Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The mucosal lining of the uterus consists of glands, stroma, and blood vessels. The function of the endometrium is to form a receptive site for pregnancy. This is initially accomplished through a nutrient effect of the glands and their secretions on the blastocyst in the 24 hours or so before implantation takes place (on or about day 7 post ovulation). Once nidation has occurred, the relationship between the conceptus and its mother is primarily between extraembryonic trophoblast and the decidualized endometrial stroma. Glandular changes are the most easily observed and common pathologic conditions in the uterus.
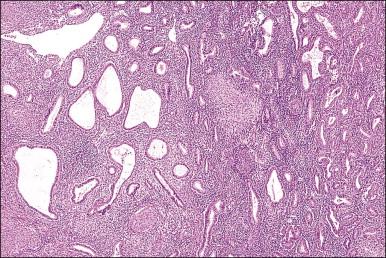
The endometrium merges with the mucosa of the fallopian tube at its upper extreme and with the endocervical epithelium at its lower end. The junction with the fallopian tube epithelium is usually abrupt, although the exact position may vary considerably. Uncommonly, endometrium may line the tube some centimeters lateral to the cornu, a condition referred to as ‘endometrialization’ (to be distinguished from endometriosis, Chapter 22 ). At the junction of the endometrium with the endocervical epithelium, however, there is a gradual transition from one type of mucosa to the other, sometimes over a distance of as much as 1 cm. This is the lower uterine segment, which contains glands with features between those of the endometrium proper and the endocervix ( Figure 14.1 ). Not uncommonly, endometrial glands will be found deep to the endocervical lining. Glands of the lower uterine segment show practically none of the morphologic effects that hormonal stimulation elicits in the fundus. Care must be taken to recognize this tissue for what it is, so that the inactivity in these pieces is not taken to mean that the endometrium as a whole is not being stimulated or is not responding. Glands in this area are prone to be partly lined by epithelium containing a mixture of undistinguished columnar cells admixed with ciliated cells. The stroma of the lower uterine segment differs from that of the endometrium proper in being rather more fibrous and displaying cells that are generally more spindled. The glands are typically flattened and slit-like and the epithelial cells lack mucus.
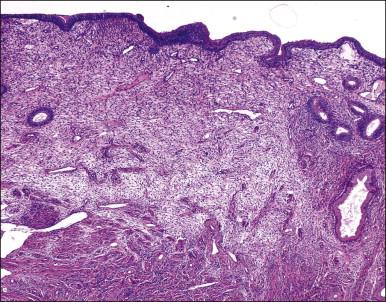
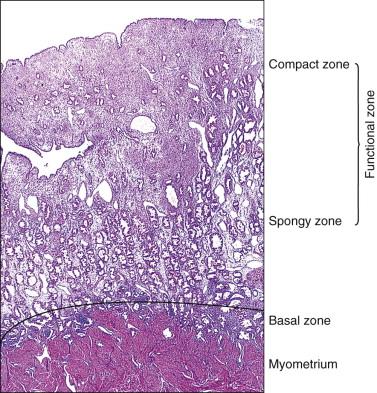
The endometrium from the uterine body and fundus is generally fairly uniform from one area to another. There is, however, variation within the endometrial thickness depending on the vertical position of the tissue in relation to the surface epithelium and the endometrial–myometrial junction ( Figure 14.2 ). These layers become more pronounced as the menstrual cycle progresses ( Figure 14.3 ). The basal layer (stratum basalis) is adjacent to the myometrium, and consists of tubular glands, occasionally branching, lined by simple to pseudostratified epithelium in a more basophilic, compact stroma ( Figure 14.4 ). The glandular epithelium shows no evidence of secretory activity whatever the phase of the cycle, and there is no or minimal mitotic activity in either glands or stroma. As the overall volume of glands is small compared with that in the functional layer, the stroma is relatively more prominent. The stroma also appears more cellular as it is composed of largely spindled nuclei with only scant, inapparent cytoplasm.
