned with the long axis of the collagen fibers.
Several other osteoblast-derived proteins are important to the mineralization process, including osteocalcin and osteonectin. Osteocalcin is a 6-kDa protein synthesized by osteoblasts at sites of new bone formation. 1,25-Dihydroxyvitamin D induces the synthesis of osteocalcin. Osteocalcin has an unusual structure: it possesses three γ-carboxylated glutamic acid residues. These residues are formed by post-translational modification of glutamic acid by vitamin K–dependent enzymes. Like other proteins with γ-carboxylated glutamic acid, osteocalcin binds Ca 2+ avidly. It binds hydroxyapatite, the crystalline mineral of bone, with even greater avidity. This observation has led to the suggestion that osteocalcin participates in the nucleation of bone mineralization at the crystal surface. Osteonectin, a 35-kDa protein, is another osteoblast product that binds to hydroxyapatite. It also binds to collagen fibers and facilitates the mineralization of collagen fibers in vitro. Additional proteins have been identified that appear to participate in the mineralization process. For instance, extracellular glycoproteins present in bone may inhibit mineralization and their removal may be necessary for mineralization to occur.
In addition to providing the proteins in osteoid, osteoblasts promote mineralization by exporting Ca 2+ and  from intracellular vesicles that have accumulated these minerals. Exocytosis of Ca 2+ and
from intracellular vesicles that have accumulated these minerals. Exocytosis of Ca 2+ and  raises the local extracellular concentration of these ions around the osteoblast to levels that are higher than in the bulk ECF, which promotes crystal nucleation and growth ( Fig. 52-4 ). Bone formation along spicules of trabecular bone occurs predominantly at sites of previous resorption by osteoclasts. The processes of bone resorption and synthesis are thus spatially coupled within an active basic multicellular unit (BMU). In adults, 1 to 2 million BMUs actively remodel bone.