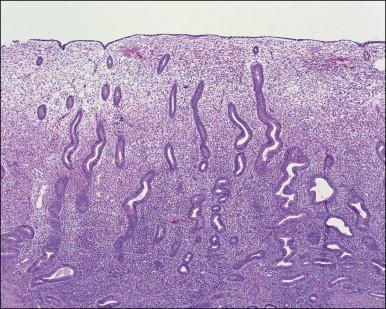
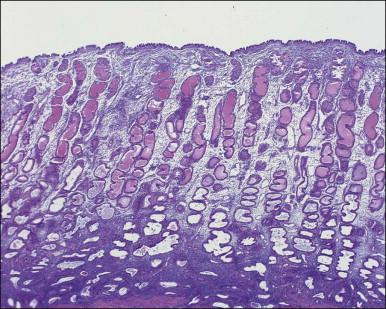
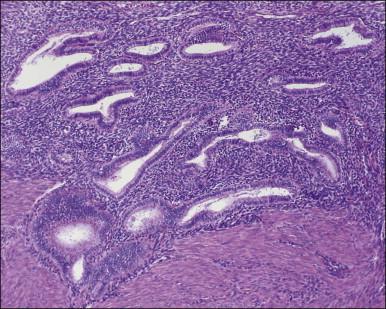
The remaining endometrium is the functional zone (stratum functionalis), which is further subdivided into the superficial compact layer (stratum compactum) and the deeper spongy layer (stratum spongiosum). This distinction only becomes striking in the late secretory (postovulatory or luteal) phase ( Figure 14.5 ). At that time the spongy layer consists of glands showing maximal secretory activity but a relatively unresponsive stroma that does not develop a good predecidual response apart from the immediate vicinity of the spiral arterioles. Stroma in the more superficial compact layer, on the other hand, responds remarkably to hormonal stimulation with a prominent predecidual reaction and numerous granulated lymphocytes (see later). Glands in this zone are stretched thin by the expanding stroma, and demonstrate less secretory activity. It is apparent therefore that the morphology of the endometrial stromal and glandular cells is a function not only of the systemic hormonal environment, but also of the position in the corpus or lower uterine segment, and vertical location within the endometrial layers. As material from all of these layers routinely appears in curettings, the pathologist must be aware of characteristic appearances at all sites throughout the menstrual cycle.
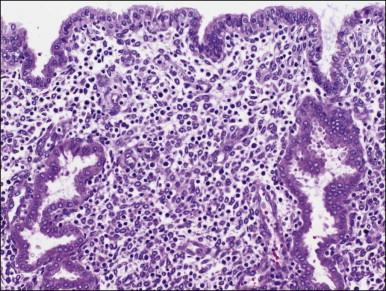
The surface epithelium of the endometrium is continuous with the glandular epithelium and is generally similar. However, the constituent cells show less marked cyclical variation than the cells in the glands ( Figure 14.6 ), responding relatively weakly to circulating sex steroids, and are frequently ciliated. Although subnuclear vacuolation and mitotic activity are seen, these features do not always accurately reflect the time of the cycle. There is usually no problem in identifying surface epithelium in curettings, but the few small epithelial strips that may constitute an entire biopsy sample in an atrophic endometrium can be difficult if not impossible to characterize with any degree of certainty.
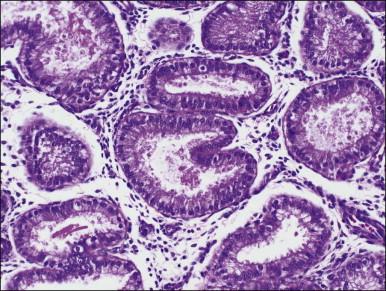
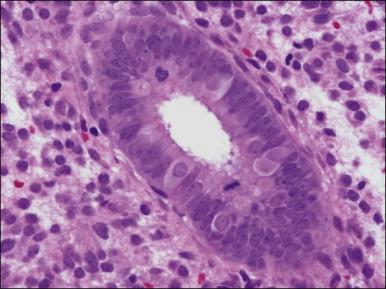
There are three types of endometrial glandular cells: the secretory cell ( Figure 14.7 ), the ciliated cell ( Figure 14.8 ), and the clear cell ( Figure 14.9 ).
The secretory cells are by far the most abundant and their morphology varies with the time of the menstrual cycle. The various appearances will be covered when the phases of the cycle are described.
The ciliated cells are more frequent near the cornua and toward the endocervix as well as being quite common in the surface epithelium. Although a normal constituent of the endometrium, the ciliated cells are particularly under the influence of estrogens and become more prominent in conditions of estrogen excess (e.g., anovulatory cycles). As ciliated cells are so common, they must be considered as normal and not as ‘ciliated metaplasia’ as described elsewhere in the literature. Furthermore, in specimens, where only strips of cells are produced, the presence of cilia is useful to suspect that an estrogenic milieu is present whether from endogenous or exogenous sources and that the specimen is not atrophic.
The clear cells are much less common and are thought to be precursors of the ciliated cells. They are most frequently seen in the proliferative phase.
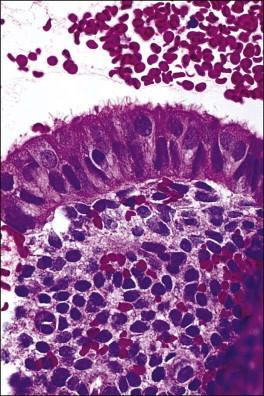
The morphology of the endometrial stromal cells varies dramatically throughout the menstrual cycle. During the proliferative phase, the stromal cells are small and mostly compact, with oval, hyperchromatic nuclei and inapparent cytoplasm. In the mid-proliferative phase, at the preovulatory peak of serum estrogen levels, stromal cells are separated by increased intercellular edema. At the end of the proliferative phase the nuclei become slightly larger and their chromatin a little less dense ( Figure 14.10 ). The stroma again becomes edematous in the middle of the secretory phase, reaching a peak at about the 22nd day of a normalized 28 day cycle (again due to an estrogen peak), after which the cells of the compact zone progressively undergo predecidual change, developing into polygonal cells with vesicular nuclei and abundant, pale cytoplasm with well-defined cell borders ( Figure 14.11 ) The terms ‘predecidua’ and ‘pseudodecidua’ are often used to describe this change in the morphology of the stromal cells as a response to endogenous and exogenous progesterone, respectively. The term ‘decidua’ is properly reserved for the change seen in pregnancy, but in practice many pathologists use the terms interchangeably. While it is true that the morphologic changes in the cells are qualitatively the same whether it has been brought about by physiologic levels of progesterone or by synthetic progestins (as in oral contraceptives), there usually is a quantitative difference in both the amount of cytoplasm present in any given cell and the proportion of stromal cells with the change. In general, decidual change is more extensive and uniform in pregnancy and in patients receiving exogenous progestins ( Figure 14.12 ), whereas in premenstrual endometrium a significant proportion of the cells are only minimally or partially affected (compare with Figure 14.11 ). Decidual change is first apparent adjacent to the spiral arterioles but toward the end of the secretory phase and in pregnancy the change becomes diffuse. The stromal cells that are situated deeper in the endometrium, lying between the active glands of the spongy layer, show little, if any, decidual change and remain fairly nondescript ( Figure 14.13 ). Occasionally, small bundles of smooth muscle may be found in the endometrial stroma ( Figure 14.14 ). The decidual cells of the endometrial stroma, together with an exponential increase in granulated lymphocytes and natural killer (NK) cells, and the synthesis of a variety of cytokines and extracellular matrix proteins including laminin and fibronectin play a central role in the process of nidation (see Chapter 32 ).