raises the local extracellular concentration of these ions around the osteoblast to levels that are higher than in the bulk ECF, which promotes crystal nucleation and growth ( Fig. 52-4 ). Bone formation along spicules of trabecular bone occurs predominantly at sites of previous resorption by osteoclasts. The processes of bone resorption and synthesis are thus spatially coupled within an active basic multicellular unit (BMU). In adults, 1 to 2 million BMUs actively remodel bone.
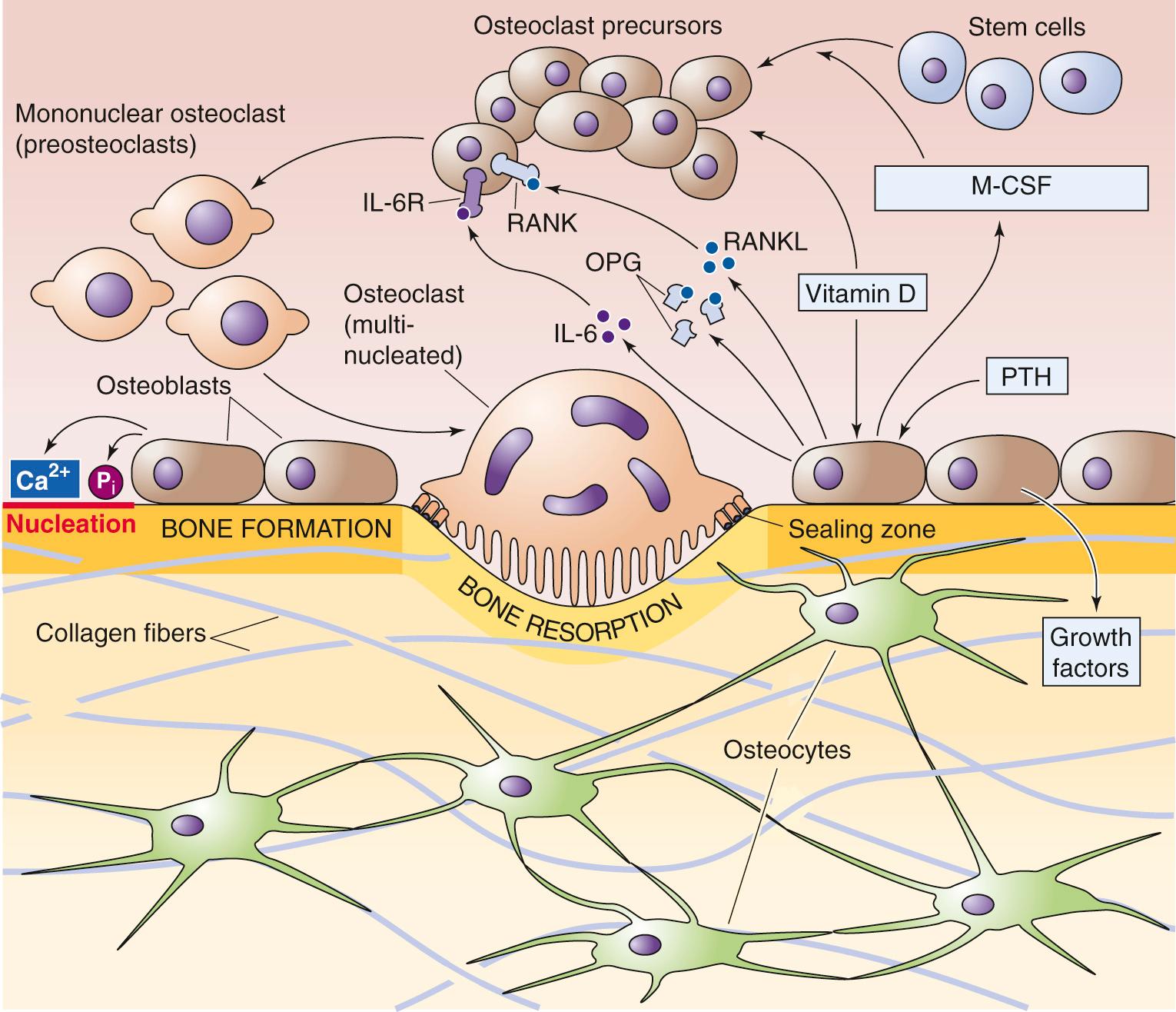
Vitamin D and PTH stimulate osteoblastic cells to secrete factors—such as macrophage colony-stimulating factor (M-CSF; see p. 431 )—that cause osteoclast precursors to proliferate (see Fig. 52-4 ). These precursors differentiate into mononuclear osteoclasts and then, with further stimulation by RANK ligand (also released by PTH-stimulated osteoblasts), fuse to become multinucleated osteoclasts. Osteoclasts resorb bone in discrete areas in contact with the “ruffled border” of the cell ( Fig. 52-5 ). The osteoclast closely attaches to the bone matrix when integrins on its membrane attach to vitronectin in the bone matrix. The osteoclast—in reality a one-cell epithelium—then secretes acid and proteases across its ruffled border membrane into a confined resorption space (the lacuna). The acid secretion is mediated by a V-type H pump (see pp. 118–119 ) and the ClC-7 Cl − channel at the ruffled border membrane. Abundant intracellular carbonic anhydrase provides the H + . Cl-HCO 3 exchangers, located in the membrane that faces the blood, remove the  formed as a byproduct by the carbonic anhydrase. The acidic environment beneath the osteoclast dissolves bone mineral and allows acid proteases to hydrolyze the exposed matrix proteins. Having reabsorbed some of the bone in a very localized area, the osteoclast moves away from the pit or trough in the bone that it has created. Osteoblastic cells replace the osteoclast and now build new bone matrix and promote its mineralization.
formed as a byproduct by the carbonic anhydrase. The acidic environment beneath the osteoclast dissolves bone mineral and allows acid proteases to hydrolyze the exposed matrix proteins. Having reabsorbed some of the bone in a very localized area, the osteoclast moves away from the pit or trough in the bone that it has created. Osteoblastic cells replace the osteoclast and now build new bone matrix and promote its mineralization.