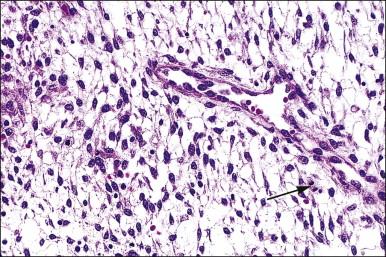
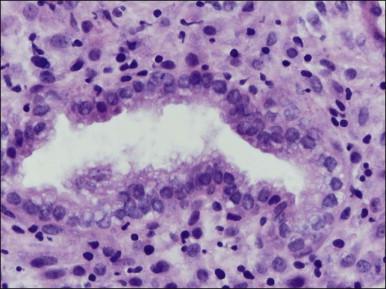
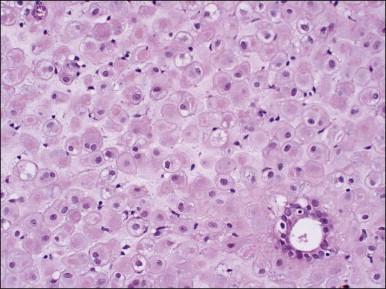
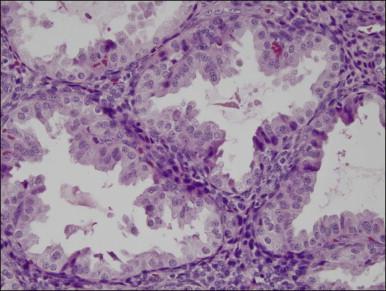
Several subpopulations of T-lymphocytes reside in the normal endometrium, and small lymphoid aggregates are normal, particularly in the basal zone ( Figure 14.15 ). CD4+ and CD8+ T-cells are randomly scattered throughout the functional layer and, in the absence of an inflammatory process, show only a modest variation in density throughout the menstrual cycle, increasing toward menstruation. In any form of endometritis, they aggregate near and around glands and can be seen within the gland lumens along with CD68+ or CD163+ macrophages. Macrophages are normally found in all areas of the functional and basal zones.
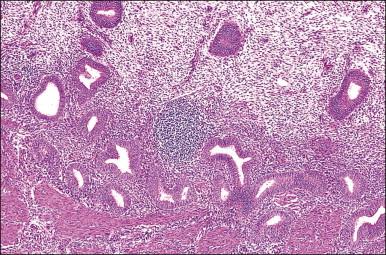
In contrast, small, rounded cells with hyperchromatic nuclei that are usually kidney-shaped or segmented increase in number in the endometrial stroma in the second half of the cycle ( Figures 14.16 and 14.17 ). These cells are known as endometrial granulocytes, endometrial granular cells, or K-cells. The cytoplasm contains eosinophilic granules of variable size, so that the cells are also often mistaken for infiltrating polymorphonuclear leukocytes, particularly in the presence of early or imminent menstrual fragmentation (neutrophils are not seen in a normal endometrium until menstruation is well established and their presence at other times of the cycle indicates inflammation). These cells are large granulated lymphocytes that, on flow cytometric analysis, exhibit the unusual T-cell phenotype CD56+, CD3−, CD16−, and also have an NK function. Endometrial NK cells increase in number dramatically in the late secretory phase of the menstrual cycle, sometimes reaching a population density exceeding 25% of all stromal cells. If conception and implantation occur, they continue to increase in number and make up about 70% of the stromal lymphocytes in the first trimester of pregnancy. Endometrial and decidual NK cells are distinct from other classes of NK cells, having both a major immune regulatory function and a critical role in remodeling of the endometrium after implantation. Our expanding understanding of the important role of decidual NK cells is leading to the possibility of infertility therapies aimed at disorders in their function.