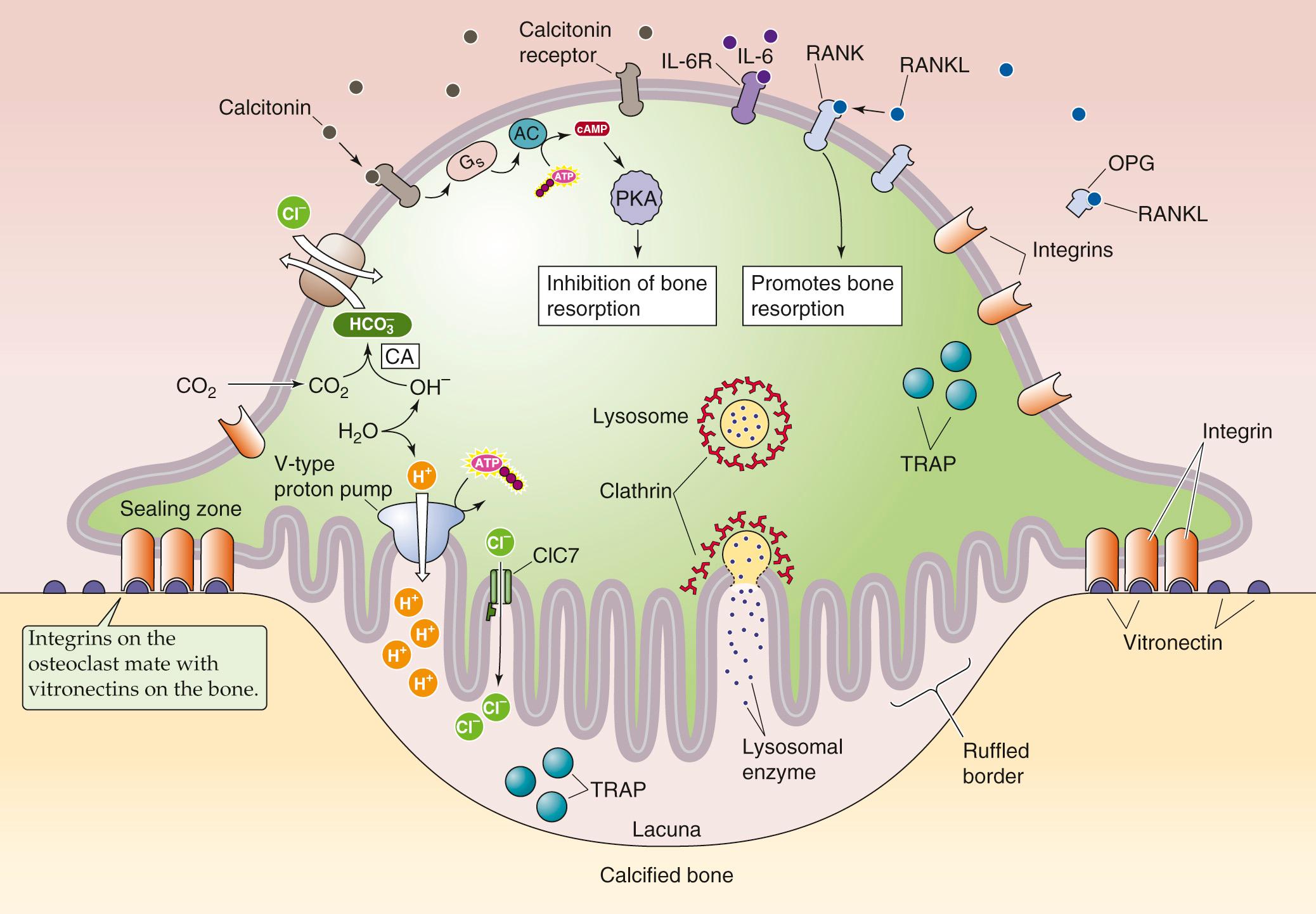
RANK ligand (RANKL), previously called osteoprotegerin ligand, appears to be a major stimulator of both the differentiation of preosteoclasts to osteoclasts (see Fig. 52-4 ) and the activity of mature osteoclasts (see Fig. 52-5 ). RANKL is a member of the tumor necrosis factor (TNF) cytokine family and exists both as a membrane-bound form (mRANKL) on the surface of stromal cells and osteoblasts, and as a soluble protein (sRANKL) secreted by these same cells. RANKL binds to and stimulates a membrane-bound receptor of the osteoclast called RANK (receptor for activation of nuclear factor κB), a member of the TNF receptor family. The interaction is essential for the formation of mature osteoclasts.