In comparison to T-lymphocytes, B-lymphocytes are normally sparse in the endometrium. Whether rare plasma cells can be seen in normal endometrium remains a controversial topic, as observations differ according to the population studied and detection method employed. One of the few studies to rigorously select normal women used a sensitive methyl-green pyronine stain to identify small numbers of plasma cells in about one-third of normal endometria.
The arterial supply of the endometrium is from the radial arteries that arise from the arcuate arteries in the myometrium. The radial arteries branch near the endometrial–myometrial interface, forming the basal arteries. As these ascend through the functionalis to the endometrial surface, they become the spiral arterioles ( Figure 14.18 ). The spiral arterioles respond to the varying levels of ovarian hormones and become prominent in the second half of the secretory phase, under the influence of progesterone. Coiling becomes most pronounced when the stromal edema is reabsorbed prior to menstruation. In the proliferative phase, the arterioles show little coiling and are confined to the deeper levels of the functionalis. There is an irregular network of venous channels with the veins frequently intersecting, forming venous lakes. Lymphatic vessels are present in normal endometrium, but disappear in decidualized endometrium during pregnancy.

The endometrium undergoes a complex and orchestrated series of changes during menstrual cycle progression that when properly interpreted can provide useful information about the patient's hormonal state. The main features throughout the cycle are summarized and illustrated in Figure 14.19 . The endometrial cycle is renewed with menses, and then customarily divided into two sequential phases, the proliferative (preovulatory or follicular) and the secretory (postovulatory or luteal) phases. This division of the cycle is related, of course, to the hormones stimulating it, with estrogen predominating in the proliferative phase and progesterone in the secretory phase. The endometrial cycle is often standardized in pathology reports as an idealized 28 day cycle beginning on the first day of clinical menses, followed by a proliferative phase with ovulation on day 14. Physiologic differences in total cycle length between women are generally caused by variation in the duration of menses and the proliferative phase, as the postovulatory temporal sequence is a remarkably consistent 14 days. In dating the endometrium it is important to remember that there is a delay of 2–3 days between ovulation and appearance of diagnostic postovulatory histologic changes. This is caused by progressively rising progesterone levels and time necessary for the endometrium to respond. Thus, although clinical ovulation is assumed to occur on day 14, there is a brief ‘interval phase’ corresponding to days 15–16, before definitive progestational effects are evident.

In the following description we summarize histopathologic endometrial features seen on particular days according to an idealized 28 day cycle (e.g., secretory endometrium, 24 days), starting with the first day of menses. The more physiologic approach of directly referencing the date of ovulation (e.g., postovulatory day 10) is an alternative.
If conception and implantation have not occurred by day 24 (postovulatory day 10), the corpus luteum involutes, leading to a marked fall in progesterone output. The late secretory phase then leads inevitably to the menstrual phase, which defines cycle day 1. This is recognized histologically by very fine crumbling or fragmentation of the stroma and glandular collapse ( Figure 14.20 ), with abundant blood and neutrophils in the background. Two very characteristic changes, especially when seen in combination, are compact balls of stromal cells which, with hematoxylin, have an unusually deep blue color ( Figure 14.21 ), and which are covered by a layer of epithelial cells that appear eosinophilic and reactive. A third change, often seen, is the presence of plump epithelial glandular cells that are frequently multilayered, the so-called ‘papillary syncytial metaplasia’ ( Figure 14.22 ).



The appearances of menstrual endometrium can confuse inexperienced pathologists because the stromal crumbling results in irregular, collapsing glands often coming together to give a ‘back-to-back’ pattern that may be misinterpreted as hyperplasia or even malignancy. Attention to the rest of the material on the slide will show the remains of secretory glands and, perhaps, some areas where the stroma remains intact and shows predecidual change ( Figures 14.21 and 14.23 ). In addition, the highly cellular stromal balls are occasionally misinterpreted as small cell carcinoma of either endometrium or cervix due to the very high nuclear to cytoplasmic ratios and prominent nuclear molding and apoptosis.