The activity of RANKL is under the control of a soluble member of the TNF receptor family called osteoprotegerin ( OPG; from the Latin osteo [bone] + protegere [to protect]). Like RANKL, OPG is produced by osteoblastic and stromal cells (see Fig. 52-4 ). By scavenging RANKL, OPG limits osteoclastogenesis, thereby protecting bone from osteoclastic activity. The precise role of RANKL, RANK, and OPG in the development of various forms of osteoporosis (diminished bone density) and osteopetrosis (increased bone density) is only beginning to be understood. However, the balance between OPG and RANKL production by the osteoblast/stromal cell appears to be a very important factor in the development of osteoporosis from either estrogen deficiency or glucocorticoid excess. In both cases, RANKL production rises and OPG production falls. In 2010, the U.S. Food and Drug Administration approved denosumab, a humanized monoclonal antibody against RANKL, for the treatment of postmenopausal osteoporosis.
In rare human monogenic syndromes, defects of the Wnt signaling system are associated with marked increases or decreases in bone mass. Moreover, targeted disruption of specific Wnt antagonists in mice reveals the major role of Wnt in osteoblastogenesis and in modulation of the activity of both osteoblasts and osteocytes. Wnt increases the differentiation of mesenchymal stem cells and preosteoblasts, thereby increasing bone-forming capacity. In addition, Wnt increases the production of OPG, which competes with RANKL and thereby decreases osteoclastogenesis. New therapies to treat or prevent bone loss based on manipulation of Wnt signaling offer substantial promise.
Humans have four parathyroid glands, two located on the posterior surface of the left lobe of the thyroid and two more on the right. Combined, these four glands weigh <500 mg. They are composed largely of chief cells, which are responsible for the synthesis and secretion of PTH. These cells, like cells that secrete other peptide hormones, are highly specialized to synthesize, process, and secrete their product. The major regulator of PTH secretion is ionized plasma Ca 2+ , although vitamin D also plays a role. Both inhibit the synthesis or release of PTH. In contrast, an increase in plasma phosphorus concentration stimulates PTH release.
The PTH gene possesses upstream regulatory elements in the 5′ region, including response elements for both vitamins D and A (see p. 90 ). The vitamin D response element binds a vitamin D receptor (VDR) when the receptor is occupied by a vitamin D metabolite, usually 1,25-dihydroxyvitamin D. The VDR is a member of the family of nuclear receptors, like the steroid hormone and thyroid hormone receptors. Like the thyroid hormone receptor, VDR forms a heterodimer with the retinoid X receptor (RXR) and acts as a transcription factor (see Table 3-6 ). The receptor has a very high affinity for the 1,25-dihydroxylated form of vitamin D ( K D ≅ 10 −10 M), less affinity for the 25-hydroxy form ( K D ≅ 10 −7 M), and little affinity for the parent vitamin (either D 2 or D 3 , see below). Binding of the vitamin D–VDR complex to the VDR response element decreases the rate of PTH transcription.
After transport of the mature PTH messenger RNA (mRNA) to the cytosol, PTH is synthesized on ribosomes of the rough endoplasmic reticulum (RER) and begins its journey through the secretory pathway (see pp. 34–35 ). PTH is transcribed as a prepro-PTH of 115 amino acids ( Fig. 52-6 ). The 25–amino-acid “pre” fragment targets PTH for transport into the lumen of the RER. This signal sequence (see p. 28 ) appears to be cleaved as PTH enters the RER. During transit through the secretory pathway, the 90–amino-acid pro-PTH is further processed to the mature, active, 84–amino-acid PTH. This cleavage appears quite efficient, and no pro-PTH appears in the storage granules. Conversely, the breakdown of 1-84 PTH or “intact” PTH into its N- and C-terminal fragments—as discussed below—already starts in the secretory granules.
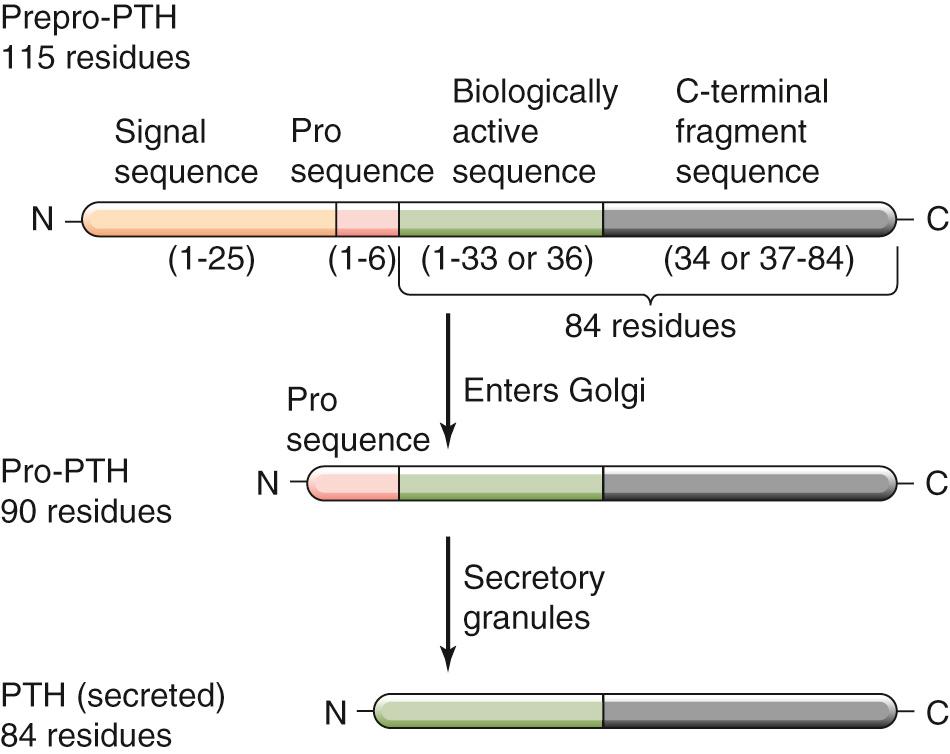
Once secreted, PTH circulates free in plasma and is rapidly metabolized; the half-life of 1-84 PTH is ~4 minutes. Beginning in the secretory granules inside the parathyroid chief cells and continuing in the circulation—predominantly in the liver—PTH is cleaved into two principal fragments, a 33– or 36–amino-acid N-terminal peptide and a larger C-terminal peptide (see Fig. 52-6 ). Virtually all the known biological activity of PTH resides in the N-terminal fragment, which is rapidly hydrolyzed, especially in the kidney. However, the half-life of the C-terminal fragment is much longer than that of either the N-terminal peptide or the intact 84–amino-acid PTH molecule. An estimated 70% to 80% of the PTH-derived peptide in the circulation is represented by the biologically inactive C-terminal fragment. The presence of a significant amount of antigenically recognized but biologically inactive C-terminal fragments in the circulation has complicated the use of the usual radioimmunoassay methods for measuring PTH in both clinical and experimental settings. This problem has been solved by the development of sensitive enzyme-linked immunosorbent assays (ELISAs) that use two antibodies that react with two distinct sites on the PTH molecule and measure only the intact PTH hormone (i.e., 84 amino acids). These assays are invaluable in the diagnosis of disorders of PTH secretion, particularly when kidney function is impaired (a circumstance that further prolongs the half-life of the inactive C-terminal metabolites). ![]() N52-1
N52-1
On pages 1059–1060 , we discuss the modern methods for assaying intact PTH. In the past, clinicians exploited the effects of PTH on renal phosphate transport and renal cAMP production to assay biologically active PTH levels. Both the renal threshold (T m ) for phosphate (see p. 786 and Fig. 36-15 ) and the rate of urinary excretion of cAMP are readily determined in humans and are indirect measures of plasma PTH levels. In the case of cAMP, one actually measures the excretion of “nephrogenous” cAMP; that is, the total urinary cAMP minus the amount of cAMP filtered in the glomeruli.
With the advent of the newer PTH assays, these tests have largely been discarded in clinical settings.
To a first approximation, and ignoring the contributions of vitamin D that are discussed below, regulation of PTH secretion by plasma Ca 2+ appears to be a simple negative-feedback loop. The major stimulus for PTH secretion is a decline in the concentration of Ca 2+ in the blood (hypocalcemia) and ECF. Hypocalcemia also stimulates synthesis of new PTH, which is necessary because the parathyroid gland contains only enough PTH to maintain a stimulated secretory response for several hours.