Histologic examination of curettings taken during menstruation is usually uninformative, as architecture is obscured by fragmentation and the true morphology of the epithelium is replaced by the degenerative changes that take place very rapidly once menstruation commences. From the configuration of the larger intact fragments, and the appearances of the glandular cells, especially by the presence of secretory change, it is often possible to indicate whether the cycle just finishing has been ovulatory. Mitotic figures are not seen at the time of menstruation, but some residual stromal aggregates remaining after cessation of clinical bleeding may coexist with occasional mitoses during transition to the proliferative phase. In general, if the curettage is undertaken to diagnose a suspected underlying hormonal abnormality or cause of infertility, it is best performed when intact tissue can be obtained, outside of menses. On the other hand, if a woman has abnormal bleeding, then curettage can be done while bleeding is occurring.
Menstruation follows a chain of events that begins with involution of the corpus luteum. Falling levels of estradiol and progesterone have direct effects upon vascular, stromal, and glandular elements, which then develop a secondary cascade of dynamic interactions. Stromal breakdown, the pathognomonic histologic feature of menstruation, is initiated by the progesterone drop, which forces stromal cells into apoptosis. At the same time, intracapillary and intravenular hydrostatic pressure declines, with reabsorption of stromal edema fluid and a rapid decrease in the endometrial thickness. Spiral arterioles, which are structured as irregular spirals, collapse and kink, resulting in ischemia of the overlying endometrial tissue. Thrombus formation is not part of the normal process. About 20 hours after menstruation starts, intense prostaglandin-induced vasoconstriction controls the blood loss.
It follows from this outline that true menstruation cannot occur in the absence of ovulation. The complete process depends on falling levels of both estrogen and progesterone. From a morphologic point of view, fragments of endometrial epithelium showing secretory changes are diagnostic of ovulation, and these samples may be designated ‘menstrual endometrium, ovulatory type.’ By late menses, even in postovulatory patients, these glands with diagnostic secretory changes may already be completely passed and it may no longer be possible to confirm ovulation. These can be diagnosed as ‘late menstrual endometrium,’ with a note or qualifier that ovulation cannot be confirmed in the late phase of shedding. One clue of a preceding anovulatory cycle is the presence of fibrin thrombi, not normally seen in a true menstrual phase endometrium, but in itself it is not diagnostic.
The remaining basal layer and a variable, narrow band of the spongy zone is then ready to begin proliferating again at the start of the next cycle. In fact, it is usually possible to appreciate an overlap between the menstrual breakdown and beginning proliferation; curettings taken after menstruation is well established will often contain crumbling tissue, intact basal endometrium, and early proliferating endometrium. A further feature of the endometrium at menstruation is the presence of a neutrophil polymorph infiltrate, which is seen as a response to necrotic tissue and does not indicate acute infection.
This part of the cycle generally lasts for 11–12 days, but may vary considerably between patients with cycles of differing lengths. Proliferative activity commences before menstrual bleeding from the previous cycle has finished, when the endometrium is thin with sparse, small, straight glands and a loose stroma of spindled cells. At 8–10 days, the endometrial thickness increases, mainly as a result of stromal edema induced by estrogen ( Figure 14.24 ), which reaches a peak at about the 10th day. Continued growth of the glands overtakes that of the stroma, so that they become slightly tortuous. This process becomes more exaggerated close to the time of ovulation ( Figure 14.25 ). Glandular epithelium takes on a ‘pseudostratified’ appearance, with nuclei staggered at various heights in the cell, although most are in the basal half. The epithelial cells have smooth, sharp luminal borders and basophilic or amphophilic cytoplasm ( Figure 14.26 ). Mitoses are frequent in both glands and stroma, and are a direct result of stimulation by estrogen. Accurate dating of the proliferative endometrium as early, mid, or late proliferative is rarely possible using morphologic criteria or, indeed, necessary, as in most cases a diagnosis of ‘proliferative endometrium’ is sufficient.



Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here