Using cultured parathyroid chief cells (which like the parathyroids in vivo respond to very small decreases in the concentration of ionized Ca 2+ ), investigators identified a Ca 2+ -sensing receptor (CaSR) that resides in the plasma membrane of the parathyroid cell ( Fig. 52-7 A ). This receptor binds Ca 2+ in a saturable manner, with an affinity profile that is similar to the concentration dependence for PTH secretion. CaSR is a member of the G protein–coupled receptor (GPCR) family (see pp. 51–66 ). Coupling of this Ca 2+ receptor to Gα q activates phospholipase C, which generates inositol 1,4,5-trisphosphate (IP 3 ) and diacylglycerol (DAG) and results in the release of Ca 2+ from internal stores and the activation of protein kinase C (PKC; see pp. 60–61 ). Unlike most endocrine tissues, in which activation of these signaling systems promotes a secretory response, in the parathyroid the rise in [Ca 2+ ] i and activation of PKC inhibit hormone secretion. (Another example is the granular cell of the juxtaglomerular apparatus, in which an increase in [Ca 2+ ] i inhibits secretion of renin; see p. 842 ). Thus, increasing levels of plasma [Ca 2+ ] decrease PTH secretion (see Fig. 52-7 B ).
![Figure 52-7, PTH secretion and its dependence on ionized Ca 2+ in the plasma. A, Four parathyroid glands lie on the posterior side of the thyroid. The chief cells synthesize, store, and secrete PTH. Increases in extracellular [Ca 2+ ] inhibit PTH secretion in the following manner: Ca 2+ binds to a receptor that is coupled to a heterotrimeric G protein, Gα q , which activates phospholipase C (PLC). This enzyme converts phosphoinositides (phosphatidylinositol 4,5-bisphosphate, or PIP 2 ) to IP 3 and DAGs. The IP 3 causes the release of Ca 2+ from internal stores, whereas the DAG stimulates PKC. Paradoxically, both the elevated [Ca 2+ ] i and the stimulated PKC inhibit release of granules containing PTH. Increased [Ca 2+ ] o also inhibits PTH synthesis. Thus, increased levels of plasma Ca 2+ lower PTH release and therefore tend to lower plasma [Ca 2+ ]. B, Small decreases in free plasma [Ca 2+ ] greatly increase the rate of PTH release. About half of the total plasma Ca 2+ is free. In patients with familial hypocalciuric hypercalcemia (FHH), the curve is shifted to the right; that is, plasma [Ca 2+ ] must rise to higher levels before inhibiting PTH secretion. As a result, these patients have normal PTH levels, but elevated plasma [Ca 2+ ]. ER, endoplasmic reticulum. Figure 52-7, PTH secretion and its dependence on ionized Ca 2+ in the plasma. A, Four parathyroid glands lie on the posterior side of the thyroid. The chief cells synthesize, store, and secrete PTH. Increases in extracellular [Ca 2+ ] inhibit PTH secretion in the following manner: Ca 2+ binds to a receptor that is coupled to a heterotrimeric G protein, Gα q , which activates phospholipase C (PLC). This enzyme converts phosphoinositides (phosphatidylinositol 4,5-bisphosphate, or PIP 2 ) to IP 3 and DAGs. The IP 3 causes the release of Ca 2+ from internal stores, whereas the DAG stimulates PKC. Paradoxically, both the elevated [Ca 2+ ] i and the stimulated PKC inhibit release of granules containing PTH. Increased [Ca 2+ ] o also inhibits PTH synthesis. Thus, increased levels of plasma Ca 2+ lower PTH release and therefore tend to lower plasma [Ca 2+ ]. B, Small decreases in free plasma [Ca 2+ ] greatly increase the rate of PTH release. About half of the total plasma Ca 2+ is free. In patients with familial hypocalciuric hypercalcemia (FHH), the curve is shifted to the right; that is, plasma [Ca 2+ ] must rise to higher levels before inhibiting PTH secretion. As a result, these patients have normal PTH levels, but elevated plasma [Ca 2+ ]. ER, endoplasmic reticulum.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TheParathyroidGlandsandVitaminD/6_3s20B9781455743773000525.jpg)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here